Abstract
Objective
Many African infants fail to receive their diagnostic HIV molecular test results and subsequently, antiretroviral therapy (ART). To determine whether a point-of-care molecular HIV test increases ART access for hospitalized Malawian infants, we simulated a point-of-care test using rapid HIV RNA polymerase chain reaction (Rapid PCR) and compared patient outcomes to an optimized standard care that included assessment with the World Health Organization (WHO) clinical algorithm for HIV infection plus a DNA PCR with a turnaround time of several weeks (standard care).
Design
Randomized controlled trial.
Methods
Hospitalized HIV-exposed Malawian infants <12 months old were randomized into Rapid PCR or standard care. Rapid PCR infants obtained molecular test results within 48 hours to facilitate immediate ART, similar to a point-of-care test. Standard care infants meeting clinical criteria were also offered inpatient ART. The primary outcome was appropriate in-hospital ART for DNA or RNA PCR-confirmed HIV-infected infants.
Results
300 infants were enrolled. A greater proportion of HIV-infected infants receiving Rapid PCR, versus standard care, started inpatient ART (72.3% vs 47.8%, p=0.016). Among molecular test-negative infants, 26.9% receiving standard care unnecessarily initiated inpatient ART, versus 0.0% receiving Rapid PCR (p<0.001). Rapid PCR modestly reduced the median days to ART (3.0 vs 6.5, p=0.001) but did not influence outpatient follow-up for HIV-infected infants (78.1% vs 82.4%, P = 0.418).
Conclusions
Rapid PCR, versus an optimized standard care, increased the proportion of hospitalized HIV-infected infants initiating ART and reduced ART exposure in molecular test-negative infants, without meaningfully impacting time to ART initiation or follow-up rates.
Keywords: point-of-care, early infant diagnosis of HIV, presumptive diagnosis, Africa, DNA PCR
Introduction
New pediatric HIV infection rates in Africa, home to >90% of the world’s 3.4 million HIV-infected children, are slowing.1 Nevertheless, substantial challenges remain. African mother-to-child HIV transmission rate targets are <5%, but most countries are not meeting this goal.2 Early antiretroviral therapy (ART) for HIV-infected infants reduces mortality,3–5 yet <25% of eligible African children receive ART.6 Low ART coverage is largely due to delayed diagnosis of infants,4 who require molecular diagnostic HIV testing with either HIV DNA polymerase chain reaction (PCR) or HIV RNA PCR. Both tests have limited availability and slow result turnaround time throughout Africa.7,8 These missed diagnostic opportunities contribute to high mortality rates for HIV-infected African infants.4
In Malawi, a southern African country with an 11% adult HIV prevalence,1 improving infant HIV care will require both community and facility-based strategies.9 Given the high HIV prevalence and advanced immunosuppression commonly present in hospitalized Malawian children,10,11 hospitals are key HIV care entry points. Routine in-hospital HIV testing, for example, can identify younger HIV-infected children and increase their access to ART.12,13 But the approach has not fulfilled its potential, as many infants miss their DNA PCR results after testing and result disclosure delays.
One solution to existing DNA PCR system gaps would be a point-of-care infant HIV test that is simple, rapidly processed, and does not require a laboratory.7 Such a point-of-care test could eliminate many of the DNA PCR system barriers, so practitioners could expediently determine an infant’s HIV status and start ART before hospital discharge. The World Health Organization (WHO) clinical algorithm for identifying symptomatic HIV-infected infants could meet these needs. However, it has not been widely implemented due to performance concerns,5,14–17 and its key clinical endpoints including ART initiation have not been rigorously studied. While preliminary studies with recently-developed molecular point-of-care infant HIV tests suggest that their sensitivity and specificity profiles will outperform the WHO algorithm,7 at this time the clinical implications of a point-of-care infant HIV test have not been thoroughly investigated.
Therefore, we conducted a randomized controlled trial to compare patient outcomes between an optimized standard care, defined as a pediatrician evaluation using the WHO algorithm in addition to an HIV DNA PCR test with results turnaround times of several weeks, versus a rapid HIV RNA PCR test with a 48-hour turn-around time (Rapid PCR), which allows patients to receive definitive test results before hospital discharge. Consequently, this Rapid PCR test serves as a surrogate point-of-care infant molecular test, since it permits the clinician to use a definitive HIV status to determine hospital ART eligibility, similar to a true point-of-care test. We hypothesized that Rapid PCR would initiate a greater proportion of HIV-infected infants on ART during hospitalization and expose fewer molecular test-negative infants to ART, compared to an optimized standard care.
Methods
Study Setting, Enrollment, and Definitions
This study occurred between February and November 2011 at Kamuzu Central Hospital (KCH) in Lilongwe, Malawi. KCH admits >13,000 children annually.10 Mother-infant pairs were offered routine HIV antibody testing in the pediatric wards,10 and were invited for enrollment if the infant was HIV-exposed and <12 months old. Study staff obtained written informed consent from caregivers. At enrollment, patients were randomized by computer-generated permutation to either optimized standard care (WHO clinical algorithm evaluation plus DNA PCR) or Rapid PCR (on-site RNA PCR, with results returned within 48 hours, instead of standard care using the WHO clinical algorithm plus DNA PCR). We chose a randomized controlled study design after carefully considering multiple factors including what we believed to be the best methodological approach for answering our primary research question along with practical issues including overall feasibility, cost, and ethical considerations. The study pediatrician and caregivers were not blinded. All infants had a physical examination and chest radiograph interpreted by the study pediatrician. Clinical- and laboratory-confirmed diagnoses and treatments were according to Malawi and WHO guidelines or consensus recommendations.18–20 Patients were excluded if they were previously documented as HIV-uninfected or HIV-infected, or if they were HIV-exposed but unlikely to be HIV-infected because their mother was adherent to ART for >1 year. The latter group was excluded because ethical review determined that both the risk and costs of empiric ART initiation outweighed its potential benefits in this cadre of likely HIV-uninfected children.
An infant could meet the definition of HIV exposure and be eligible for enrollment in two ways. First, HIV exposure included children, irrespective of breastfeeding status, never previously tested for HIV and born to an HIV-infected mother. Second, a child was also HIV-exposed if HIV DNA PCR negative or with an undetectable quantity of HIV virus on RNA PCR (<400 copies/ml) and breastfeeding from an HIV-infected mother <6 weeks after the date of either test. This HIV exposure definition did not rely upon HIV antibody positivity since both severely ill HIV-infected children and acutely HIV-infected children could be HIV antibody-negative. We defined HIV infection as infants with a positive HIV DNA PCR (standard care) or >10,000 copies/ml of HIV virus on RNA PCR (Rapid PCR). HIV molecular test-negative infants were participants that had either a negative HIV DNA PCR (standard care) or undetectable quantity of HIV virus on RNA PCR (<400 copies/ml [Rapid PCR]) irrespective of ongoing breastfeeding status. See Supplemental Digital Content 1, for additional definitions.
Laboratory Procedures
Blood for HIV antibodies (Alere, Trinity Biotech) and either dried blood spot HIV DNA PCR (Amplicor HIV-1 DNA Test, version 1.5® [Roche Molecular Systems, Inc.]) or RNA PCR (Amplicor HIV-1 Monitor, version 1.5® [Roche Molecular Systems, Inc.]) were drawn from standard care and Rapid PCR patients, respectively, based upon randomization results. DNA PCR tests were routinely processed at the government KCH laboratory while RNA PCR tests were processed as prescribed by the manufacturer using manual extraction and PCR set-up (see manufacturer package insert for procedure details) at the University of North Carolina Project laboratory at KCH. A complete blood count with differential, malaria blood smear, blood culture, and a CD4 cell count percentage were collected on all infants and analyzed at the study laboratory.
Each child received a tuberculin skin test, which was assessed 48–72 hours after placement. Children at high risk for tuberculosis (see Supplemental Digital Content 1) had an induced sputum and gastric aspirate microscopy, evaluated by fluorescent microscopy, and culture using MGIT liquid media (Becton, Dickinson and Company) or Lowenstein-Jensen solid agar (Remel). Among children meeting WHO-defined pneumonia criteria or the Pneumocystis jirovecii pneumonia case definition (see Supplemental Digital Content 1), sputum was analyzed by immunofluorescence for P.jirovecii (Biorad). Sputum specimen quality was not evaluated, because no pediatric standards existed at the time of the study.21
WHO Algorithm Evaluation and ART Eligibility
Standard care infants were evaluated per the WHO algorithm (see Supplemental Digital Content 2). WHO algorithm-positive infants were HIV antibody-positive and had >2 conditions (severe sepsis, severe or very severe pneumonia, or oral thrush). Alternatively, infants not meeting criteria of >2 conditions could still be WHO algorithm-positive if found with one AIDS-specific condition (P.jirovecii pneumonia, esophageal candidiasis, treatment-unresponsive severe malnutrition, extra-pulmonary tuberculosis, Kaposi sarcoma, cerebral toxoplasmosis, or cryptococcal meningitis). We classified HIV-exposed infants that tested HIV antibody-negative as ineligible for evaluation by the WHO algorithm, per their antibody-negative status.
If an infant was WHO algorithm-positive, caregivers were counseled that their infant was likely HIV-infected and would benefit from ART (stavudine, lamivudine, and nevirapine). The risks and benefits of commencing ART in a WHO algorithm-positive child were discussed with caregivers. Caregivers of Rapid PCR-positive infants were similarly counseled regarding ART.
Follow-up
Study staff followed infants, irrespective of HIV status, for at least one month after hospital discharge or ART initiation in a hospital-based study clinic to ensure receipt of molecular test results, re-review ART eligibility, and assess for adverse outcomes. ART was continued in WHO algorithm-positive infants taking ART subsequently found to be DNA PCR-positive during follow-up. ART was stopped in DNA PCR-negative infants previously started on ART due to their in-hospital WHO algorithm-positive status. Caregivers of WHO algorithm-negative infants testing DNA PCR-positive were re-counseled regarding HIV infection and ART. Immune reconstitution inflammatory syndrome (IRIS) was defined as infants with a new illness after ART and clinical signs as previously defined.22 HIV-infected and molecular test-negative infants still breastfeeding were transferred to a government HIV clinic once stable with a confirmed HIV status.
Ethical Approval
The Malawi National Health Science Research Committee, UNC-Chapel Hill School of Medicine and Baylor College of Medicine Institutional Review Boards approved this study. This trial was registered at ClinicalTrials.gov NCT01388452.
Statistical Analysis
Data were tested for normality using Q-Q plots and Shapiro-Wilk tests. Normally-distributed data were described with mean and standard deviation, and were compared using a student’s t test. Non-parametric data were described with median and interquartile range, and were compared using the Mann-Whitney U test. Proportions were compared using Pearson’s chi-square or Fisher’s exact test; when a covariate had three or more categories, a global chi-square test was performed, and when significant, post-hoc individual pair-wise testing was performed, with alpha adjusted according to Bonferroni’s correction. All statistical analyses were performed using IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY).
Results
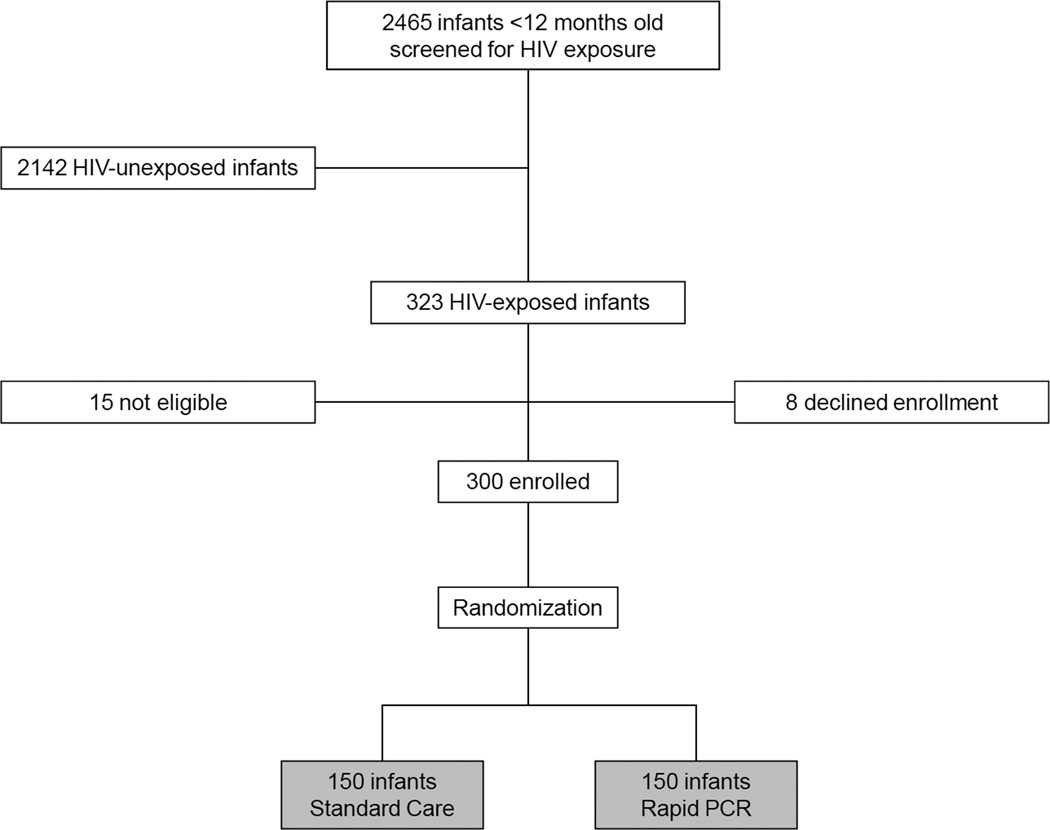
In all, 300 infants were randomized (150 per group [Figure 1]). Enrollment characteristics of both groups were similar (Table 1). The median age of all 300 subjects was 4.6 months, and 153 (51%) were female. Although nearly 2/3 of mother-infant pairs, regardless of randomization, reported receipt of HIV prevention services, just 76 (25.3%) were receiving HIV care. We found that 234 (78.0%) subjects tested HIV antibody-positive with a normal mean CD4% (31.9%). The cohort’s overall HIV prevalence was 31.0% (either DNA PCR-positive [standard care] or >10,000 copies/ml on RNA PCR [Rapid PCR]), with no prevalence difference between groups. The median age of the 66 infants who tested HIV antibody negative was 8.5 months (IQR 6.9–10.7); 50 (75.8%) of these infants were breastfeeding and six (9.1%) were HIV molecular test-positive. The majority of all patients reported cough (67.3%), difficulty breathing (51.7%), and fever (65.7%) at presentation, and 52.6% had a WHO-defined abnormal chest radiograph.23
Figure 1.
Study Overview. HIV indicates human immunodeficiency virus; PCR, polymerase chain reaction.
Table 1.
Baseline infant characteristics
| Variable | Standard Care N= 150 |
Rapid PCR N= 150 |
p-value | |
|---|---|---|---|---|
| Demographic | Age in months, median (IQR) | 5.1 (2.3 – 8.7) | 4.2 (1.7 – 8.0) | 0.299 |
| Females, n (%) | 74 (49.3) | 79 (52.7) | 0.564 | |
| Breastfeeding, n (%) | 123 (82.0) | 124 (82.7) | 0.880 | |
| Vaccinations up-to-date, n (%) | 85/149 (57.0) | 79/147 (53.7) | 0.567 | |
| Infant enrolled in HIV care, n (%) | 38 (25.3) | 38 (25.3) | 1.000 | |
| PMTCT received by mother, n (%) | 96/148 (64.9) | 93/148 (62.8) | 0.717 | |
| PMTCT received by infant, n (%) | 96 (64.0) | 91 (60.7) | 0.551 | |
| Presenting clinical signs | Temperature in °C, mean (SD) | 37.0 (0.8) | 37.0 (0.8) | 0.787 |
| Heart rate (beats per minute), mean (SD) | 149 (23) | 149 (20) | 0.898 | |
| Respiratory rate (breaths per minute), mean (SD) | 55 (19) | 55 (16) | 0.726 | |
| Oxygen saturation, median (IQR) | 97 (94 – 100) | 98 (95 – 100) | 0.452 | |
| Weight for height z score, mean (SD) | −1.05 (2.04) | −0.98 (1.81) | 0.781 | |
| Laboratories | HIV antibody test positive, n (%) | 119 (79.3) | 115 (76.7) | 0.577 |
| HIV molecular test positive, n (%)1 | 46 (30.7) | 47 (31.3) | 0.901 | |
| CD4%, mean (SD) | 30.4 (14.1) | 33.4 (14.3) | 0.072 | |
| Hemoglobin (g/dL), mean (SD) | 9.7 (2.4) | 10.2 (2.8) | 0.086 | |
| Malaria blood film positive, n (%) | 5/148 (3.4) | 6/150 (4.0) | 1.000 | |
| Blood culture positive, n (%) | 9/149 (6.0) | 7/150 (4.7) | 0.598 | |
| Radiography, n (%) | Abnormal chest radiograph | 81/146 (55.5) | 71/143 (49.7) | 0.321 |
PCR indicates polymerase chain reaction; IQR, interquartile range; HIV, human immunodeficiency virus; PMTCT, prevention of mother-to-child transmission; SD, standard deviation.
46 standard care patients were DNA PCR-positive and 47 Rapid PCR patients had >10,000 viral copies/ml on RNA PCR
There were also no differences between the groups regarding hospital diagnoses or inpatient disposition (Table 2). Forty-four percent of the 119 HIV antibody-positive patients in the standard care group were WHO algorithm-positive and thus inpatient ART-eligible. The WHO algorithm achieved a sensitivity of 53.5%, specificity of 60.5%, and positive and negative predictive values of 43.4% and 69.7% for the 119 HIV antibody-positive standard care infants eligible for WHO algorithm evaluation. More than 60% of all infants met WHO pneumonia criteria. Sepsis was also common (44.7% standard care; 42.0% Rapid PCR), and P. jirovecii pneumonia, malaria, and gastroenteritis were each found in one in four children. In all, 9.3% of patients died while hospitalized.
Table 2.
Hospital Outcomes
| Variable | Standard Care N=150 |
Rapid PCR N=150 |
p-value | |
|---|---|---|---|---|
| Final Diagnoses, n (%) | WHO clinical algorithm-positive1 | 53/119 (44.5) | NA | |
| Rapid PCR-positive | NA | 47 (31.3) | - | |
| Severe Pneumonia | 63 (42.0) | 61 (40.7) | 0.815 | |
| Very severe pneumonia | 29 (19.3) | 31 (20.7) | 0.773 | |
| Pneumocystis jirovecii pneumonia2 | 40 (26.7) | 37 (24.7) | 0.692 | |
| Pulmonary Tuberculosis3 | 19 (12.7) | 14 (9.3) | 0.356 | |
| Severe Acute Malnutrition | 32 (21.3) | 29 (19.3) | 0.667 | |
| Sepsis4 | 67 (44.7) | 63 (42.0) | 0.641 | |
| Malaria5 | 35 (23.3) | 38 (25.3) | 0.686 | |
| Meningitis6 | 5 (3.3) | 5 (3.3) | 1.000 | |
| Gastroenteritis | 38 (25.3) | 38 (25.3) | 1.000 | |
| Esophageal candida | 8 (5.3) | 5 (3.3) | 0.572 | |
| Length of hospitalization in days, median (IQR) | 4 (4 – 7) | 5 (4 – 6) | 0.133 | |
| Hospital Outcome, n (%) | 0.492 | |||
| Discharged | 137 (91.3) | 131 (87.3) | ||
| Died | 11 (7.3) | 17 (11.3) | ||
| Absconded | 2 (1.3) | 2 (1.3) |
PCR indicates polymerase chain reaction; WHO, World Health Organization; NA, not applicable; HIV, human immunodeficiency virus; IQR, interquartile range; ART, antiretroviral therapy.
31 standard care infants tested HIV antibody-negative and were not eligible to be evaluated by the WHO algorithm.
3 and 37 standard care patients were confirmed and probable Pneumocystis jirovecii cases compared to 6 and 31 Rapid PCR patients, respectively.
1 and 18 standard care patients were confirmed and probable pulmonary tuberculosis cases compared to 1 and 13 Rapid PCR patients, respectively.
7 and 60 standard care patients were confirmed and probable sepsis cases compared to 9 and 54 Rapid PCR patients, respectively.
6 and 29 standard care patients were confirmed and probable malaria cases compared to 5 and 33 Rapid PCR patients, respectively.
1 and 4 standard care patients were confirmed and probable bacterial meningitis cases compared to 0 and 5 Rapid PCR patients, respectively.
Rapid RNA PCR results were returned a mean of 30 days earlier than DNA PCR results (Table 3, p<0.001). In addition, a greater proportion of HIV-infected infants in the Rapid PCR group initiated ART in the hospital compared to standard care (34/47 [72.3%] vs 22/46 [47.8%], p=0.016). Because of early deaths shortly after enrollment, not all HIV-infected infants in the Rapid PCR group received ART. No molecular test-negative infants randomized to Rapid PCR were incorrectly initiated on ART while hospitalized, compared to 28/104 (26.9%) receiving standard care (p<0.001). Of the 24 standard care DNA PCR-positive infants who did not receive in-hospital ART, one (4.2%) WHO algorithm-positive infant died in the hospital before ART could be initiated, three (12.5%) were ineligible for inpatient ART due to a negative HIV antibody test, and twenty (83.3%) were ART-ineligible per negative inpatient algorithm status. Fourteen of the 20 (70.0%) antibody or algorithm-negative PCR-positive infants who survived to hospital discharge also returned for follow-up, all of whom initiated outpatient ART. The median time between molecular HIV testing and ART was reduced by 3.5 days for HIV-infected infants in the Rapid PCR group versus the standard care group (p=0.001), reflecting the fact that HIV-infected standard care infants received ART in a bimodal fashion (e.g., hospital-initiated if WHO algorithm-positive or outpatient-initiated if algorithm-negative but DNA PCR-positive).
Table 3.
HIV-associated Outcomes
| Variable | Standard Care N=150 |
Rapid PCR N=150 |
p-value | |
|---|---|---|---|---|
| HIV molecular test status | 0.901 | |||
| Positive1 | 46 (30.7) | 47 (31.3) | ||
| Negative2 | 104 (69.3) | 103 (68.7) | ||
| Days to molecular test results, mean (SD) | All | 32.5 (9.2) | 2.5 (0.9) | <0.001 |
| Hospital ART initiation, n (%) | HIV molecular test positive1,3 | 22/46 (47.8) | 34/47 (72.3) | 0.016 |
| HIV molecular test negative2,4 | 28/104 (26.9) | 0/103 (0.0) | <0.001 | |
| Days to ART, median (IQR) | HIV molecular test positive1,5 | 6.5 (3 – 28) | 3.0 (3 – 4) | 0.001 |
| Outpatient follow-up, n (%) | All | 113/137 (82.5) | 108/131 (82.4) | 0.993 |
HIV indicates human immunodeficiency virus; PCR, polymerase chain reaction; SD, standard deviation; ART, antiretroviral therapy.
Patients were either DNA PCR-positive (standard care) or had >10,000 viral copies/ml on RNA PCR (Rapid PCR).
Patients were either DNA PCR-negative (standard care) or had <400 viral copies/ml on RNA PCR (Rapid PCR).
1 and 2 HIV-infected infants in the standard care group that met the WHO clinical algorithm criteria either died or absconded before inpatient ART initiation, respectively. 13 and 0 HIV-infected infants in the Rapid PCR group either died or absconded before inpatient ART initiation, respectively.
Zero DNA PCR-negative infants in the standard care group that did not meet WHO clinical algorithm criteria died before hospital discharge. 4 and 2 infants with <400 viral copies/ml on RNA PCR in the Rapid PCR group either died or absconded before hospital discharge, respectively.
HIV-infected standard care infants were started on ART either in the hospital if WHO algorithm-positive or as an outpatient if algorithm-negative but DNA PCR-positive. Rapid PCR infants were started on ART in the hospital only.
In a subgroup analysis of WHO algorithm-positive and Rapid PCR-positive infants (data not shown), we found no difference between the groups in the average time from testing to in-hospital ART (3.8 days, algorithm-positive vs 3.9 days, Rapid PCR-positive, p=0.884). Nor were any differences found in outpatient follow-up rates, including WHO algorithm and Rapid PCR-positive infants who were initiated on ART while hospitalized [37/43 (86.0%) vs 28/34 (82.4%), P = 0.631].
Lastly, of all 41 HIV-infected infants (DNA PCR-positive or >10,000 copies/ml on RNA PCR) started on inpatient ART and in care, three developed IRIS and one had a mild nevirapine rash at follow-up (data not shown). ART was continued in these patients. Furthermore, no differences in outpatient mortality or default rates were found among all HIV-infected infants stratified by inpatient ART (p=0.785, Table 4), suggesting that factors unassociated with ART may have influenced these unfavorable outcomes. We also examined the implications of ART in DNA PCR-negative infants. Of the 22 WHO algorithm-positive children on ART who were PCR-negative, 1 died shortly after ART initiation because of primary illness, 2 defaulted, and 1 developed a nevirapine-associated rash. These default and death rates were no different from ART naïve molecular test-negative infants (Table 4).
Table 4.
Influence of ART on short-term patient outcomes.
| Variable | HIV molecular test-positive infants | HIV molecular test-negative infants | |||||
|---|---|---|---|---|---|---|---|
| Inpatient ART N= 55 |
No ART N= 20 |
p-value | Inpatient ART N= 22 |
No ART N= 146 |
p-value | ||
| Outpatient follow-up, n (%) | 0.785 | 0.194 | |||||
| Yes | 41 (74.5) | 13 (65.0) | 21 (95.5) | 116 (79.5) | |||
| No, default | 9 (16.4) | 5 (25.0) | 1 (4.5) | 27 (18.5) | |||
| No, death | 5 (9.1) | 2 (10.0) | 0 (0.0) | 3 (2.1) | |||
ART indicates antiretroviral therapy; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
Patients were either DNA PCR-positive (standard care) or had >10,000 viral copies/ml on RNA PCR (Rapid PCR).
Patients were either DNA PCR-negative (standard care) or had <400 viral copies/ml on RNA PCR (Rapid PCR).
Discussion
The widespread availability of a point-of-care molecular HIV test will revolutionize the care of African infants. However, this hope is largely inferred from studies showing the limitations of current care, rather than direct evaluation of key clinical endpoints of a point-of-care test.4 Although several promising molecular point-of-care tests are in development, none are yet approved for routine use.7 In this study, we accelerated the PCR reporting time from more than one month to just 48 hours, enabling us to simulate, and thus evaluate, a rapid molecular HIV test and its clinical implications for hospitalized infants. The Rapid PCR test we used here successfully achieved the same clinical endpoints that would be targeted by a point-of-care test performed in the hospital, including that all Rapid PCR subjects received their results and were offered ART if HIV-infected before discharge. Our data demonstrates that a rapid PCR test in hospitalized African infants, compared to an idealized standard care, greatly increases ART access but only modestly reduces the time between HIV testing and ART initiation for infants found to be HIV-infected. Rapid molecular testing also more effectively utilizes resources by eliminating unnecessary ART exposure for patients with a negative molecular HIV test.
Point-of-care serologic HIV tests have been in use for >10 years and offer a glimpse of what could be achieved with widespread implementation of an infant point-of-care molecular HIV test. Point-of-care serologic testing has been a key driver in the dramatic increase in HIV testing access for African adults in areas with limited laboratory support,24 and have ensured that almost every HIV-infected adult receives their test results, and hence a definitive HIV diagnosis, so that they can consider ART immediately.25–27 In our study, clinical staff consistently expressed an increased motivation to test Malawian infants for HIV since a rapid molecular test was available. This observation is supported by the unexpectedly short enrollment time-frame and suggests that a scale-up of point-of-care molecular testing should further increase infant access to HIV diagnostics. Our results also demonstrate for the first time that a high proportion of HIV-infected infants with positive rapid molecular tests received their results and commenced ART expediently.
Our data show that a Rapid PCR test, compared to optimized standard care, will enable clinicians to more proficiently prescribe ART to hospitalized HIV-infected infants. However, it was not yet known whether commencing ART during hospitalization is either a judicious use of resources, given historical 31.7%-32.7% initial attrition rates after discharge in ART-naïve patients,10,13 or safe, considering the likelihood of IRIS when starting ART in an acutely ill infant.28 Our results suggest that ART initiation should not be deferred until outpatient follow-up solely on the basis of initial default rates, as 82.4% of HIV-infected infants in the Rapid PCR group on ART returned for outpatient care. These initial follow-up rates are higher than previously reported,10,13 and could reflect greater caregiver motivation to follow-up after definitive infant diagnosis and prescription for ART. Recent evidence shows that hospital-identified HIV-infected children, once established in outpatient care, have one-year attrition rates no different than their community-identified counterparts.12 On the other hand, our high follow-up rates may partly reflect the controlled, more rigorous conditions of our study design and might not be replicable in a routine programmatic setting. We also found little reason to defer in-hospital ART initiation on the basis of a high incidence of IRIS or acute ART toxicities. These findings contrast with current clinical practice in Africa, by which providers delay ART initiation until outpatient care, largely on these concerns. Although we acknowledge that our follow-up duration was short, evidence suggests the majority of IRIS cases usually occur soon after ART initiation and within this study’s follow-up time-frame.28 Given that the hospital will be an important ART access point for African infants for the foreseeable future, our results warrant additional study of longer-term care retention for HIV-affected infants first identified in the hospital, and of rates and severity of IRIS in HIV-infected hospitalized infants initiated on ART while recovering from an acute illness.
Our study applied the WHO clinical algorithm to all hospitalized HIV antibody-positive standard care subjects and offered ART if they were algorithm-positive. Standard program conditions throughout most African hospitals do not typically include routine use of the WHO clinical algorithm as implemented during this study. Instead, real-world standard care consists of infants waiting weeks for their DNA PCR results, with the majority defaulting before receiving their results and entering care.4 These data demonstrate that despite its shortcomings compared to Rapid PCR, routine use of the WHO algorithm can serve as a useful bridge between the currently-used DNA PCR testing system and the next-generation point-of-care molecular tests under development. Three aspects of our data support this position. First, nearly 50% of HIV-infected infants were initiated on ART in the standard care group before hospital discharge. Current evidence suggests that most of these infants may otherwise not have gained access to these life-saving medicines, especially if they were forced to wait at least one month for their DNA PCR results.4,13 Second, our data implies that starting ART on acutely ill HIV-infected infants may be safe, and that temporarily initiating ART on molecular test-negative infants could be equally benign. However, given our relatively small sample size, additional confirmatory research is needed to determine the true rates of IRIS and ART toxicities. Lastly, we found that the timeliness of ART initiation in WHO algorithm-positive infants (3.8 days) compared favorably with Rapid PCR-positive patients (3.9 days), thus allowing ART initiation prior to hospital discharge. Taken together, these data indicate that without a point-of-care molecular test, the WHO algorithm is still a useful tool that can help alleviate some of the inherent drawbacks of current infant HIV DNA PCR testing systems.
Our study has several weaknesses. We likely underestimated the prevalence of ART toxicity since we relied upon clinical screening for side-effects followed by laboratory confirmation on suspected cases only. With this approach, we found no cases of nevirapine-associated hepatotoxicity, for example, although subclinical cases could have been missed. However, the effect of any subclinical toxicity was likely minimal, since outcomes were no different when we controlled for ART exposure. This study is also limited by our unblinded study design, which increases the likelihood of observer and participant bias. However, a blinded design would be unethical, given that it would be inappropriate to counsel algorithm-positive and -negative patients identically to Rapid PCR patients in regard to HIV status. Lastly, our hospital-based results may not reflect outpatient performance of either a point-of-care molecular diagnostic or the WHO algorithm, for two reasons. First, our 48-hour Rapid PCR test, if used in outpatient clinics, would require two separate visits (one for blood collection and another to deliver results), whereas only one visit is needed for a true point-of-care test. Second, outpatient HIV prevalence is lower and patients are generally less ill than in the hospital, such that fewer HIV-infected infants meet WHO clinical criteria in a clinic setting. Thus, the generalizability of our results to the outpatient setting would require further study.
In summary, we simulated a point-of-care molecular HIV test in a hospital setting, and found that Rapid PCR, compared to an optimized standard care predicated on routine use of the WHO clinical algorithm, greatly improves ART access for HIV-infected infants, while minimizing unnecessary ART exposure for infants with negative molecular HIV test results. The WHO algorithm compared favorably to Rapid PCR from the perspective of ART timing and initial outpatient follow-up for Rapid PCR and WHO algorithm-positive infants. Therefore, until a point-of-care molecular HIV test is widely available, we recommend the routine use of the WHO clinical algorithm for hospitalized African infants in resource-constrained settings.
Supplementary Material
Acknowledgements
The authors express their gratitude to the children and caregivers who participated in the study. We also thank, in particular, the Malawi Ministry of Health for their contributions to this project.
Sources of Support
This work was supported by the National Institutes of Health through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University [R24 TW007988 to E.D.M., M.M., D.O.] and through the National Heart Lung and Blood Institute [T32 HL072748-11 to E.D.M.]. The University of North Carolina Center for AIDS Research [5 P30-AI50410 to E.D.M.] also supported this work.
Footnotes
Abstract number LBPE13 at the 19th International AIDS Conference in Washington, D.C., USA.
The authors declare no conflicts of interest.
References
- 1.United Nations Joint Programme on HIV/AIDS (UNAIDS) UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: World Health Organization; 2012. [Accessed 26 February 2013]. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_en.pdf. [Google Scholar]
- 2.United Nations Joint Programme on HIV/AIDS (UNAIDS) Global Plan Towards The Elimination of New HIV Infections Among Children By 2015 and Keeping Their Mothers Alive. Geneva, Switzerland: World Health Organization; 2011. [Accessed 26 February 2013]. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/20110609_JC2137_Global-Plan-elimination-HIv-Children_en.pdf. [Google Scholar]
- 3.Violari A, Cotton M, Gibb D, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun M, Kabue MM, McCollum ED, et al. Inadequate Coordination of Maternal and Infant HIV Services Detrimentally Affects Early Infant Diagnosis Outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access. Geneva, Switzerland: World Health Organization; 2010. [Accessed 26 February 2013]. Available at: http://whqlibdoc.who.int/publications/2010/9789241599801_eng.pdf. [PubMed] [Google Scholar]
- 6.World Health Organization. Progress Report on Global HIV/AIDS Response. Geneva, Switzerland: World Health Organization; 2011. [Accessed 26 February 2013]. Available at: http://whqlibdoc.who.int/publications/2011/9789241502986_eng.pdf. [Google Scholar]
- 7.Schito ML, D'Souza MP, Owen SM, et al. Challenges for rapid molecular HIV diagnostics. J Infect Dis. 2010;201:S1–S6. doi: 10.1086/650394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Creek TL, Sherman GG, Nkengasong J, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197:S64–S71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Kellerman S, Essajee S. HIV Testing for Children in Resource-Limited Settings: What Are We Waiting For? PloS Medicine. 2010;7:e10000285. doi: 10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCollum ED, Preidis GA, Kabue MM, et al. Task shifting routine inpatient pediatric HIV testing improves program outcomes in urban Malawi: a retrospective observational study. PloS One. 2010;5:e9626. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogerson SR, Gladstone M, Callaghan M, et al. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans R Soc Trop Med Hyg. 2004;98:544–552. doi: 10.1016/j.trstmh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Preidis GA, McCollum ED, Kamiyango W, et al. Routine Inpatient Provider-Initiated HIV Testing in Malawi, Compared to Client-Initiated Community-Based Testing, Identifies Younger Children at Higher Risk of Early Mortality. J Acquir Immune Defic Syndr. 2013;63:e16–e22. doi: 10.1097/QAI.0b013e318288aad6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCollum ED, Preidis GA, Golitko CL, et al. Routine inpatient human immunodeficiency virus testing system increases access to pediatric human immunodeficiency virus care in sub-Saharan Africa. Pediatr Infect Dis J. 2011;30:e75–e81. doi: 10.1097/INF.0b013e3182103f8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inwani I, Mbori-Ngacha D, Nduati R, et al. Performance of clinical algorithms for HIV-1 diagnosis and antiretroviral initiation among HIV-1-exposed children aged less than 18 months in Kenya. J Acquir Immune Defic Syndr. 2009;50:492–498. doi: 10.1097/QAI.0b013e318198a8a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundmann N, Iliff P, Stringer J, et al. Presumptive diagnosis of severe HIV infection to determine the need for antiretroviral therapy in children less than 18 months of age. Bull World Health Organ. 2011;89:513–520. doi: 10.2471/BLT.11.085977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwood C, Liebeschuetz S, Blaauw D, et al. Diagnosis of paediatric HIV infection in a primary health care setting with a clinical algorithm. Bull World Health Organ. 2003;81:858–866. [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SA, Sherman GG, Coovadia AH. Can clinical algorithms deliver an accurate diagnosis of HIV infection in infancy? Bull World Health Organ. 2005;83:559–560. [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips JA, Kazembe PN, Nelson EAS, et al. A Paediatric Handbook for Malawi. 3rd ed. Limbe: Montford Press; 2008. [Accessed 26 February 2013]. Available at: http://www.medcol.mw/pharmacy/downloads/malawian_handbook_paediatrics.pdf. [Google Scholar]
- 19.World Health Organization. Pocketbook of Hospital care for children. Geneva, Switzerland: World Health Organization; 2005. [Accessed 26 February 2013]. Available at: http://whqlibdoc.who.int/publications/2005/9241546700.pdf. [Google Scholar]
- 20.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis. 2012;205:S199–S208. doi: 10.1093/infdis/jis008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant LR, Hammitt LL, Murdoch DR, et al. Procedures for collection of induced sputum specimens from children. Clin Infect Dis. 2012;54:S140–S145. doi: 10.1093/cid/cir1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puthanakit T, Oberdorfer P, Akarathum N, et al. Immune Reconstitution Syndrome After Highly Active Antiretroviral Therapy in Human Immunodeficiency Virus-Infected Thai Children. Pediatr Infect Dis J. 2006;25:53–58. doi: 10.1097/01.inf.0000195618.55453.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Pneumonia Vaccine Trial Investigators Group. Standardization of interpretation of chest radiographs for the diagnosis of pneumonia in children. Geneva, Switzerland: World Health Organization; 2001. [Accessed 26 February 2013]. Available at: http://www.who.int/vaccine_research/documents/en/pneumonia_children.pdf. [Google Scholar]
- 24.Pilcher CD, Christopoulos KA, Golden M. Public health rationale for rapid nucleic acid or p24 antigen tests for HIV. J Infect Dis. 2010;201:S7–S15. doi: 10.1086/650393. [DOI] [PubMed] [Google Scholar]
- 25.Puren A, Gerlach JL, Weigl BH, et al. Laboratory operations, specimen processing, and handling for viral load testing and surveillance. J Infect Dis. 2010;201:S27–S36. doi: 10.1086/650390. [DOI] [PubMed] [Google Scholar]
- 26.Kendrick SR, Kroc KA, Couture E, et al. Comparison of point-of-care rapid HIV testing in three clinical venues. AIDS. 2004;18:2208–2210. doi: 10.1097/00002030-200411050-00017. [DOI] [PubMed] [Google Scholar]
- 27.Kendrick SR, Kroc KA, Withum D, et al. Outcomes of offering rapid point-of-care HIV testing in a sexually transmitted disease clinic. J Acquir Immune Defic Syndr. 2005;38:142–146. doi: 10.1097/00126334-200502010-00004. [DOI] [PubMed] [Google Scholar]
- 28.Boulware DR, Callens S, Pahwa S. Pediatric HIV immune reconstitution inflammatory syndrome. Curr Opin HIV AIDS. 2008;3:461–467. doi: 10.1097/COH.0b013e3282fe9693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.