Abstract
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is a multisystemic autosomal recessive disease due to primary thymidine phosphorylase (TP) deficiency. To restore TP activity, we performed reduced intensity allogeneic stem cell transplantations (alloSCTs) in two patients. In the first, alloSCT failed to engraft, but the second achieved mixed donor chimerism, which partially restored buffy coat TP activity and lowered plasma nucleosides. Thus, alloSCT can correct biochemical abnormalities in the blood of patients with MNGIE, but clinical efficacy remains unproven.
Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) is an autosomal recessive disease caused by mutations in the ECGF1 gene encoding thymidine phosphorylase (TP).1–3 The disease is characterized by ophthalmoplegia, gastrointestinal dysmotility, cachexia, peripheral neuropathy, and leukoencephalopathy and typically causes death in early to mid-adulthood.1,3,4 Laboratory studies reveal defects of the respiratory chain and mitochondrial DNA (mtDNA) alterations.1,3,4
In patients with MNGIE, complete or near total absence of TP activity (buffy coat TP [bcTP] 10 ± 15 [mean ± SD] nmol thymine/h/mg protein; normal control subjects 634 ± 217 nmol/h/mg) causes accumulations of its major substrates, thymidine (dThd) (patients 8.6 ± 3.4 µM; normal control subjects <0.05 µM) and deoxyuridine (dUrd) (patients 14.2 ± 4.4 µM; normal control subjects <0.05 µM).2,5,6 Elevated levels of these nucleosides may lead to nucleotide pool imbalances, which, in turn, cause instability of mtDNA.2,6 Reductions of dThd and dUrd may be therapeutic for MNGIE.6 We have successfully performed reduced intensity conditioning and allogeneic stem cell transplantations (alloSCTs) in adolescents and young adults with non-malignant diseases.7 To attempt to restore TP activity and eliminate circulating dThd and dUrd, we performed alloSCT in two patients with MNGIE.
Case reports
Patient 1, a 21-year-old man with consanguineous parents, developed weakness in his left first toe at age 18. Nerve conduction studies were consistent with a demyelinating peripheral neuropathy, and brain MRI revealed leukoencephalopathy. Two years later, he developed abdominal pain, early satiety, and recurrent vomiting and was found to have gastroparesis. The diagnosis of MNGIE was suspected and confirmed by deficiency of bcTP activity, elevated plasma dThd and dUrd, and a novel homozygous T3359C transition in the ECGF1 gene. The mutation changes amino acid 285 from leucine to proline and was absent in 100 control alleles.
The peripheral neuropathy and gastrointestinal dysmotility worsened, and by age 21, he was wheelchair bound and required parenteral nutrition. Examination revealed cachexia (height 168 cm, weight 41 kg), hepatomegaly, ptosis, ophthalmoparesis, predominantly distal limb weakness, stocking-glove sensory loss, and areflexia except for trace knee jerks. Because of the patient’s medical condition, risk-adapted reduced-intensity alloSCT was recommended, and because no family donor was identified, the donor source was a human leukocyte antigen (HLA) 4/6–matched unrelated placental cord blood unit (4.7 × 107 total nucleated cells/kg recipient body wt). He was enrolled in a Columbia University Medical Center Institutional Review Board–approved protocol and conditioned with cyclophosphamide 60 mg/kg, fludarabine 180 mg/m2, and thymoglobulin 8 mg/kg. Graft-vs-host disease prophylaxis consisted of tacrolimus and mycophenolate. The patient had primary nonengraftment of donor cells with spontaneous autologous recovery and died 86 days after transplant from disease progression complicated by sepsis and respiratory failure.
Patient 2, a 30-year-old Italian woman, at age 20 days was noted to have a poor appetite and vomiting. In childhood, she was short and thin and developed early satiety, borborgymi, persistent diarrhea, and diplopia. In her early 20s, she developed ptosis, stocking-glove paresthesias and numbness, and gastrointestinal dysmotility, which prompted supplemental parenteral nutrition. At age 24, electrophysiologic studies demonstrated peripheral neuropathy, and brain MRI revealed diffuse leukoencephalopathy leading to the diagnosis of MNGIE, which was confirmed by undetectable bcTP activity, elevated plasma dThd and dUrd, and identification of compound heterozygous mutations in ECGF1, IVS8-1A→G, and nucleotide 1,382 insertion C; both mutations were absent in 100 control alleles. On examination at age 30, she was short and cachetic (height 158 cm, weight 25 kg). She had prominent borborygmi, ptosis, mild ophthalmoparesis, impaired hearing, proximal limb weakness, stocking-glove sensory loss, and areflexia.
This patient was treated with the same alloSCT protocol, but methylprednisolone was limited to 5 days and, to avoid potential iatrogenic neurotoxicity, tacrolimus was replaced by sirolimus. The donor was her healthy HLA 6/6–matched 32-year-old brother, who does not carry a TP mutation. A total of 8 × 106 CD34-positive peripheral blood progenitor cells/kg recipient body wt was infused. Neutrophil engraftment was detected 11 days after transplant. Due to concerns that the donor cells were being suppressed by medications, immunosuppression was discontinued 3 months after the transplant.
At 6.5 months after the transplant, the patient reported less severe abdominal pain, improved swallowing ability, and decreased numbness in her hands and feet. Previously unelicitable tendon reflexes were weakly present at the biceps and ankles; otherwise her examination was unchanged.
Methods
Plasma dThd and dUrd concentrations were determined by high performance liquid chromatography (HPLC).5 The ECGF1 gene was sequenced as described.2 To determine TP activity, buffy coat homogenates were incubated for 1 hour with substrate dThd, and thymine formed was quantified by ultraviolet detection after separation by HPLC.2,6
Results
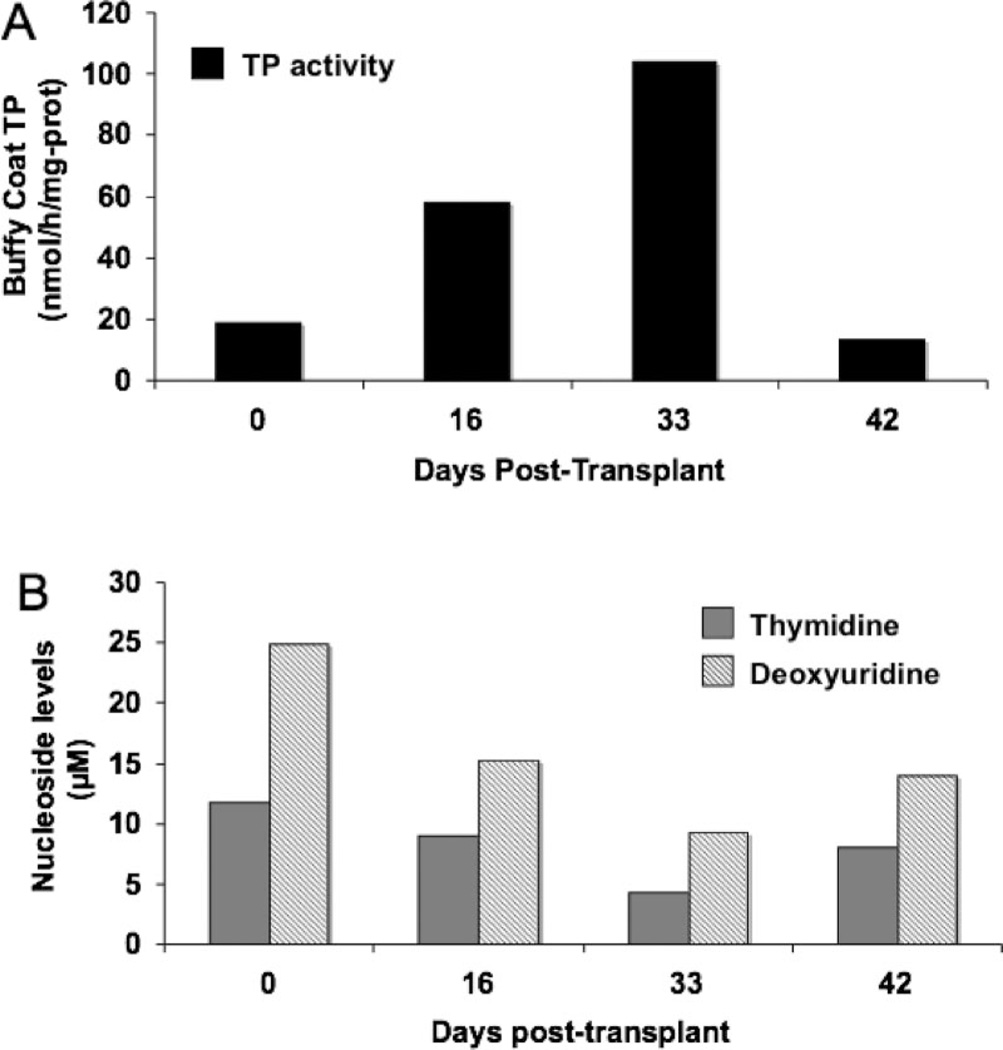
In Patient 1, baseline TP activity in buffy coat was 28 nmol/h/mg protein, plasma dThd 11.7 µM, and dUrd 24.9 µM (figure 1). Two weeks after transplantation, donor cell chimerism in peripheral blood peaked at 4% and slight biochemical improvements were noted. One month post transplant, circulating donor cell chimerism decreased to 1%, but biochemical abnormalities showed further modest improvements; bcTP activity was 104 nmol/h/mg protein, plasma dThd 4.3 µM, and dUrd 9.2 µM. Six weeks post transplantation, the patient’s bcTP and levels of pyrimidine nucleosides reverted toward baseline levels.
Figure 1.
Patient 1 pre– and post–allogeneic stem cell transplantation buffy coat thymidine phosphorylase (TP) activity in nmol/h/mg protein (A) and plasma thymidine and deoxyuridine levels in µM (B).
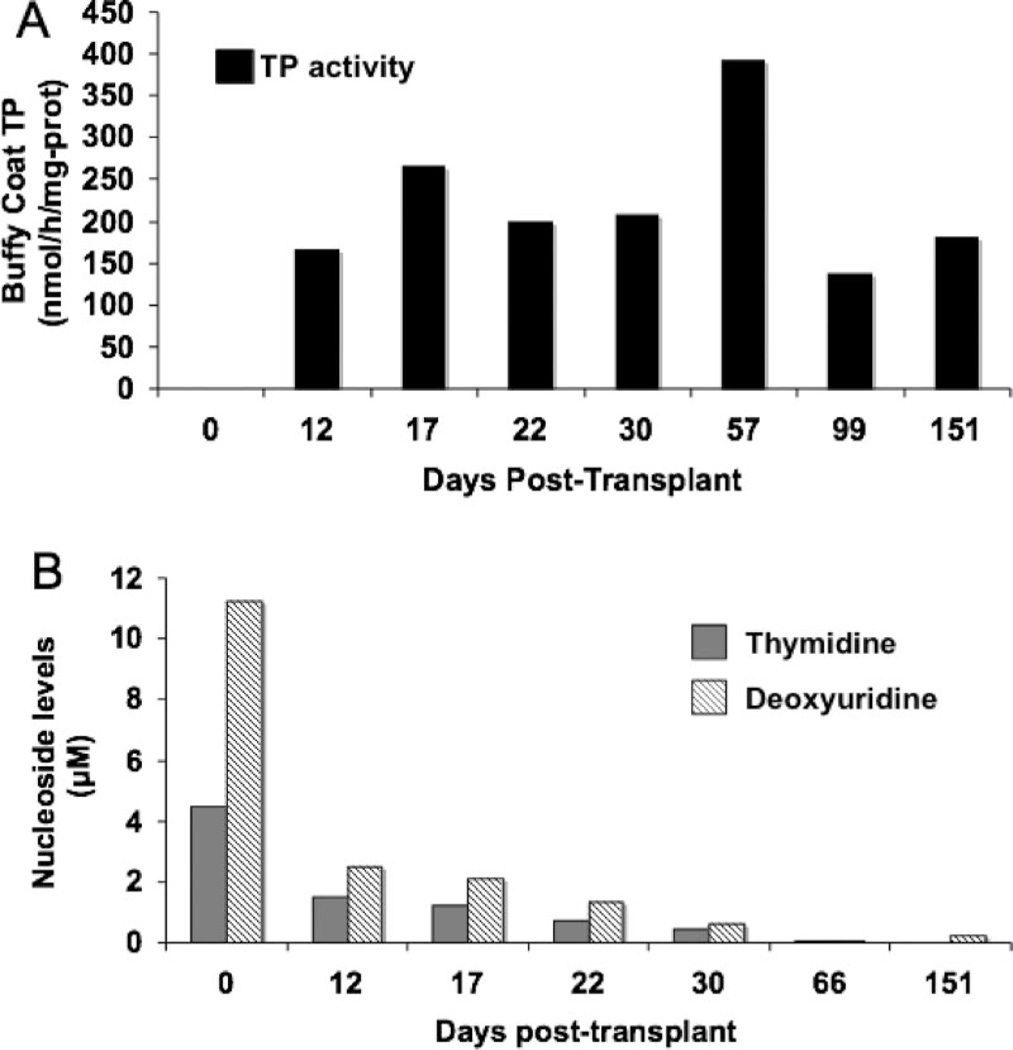
Prior to transplantation, Patient 2 had undetectable bcTP activity and elevated plasma dThd (4.5 µM) and dUrd (11.2 µM) (figure 2). Two weeks post transplant, circulating nucleated donor cell chimerism was 36% with significant bcTP activity (165 nmol/h/mg protein) and reductions in plasma dThd (1.5 µM) and dUrd (2.5 µM). One week later, donor chimerism peaked at 56%. Two months after transplantation, bcTP activity was 392 nmol/h/mg protein and dThd and dUrd in plasma were undetectable (<0.05 µM). Three months post transplant, decreased donor chimerism (14%) and bcTP activity (136 nmol/h/mg protein) prompted discontinuation of immunosuppression. Five months after transplant, donor cell chimerism increased to 26% and bcTP activity rose to 181 nmol/h/mg protein, while plasma dThd remained undetectable and dUrd was 0.2 µM.
Figure 2.
Patient 2 pre– and post–allogeneic stem cell transplantation buffy coat thymidine phosphorylase (TP) activity in nmol/h/mg protein (A) and plasma thymidine and deoxyuridine levels in µM (B).
Discussion
Patients with MNGIE and their asymptomatic relatives carrying ECGF1 mutations have revealed correlations between the biochemical and clinical phenotypes.1–6 Typical MNGIE patients manifest symptoms at about age 18 and have absent or very low bcTP activity (<8% of control mean) and markedly elevated dThd (>3 µM, normal <0.05) and dUrd (>5 µM, normal <0.05).1–6 In contrast, three late-onset MNGIE patients, who developed symptoms in their fifth decade, had less severe bcTP deficiencies (10 to 15% of control mean) and moderate increases of plasma nucleosides (dThd and dUrd levels 0.8 to 1.4 µM).8 Carriers of ECGF1 mutations, who are asymptomatic, have moderate reductions of bcTP activity (26 to 35% of control mean) and undetectable levels of dThd and dUrd in plasma.2,6,8 These observations suggest that partial restoration of TP activity to MNGIE patients can ameliorate the disease.
Based on the “muscle paradox,” normal muscle does not express TP, and yet muscle from MNGIE patients shows alterations of mtDNA, cytochrome c oxidase–deficient and ragged-red fibers and respiratory chain defects.2 We postulate that lack of intracellular TP per se does not cause mitochondrial dysfunction, but rather extracellular dThd and dUrd are incorporated by cells where the nucleosides exert toxic effects. This hypothetical pathomechanism is supported by in vitro studies demonstrating that high concentrations of extracellular dThd (10 to 50 µM) unbalance nucleotide pools both in cytosol and in mitochondria and cause alterations of mtDNA.9 Thus, observations and experimental data in patients and in cultured cells indicate that restoration of intracellular TP activity may not be necessary to protect tissue mitochondrial toxicity in MNGIE.
We therefore proposed that reduction of circulating nucleosides would be therapeutic in MNGIE.6 Hemodialysis in two MNGIE patients and platelet infusions in two other patients transiently reduced plasma nucleoside levels.6,10 The results of the platelet infusions encouraged us to attempt alloSCT to provide long-term TP enzyme replacement to patients with MNGIE.
In the first patient, umbilical cord SCT generated a peak of only 4% circulating nucleated donor cell chimerism, but bcTP activity rose modestly and plasma dThd and dUrd decreased partially (figure 1). Unfortunately, in this patient, who was severely debilitated by MNGIE, donor cells failed to engraft and he died 3 months after transplant. By contrast, in the second patient, donor cells from the HLA-identical brother generated a maximum circulating donor chimerism of 56%, which increased in bcTP activity from undetectable at baseline to 392 nmol/h/mg protein and decreased plasma dThd and dUrd to <0.05 µM (figure 2). By 6.5 months post transplant, the patient reported subjective improvement of her abdominal pain, swallowing, and distal limb numbness with return of tendon reflexes at the biceps and ankles. Thus, both transplants demonstrated that alloSCT can restore TP activity and reduce extracellular levels of nucleosides, but reduced intensity conditioning failed to induce high donor chimerism and clinical efficacy remains uncertain.
Based on experimental data, we hypothesize that in MNGIE, mitochondrial dysfunction and clinical symptoms are produced after years of cumulative toxic effects of excessive nucleosides on mtDNA. We anticipate that the process can be halted through the reduction of dThd and dUrd to normal or nearly normal levels. Unfortunately, because somatic mtDNA mutations that accumulate in postmitotic cells of patients are unlikely to be reversible, treatments for MNGIE based on reduction of dThd and dUrd should be initiated early in the course of the disease to prevent mitochondrial damage and minimize co-morbidities. Increased intensity of the conditioning regimen may produce higher chimerism.
Acknowledgment
The authors thank the patients and their families for their participation.
Supported by grants from the NIH (P01NS11766), Muscular Dystrophy Association, the Marriott Mitochondrial Disorder Clinical Research Fund (MMDCRF) (M.H.), and Swim Across America (J.H.G.), and the Pediatric Cancer Research Foundation (M.S.C.).
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Hirano M, Silvestri G, Blake DM, et al. Mitochondrial neurogastrointestinal encephalomyopathy (MNGIE): clinical, biochemical, and genetic features of an autosomal recessive mitochondrial disorder. Neurology. 1994;44:721–727. doi: 10.1212/wnl.44.4.721. [DOI] [PubMed] [Google Scholar]
- 2.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–692. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 3.Nishino I, Spinazzola A, Papadimitriou A, et al. MNGIE: an autosomal recessive disorder due to thymidine phosphorylase mutations. Ann Neurol. 2000;47:792–800. [PubMed] [Google Scholar]
- 4.Hirano M, Nishigaki Y, Martí R. MNGIE: a disease of two genomes. Neurologist. 2004;10:8–17. doi: 10.1097/01.nrl.0000106919.06469.04. [DOI] [PubMed] [Google Scholar]
- 5.Martí R, Nishigaki Y, Hirano M. Elevated plasma deoxyuridine in patients with thymidine phosphorylase deficiency. Biochem Biophys Res Commun. 2003;303:14–18. doi: 10.1016/s0006-291x(03)00294-8. [DOI] [PubMed] [Google Scholar]
- 6.Spinazzola A, Marti R, Nishino I, et al. Altered thymidine metabolism due to defects of thymidine phosphorylase. J Biol Chem. 2002;277:4128–4133. doi: 10.1074/jbc.M111028200. [DOI] [PubMed] [Google Scholar]
- 7.Pel Toro G, Satwani P, Harrison L, et al. A pilot study of reduced intensity conditioning and allogeneic stem cell transplantation from unrelated cord blood and matched family donors in children and adolescent recipients. Bone Marrow Transplant. 2004;33:613–622. doi: 10.1038/sj.bmt.1704399. [DOI] [PubMed] [Google Scholar]
- 8.Marti R, Verschuuren JJ, Buchman A, et al. Late-onset MNGIE due to partial loss of thymidine phosphorylase activity. Ann Neurol. 2005;58:649–652. doi: 10.1002/ana.20615. [DOI] [PubMed] [Google Scholar]
- 9.Pontarin G, Gallinaro L, Ferraro P, Reichard P, Bianchi V. Origins of mitochondrial thymidine triphosphate: dynamic relations to cytosolic pools. Proc Natl Acad Sci USA. 2003;100:12159–12164. doi: 10.1073/pnas.1635259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lara MC, Weiss B, Illa I, et al. Infusion of platelets transiently reduces nucleoside overload in MNGIE. Neurology. 2006;67:1461–1463. doi: 10.1212/01.wnl.0000239824.95411.52. [DOI] [PubMed] [Google Scholar]