Abstract
Objective
We evaluated the effect of time to surgery on tumor growth by comparing initial imaging and pathologic tumor size estimates. We also determined predictors of delay to surgery.
Summary Background Data
Preoperative work-up, coordination of reconstructive surgery, and referral to tertiary care centers can delay surgical treatment of breast cancer. Whether these delays are associated with interim tumor progression is unknown.
Methods
We identified 818 clinically node-negative breast cancer patients at our cancer center who had undergone surgery as their first therapeutic modality for invasive breast cancer from 9/2003 to 12/2006. Baseline tumor size was determined by mammography and sonography; tumor size at surgery was determined from pathology reports.
Results
The median time from imaging to surgery was 21 days (1 to 132 days). In multivariate analysis, increased time to surgery was associated with older age, total mastectomy versus breast-conserving surgery, and reconstructive surgery. The median difference from baseline mammographic tumor size to surgery was 0 cm (8.6 cm smaller to 7.3 cm larger at surgery). The median difference from baseline sonographic tumor size to surgery was 0.1 cm (7.5 cm smaller to 8.3 cm larger at surgery). Neither of these differences was significantly associated with time to surgery. Time to surgery was associated with positive lymph nodes at surgery; however, no association was found after controlling for other prognostic factors.
Conclusions
Modest time intervals from imaging to surgery are not significantly associated with change in tumor size; thus, patients may undergo preoperative work-up without experiencing significant disease progression.
Introduction
The recent focus on health care policy has stimulated interest in optimizing multidisciplinary breast cancer care, with one emphasis being on quality assessment. The wait time from diagnosis to treatment is a component of these discussions and had been proposed as a quality measure. This issue is likely to become increasingly relevant as our population ages and there are a greater number of at-risk women aged 50 years or more.1 The aging of our population is likely to translate into an increased number of breast cancer diagnoses and the number of patients requiring surgical management of their disease. It is anticipated that this could burden health care systems that have not secured the resources required to meet this increasing demand for surgical care. In addition to an increase in the number of patients requiring surgery, other considerations/issues that can delay the surgical treatment of breast cancer include coordination of reconstructive surgery, co-morbidity evaluation, and referrals to tertiary care centers.
Whether modest treatment delays are associated with disease progression is unknown. Weedon-Fekjaer et. al.9 developed a model that estimates tumor progression by calculating tumor size as a function of size at mammography (the gold standard for breast cancer screening) vs. size at clinical detection. A mean growth rate of 1.7 years was found for an increase from 10 mm to 20 mm. However, large variations existed in tumor growth and tumor doubling times at 15 mm, varying from 41 days for the first quartile to 234 days for the fourth quartile. Older women had significantly slower growing tumors. Thus, patient and tumor characteristics may be predictive of tumor growth rates during delays to surgical treatment.
In this retrospective study, we determined the difference in tumor sizes at initial imaging performed at our cancer center and at surgery in patients undergoing treatment for breast cancer. We also determined predictors of delays to surgery.
Patients and Methods
The Breast Surgical Oncology prospective database at The University of Texas M. D. Anderson Cancer Center (Houston, Texas) was used to identify patients who had been diagnosed with invasive breast carcinoma from September 2003 to December 2006. Electronic records of reports were reviewed to obtain tumor sizes on mammography or sonography performed at outside facilities prior to presentation at our institution. All patients in this study had undergone repeat diagnostic imaging to include mammogram or ultrasound at the M. D. Anderson Cancer Center, followed by primary surgical treatment. Patients were excluded from the study if they had undergone neoadjuvant systemic therapy, were clinically node positive at presentation, or had undergone surgical excision of the primary tumor prior to presenting at M. D. Anderson. The final tumor size was defined as the pathologic tumor size at surgery. This study was approved by the M. D. Anderson institutional review board with waiver of informed consent.
Electronic records were reviewed to extract the relevant clinicopathologic features. Baseline tumor size was determined by mammography or sonography. Patient and tumor characteristics were summarized using medians and frequencies. The change in tumor size was defined as the pathologic tumor size minus the sonographic tumor size or the pathologic tumor size minus the mammographic tumor size. Equal tumor size estimates were reported as the “same.” The time from imaging performed at our institution to surgery was calculated in days. The time interval was evaluated as a predictor of tumor size change in univariate and multivariate generalized linear models for an unbalanced analysis of variance. In the multivariate model, we controlled for patient age; baseline sonographic or mammographic tumor size; estrogen receptor (ER), progesterone receptor (PR), and HER 2 status; and tumor grade and histologic type. Independent variables included in the model of time from imaging to surgery included patient age, surgical procedure, number of procedures, and reconstructive surgery. We also used univariate and multivariate logistic regression analyses to determine whether time from imaging to surgery was associated with positive nodal status at surgery and to compute odds ratios and 95% confidence intervals (CIs). In the multivariate model, we controlled for patient age; imaging tumor size; tumor grade; ER, PR, and HER 2 status; lymphovascular invasion; tumor histologic type; tumor focality; and time from imaging to surgery. Calculations were performed using SAS software version 9.2. All P values were two-tailed and considered statistically significant at less than 0.05.
Results
Patient characteristics
We identified 818 patients who had been diagnosed with clinically node-negative invasive breast carcinoma and underwent initial surgical intervention at M. D. Anderson. Patient and tumor characteristics are summarized in Table 1. Most patients (77%) had invasive ductal carcinoma, 16% had invasive lobular carcinoma, and 7% had other types of invasive carcinomas. The median age at diagnosis was 58 years (range, 29-88 years). Initial surgical interventions included breast-conserving surgery (571 [70%]) or total mastectomy (247 [30%]). One hundred forty-three (18%) patients underwent immediate breast reconstruction. Twenty-four (4%) of the 571 patients who underwent breast-conserving surgery and 119 (48%) of the 247 patients who had undergone total mastectomy also underwent reconstructive surgery. The median time from initial imaging at M. D. Anderson Cancer Center to surgery was 21 days (range, 1 to 132 days).
Table 1. Patient characteristics.
| Characteristic | Result n=818 |
|---|---|
| Median age, years (range) | 58 (29-88) |
| Histologic carcinoma type, n (%) | |
| Invasive ductal | 632 (77.3) |
| Invasive lobular | 129 (15.8) |
| Other invasive | 57 (7.0) |
| Tumor grade, n (%) | |
| I | 119 (14.6) |
| II | 468 (57.2) |
| III | 229 (28.0) |
| Unknown | 2 (0.2) |
| ER status, n (%) | |
| Positive | 644 (78.7) |
| Negative | 149 (18.2) |
| Unknown | 25 (3.1) |
| PR status, n (%) | |
| Positive | 537 (65.6) |
| Negative | 246 (30.1) |
| Unknown | 35 (4.3) |
| HER2 status, n (%) | |
| Positive | 82 (10.0) |
| Negative | 675 (82.5) |
| Unknown | 61 (7.5) |
| Initial surgical procedure, n (%) | |
| Breast-conserving surgery | 571 (69.8) |
| Total mastectomy | 247 (30.2) |
| Reconstructive surgery, n (%) | |
| Yes | 143 (17.5) |
| No | 675 (82.5) |
Comparison of imaging and pathologic tumor sizes
We first determined the difference between mammographic and pathologic tumor sizes. Six hundred thirty-five patients underwent mammography at our institution. The pathologic tumor size was the same as the mammographic size in 14% of patients (n=88), smaller in 49% (n=311), and larger 37% (n=236) (Figure 1A). Pathologic and mammographic tumor sizes differed by ≤ 0.5 cm in 70% of cases. The median change in tumor size from mammography at our institution to surgery was 0 cm (range, 8.6 cm smaller to 7.3 cm larger at surgery). The time from imaging to surgery was not significantly associated with the difference in mammographic and pathologic tumor size estimates, either in univariate analysis or in a multivariate model that controlled for patient age; tumor histologic type; tumor grade; ER, PR, and HER2 status; and mammographic tumor size.
Figure 1.
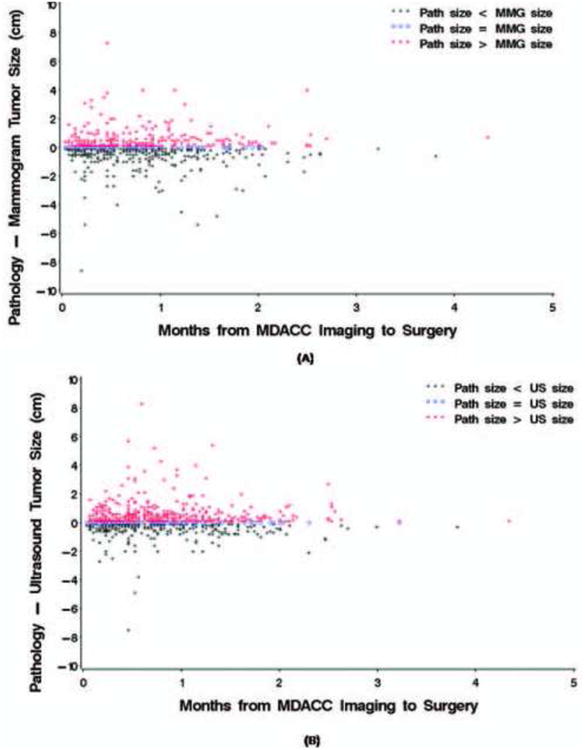
Estimated difference between imaging and pathologic tumor sizes from imaging to surgery. (A) Mammography. (B) Sonography.
We next determined the difference between sonographic and pathologic tumor sizes. Seven hundred seventy-eight patients had undergone sonography at our institution. Pathologic tumor sizes were the same as sonographic size estimates in 11% of patients (n=82), smaller in 38% (n=294), and larger in 52% (n=402) (Figure 1B). Pathologic and sonographic tumor sizes differed by ≤ 0.5 cm in 72% of cases. The median difference between baseline sonographic and pathologic tumor size from was 0.1 cm (range, 7.5 cm smaller to 8.3 cm larger at surgery). In univariate and multivariate analysis, the time interval to surgery was not significantly associated with differences between sonographic and pathologic tumor size. In addition to time from imaging at our institution until surgery, we calculated time from first imaging, whether at an outside facility or M. D. Anderson, until surgery and change in tumor size over that period of time. In both univariate and multivariate models, no significant association was detected between time from first imaging until surgery and the change in tumor size according to the mammogram and ultrasound (Figures 3A & B).
Figure 3.
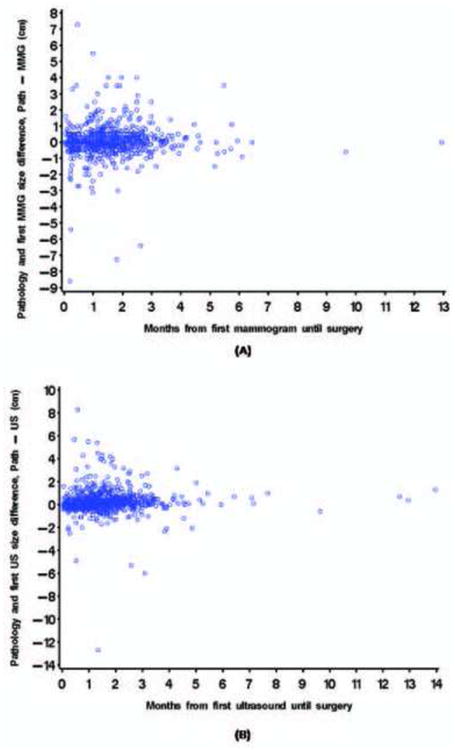
Estimated difference between first imaging and pathologic tumor sizes from first imaging to surgery (A) Mammography (B) Sonography.
Comparison of imaging tumor sizes between institutions
Tumor size estimates for 620 patients with imaging performed at outside facilities were compared with those from our institution. Mammographic tumor size estimates at our institution were the same as those at outside facilities in 32% of cases, smaller in 21%, and larger in 48% (Figure 2A). Sonography estimates at our institution were the same in 11%, smaller in 25%, and larger in 65% (Figure 2B). Among patients who had tumor size estimates, the median time between imaging at outside facilities and at our institution was 33 days (range, 1 to 418 days). No statistically significant association was found between the differences in the outside facilities' and our institution's tumor size estimates and time lapse between the imaging at the separate facilities.
Figure 2.
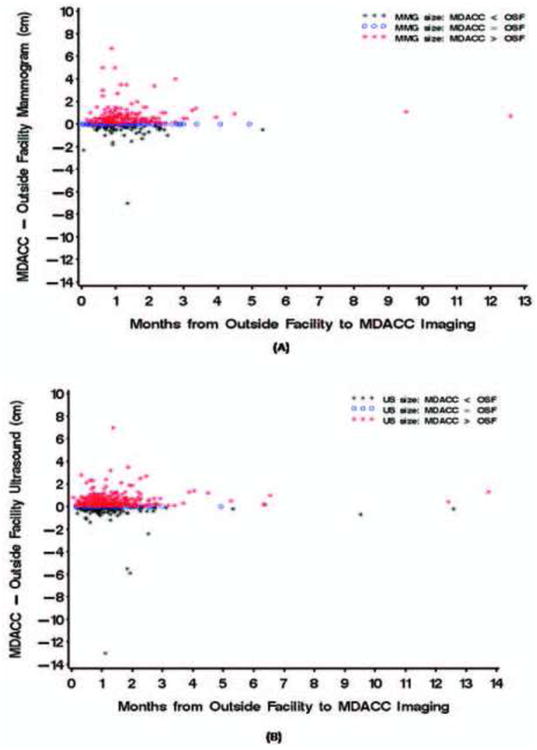
Estimated difference between outside facility and M. D. Anderson imaging tumor sizes by time between imaging. (A) Mammography. (B) Sonography.
Predictors of Delays to Surgery
In univariate analysis, initial surgical intervention type (P<0.0001) and reconstructive surgery (P<0.0001) were significantly associated with time from imaging at M. D. Anderson to surgery. Patients who underwent total mastectomy had a longer time to surgery (median, 26 days; range, 2 to 132 days) than did those who underwent breast-conserving surgery (median, 17 days; range, 1 to 116 days). In addition, patients who underwent reconstructive surgery had a median delay of 29 days (range, 4 to 124 days) vs. 19 days (range, 1 to 132 days) in those who had not undergone reconstructive surgery. A nonsignificant trend was found between older patient age and increased time to surgery (P=0.0752). The results of multivariate modeling indicated that total mastectomy (P=0.0121), reconstructive surgery (P=0.0005), and older age (P=0.0022) were significant predictors of increased time from initial M. D. Anderson imaging to surgery. In addition to analyzing delay from imaging until surgery for the entire sample, we performed several subgroup analyses based on the time from imaging until surgery. We examined each of the following subsets; those with a delay over the median of 21 days, those with a delay over 35 days (3rd quartile), 49 days (top 10%), and 59 days (top 5%). No association between the delay from M. D. Anderson imaging until surgery and change in tumor size was found for any of these subgroups (Figures 4A & B).
Figure 4.
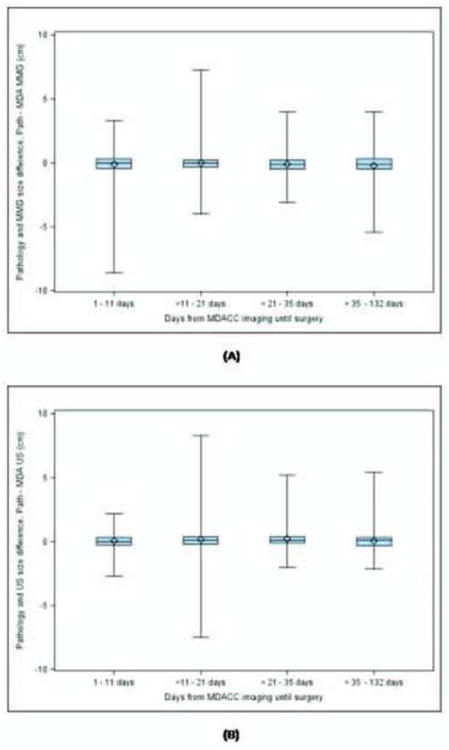
Difference between imaging and pathologic tumor size by quartiles of time from M. D. Anderson imaging to surgery (A) Mammography (b) Sonography.
Predictors of nodal metastasis
We also identified variables associated with positive lymph nodes at surgery. All 818 patients included in this study were clinically node negative at the time of staging sonography; 193 (24%) had nodal metastasis at surgery. In univariate analysis, the delay between imaging at our institution and surgery was significantly associated with nodal metastasis (P=0.0110). In addition, young age at diagnosis (P=0.0151), large tumor size on imaging (P=0.0014), high tumor grade (P=0.0194), ER-positive status (P=0.0062), lymphovascular invasion (P<0.0001), invasive lobular tumor histologic type (vs. ductal, P=0.0020), other invasive carcinoma (vs. ductal, P=0.0102), and tumor multifocality on sonography (P=0.0384) were significantly associated with nodal metastasis. PR status and HER2 status were not significantly associated with nodal status at surgery. In multivariate analysis, however, time to surgery was not significantly associated with nodal status after we controlled for patient age; tumor grade and histologic type; ER, PR, and HER2 status; lymphovascular invasion; and baseline imaging size at M. D. Anderson (Table 2). Furthermore, no association was detected between time delay between time from first imaging, performed either at an outside facility or at M. D. Anderson, until surgery, and nodal status.
Table 2.
Multivariate logistic regression analyses to predict positive lymph node status at time of surgery in breast cancer patients (n=666*).
| Variable | Multivariate | |
|---|---|---|
|
| ||
| OR (95% CI) | P value | |
| Months from imaging to surgery | 1.31 (0.95-1.82) | 0.1065 |
| Age at diagnosis (years) | 0.99 (0.97-1.01) | 0.1914 |
| Tumor grade | ||
| II or III | 1.14 (0.62-2.10) | 0.6802 |
| I | 1.00 | |
| ER status | ||
| Positive | 2.41 (1.21-4.80) | 0.0127 |
| Negative | 1.00 | |
| PR status | ||
| Positive | 0.94 (0.56-1.56) | 0.8024 |
| Negative | 1.00 | |
| HER2 status | ||
| Positive | 1.50 (0.83-2.71) | 0.1768 |
| Negative | 1.00 | |
| Lymphovascular invasion | ||
| Yes | 3.57 (2.33-5.47) | <.0001 |
| No | 1.00 | |
| Histologic carcinoma type | ||
| Infiltrating lobular | 1.77 (1.09-2.88) | 0.0205 |
| Other invasive | 0.36 (0.10-1.25) | 0.1068 |
| Infiltrating ductal | 1.00 | |
| Sonographic focality | ||
| Multifocal | 1.30 (0.77-2.19) | 0.3226 |
| Unifocal or multicentric | 1.00 | |
| Imaging size (sonography or mammography if no sonography), cm | 1.40 (1.14-1.70) | 0.0011 |
Analysis limited to the 666 patients with known variables.
Discussion
Breast cancer growth occurs over years before it can be detected with standard imaging studies; however, tumor growth rates may vary.2 Therefore, in this study, we determined whether delays from imaging to surgery were associated with tumor growth, as determined by differences between pathologic and imaging tumor sizes. The median time from imaging at our facility to surgery was 21 days. Discrepancies between pathologic and imaging tumor sizes did not follow a pattern that would indicate interim tumor growth had occurred. This finding suggests that modest treatment delays are not associated with significant tumor growth.
Increasing interest in optimizing health care quality has led to an emphasis on reducing time to treatment initiation. Several groups have proposed that time from diagnosis to surgery be used as a quality measure for breast cancer treatment.3,4 However, patients often seek second opinions and care at tertiary care centers or immediate reconstructive surgery, all of which may ensure or enhance quality but also delay time to initiation of therapy. Furthermore, it is not uncommon for patients to request surgical delays of a few weeks to coordinate travel of relatives, work schedules or for other personal reasons.
National health care systems have been referenced as potential models of standards of care. In the United Kingdom, the Department of Health stated that survival rates for women with breast cancer would improve if regional variations in the delivery of relevant services were reduced; thus, it recommended that breast cancer be treated only in specialist cancer units.5 In 1999, they began an initiative in which all patients with suspected breast cancer are evaluated by a specialist within 2 weeks of their general practitioner's request for an appointment.6 In our study, the median time from imaging at outside facilities to imaging at our institution was 33 days, illustrating that although specialist referrals may ensure quality care, they also confer additional delays.
In its position statement, the Canadian SSO recommends that patients undergo treatment within 2 weeks of completing any necessary preoperative tests. However, the 2-week recommendation has been scrutinized. Bardell et. al.4 found that wait times in Ontario increased from 12 days in 1984-1987 to 27 days in 1998-2000. The median wait time from first diagnostic procedure to first surgery in Quebec was 42 days in 1998, an increase from 29 days in 1992. Furthermore, Reed et al.2 found that wait times increased 2 days per year from 1997 to 2000. These increases may reflect increases in breast cancer incidence; practice pattern changes, including preoperative work-up, care access and staff and operating room availability. All of these remain important factors in the United States as well, and the impending shortage of general surgeons in U.S. is likely to result in additional delays nationwide.7
Several patient factors have been associated with diagnostic delays, including older patient age, full-time employment, and somewhat surprisingly, higher education and family history of breast cancer.8 Studies have also addressed the reasons for delay of adjuvant therapy after surgery. Hershman et. al9 reported that delays in initiating chemotherapy were associated with factors such as older age, rural location, being unmarried, early tumor stage, hormone receptor positivity, use of mastectomy, and not undergoing radiation therapy. Similarly, in our study, older age was associated with longer time to surgery. The retrospective nature of our study did not allow us to determine the reason for this association, but it may be due to patient-related medical factors, such as preoperative work-up of co-morbidities, or personal factors, such as postoperative care arrangements. Alternatively, it may be provider related. In our study, mastectomy and reconstructive surgery were associated with treatment delays. Most patients with early stage breast cancer at our institution have the opportunity to undergo immediate reconstructive surgery at the time of mastectomy and scheduling a coordinated procedure with two surgeons may contribute to a delay in treatment. However, immediate reconstruction is advantageous, both from an esthetic standpoint and a psychosocial standpoint.10 Our results suggest that modest delays in scheduling are unlikely to result in significant interim tumor growth.
Whether delays in breast cancer diagnosis and treatment adversely affect prognosis has been a subject of debate. In a systematic review of published studies, Richards et al11 reported that delays of more than 3 months between symptom onset and treatment were associated with a 12% reduction in 5-year survival rates. However, upon further investigation through a single center retrospective analysis, Richards et al12 reported that delayed presentation was not significantly associated with survival when it was included with tumor size and stage in a multivariate model. Delayed initiation of chemotherapy beyond 3 months after surgery has also been associated with increased disease-specific mortality (hazard ratio=1.69, 95% CI=1.31-2.19) and overall mortality (hazard ratio=1.49, 95% CI=1.21-1.75).9 The results of some studies have suggested that a delay in radiation therapy after breast-conserving surgery does not affect outcomes,13 but a systematic review of eight studies that compared local disease control among patients treated within 8 weeks of surgery with that among patients treated more than 8 weeks after surgery demonstrated that 5-year local recurrence rates were significantly higher in patients whose treatment was delayed (odds ratio=1.62, 95% CI=1.21-2.16).14 In all of these studies, whether the adverse outcome was due to the delay or to factors associated with the delay is unclear. Regardless, treatment delays may result in genuine risks for adverse outcomes. The patient series reported in this study was relatively recent; thus, we do not have enough follow-up data to assess long-term outcomes. Further study is needed to determine the relative oncologic safety of modest operative delays on distant disease-free and overall survival.
Predictors of nodal metastasis have been well described in the medical literature. Large tumor size, lymphovascular invasion, and high tumor grade have consistently been shown to be significant predictors of axillary lymph node metastasis.15,16,17,18 Similarly, we found that large tumor size on imaging, lymphovascular invasion, invasive ductal and invasive lobular tumor histologic type, ER positivity, and tumor multifocality were significantly associated with nodal metastasis at surgery. Time to surgery was associated with nodal positivity in univariate analysis, but it was not in multivariate analysis. Both nodal positivity and mastectomy and reconstruction (and thus time to surgery) may be associated with a common variable (e.g., large tumor size). However, further studies are needed to confirm that no association exists between time to surgery and nodal progression.
The current study has several limitations. First, we used initial imaging size-pathologic size discordance as a surrogate for tumor growth. Unfortunately, our current imaging methods are limited in their ability to accurately predict tumor size. As in other reports,19,20mammographic and sonographic tumor size were modestly correlated with pathologic size. Differences between outside facility and M. D. Anderson estimates may have resulted from the use of analog vs. digital mammographic technology. Magnetic resonance imaging (MRI) has been reported to be more accurate at assessing tumor size, but it is not routinely used at our institution.20,21 However, it is notable that MRI use itself has been associated with a 22.4-day delay in pretreatment evaluation (P=0.011).22 Second, assessing the change in tumor size as measured by imaging at diagnosis to the size on imaging immediately prior to surgery may allow more uniform comparison of disease progression. Needle localization for breast conserving surgery is the only imaging performed prior to surgery at our institution. However, tumor size estimates are not evaluated or recorded. Patients that undergo mastectomy do not receive preoperative imaging, therefore, no imaging size estimates are available to perform this type of analysis in a retrospective fashion. Third, our study had patient selection limitations: as our primary endpoint relied on pathology-imaging correlation, our study cohort was limited to patients who had undergone surgery first. During the time period of this study, neoadjuvant chemotherapy was widely used at our institution, with many patients with stage II and III tumors being treated with chemotherapy first. Thus, patients with more aggressive tumors may not have been included in our study. Fourth, because of the retrospective nature of our study, we were not able to determine the reasons for surgical delays. Finally, this was a recent patient series: it reflects our current practice but does not allow us to determine the effect of delays on disease-free and overall survival. Continuing to gather vital data from segregated studies will provide the information necessary to optimize breast cancer treatment.
We recommend patients with palpable masses, symptoms, or imaging findings that are suggestive of breast cancer, be appropriately evaluated and treated. Our findings suggest that in patients who present with operable disease, modest delays associated with therapeutic planning are not associated with primary tumor growth. Therefore, patients may seek specialized care, including reconstructive surgery. When assessing breast cancer care quality, time to surgery should be evaluated in the context of surgical outcome quality. However, further study is needed into strategies to shorten time to surgery.
Footnotes
The authors indicate no potential conflicts of interest.
Jamie L. Wagner: First author substantially contributed to conception and design, acquisition and interpretation of data, drafting the article and gave final approval for manuscript publication.
Carla L. Warneke: Substantially contributed to analysis and interpretation of data, participated in revising of the manuscript and gave final approval for publication.
Elizabeth A. Mittendorf: Substantially contributed to concept, interpretation of data, critical revising of the manuscript and gave final approval for publication.
Isabelle Bedrosian: Substantially contributed to concept, interpretation of data, critical revising of the manuscript and gave final approval for publication.
Gildy V. Babiera: Substantially contributed to interpretation of data, critical revising of the manuscript and gave final approval for publication.
Henry M. Kuerer: Substantially contributed to interpretation of data, critical revising of the manuscript and gave final approval for publication.
Kelly K. Hunt: Substantially contributed to concept, interpretation of data, critical revising of the manuscript and gave final approval for publication.
Wei Yang: Substantially contributed to interpretation of data, critical revising of the manuscript and gave final approval for publication.
Aysegul A. Sahin: Substantially contributed to interpretation of data, critical revising of the manuscript and gave final approval for publication.
Funda Meric-Bernstam: Senior author substantially contributed to conception and design, aquisition of data, interpretation of data, drafting and revising the manuscript and gave final approval for publication.
There are no financial disclosures from any of the authors
There is no commercial sponsorship
References
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009 Jun 10;27(17):2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Weedon-Fekjaer H, Lindqvist BH, Vatten LJ, Aalen OO, Tretli S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008;10(3):R41. doi: 10.1186/bcr2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCahill LE, Privette A, James T, Sheehey-Jones J, Ratliff J, Majercik D, et al. Quality measures for breast cancer surgery: initial validation of feasibility and assessment of variation among surgeons. Arch Surg May. 2009;144(5):455–462. doi: 10.1001/archsurg.2009.56. discussion 462-453. [DOI] [PubMed] [Google Scholar]
- 4.National Quality Measures for Breast Centers™. [Accessed February 13 2010];A Quality Initiative of the National Consortium of Breast Centers, Inc. Website. Available at: http://www.nqmbc.org/List_of_all_NQMBC_Measures.pdf:
- 5.Lancaster T. Crown Copyright; 1996. [Accessed February]. Guidance for general practitioners and primary care teams improving outcomes in breast cancer. Department of Health website. Available at: http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4006778. 4006713, 4002010. [Google Scholar]
- 6.Department of Health. Crown Copyright; Dec 19-22, 1998. HSC 1998/1242: Breast cancer waiting times achieving the two week target. Available at: http://www.dh.gov.uk/dr_consum_dh/groups/dh_digitalassets/@dh/@en/documents/digitalasset/dh_4011903.pdf. Accessed February 4011913, 4012010. [Google Scholar]
- 7.Williams TE, Jr, Satiani B, Thomas A, Ellison EC. The Impending Shortage and the Estimated Cost of Training the Future Surgical Workforce. Ann Surg. 2009 Aug 27; doi: 10.1097/SLA.0b013e3181b6c90b. [DOI] [PubMed] [Google Scholar]
- 8.Montella M, Crispo A, D'Aiuto G, De Marco M, de Bellis G, Fabbrocini G, et al. Determinant factors for diagnostic delay in operable breast cancer patients. Eur J Cancer Prev. 2001 Feb;10(1):53–59. doi: 10.1097/00008469-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat Oct. 2006;99(3):313–321. doi: 10.1007/s10549-006-9206-z. [DOI] [PubMed] [Google Scholar]
- 10.Chevray PM. Timing of breast reconstruction: immediate versus delayed. Cancer J. 2008 Jul-Aug;14(4):223–229. doi: 10.1097/PPO.0b013e3181824e37. [DOI] [PubMed] [Google Scholar]
- 11.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999 Apr 3;353(9159):1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 12.Richards MA, Smith P, Ramirez AJ, Fentiman IS, Rubens RD. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999 Feb;79(5-6):858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vujovic O, Perera F, Dar AR, Stitt L, Yu E, Voruganti SM, et al. Does delay in breast irradiation following conservative breast surgery in node-negative breast cancer patients have an impact on risk of recurrence? Int J Radiat Oncol Biol Phys. 1998 Mar 1;40(4):869–874. doi: 10.1016/s0360-3016(97)00922-x. [DOI] [PubMed] [Google Scholar]
- 14.Huang J, Barbera L, Brouwers M, Browman G, Mackillop WJ. Does delay in starting treatment affect the outcomes of radiotherapy? A systematic review. J Clin Oncol. 2003 Feb 1;21(3):555–563. doi: 10.1200/JCO.2003.04.171. [DOI] [PubMed] [Google Scholar]
- 15.Yiangou C, Shousha S, Sinnett HD. Primary tumour characteristics and axillary lymph node status in breast cancer. Br J Cancer. 1999 Aug;80(12):1974–1978. doi: 10.1038/sj.bjc.6690629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenin DR, Manasseh DM, El-Tamer M, Troxel A, Schnabel F, Ditkoff BA, et al. Factors correlating with lymph node metastases in patients with T1 breast cancer. Ann Surg Oncol. 2001 Jun;8(5):432–437. doi: 10.1007/s10434-001-0432-7. [DOI] [PubMed] [Google Scholar]
- 17.Barth A, Craig PH, Silverstein MJ. Predictors of axillary lymph node metastases in patients with T1 breast carcinoma. Cancer. 1997 May 15;79(10):1918–1922. [PubMed] [Google Scholar]
- 18.Theodosiou Z, Kasampalidis IN, Karayannopoulou G, Kostopoulos I, Bobos M, Bevilacqua G, et al. Evaluation of FISH image analysis system on assessing HER2 amplification in breast carcinoma cases. Breast. 2008 Feb;17(1):80–84. doi: 10.1016/j.breast.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 19.Golshan M, Fung BB, Wiley E, Wolfman J, Rademaker A, Morrow M. Prediction of breast cancer size by ultrasound, mammography and core biopsy. Breast. 2004 Aug;13(4):265–271. doi: 10.1016/j.breast.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Wasif N, Garreau J, Terando A, Kirsch D, Mund DF, Giuliano AE. MRI versus ultrasonography and mammography for preoperative assessment of breast cancer. Am Surg. 2009 Oct;75(10):970–975. [PubMed] [Google Scholar]
- 21.Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007 Dec;16(Suppl 2):S34–44. doi: 10.1016/j.breast.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 22.Bleicher RJ, Ciocca RM, Egleston BL, Sesa L, Evers K, Sigurdson ER, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg. 2009 Aug;209(2):180–187. doi: 10.1016/j.jamcollsurg.2009.04.010. quiz 294-185. [DOI] [PMC free article] [PubMed] [Google Scholar]


