Abstract
Objective
Injections for spinal pain have high failure rates, emphasizing the importance of patient selection. It is possible that detecting the presence of a fibromyalgia-like phenotype could aid in prediction, because in these individuals a peripheral injection would not address pain due to alterations in central neurotransmission. We hypothesized that spine pain patients meeting survey criteria for fibromyalgia would be phenotypically distinct from those who do not meet criteria.
Methods
548 patients with a primary spine pain diagnosis were studied. All patients completed validated self-report questionnaires, including the Brief Pain Inventory, PainDETECT, Hospital Anxiety and Depression Scale, measures of physical function, and the American College of Rheumatology survey criteria for fibromyalgia.
Results
42% met survey criteria for fibromyalgia (FM+). When compared with criteria negative patients, FM+ patients were more likely to be younger, unemployed, receiving compensation, have greater pain intensity, pain interference and neuropathic pain descriptors, as well as higher levels of depression and anxiety, and lower level of physical function (p < 0.0001 for each comparison). Gender, neuropathic pain, pain interference, physical function, and anxiety were independently predictive of fibromyalgia status in a multivariate analysis (p < 0.01, all variables). ROC analysis showed the strength of association of 0.81 as measured by the cross-validated C-statistic.
Conclusion
Using the survey criteria for fibromyalgia, we demonstrated profound phenotypic differences in a spine pain population. Although centralized pain cannot be confirmed with a survey alone, the pathophysiology of fibromyalgia may help explain a portion of the variability of responses to spine interventions.
Introduction
Spine pain is one of the most common causes of disability in the world. It is estimated that 10-15% of the US population seeks care for low back pain (LBP) each year.(1) Second only to the treatment of joint pain, spine pain is considered the most expensive musculoskeletal condition; estimates exceed $140 billion in annual lost wages and treatment costs.(1, 2) Recently, there has been an explosion in the use of minimally invasive spine therapies for the treatment of spine pain. Between 1997-2006 in the Medicare population, facet joint interventions increased by 543%,(3) and epidural steroid injections by 102%.(4) These and other minimally invasive therapies have high failure rates implying that patient selection may play a crucial role.(5, 6)
Some patient risk factors predictive of poor outcomes from epidural steroid and facet interventions include long duration of pain, opioid consumption, previous spine surgery, younger age, increased pain sensitivity, depression, and anxiety.(5, 7-12) Similarly, pain in other locations, depression, catastrophizing, and somatization all have been described as predictors of lesser analgesic response from lower extremity joint arthroplasty.(13) It is possible that this collection of patient risk factors can be explained by a common pathophysiologic mechanism. There is a growing appreciation of the importance of augmented central nervous system (CNS) processing of pain and other symptoms in several chronic pain states.(14) Such states lack clear peripheral pathology and have been given specific names, including fibromyalgia, irritable bowel syndrome, and interstitial cystitis.(14-17) Arguably the best studied of these, fibromyalgia, is characterized by widespread body pain and comorbid symptoms (e.g. fatigue, trouble thinking, depression) without apparent peripheral pathology. Instead, alterations in central neurotransmission have been associated with pain sensitivity and neuropathic pain symptoms.(15, 18-22)
Experimental pain testing and functional neuroimaging studies have shown that subsets of individuals with classically described “peripheral” pain conditions, such as osteoarthritis and rheumatoid arthritis, demonstrate similar patterns of augmented CNS pain processing as those seen in conditions like fibromyalgia, and thus potentially have a component of “centralized pain.”(23, 24) The few experimental studies conducted in spine pain support the same conclusion. Pain threshold has been shown to be a robust predictor of pain response and physical function,(25) and functional magnetic resonance imaging in LBP has demonstrated similar patterns of augmented central pain processing to those seen with fibromyalgia.(26) However, the frequency with which “centralized pain” exists in a population of general spine patients is not known.
In 2011, fibromyalgia criteria and severity scales were introduced for use in clinical and epidemiological studies.(27) These “survey criteria” rely on the completion of a self-report questionnaire and, like the American College of Rheumatology (ACR) preliminary diagnostic criteria introduced in 2010, do not require a tender point examination.(27) The aim of the present study was to determine whether the ACR survey criteria for fibromyalgia could differentiate spine pain patients in terms of measures of pain, affect and function. Fibromyalgia is rarely diagnosed in this population and patients are generally treated as having pain that is predominately or solely due to peripheral pathology of the spine. We hypothesized that spine pain patients meeting ACR survey criteria for fibromyalgia,(27-29) which were used as a surrogate of centralized pain in this study, would report pain that is more neuropathic in nature and have higher levels of pain, depression, anxiety, and disability than those who do not meet criteria. The clinical implications are that a brief self-report measure (e.g., ACR fibromyalgia survey criteria) could eventually be used to guide patient selection for various interventions based, in part, on underlying pain mechanisms.
Methods
Institutional Review Board (Ann Arbor, MI) approval was obtained. New patients (age ≥ 18 years old) presenting to the University of Michigan Back & Pain Center (Department of Anesthesiology) from November 2010 to March 2012 were included. As previously described,(30) all new patients presenting for treatment at our academic outpatient pain clinic complete an intake packet that includes validated self-report measures of pain, psychological status, physical function, and demographic information (including age, gender, race, ethnicity, marital status, employment status, and compensation for pain, as reported by the patient from a predefined list). A coversheet for the intake packet explains that the information will be used for clinical care and research, and a waiver of written informed consent was obtained from the Institutional Review Board. The International Classification of Diseases code (ICD-9) assigned by the treating physician was used to define the spine cohort. Disorders of the axial neck, mid and low back, radicular pain, failed spine surgery, or surrounding spine structures were all included (Table 1). A three-month audit of the number of new patients evaluated with phenotyping included in the dataset demonstrated that 85.7% of the patients were captured (data not shown).
Table 1. Diagnostic code inclusions.
Spine pain was defined by the primary diagnosis assigned by the treating physician during the new patient encounter using the International Classification of Diseases codes (ICD-9).
| Diagnosis | ICD-9 Code |
Frequency (n) |
Percent (%) |
|
|---|---|---|---|---|
| Neck and Upper Extremities |
Cervicalgia, Neck | 723.1 | 84 | 15.3 |
| Facet Arthropathy Cervical OR Spondylosis w/o Myelopathy Cervical |
721 | 12 | 2.2 | |
| Degenerative Disc Cervical | 722.4 | 3 | 0.5 | |
| Radiculopathy Cervical OR Cervical- radiculitis/neck |
723.4 | 7 | 1.3 | |
| Spondylosis w Myelopathy Cervical | 721.1 | 1 | 0.2 | |
| Post Laminectomy Syndrome Cervical | 722.81 | 2 | 0.4 | |
| Spinal Stenosis Cervical | 723 | 3 | 0.5 | |
| Mid-Back | Thoracic Spine- Mid-back | 724.1 | 26 | 4.7 |
| Herniated Nucleus Pulposus- Thoracic | 722.11 | 0 | 0.0 | |
| Low Back and Lower Extremities |
Low back/Lumbago | 724.2 | 269 | 49.1 |
| Degenerative Disc Lumbar | 722.52 | 11 | 2.0 | |
| Facet Arthropathy Lumbar OR Spondylosis w/o Myelopathy Lumbosacral |
721.3 | 13 | 2.4 | |
| Herniated Nucleus Pulposus Lumbar | 722.1 | 17 | 3.1 | |
| Radiculopathy Thoracic/Lumbar | 724.4 | 30 | 5.5 | |
| Spinal Stenosis Lumbar | 724.02 | 27 | 4.9 | |
| Post Laminectomy Syndrome Lumbar | 722.83 | 32 | 5.8 | |
| Sacroiliac Joint Dysfunction | 739.4 | 9 | 1.6 | |
| Sacroilitis | 720.2 | 2 | 0.4 | |
| Total | 548 |
The ACR survey criteria for fibromyalgia consist of an assessment of widespread pain and symptom severity.(27, 29) The Widespread Pain Index (WPI) was calculated using the Michigan Body Map(31) to assess the 19 specific body areas described in the ACR survey criteria (score 0-19). The second aspect of the criteria was evaluated using the Symptom Severity (SS) scale (score 0-12). As per the ACR criteria, patients were classified as fibromyalgia positive (FM+) if their scores were WPI ≥ 7 and SS ≥ 5 or WPI = 3-6 and SS ≥ 9. The validity of the survey criteria has been established both when compared with the 1990 ACR criteria (which included the tender point examination) (28) and the 2010 preliminary diagnostic criteria which include physician assessment.(29, 32) Patients missing fibromyalgia survey criteria data were excluded from analysis. Additional phenotyping measures were completed, including the Brief Pain Inventory (BPI; pain severity and interference),(33) PainDETECT (neuropathic pain),(34) Oswestry Disability Index (ODI; physical function),(35) Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function Short Form 1 (physical function),(36) Hospital Anxiety and Depression Scale (HADS; depressive symptoms and anxiety symptoms),(37) and duration of pain.
Statistical analysis
Data were entered into the Assessment of Pain Outcomes Longitudinal (APOLO) Electronic Data Capture system.(30) Missing data for the validated instruments were handled as described by instrument authors.(34, 37, 38) As noted above, patients who did not complete all the components of the ACR survey criteria for fibromyalgia were not included in the analysis. Additionally, patients missing more than one item on the BPI subscales, PainDETECT, and ODI were excluded. The PROMIS Physical Function Short Form 1 requires complete data on all 10 items so patients missing any items were excluded. For the HADS, when 6 of the 7 questions were answered, a single value for the missing item was inferred by imputation of the mean of the other 6 values as recommended. For the other instruments, only one missing question was allowed; however, other completed questionnaires were allowed (e.g. patients were not completely excluded from the analysis for having one incomplete questionnaire).
Data were analyzed using R 2.15.0 and SPSS (version 19; SPSS Inc, Chicago, IL, USA). Between-group comparisons were performed using a t-test for continuous variables, Chi-square test for binary variables and Wilcoxon test for ordered categorical variables. Significance level was set at 0.025 to account for the previous analysis on a smaller dataset conducted for the presented abstract. We studied patterns of association between the observed phenotype, and their relationship with the centralized pain phenotype, using binary categorization of fibromyalgia status by the previously described ACR survey criteria (FM+ vs. FM−)(27, 29) and continuous “fibromyalgia-ness” score (sum of WPI and SS scales). Multivariate linear regression was used to study association between the continuous fibromyalgia score and measured phenotype and pain variables. Association of the phenotype and pain variable panel with the binary fibromyalgia status was analyzed using logistic regression. Best model selection using Akaike information criterion (AIC) and likelihood ratio tests was used to select the best set of predictor variables. Prediction strength of the logistic model was analyzed using receiver operating characteristics (ROC). The ROC analysis was cross-validated (10-fold) to ensure reproducibility of predictions and avoid model over-fitting. Cross-validated estimate of the area under the ROC (C-statistic) was used to characterize the predictive performance of the phenotype and pain profile with respect to the fibromyalgia categorization (FM+ or FM−). Model adequacy was tested using a variety of standardized residual plots (not shown).
Radicular pain and spinal stenosis are frequently associated with neuropathic pain complaints and are often differentiated from axial spine pain. In order to specifically analyze the portion of the cohort with axial spine pain (neck, mid back and low back pain), the multivariate analyses noted above were repeated after excluding patients with radicular or non-axial spine disorders (Excluded ICD-9 codes 723.4 [cervical radiculopathy], 721.1 [cervical spondylosis], 722.81, 723 [cervical spinal stenosis], 722.11 [thoracic herniated nucleus pulposus], 722.1 [lumbar herniated nucleus pulposus], 724.4 [thoracic or lumbar radiculopathy], 724.02 [lumbar spinal stenosis], 722.83 [lumbar post-laminectomy syndrome], 739.4 [sacroiliac joint dysfunction], 720.2 [sacroilitis]; Total n = 130; See Table 1).
Results
The total number of new patients seen in the pain clinic over the defined time period was 1208, with primary spine diagnoses in 548 patients. After exclusion of patients who did not have complete data for the ACR survey criteria for fibromyalgia, 443 were retained for the analysis (Table 2). Of these patients, 186 (42%) met survey criteria for fibromyalgia (FM+). In univariate analyses, FM+ patients were younger (p = 0.001), less likely to be employed (p = 0.0005), and more likely to be receiving compensation for their pain (p = 0.0005), but there were no other significant differences between the two groups with respect to demographic variables (Table 2). Compared to FM− patients, FM+ patients were more likely to have pain of a longer duration (p = 0.0096) and had higher scores on measures of pain severity, pain interference, and neuropathic pain (p < 0.0001). FM+ patients also reported lower physical function and showed higher levels of anxiety and depressive symptoms (p < 0.0001).
Table 2. Between Group Analyses.
Differences between groups are noted when patients were categorized by the 2011 American College of Rheumatology survey criteria for fibromyalgia (FM). Except for age, employment status, and compensation for pain,,demographic variables were well balanced between the two groups. Patients categorized as fibromyalgia positive (FM+) using the ACR survey criteria showed profound phenotypic differences for pain intensity, pain interference, neuropathic pain, physical function, depression, anxiety, and duration of pain.
|
FM− (n = 257) |
FM+ (n = 186) |
P value | |
|---|---|---|---|
| Fibromyalgia Score | 8.46 (2.79) | 17.2 (3.89) | < 0.0001 |
| Demographics | |||
| Age (years) | 51.9 (17.5) | 46.9 (13.6) | 0.001 |
| Gender (% Female) | 54.1 | 59.1 | 0.29 |
| Race (% Caucasian) | 89.2 | 85.2 | 0.22 |
| Education (% with college education) | 46.0 | 45.3 | 0.89 |
| Marital Status (% married) | 59.6 | 53.8 | 0.23 |
| Employment Status (% employed) | 41.2 | 25.4 | 0.0005 |
| Compensation for Pain (% Yes) | 18.1 | 33.1 | 0.0005 |
| Type of Compensation (% patients) | |||
| Social Security | 51.2 | 56.4 | 0.20 |
| Long-term Disability | 7.3 | 10.9 | |
| Workers’ Compensation | 14.6 | 9.1 | |
| Sick Leave | 12.2 | 1.8 | |
| No Fault Insurance | 14.6 | 21.8 | |
| Pain Phenotype | |||
| Pain Intensity (BPI) | 6.02 (1.83) | 7.2 (1.54) | < 0.0001 |
| Pain Interference (BPI) | 6.33 (2.11) | 7.95 (1.69) | < 0.0001 |
|
Neuropathic pain descriptors
(PainDETECT) |
14 (7.85) | 22.1 (7.79) | < 0.0001 |
| Physical function (ODI) | 40.3 (16.6) | 55.3 (15.8) | < 0.0001 |
| Physical function (PROMIS) | 31.9 (7.42) | 26.9 (7.81) | < 0.0001 |
| Anxiety (HADS) | 6.61 (3.67) | 11.7 (4.65) | < 0.0001 |
| Depression (HADS) | 7.15 (4.23) | 11.5 (4.4) | < 0.0001 |
|
Duration of Pain (%
patients/category) |
|||
| < 3 months | 8.1 | 1.0 | 0.0096 |
| 3-6 months | 11.4 | 3.1 | |
| 7-12 months | 12.2 | 12.5 | |
| 1-5 years | 35.0 | 42.7 | |
| > 5 years | 33.3 | 40.6 | |
Univariate analyses of differences by the FM status; t-test used with continuous variables (means), chi-square test with binary (%), and Wilcoxon test with ordinal data. Data presented as mean (standard deviation) or percentages, as appropriate.
BPI = Brief Pain Inventory, Pain intensity = mean score of 4 questions regarding pain intensity current, worst, least, and average over last 7 days (0-10, 0 = no pain, 10 = worst pain imaginable); Pain interference = mean score of the seven interference questions of BPI (0-10, 0 = does not interfere, 10 = totally interferes); HADS = Hospital Anxiety and Depression Scalehigher scores indicate more anxiety (0-21) and depressive symptoms (0-21); ODI = Oswestry Disability Index (0-100)- higher score indicates more disability; PainDETECT ([-1]- 38)- higher scores indicate more neuropathic pain; PROMIS = Patient Reported Outcomes Measurement Information System physical function scale short form (10-50)- lower scores indicate lower physical function.
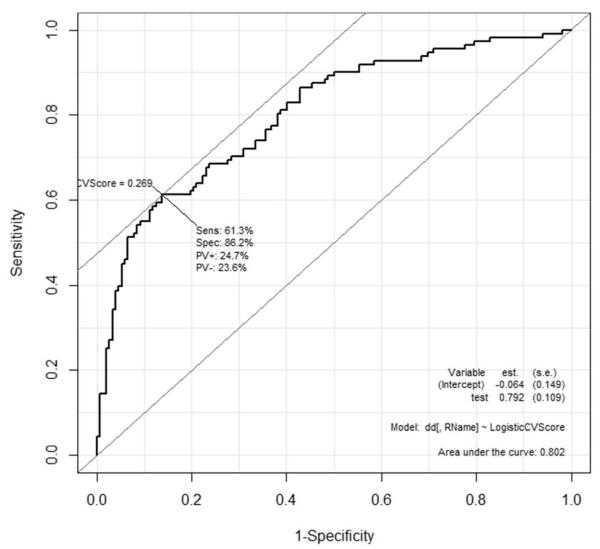
The multivariate analysis of the association between the observed demographic and pain/mood/function phenotype and continuous fibromyalgia score and binary fibromyalgia classification are shown in Table 3. Fibromyalgia status could be best-predicted using neuropathic pain (PainDETECT), physical function (ODI), anxiety (HADS), and compensation for pain with a high area under the ROC curve (C-statistic, AUC=0.80). Best model search using AIC resulted in the same model as the cross-validated ROC analysis (Figure 1). Multivariate prediction of the fibromyalgia score as a continuous score reported a similar set of highly significant best model predictors, gender (male vs. female), pain interference (BPI), neuropathic pain, and anxiety (p < 0.005 for each significant predictor, Table 3).
Table 3. Multivariate Analyses.
The association between pain and phenotypic variables and fibromyalgia status measured as a continuous score (linear regression) or dichotomized (logistic regression) using the 2011 American College of Rheumatology survey criteria for fibromyalgia. The best models after variable selection are presented.
| Model | Predictor variable | Estimate | STE | p-value |
|---|---|---|---|---|
| Linear | Intercept | 2.711 | 0.765 | 0.0004 |
| Gender (male vs. female) | −1.567 | 0.422 | 0.0002 | |
| Physical Function (ODI) | 0.028 | 0.018 | 0.12 | |
| Pain Interference (BPI) | 0.432 | 0.152 | 0.0047 | |
| Neuropathic Pain (PainDETECT) |
0.129 | 0.030 | < 0.0001 | |
| Anxiety (HADS) | 0.412 | 0.051 | < 0.0001 | |
| Logistic | Intercept | −4.672 | 0.518 | < 0.0001 |
| Neuropathic Pain (PainDETECT) |
0.069 | 0.018 | 0.0002 | |
| Physical Function (ODI) | 0.021 | 0.009 | 0.024 | |
| Anxiety (HADS) | 0.221 | 0.034 | < 0.0001 | |
| Compensation for Pain | 0.485 | 0.299 | 0.11 |
BPI = pain interference questions from the Brief Pain Inventory; HADS = Hospital Anxiety and Depression Scale; ODI = Oswestry Disability Scale.
Figure 1.
Receiver-operating characteristic for predicting the FM status (per 2011 American College of Rheumatology survey criteria for fibromyalgia definition) using a panel of demographic, pain and other phenotypic predictor variables.
A sub-analysis of the axial spine cohort was conducted, which resulted in a reduction in the number of patients (n = 418) available for the analysis (See methods, statistics section for additional details). Descriptive between-group analysis in the axial spine subset yielded a very similar outcome to Table 1 (not shown). The smaller sample size resulted in a reduced panel of pain and phenotype variables in the trimmed multivariate models. Pain interference, neuropathic pain and anxiety represent the set of best predictors after variable selection using linear (continuous fibromyalgia status) and logistic (binary fibromyalgia status) models (p < 0.001 for all variables). The analysis yielded the same area under the curve (C-statistic) of 0.81 (not shown).
Discussion
We found that 42% of the patients presenting to a tertiary care facility with a primary spine diagnosis met ACR survey criteria for fibromyalgia, which suggests the presence of centralized pain. To our knowledge, this is the first time that the ACR survey criteria for fibromyalgia has been used to differentiate a spine pain cohort. Patients meeting fibromyalgia survey criteria reported a strikingly different pain phenotype than those who did not meet criteria (e.g., in univariate analyses the former were more likely to be female, younger, unemployed, receiving compensation for their pain, report greater pain severity and interference, anxious, depressed, use neuropathic pain descriptors, and report diminished functioning; Table 2). Analyzing the groups using the ACR survey criteria score as a dichotomous (logistic) or continuous (linear) measure further demonstrated that neuropathic pain descriptors, anxiety, pain interference, and physical function were independently predictive of fibromyalgia survey criteria score (Table 3), which is consistent with what has previously been described in patients diagnosed with fibromyalgia using the 1990 criteria.(39) The independent predictors from the multivariate models are some of the most commonly described predictors of poor outcomes in minimally invasive spine interventions and post-surgical pain. Hence, there may be a common underlying pathophysiology or “diagnosis” driving these findings.
The Use of the American College of Rheumatology Survey Criteria for Fibromyalgia
The diagnosis of fibromyalgia has been a point of controversy in the pain community for years, as there is no definitive diagnostic test. Meeting ACR survey criteria for fibromyalgia does not confirm categorical diagnosis. Instead, a history, physical examination, and laboratory testing (e.g., blood count, erythrocyte sedimentation rate, C-reactive protein, creatine kinase, and thyroid stimulating hormone) by an experienced provider are required to make the diagnosis of fibromyalgia.φ However, comorbid fibromyalgia is common in nearly all other musculoskeletal chronic pain conditions, including osteoarthritis and rheumatoid arthritis (estimates range from 20-30%).(23, 24) The validity of the survey criteria when compared with the 1990 ACR criteria (which included the tender point examination)(28) and the 2010 preliminary diagnostic criteria which include physician assessment has been established.(29, 32) As was previously recommended, the present study applied this validated self-report measure in an epidemiological fashion to detect widespread body pain and comorbid symptoms.
Centralized Pain Phenotype in a Spine Cohort
These data and those from experimental studies(26, 40, 41) suggest that centralized pain may be very common in spine pain. For example, two studies have shown that a sizable proportion of individuals with chronic LBP display diffuse tenderness (e.g., mechanical hyperalgesia) and have functional magnetic resonance imaging findings consistent with fibromyalgia or similar centralized pain states.(25, 26) A retrospective study found that female patients with higher pain severity, family history of chronic widespread pain, and more painful comorbidities were more likely to transition from neck and back pain to chronic widespread pain using the four quadrant pain described in the ACR 1990 criteria for fibromyalgia.(42) In the present study, independent predictors of meeting fibromyalgia survey criteria included sex (female), higher levels of neuropathic pain descriptors, anxiety, more pain interference, and lower levels of physical function (Table 3). Neuropathic pain descriptors in particular are thought to be strongly associated with the fibromyalgia pain phenotype and have been shown to correlate with the number of tender points and pain sensitivity during experimental pain testing.(20, 21) Because radicular pain would be expected to present with more neuropathic pain symptoms, the analyses were also re-run after excluding radicular pain and non-axial spine pain diagnoses, and the results did not change.
Most axial spine diagnoses (e.g. facet arthropathy, lumbago) are not normally thought to be associated with high levels of neuropathic pain, yet the mean value for patients meeting fibromyalgia survey criteria for the PainDETECT measure exceeded the instrument’s defined cut-point for neuropathic pain - with or without patients with radicular pain diagnoses included.(34) Although there is certainly overlap between that which is described by patients meeting criteria for neuropathic pain using the PainDETECT, the phenotype of patients meeting ACR survey criteria for fibromyalgia is distinct.
Pathophysiology of Fibromyalgia and Possible Implications for Spine Interventions
The finding that many spine pain patients complain of widespread body pain and comorbid symptoms, such as fatigue, trouble thinking, anxiety and/or depression, could have important treatment implications and may explain a portion of the high failure rates described for some of the most common spine interventions. Injections and peripherally targeted analgesics would be expected to provide less benefit in a patient with altered central pain processing than in those with predominantly peripheral pathology. Fibromyalgia is associated with lower levels of neurotransmitters that inhibit pain, including norepinephrine, serotonin, and γ-Aminobutyric acid (GABA), along with higher levels of neurotransmitters that increase pain, including glutamate.(15, 43) Fibromyalgia patients also have lower endogenous opioid receptor binding availability and high levels of cerebrospinal fluid opioids,(44) together suggesting that the endogenous opioid system is already activated in fibromyalgia and thus perhaps explaining the anecdotal sense that opioids are ineffective in most of these patients.(45)
The use of minimally invasive interventions for spine pain, such as epidural steroid and facet joint interventions, has increased dramatically in recent years.(3) A recent well-publicized meta-analysis has called into question the long-term efficacy of epidural steroid injections,(46) and studies of response prediction estimate rates of treatment failure to be between 25-45%.(47-49) Similarly, although the efficacy of facet interventions has been established in tightly selected patient populations,(50, 51) higher failure rates have been shown in retrospective studies (39-47% failure)(5, 6) and effectiveness in standard clinical care is not known. Back and neck pain are frequently described in fibromyalgia, and it is possible that the high failure rate for these interventions is in part due to intervention on a peripheral target in the spine when the nature of the patient’s pain is at least in part due to brain and spinal cord dysfunction. Taken together, these data make a compelling case for the study of a modified treatment approach. For example, previous studies have demonstrated efficacy for serotonin-norepinephrine reuptake inhibitors in chronic LBP.(52) Non-pharmacologic interventions, such as cognitive behavioral therapy and exercise, have also demonstrated excellent effect sizes that often exceed pharmacologic interventions in fibromyalgia and other pain states.(53)
Limitations
The location of the study was a single, large, tertiary care pain clinic, and these results may not be generalizable. The present study is largely hypothesis generating as it is cross-sectional in nature. Despite the profound phenotypic differences demonstrated in this study, additional research is needed to evaluate the presence of centralized pain (e.g., quantitative sensory testing, neuroimaging) and then to better understand the impact of centralized pain on treatment outcomes. Prospective studies are required to examine whether some of these outcome measures are better than an otherwise detailed history and physical and review of radiographic findings at predicting which patients with axial pain respond best to peripherally-directed procedures, regardless of “diagnosis” (e.g., lumbago, facet arthropathy). As with any dataset, this study was limited by the variables included in the patient-completed phenotype.
Conclusions
New patients presenting to a tertiary care pain clinic with a primary spine pain diagnosis commonly meet ACR survey criteria for fibromyalgia indicating that more widespread pain is present along with a constellation of associated symptoms that are presumed to be largely due to alterations in central neurotransmission. Spine pain patients who met ACR survey criteria for fibromyalgia described profound phenotypic differences when compared to those not meeting criteria, including more neuropathic pain descriptors, higher levels of anxiety symptoms, greater pain interference, and lower physical functioning. These factors largely overlap with those found to be predictive of poor outcomes in spine pain interventions. It is possible that a simple self-report measure could aid in the prediction of outcomes in some of the most common minimally invasive spine interventions.
Acknowledgements
We thank David A. Williams, Ph.D. (Professor, Department of Anesthesiology) and Kevin K. Tremper, Ph.D., M.D. (Professor and Chairman, Department of Anesthesiology) for guidance and support.
Funding: Study was supported by R01 AR060392 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH, Bethesda, MD; Co-PIs Brummett and Clauw), and the American Society of Regional Anesthesia and Pain Medicine Chronic Pain Research Grant (PI Brummett). Additional funding was from the Department of Anesthesiology, University of Michigan.
Footnotes
2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome; http://www.canadianpainsociety.ca/pdf/Fibromyalgia_Guidelines_2012.pdf; Last accessed February 6, 2013
Disclosures: Dr. Brummett is a consultant for Purdue Pharma L.P. (Stamford, CT). Dr. Clauw receives grant funding from Merck and Co. (Whitehouse Station, NJ), Forest Laboratories (New York, NY), and Nova (Baulkham Hills, Australia). In addition, Dr. Clauw is a consultant for Forest Laboratories, Merck & Co., Pierre Fabre Pharmaceuticals Inc (Parsippany, NJ), Jazz Pharmaceuticals (Palo Alto, CA), Eli Lilly & Co Inc (Indianapolis, IN), UCB Inc (Smyrna, GA), Purdue Pharma, Pfizer Inc (New York, NY), and Nuvo. Dr. Hassett receives research funding from Bristol-Myers Squibb and Pfizer. In addition, Dr. Hassett is a consultant for Bristol-Myers Squibb and Pfizer. There are otherwise no relevant disclosures.
Previous presentations: Preliminary data presented at the Best Abstracts session at the American Society of Anesthesiologists Annual Meeting October 18, 2011.
Permissions for Use of Copyrighted Surveys: Permission was obtained for use of all of the copyrighted self-report measures presented in this study, including the Brief Pain Inventory, PainDETECT, and Hospital Anxiety and Depression Scale.
References
- 1.The Burden of Musculoskeletal Diseases in the United States. American Academy of Orthopaedic Surgeons; Rosemont, IL: 2011. Spine: Low Back and Neck Pain. United States Bone and Joint Initiative. [Google Scholar]
- 2.Katz JN. Lumbar disc disorders and low-back pain: socioeconomic factors and consequences. J Bone Joint Surg Am. 2006;88(Suppl 2):21–4. doi: 10.2106/JBJS.E.01273. [DOI] [PubMed] [Google Scholar]
- 3.Manchikanti L, Pampati V, Singh V, Boswell MV, Smith HS, Hirsch JA. Explosive growth of facet joint interventions in the medicare population in the United States: a comparative evaluation of 1997, 2002, and 2006 data. BMC Health Serv Res. 2010;10:84. doi: 10.1186/1472-6963-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manchikanti L, Pampati V, Boswell MV, Smith HS, Hirsch JA. Analysis of the growth of epidural injections and costs in the Medicare population: a comparative evaluation of 1997, 2002, and 2006 data. Pain Physician. 2010;13(3):199–212. [PubMed] [Google Scholar]
- 5.Cohen SP, Bajwa ZH, Kraemer JJ, Dragovich A, Williams KA, Stream J, et al. Factors predicting success and failure for cervical facet radiofrequency denervation: a multi-center analysis. Reg Anesth Pain Med. 2007;32(6):495–503. doi: 10.1016/j.rapm.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Cohen SP, Stojanovic MP, Crooks M, Kim P, Schmidt RK, Shields CH, et al. Lumbar zygapophysial (facet) joint radiofrequency denervation success as a function of pain relief during diagnostic medial branch blocks: a multicenter analysis. Spine J. 2008;8(3):498–504. doi: 10.1016/j.spinee.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Cohen SP, Hurley RW, Christo PJ, Winkley J, Mohiuddin MM, Stojanovic MP. Clinical predictors of success and failure for lumbar facet radiofrequency denervation. Clin J Pain. 2007;23(1):45–52. doi: 10.1097/01.ajp.0000210941.04182.ea. [DOI] [PubMed] [Google Scholar]
- 8.Stojanovic MP, Sethee J, Mohiuddin M, Cheng J, Barker A, Wang J, et al. MRI analysis of the lumbar spine: can it predict response to diagnostic and therapeutic facet procedures? Clin J Pain. 2010;26(2):110–5. doi: 10.1097/AJP.0b013e3181b8cd4d. [DOI] [PubMed] [Google Scholar]
- 9.Manchikanti L, Cash KA, Pampati V, Fellows B. Influence of psychological variables on the diagnosis of facet joint involvement in chronic spinal pain. Pain Physician. 2008;11(2):145–60. [PubMed] [Google Scholar]
- 10.Schiff E, Eisenberg E. Can quantitative sensory testing predict the outcome of epidural steroid injections in sciatica? A preliminary study. Anesth Analg. 2003;97(3):828–32. doi: 10.1213/01.ANE.0000078583.47735.69. [DOI] [PubMed] [Google Scholar]
- 11.Revel M, Poiraudeau S, Auleley GR, Payan C, Denke A, Nguyen M, et al. Capacity of the clinical picture to characterize low back pain relieved by facet joint anesthesia. Proposed criteria to identify patients with painful facet joints. Spine (Phila Pa 1976) 1998;23(18):1972–6. doi: 10.1097/00007632-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 12.Revel ME, Listrat VM, Chevalier XJ, Dougados M, N’Guyen MP, Vallee C, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73(9):824–8. [PubMed] [Google Scholar]
- 13.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–72. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams DA, Clauw DJ. Understanding fibromyalgia: lessons from the broader pain research community. J Pain. 2009;10(8):777–91. doi: 10.1016/j.jpain.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauw DJ, Schmidt M, Radulovic D, Singer A, Katz P, Bresette J. The relationship between fibromyalgia and interstitial cystitis. J Psychiatr Res. 1997;31(1):125–31. doi: 10.1016/s0022-3956(96)00051-9. [DOI] [PubMed] [Google Scholar]
- 17.Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best Pract Res Clin Rheumatol. 2011;25(2):133–9. doi: 10.1016/j.berh.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 18.El-Gabalawy H, Ryner L. Central nervous system abnormalities in fibromyalgia: assessment using proton magnetic resonance spectroscopy. J Rheumatol. 2008;35(7):1242–4. [PubMed] [Google Scholar]
- 19.Schmidt-Wilcke T, Clauw DJ. Pharmacotherapy in fibromyalgia (FM) - Implications for the underlying pathophysiology. PharmacolTher. 2010;127(3):283–94. doi: 10.1016/j.pharmthera.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Amris K, Jespersen A, Bliddal H. Self-reported somatosensory symptoms of neuropathic pain in fibromyalgia and chronic widespread pain correlate with tender point count and pressure-pain thresholds. Pain. 2010;151(3):664–9. doi: 10.1016/j.pain.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Rehm SE, Koroschetz J, Gockel U, Brosz M, Freynhagen R, Tolle TR, et al. A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology (Oxford) 2010;49(6):1146–52. doi: 10.1093/rheumatology/keq066. [DOI] [PubMed] [Google Scholar]
- 22.Fitzcharles MA, Yunus MB. The clinical concept of fibromyalgia as a changing paradigm in the past 20 years. Pain Res Treat. 2012;2012:184835. doi: 10.1155/2012/184835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clauw DJ, Witter J. Pain and rheumatology: thinking outside the joint. Arthritis Rheum. 2009;60(2):321–4. doi: 10.1002/art.24326. [DOI] [PubMed] [Google Scholar]
- 24.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, et al. Pain sensitivity as a correlate of clinical status in individuals with chronic low back pain. Spine. 1999;24(19):2035–41. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- 26.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50(2):613–23. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–22. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 28.Hauser W, Jung E, Erbsloh-Moller B, Gesmann M, Kuhn-Becker H, Petermann F, et al. Validation of the Fibromyalgia Survey Questionnaire within a Cross-Sectional Survey. PLoS One. 2012;7(5):e37504. doi: 10.1371/journal.pone.0037504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 30.Hassett AL, Wasserman R, Goesling J, Rakovitis K, Shi B, Brummett CM. Longitudinal Assessment of Pain Outcomes in the Clinical Setting: Development of the “APOLO” Electronic Data Capture System. Reg Anesth Pain Med. 2012;37(4):398–402. doi: 10.1097/AAP.0b013e3182524672. [DOI] [PubMed] [Google Scholar]
- 31.Brummett CM, Hassett AL, Brummett KA, Clauw DJ, Williams DA. The Michigan Body Map and Its Use in Assessing the American College of Rheumatology Survey Criteria for Fibromyalgia. Arthritis & Rheumatism. 2011;63(10 supplement):S368. [Google Scholar]
- 32.Vincent A, Lahr BD, Wolfe F, Clauw DJ, Whipple MO, Oh TH, et al. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology project. Arthritis Care Res (Hoboken) 2012;65(5):786–92. doi: 10.1002/acr.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–7. doi: 10.1016/j.jpain.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Freynhagen R, Baron R, Gockel U, Tolle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22(10):1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 35.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976) 2000;25(22):2940–52. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 36.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 38.Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–18. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 40.George SZ, Wittmer VT, Fillingim RB, Robinson ME. Sex and pain-related psychological variables are associated with thermal pain sensitivity for patients with chronic low back pain. J Pain. 2007;8(1):2–10. doi: 10.1016/j.jpain.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Puta C, Schulz B, Schoeler S, Magerl W, Gabriel B, Gabriel HH, et al. Enhanced sensitivity to punctate painful stimuli in female patients with chronic low back pain. BMC Neurol. 2012;12:98. doi: 10.1186/1471-2377-12-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kindler LL, Jones KD, Perrin N, Bennett RM. Risk factors predicting the development of widespread pain from chronic back or neck pain. J Pain. 2010;11(12):1320–8. doi: 10.1016/j.jpain.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60(10):3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mease PJ, Dundon K, Sarzi-Puttini P. Pharmacotherapy of fibromyalgia. Best Pract Res Clin Rheumatol. 2011;25(2):285–97. doi: 10.1016/j.berh.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Pinto R, Maher CG, M.L. F, Hancock M, Oliveira VC, McLachlan AJ, et al. Epidural corticoid injections in the management of sciatica. Ann Intern Med. 2012;157:865–77. doi: 10.7326/0003-4819-157-12-201212180-00564. [DOI] [PubMed] [Google Scholar]
- 47.Iversen T, Solberg TK, Romner B, Wilsgaard T, Twisk J, Anke A, et al. Effect of caudal epidural steroid or saline injection in chronic lumbar radiculopathy: multicentre, blinded, randomised controlled trial. BMJ. 2011;343:d5278. doi: 10.1136/bmj.d5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sayegh FE, Kenanidis EI, Papavasiliou KA, Potoupnis ME, Kirkos JM, Kapetanos GA. Efficacy of steroid and nonsteroid caudal epidural injections for low back pain and sciatica: a prospective, randomized, double-blind clinical trial. Spine (Phila Pa 1976) 2009;34(14):1441–7. doi: 10.1097/BRS.0b013e3181a4804a. [DOI] [PubMed] [Google Scholar]
- 49.Wilkinson IM, Cohen SP. Epidural steroid injections. Curr Pain Headache Rep. 2012;16(1):50–9. doi: 10.1007/s11916-011-0236-9. [DOI] [PubMed] [Google Scholar]
- 50.Dreyfuss P, Halbrook B, Pauza K, Joshi A, McLarty J, Bogduk N. Efficacy and validity of radiofrequency neurotomy for chronic lumbar zygapophysial joint pain. Spine (Phila Pa 1976) 2000;25(10):1270–7. doi: 10.1097/00007632-200005150-00012. [DOI] [PubMed] [Google Scholar]
- 51.Lord SM, Barnsley L, Wallis BJ, McDonald GJ, Bogduk N. Percutaneous radio-frequency neurotomy for chronic cervical zygapophyseal-joint pain. N Engl J Med. 1996;335(23):1721–6. doi: 10.1056/NEJM199612053352302. [DOI] [PubMed] [Google Scholar]
- 52.Skljarevski V, Desaiah D, Liu-Seifert H, Zhang Q, Chappell AS, Detke MJ, et al. Efficacy and safety of duloxetine in patients with chronic low back pain. Spine (Phila Pa 1976) 2010;35(13):E578–85. doi: 10.1097/BRS.0b013e3181d3cef6. [DOI] [PubMed] [Google Scholar]
- 53.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151(2):280–95. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]