Abstract
Statement of the Problem
In clinical situations, Calcium-Enriched Mixture (CEM) comes into direct contact or even mixes with blood during or after placement.
Purpose
The aim of this study was to evaluate the effect of blood contamination on the compressive strength of CEM.
Materials and Method
Three experimental groups were included in this study. In the first group, CEM was mixed with distilled water and was exposed to normal saline (control group). In the second group, CEM cement was mixed with distilled water and then was exposed to blood. In the third group, CEM was mixed with and exposed to blood. Nine custom-made two-part split Plexiglas molds with five holes were used to form CEM samples for compressive strength testing (15 samples in each group). After 7 days of incubation, compressive bond strength testing was performed using a universal testing machine. Data were statistically analyzed using the Mann–Whitney U test with a significance level of p< 0.05.
Results
Nine samples from group 3 were fractured during removal from the molds; the other six blocks had some cracks on their surfaces. Therefore, a compressive strength measurement was not obtainable for this group. No statistically significant difference was found between groups 1 and 2 (p> 0.05).
Conclusion
It can be concluded that exposure to blood does not adversely affect the compressive strength of CEM, but incorporation of blood makes the cement very brittle.
Key Words: Blood contamination, Compressive strength, Calcium-enriched mixture, CEM
Introduction
Mineral trioxide aggregate (MTA) was introduced to endodontics in 1993 [1] and has been widely used in the repair of root perforations, [2-3] pulp capping, [4-5] and creating an apical barrier in teeth with open apices. [6] MTA has excellent sealing ability, [7-8] biocompatibility, [9] and the ability to stimulate osteoblasts; [10] in addition, it sets in wet environment. [11] However, its disadvantages include extended setting time, [11] poor handling, [12] and relatively high cost.
Calcium-Enriched Mixture (CEM) was introduced in 2006 as a root-end filling material. [13] This novel endodontic cement has similarities to MTA in terms of pH, increased flow, decreased working time and film thickness and is less costly. [14-15] CEM also has low cytotoxicity, excellent biocompatibility, and sealing ability. [13] It has been used in clinical situations such as pulp capping, pulpotomy, perforation repair, apical plug, root resorption, and periradicular surgery. [16-20]
Although in studies on its physical properties, CEM was not allowed to set in contact with blood;13-14, 20-22 in clinical situations, CEM comes into direct contact or even mixes with blood during or after placement. It has been shown that blood contamination can affect the physical properties of MTA.
An in vitro study showed that exposure to blood during setting has an adverse effect on marginal adaptation and the surface microstructure of MTA. [23]
Two separate studies evaluated the effect of blood contamination on the compressive strength and surface microstructure of MTA. It was concluded that blood incorporation into MTA structure reduced the compressive strength of the material. [24-25] Vanderweele et al. reported that contamination of perforation sites with blood before MTA application significantly reduced resistance to displacement. [26]
Considering the similar applications of CEM and MTA, the question is whether contamination with blood influences the physical properties of CEM. Therefore, the present study was designed to evaluate the effect of blood contamination on the compressive strength of CEM.
Materials and Method
The material investigated was CEM (BioniqueDent; Tehran, Iran). Fresh human blood from a healthy volunteer in our research team was obtained by phlebotomy, performed by a trained individual in accordance with Helsinki ethical principles for medical research involving human subjects.
Nine custom-made two-part split Plexiglass molds were used in this experiment. Each mold had five holes with internal diameter of 4±0.1 mm and height of 6±0.1 mm.The molds were randomly allocated into three groups, prior to being filled with CEM.The groups (15 holes/sample in each) comprised:
Group 1: CEM mixed with distilled water and exposed to normal saline.
Group 2: CEM mixed with distilled water and exposed to blood.
Group 3: CEM mixed with blood and exposed to blood.
CEM was prepared according to the manufacturer’s instructions and was then homogenized and positioned incrementally into the molds by amalgam carrier. After gentle packing and compacting with condensers, excess material was removed with wet cotton pellets (Figure 1).
Figure 1.
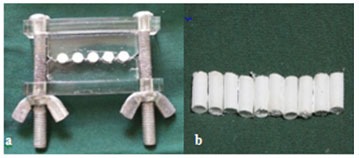
a: The experimental molds filled with the mixture of CEM and distilled water. b: The samples were removed from the molds.
In test groups 2 and 3, before placement of CEM cement, the molds were soaked in human blood and then removed to leave a small coating of blood on the internal surface of each hole in the molds.
In group 3, CEM powder was prepared according to the manufacturer’s instructions on powder-to-liquid ratio, but blood was used instead of distilled water. After the placement of CEM, the molds were placed in Petri dishes containing appropriate medium in the lower part. The medium was normal saline in group 1 and heparinized blood in groups 2 and 3. Wet pieces of gauze were then placed above the molds but without coming into close contact with the CEM surface to produce fully saturated humidity. The plates were sealed and then placed in an incubator at 37°C.
After 7 days, the samples were removed from the incubator and the molds were split. The set CEM blocks were removed carefully by applying light force, taking care not to damage the CEM samples. After removal, the samples were evaluated for voids or cracks.
To test the compressive strength, we placed the samples lengthwise between the platens of a universal testing machine (Zwick Roell Group; Germany) (Figure 2).
Figure 2.

Universal testing machine for testing the compressive strength of the samples
The samples were compressed at the speed of 1 mm/min, and the load at fracture was recorded in mega Pascals (MPa). The mean compressive strengths and standard deviation values were calculated for the groups and analyzed using the Mann–Whitney U test with a significance level of p< 0.05.
Results
Nine samples from group 3 were fractured while being removed from the molds; the other six blocks had some cracks on their surfaces. Therefore, compressive strength measurement was not obtainable for this group. The means and standard deviations of the compressive strength of the groups are represented in Table 1.
Table 1.
The means and standard deviations of compressive strengths (in MPa) of the experimental groups. Groups with the same letters were not significantly different.
| Group | Mean | Standard deviation |
|---|---|---|
| Group 1 | 3.6000a | 0.96749 |
| Group 2 | 4.0220a | 1.32033 |
| Group 3 | - | - |
No statistically significant difference was found between groups 1 and 2 (p>0.05) (Figure 3).
Figure 3.
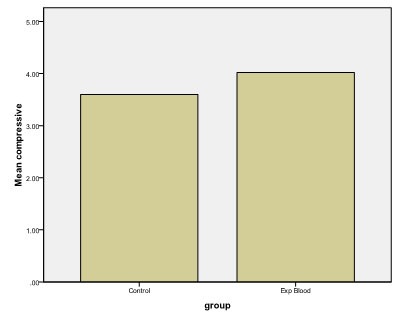
The compressive strengths of the experimental groups. (in MPa)
Discussion
In this study, the effect of blood contamination on the compressive strength of CEM was evaluated. Compressive strength is one of the indicators of the setting and strength of a material. [11, 27] Although mechanical tests are unable to reflect the clinical situation, they can show the effects of different mixing liquids and setting conditions on different types of cement.
According to ISO 9917-1 (2003) standards, for the compressive strength test, a split mold design (made of a material that will not be affected by the cement) has been advised. In studies on the compressive strength of MTA, one-piece plastic cylindrical molds, [28] one-piece polycarbonate cylindrical molds, [29] plastic split molds, [30] stainless steel split molds, [27] and one-piece borosilicate glass molds [24] have been used. In this study, two-part split Plexiglass molds were used to form CEM samples. A pilot study prior to this study showed that samples required a light force to allow removal. Although it has been reported that the setting time of CEM is shorter than that for MTA, [14] the aforementioned pilot study showed that the CEM samples were not solid before 7 days. Therefore, the compressive strength test was performed on day 7, while the studies on MTA evaluated compressive strength after 3 or 4 days. [24, 30-31]
CEM has been suggested for being used in pulp capping, pulpotomy, perforation repair, apical plug, root resorption, and periradicular surgery. [16-20] Therefore, in the majority of its clinical applications, CEM comes into contact and may mix with blood.
In this study, for simulation of the clinical situation whereby bleeding has been controlled, CEM was mixed with distilled water and exposed to fresh human blood. For simulation of situations with excessive bleeding in which blood may be incorporated into the material, CEM was first mixed with, and then exposed to blood. In the control group, CEM powder was mixed with distilled water and exposed to normal saline. It is noteworthy that mixing the endodontic cements with human blood as a model to replicate the clinical situation in which blood becomes incorporated into the cements has been previously reported. Nekoofar et al. mixed MTA with blood to investigate the effect of blood contamination on the compressive strength of two types of MTA. [24] Similarly in two separate studies, MTA was mixed with blood to evaluate its effect on the surface microhardness [32] and the microstructure of MTA. [33]
Despite using two-part split molds that required only light force for removal of the CEM samples, nine out of the 15 samples in group 3 were fractured during removal. Moreover, the other six samples had some cracks on their surfaces indicating that in the clinical situation, bleeding should be controlled before the placement of CEM to avoid unfavorable clinical outcomes.
In an in vitro study, Jasiczak and Zielinski demonstrated that mixing the powdered red blood cells with Portland cement reduced the compressive strength and increased the setting time of the cement. [34] Remadnia et al. also showed that hemoglobin or whole blood increased the porosity of Portland cement. [35] The findings of the present study, which demonstrated a decreased compressive strength of CEM mixed with blood, can be explained by the air entrapment effects of blood [34] and increased porosity of the cement. [35] Moreover, blood proteins may also affect the physical properties of CEM and interfere with hardening of the cement. Further studies should examine the effect of blood contamination on the structural porosity and formation of CEM crystals.
Although in the present study, exposure to blood did not reduce the compressive strength of CEM, Nekoofar et al. [24] reported that both exposure and mixing of MTA with blood could adversely affect its compressive strength. Since comparing the results of two separate studies with different methodologies is difficult, a new study is recommended for the comparison of the physical behaviors of MTA and CEM when contaminated with blood.
Conclusion
Within the limitations of this in vitro study, it can be concluded that exposure to blood does not adversely affect the compressive strength of CEM; however, incorporation of blood makes the cement very brittle.
Acknowledgment
The authors thank the Vice-Chancellery of Shiraz University of Medical Science for supporting this research (Grant No. 1578).This article is based on the thesis by Dr Saied Hashemzade. The authors thank Dr Mehrdad Vosoughi for the statistical analysis.
Conflict of Interest: The authors report no conflicts of interest related to this study.
References
- 1.Ford TR, Torabinejad M, McKendry DJ, Hong CU, Kariyawasam SP. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995; 79: 756–763. doi: 10.1016/s1079-2104(05)80313-0. [DOI] [PubMed] [Google Scholar]
- 2.Main C, Mirzayan N, Shabahang S, Torabinejad M. Repair of root perforations using mineral trioxide aggregate: a long-term study. J Endod. 2004; 30: 80–83. doi: 10.1097/00004770-200402000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Holland R, Filho JA, de Souza V, Nery MJ, Bernabé PF, Junior ED. Mineral trioxide aggregate repair of lateral root perforations. J Endod. 2001; 27: 281–284. doi: 10.1097/00004770-200104000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Eghbal MJ, Asgary S, Baglue RA, Parirokh M, Ghoddusi J. MTA pulpotomy of human permanent molars with irreversible pulpitis. Aust Endod J. 2009; 35: 4–8. doi: 10.1111/j.1747-4477.2009.00166.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrou MA, Alhamoui FA, Welk A, Altarabulsi MB, Alkilzy M, Splieth CH. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin Oral Investig. 2014; 18: 1383–1389. doi: 10.1007/s00784-013-1107-z. [DOI] [PubMed] [Google Scholar]
- 6.Camp JH. Diagnosis dilemmas in vital pulp therapy: treatment for the toothache is changing, especially in young, immature teeth. J Endod. 2008; 34: S6–12. doi: 10.1016/j.joen.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 7.Fischer EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material, and Super-EBA as a root-end filling material. J Endod. 1998; 24: 176–179. doi: 10.1016/S0099-2399(98)80178-7. [DOI] [PubMed] [Google Scholar]
- 8.Torabinejad M, Smith PW, Kettering JD, Pitt Ford. Comparative investigation of marginal adaptation of mineral trioxide aggregate and other commonly used root-end filling materials. J Endod. 1995; 21: 295–299. doi: 10.1016/S0099-2399(06)81004-6. [DOI] [PubMed] [Google Scholar]
- 9.Torabinejad M, Ford TR, Abedi HR, Kariyawasam SP, Tang HM. Tissue reaction to implanted root-end filling materials in the tibia and mandible of guinea pigs. J Endod. 1998; 24: 468–471. doi: 10.1016/s0099-2399(98)80048-4. [DOI] [PubMed] [Google Scholar]
- 10.Koh ET, Torabinejad M, Pitt Ford TR, Brady K, McDonald F. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997; 37: 432–439. doi: 10.1002/(sici)1097-4636(19971205)37:3<432::aid-jbm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 11.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995; 21: 349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 12.Chng HK, Islam I, Yap AU, Tong YW, Koh ET. Properties of a new root-end filling material. J Endod. 2005; 31: 665–668. doi: 10.1097/01.don.0000157993.89164.be. [DOI] [PubMed] [Google Scholar]
- 13.Asgary S, Eghbal MJ, Parirokh M, Torabzadeh H. Sealing ability of three commercial mineral trioxide aggregates and an experimental root-end filling material. Iran Endod J. 2006; 1: 101–105. [PMC free article] [PubMed] [Google Scholar]
- 14.Asgary S, Shahabi S, Jafarzadeh T, Amini S, Kheirieh S. The properties of a new endodontic material. J Endod. 2008; 34: 990–993. doi: 10.1016/j.joen.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Samiee M, Eghbal MJ, Parirokh M, Abbas FM, Asgary S. Repair of furcal perforation using a new endodontic cement. Clin Oral Investig. 2010; 14: 653–658. doi: 10.1007/s00784-009-0351-8. [DOI] [PubMed] [Google Scholar]
- 16.Asgary S, Nosrat A, Seifi A. Management of inflammatory external root resorption by using calcium-enriched mixture cement: a case report. J Endod. 2011; 37: 411–413. doi: 10.1016/j.joen.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Asgary S, Eghbal MJ. The effect of pulpotomy using a calcium-enriched mixture cement versus one-visit root canal therapy on postoperative pain relief in irreversible pulpitis: a randomized clinical trial. Odontology. 2010; 98: 126–33. doi: 10.1007/s10266-010-0127-2. [DOI] [PubMed] [Google Scholar]
- 18.Asgary S, Eghbal MJ, Ehsani S. Periradicular regeneration after endodontic surgery with calcium-enriched mixture cement in dogs. J Endod. 2010; 36: 837–841. doi: 10.1016/j.joen.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Asgary S, Eghbal MJ, Parirokh M, Ghanavati F, Rahimi H. A comparative study of histologic re-sponse to different pulp capping materials and a novel endodontic cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008; 106: 609–614. doi: 10.1016/j.tripleo.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Nosrat A, Seifi A, Asgary S. Regenerative endodontic treatment (revascularization) for necrotic immature permanent molars: a review and report of two cases with a new biomaterial. J Endod. 2011; 37: 562–567. doi: 10.1016/j.joen.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Haghgoo R, Arfa S, Asgary S. Microleakage of CEM Cement and ProRoot MTA as Furcal Perforation Repair Materials in Primary Teeth. Iran Endod J. 2013; 8: 187–190. [PMC free article] [PubMed] [Google Scholar]
- 22.Ghorbani Z, Kheirieh S, Shadman B, Eghbal MJ, Asgary S. Microleakage of CEM cement in two different media. Iran Endod J. 2009; 4: 87–90. [PMC free article] [PubMed] [Google Scholar]
- 23.Salem Milani A, Rahimi S, Froughreyhani M, Vahid Pakdel M. Effect of Blood Contamination on Marginal Adaptation and Surface Microstructure of Mineral Trioxide Aggregate: A SEM Study. J Dent Res Dent Clin Dent Prospects. 2013; 7: 157–163. doi: 10.5681/joddd.2013.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nekoofar MH, Stone DF, Dummer PM. The effect of blood contamination on the compressive strength and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010; 43: 782–791. doi: 10.1111/j.1365-2591.2010.01745.x. [DOI] [PubMed] [Google Scholar]
- 25.Oloomi K, Saberi E, Mokhtari H, Mokhtari Zonouzi HR, Nosrat A, Nekoofar MH, et al. Evaluation of the effect of blood contamination on the compressive strength of MTA modified with hydration accelerators. Restor Dent Endod. 2013; 38: 128–133. doi: 10.5395/rde.2013.38.3.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderweele RA, Schwartz SA, Beeson TJ. Effect of blood contamination on retention characteristics of MTA when mixed with different liquids. J Endod. 2006; 32: 421–424. doi: 10.1016/j.joen.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Kayahan MB, Nekoofar MH, Kazandağ M, Canpolat C, Malkondu O, Kaptan F, et al. M, Canpolat C, Malkondu O, Kaptan F, et al, authors. Effect of acid-etching procedure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2009; 42: 1004–1014. doi: 10.1111/j.1365-2591.2009.01610.x. [DOI] [PubMed] [Google Scholar]
- 28.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32: 569–572. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Nekoofar MH, Adusei G, Sheykhrezae MS, Hayes SJ, Bryant ST, Dummer PM. The effect of condensation pressure on selected physical properties of mineral trioxide aggregate. Int Endod J. 2007; 40: 453–461. doi: 10.1111/j.1365-2591.2007.01236.x. [DOI] [PubMed] [Google Scholar]
- 30.Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE. The anti-microbial effect against enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. J Endod. 2007; 33: 844–847. doi: 10.1016/j.joen.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Basturk FB, Nekoofar MH, Günday M, Dummer PM. The effect of various mixing and placement techniques on the compressive strength of mineral trioxide aggregate. J Endod. 2013; 39: 111–114. doi: 10.1016/j.joen.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Nekoofar MH, Oloomi K, Sheykhrezae MS, Tabor R, Stone DF, Dummer PM. An evaluation of the effect of blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010; 43: 849–858. doi: 10.1111/j.1365-2591.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 33.Nekoofar MH, Davies TE, Stone D, Basturk FB, Dummer PM. Microstructure and chemical analysis of blood-contaminated mineral trioxide aggregate. Int Endod J. 2011; 44: 1011–1018. doi: 10.1111/j.1365-2591.2011.01909.x. [DOI] [PubMed] [Google Scholar]
- 34.Jasiczak J, Zielinski K. Effect of protein additive on properties of mortar. Cement and Concrete Composites. 2006; 28: 451–457. [Google Scholar]
- 35.Remadnia A, Dheilly RM, Laidoudi B, Queneudec M. Use of animal proteins as foaming agent in cementitious concrete composites manufactured with recycled PET aggregates. Constr Build Mater. 2009; 23: 3118–31231. [Google Scholar]


