Abstract
To assess the interaction of human casein kinase II (CKII) with the heat shock protein 90 (HSP90) class of chaperone proteins, human CKII alpha and beta subunits and beta S2A mutant were expressed and purified separately or from a tandem coexpression construct in Escherichia coli. Recombinant human HSP90 beta and recombinant yeast HSP90 as His6 constructs were also expressed in and purified from E. coli. The rhCKII S2A mutant removed the regulatory beta subunit autophosphorylation site but had no effect on catalytic efficiency with peptide or protein substrates. As a CKII substrate, recombinant hHSP90 beta displayed a Km of 9.8 microM and a kcat of 4.1 min-1 and was phosphorylated to 1.5 mol/mol, whereas ryHSP90, lacking the known serine CKII sites of hHSP90, was phosphorylated at a 19-fold lower kcat/Km ratio to levels of 0.8 mol/mol. The endoplasmic reticulum HSP90 family member Grp94 was phosphorylated to 1.4 mol/mol but, in contrast, HSC70 and FKBP25 chaperones were phosphorylated to < 0.01 mol/mol. Neither phospho nor dephospho forms of hHSP90 showed significant activation of CKII toward the peptide substrate RRREEETEEE in contrast to a previous report that activation was observed at high molar ratios of chaperone to kinase.
Full text
PDF

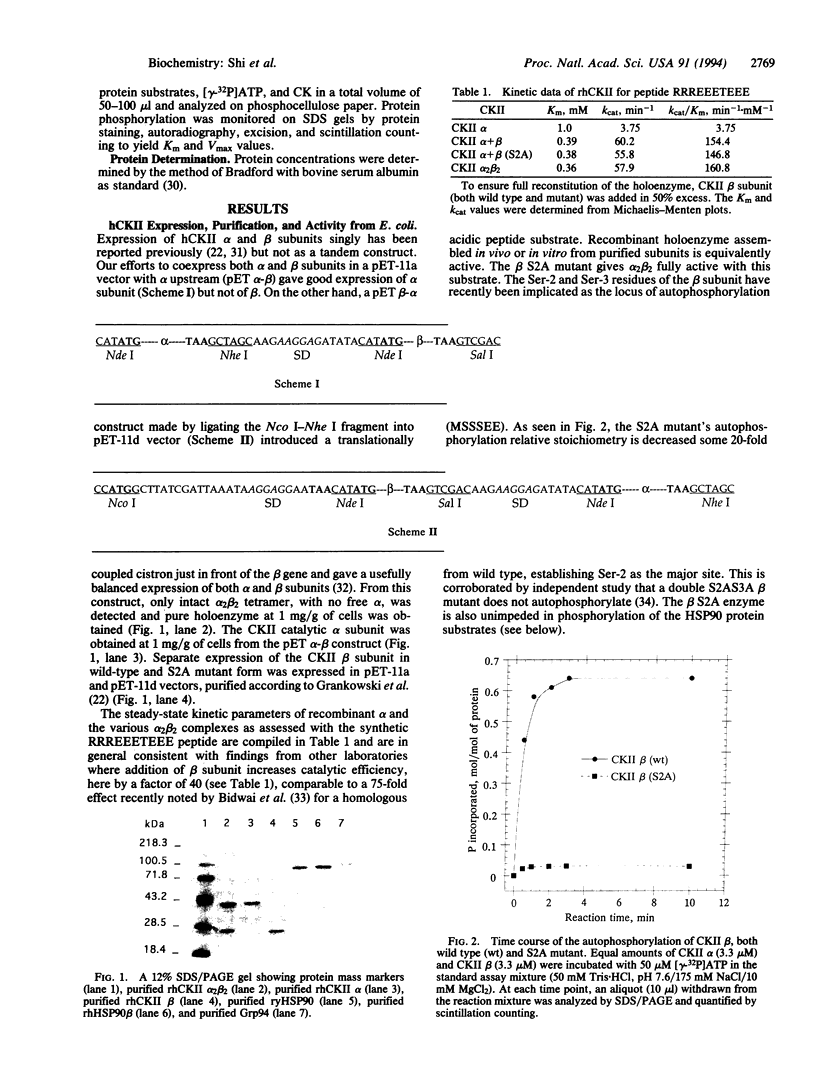


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidwai A. P., Hanna D. E., Glover C. V. Purification and characterization of casein kinase II (CKII) from delta cka1 delta cka2 Saccharomyces cerevisiae rescued by Drosophila CKII subunits. The free catalytic subunit of casein kinase II is not toxic in vivo. J Biol Chem. 1992 Sep 15;267(26):18790–18796. [PubMed] [Google Scholar]
- Bidwai A. P., Reed J. C., Glover C. V. Phosphorylation of calmodulin by the catalytic subunit of casein kinase II is inhibited by the regulatory subunit. Arch Biochem Biophys. 1993 Jan;300(1):265–270. doi: 10.1006/abbi.1993.1037. [DOI] [PubMed] [Google Scholar]
- Boldyreff B., Meggio F., Pinna L. A., Issinger O. G. Casein kinase-2 structure-function relationship: creation of a set of mutants of the beta subunit that variably surrogate the wildtype beta subunit function. Biochem Biophys Res Commun. 1992 Oct 15;188(1):228–234. doi: 10.1016/0006-291x(92)92374-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Callebaut I., Renoir J. M., Lebeau M. C., Massol N., Burny A., Baulieu E. E., Mornon J. P. An immunophilin that binds M(r) 90,000 heat shock protein: main structural features of a mammalian p59 protein. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet C., Chambaz E. M. Oligomeric structure and catalytic activity of G type casein kinase. Isolation of the two subunits and renaturation experiments. J Biol Chem. 1983 Feb 10;258(3):1403–1406. [PubMed] [Google Scholar]
- Dougherty J. J., Rabideau D. A., Iannotti A. M., Sullivan W. P., Toft D. O. Identification of the 90 kDa substrate of rat liver type II casein kinase with the heat shock protein which binds steroid receptors. Biochim Biophys Acta. 1987 Jan 19;927(1):74–80. doi: 10.1016/0167-4889(87)90067-x. [DOI] [PubMed] [Google Scholar]
- Farrelly F. W., Finkelstein D. B. Complete sequence of the heat shock-inducible HSP90 gene of Saccharomyces cerevisiae. J Biol Chem. 1984 May 10;259(9):5745–5751. [PubMed] [Google Scholar]
- Filhol O., Cochet C., Chambaz E. M. Cytoplasmic and nuclear distribution of casein kinase II: characterization of the enzyme uptake by bovine adrenocortical nuclear preparation. Biochemistry. 1990 Oct 23;29(42):9928–9936. doi: 10.1021/bi00494a025. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Protein folding in the cell. Nature. 1992 Jan 2;355(6355):33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grankowski N., Boldyreff B., Issinger O. G. Isolation and characterization of recombinant human casein kinase II subunits alpha and beta from bacteria. Eur J Biochem. 1991 May 23;198(1):25–30. doi: 10.1111/j.1432-1033.1991.tb15982.x. [DOI] [PubMed] [Google Scholar]
- Heller-Harrison R. A., Meisner H., Czech M. P. Cloning and characterization of a cDNA encoding the beta subunit of human casein kinase II. Biochemistry. 1989 Nov 14;28(23):9053–9058. doi: 10.1021/bi00449a014. [DOI] [PubMed] [Google Scholar]
- Hinrichs M. V., Jedlicki A., Tellez R., Pongor S., Gatica M., Allende C. C., Allende J. E. Activity of recombinant alpha and beta subunits of casein kinase II from Xenopus laevis. Biochemistry. 1993 Jul 20;32(28):7310–7316. doi: 10.1021/bi00079a030. [DOI] [PubMed] [Google Scholar]
- Jakobi R., Traugh J. A. Characterization of the phosphotransferase domain of casein kinase II by site-directed mutagenesis and expression in Escherichia coli. J Biol Chem. 1992 Nov 25;267(33):23894–23902. [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J., Bierer B. E. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992 Jun 5;267(16):10942–10945. [PubMed] [Google Scholar]
- Jin Y. J., Burakoff S. J. The 25-kDa FK506-binding protein is localized in the nucleus and associates with casein kinase II and nucleolin. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7769–7773. doi: 10.1073/pnas.90.16.7769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. S., Welch W. J. Characterization and purification of the 94-kDa glucose-regulated protein. J Biol Chem. 1991 Mar 25;266(9):5643–5649. [PubMed] [Google Scholar]
- Lees-Miller S. P., Anderson C. W. Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem. 1989 Feb 15;264(5):2431–2437. [PubMed] [Google Scholar]
- Lilie H., Lang K., Rudolph R., Buchner J. Prolyl isomerases catalyze antibody folding in vitro. Protein Sci. 1993 Sep;2(9):1490–1496. doi: 10.1002/pro.5560020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litchfield D. W., Lozeman F. J., Piening C., Sommercorn J., Takio K., Walsh K. A., Krebs E. G. Subunit structure of casein kinase II from bovine testis. Demonstration that the alpha and alpha' subunits are distinct polypeptides. J Biol Chem. 1990 May 5;265(13):7638–7644. [PubMed] [Google Scholar]
- Liu J., Albers M. W., Chen C. M., Schreiber S. L., Walsh C. T. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarella R. A., Green M. ERp99, an abundant, conserved glycoprotein of the endoplasmic reticulum, is homologous to the 90-kDa heat shock protein (hsp90) and the 94-kDa glucose regulated protein (GRP94). J Biol Chem. 1987 Jun 25;262(18):8875–8883. [PubMed] [Google Scholar]
- Meggio F., Brunati A. M., Pinna L. A. Autophosphorylation of type 2 casein kinase TS at both its alpha- and beta-subunits. Influence of different effectors. FEBS Lett. 1983 Aug 22;160(1-2):203–208. doi: 10.1016/0014-5793(83)80967-3. [DOI] [PubMed] [Google Scholar]
- Meggio F., Pinna L. A. Subunit structure and autophosphorylation mechanism of casein kinase-TS (type-2) from rat liver cytosol. Eur J Biochem. 1984 Dec 17;145(3):593–599. doi: 10.1111/j.1432-1033.1984.tb08598.x. [DOI] [PubMed] [Google Scholar]
- Meisner H., Heller-Harrison R., Buxton J., Czech M. P. Molecular cloning of the human casein kinase II alpha subunit. Biochemistry. 1989 May 2;28(9):4072–4076. doi: 10.1021/bi00435a066. [DOI] [PubMed] [Google Scholar]
- Melnick J., Aviel S., Argon Y. The endoplasmic reticulum stress protein GRP94, in addition to BiP, associates with unassembled immunoglobulin chains. J Biol Chem. 1992 Oct 25;267(30):21303–21306. [PubMed] [Google Scholar]
- Miyata Y., Yahara I. The 90-kDa heat shock protein, HSP90, binds and protects casein kinase II from self-aggregation and enhances its kinase activity. J Biol Chem. 1992 Apr 5;267(10):7042–7047. [PubMed] [Google Scholar]
- Nadeau K., Das A., Walsh C. T. Hsp90 chaperonins possess ATPase activity and bind heat shock transcription factors and peptidyl prolyl isomerases. J Biol Chem. 1993 Jan 15;268(2):1479–1487. [PubMed] [Google Scholar]
- Ozols J. Preparation of membrane fractions. Methods Enzymol. 1990;182:225–235. doi: 10.1016/0076-6879(90)82019-x. [DOI] [PubMed] [Google Scholar]
- Pei D., Neel B. G., Walsh C. T. Overexpression, purification, and characterization of SHPTP1, a Src homology 2-containing protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):1092–1096. doi: 10.1073/pnas.90.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna L. A. Casein kinase 2: an 'eminence grise' in cellular regulation? Biochim Biophys Acta. 1990 Sep 24;1054(3):267–284. doi: 10.1016/0167-4889(90)90098-x. [DOI] [PubMed] [Google Scholar]
- Pratt W. B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993 Oct 15;268(29):21455–21458. [PubMed] [Google Scholar]
- Price E. R., Zydowsky L. D., Jin M. J., Baker C. H., McKeon F. D., Walsh C. T. Human cyclophilin B: a second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebbe N. F., Ware J., Bertina R. M., Modrich P., Stafford D. W. Nucleotide sequence of a cDNA for a member of the human 90-kDa heat-shock protein family. Gene. 1987;53(2-3):235–245. doi: 10.1016/0378-1119(87)90012-6. [DOI] [PubMed] [Google Scholar]
- Renoir J. M., Radanyi C., Faber L. E., Baulieu E. E. The non-DNA-binding heterooligomeric form of mammalian steroid hormone receptors contains a hsp90-bound 59-kilodalton protein. J Biol Chem. 1990 Jun 25;265(18):10740–10745. [PubMed] [Google Scholar]
- Schoner B. E., Belagaje R. M., Schoner R. G. Enhanced translational efficiency with two-cistron expression system. Methods Enzymol. 1990;185:94–103. doi: 10.1016/0076-6879(90)85010-l. [DOI] [PubMed] [Google Scholar]
- Sommercorn J., Krebs E. G. Induction of casein kinase II during differentiation of 3T3-L1 cells. J Biol Chem. 1987 Mar 15;262(8):3839–3843. [PubMed] [Google Scholar]
- Szyszka R., Kramer G., Hardesty B. The phosphorylation state of the reticulocyte 90-kDa heat shock protein affects its ability to increase phosphorylation of peptide initiation factor 2 alpha subunit by the heme-sensitive kinase. Biochemistry. 1989 Feb 21;28(4):1435–1438. doi: 10.1021/bi00430a001. [DOI] [PubMed] [Google Scholar]
- Tai P. K., Albers M. W., Chang H., Faber L. E., Schreiber S. L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992 May 29;256(5061):1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- Tanasijevic M. J., Myers M. G., Jr, Thoma R. S., Crimmins D. L., White M. F., Sacks D. B. Phosphorylation of the insulin receptor substrate IRS-1 by casein kinase II. J Biol Chem. 1993 Aug 25;268(24):18157–18166. [PubMed] [Google Scholar]
- Van P. N., Peter F., Söling H. D. Four intracisternal calcium-binding glycoproteins from rat liver microsomes with high affinity for calcium. No indication for calsequestrin-like proteins in inositol 1,4,5-trisphosphate-sensitive calcium sequestering rat liver vesicles. J Biol Chem. 1989 Oct 15;264(29):17494–17501. [PubMed] [Google Scholar]
- Walsh C. T., Zydowsky L. D., McKeon F. D. Cyclosporin A, the cyclophilin class of peptidylprolyl isomerases, and blockade of T cell signal transduction. J Biol Chem. 1992 Jul 5;267(19):13115–13118. [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Purification of the major mammalian heat shock proteins. J Biol Chem. 1982 Dec 25;257(24):14949–14959. [PubMed] [Google Scholar]