Abstract
Objective
In an effort to address earliest detection of Mild Cognitive Impairment (MCI), we examined hippocampal volumes and atrophy in middle-aged males to explore neuroanatomical support for different neuropsychological definitions of MCI.
Methods
460 men ages 51-60 underwent neuropsychological testing and an MRI. MCI was defined according to five criteria sets. MRI-derived hippocampal volume and hippocampal occupancy (HOC) were obtained via FreeSurfer. Statistical analyses were performed using linear mixed models.
Results
Differences in HOC between normal cognitive functioning, amnestic, and non-amnestic MCI were observed using MCI criteria that required one impaired (>1.5 SD) cognitive measure in a given cognitive domain or a cognitive composite score method with a cut-point 2 SD below the mean. Differences in standard hippocampal volume were only found between normal and amnestic presentations and only when using the composite score method.
Conclusions
Results provide empirical support for detection of pre-MCI in younger cohorts. Convergence of neuropsychological and neuroanatomical data, particularly HOC (as opposed to standard cross-sectional volume), supports early identification of MCI as defined by some neuropsychological criteria.
Keywords: MCI, neuropsychology, MRI
Objective
Mild cognitive impairment (MCI) is a known risk factor for progression to dementia. Early identification of MCI is essential for early intervention given that subtle cognitive and pathophysiological changes precede decline by many years [1-4]. Despite great interest in the earliest identification of risk factors for cognitive decline, most research on MCI and dementia has focused on individuals over age 65 [5, 6] with relatively few reports targeting younger cohorts in the literature [7-10]. To find ‘transition points’ in which someone moves from asymptomatic to symptomatic, particularly in a slowly progressive but often unstable disorder such as MCI, understanding the full longitudinal course of cognitive functioning is essential to tracking clinical progression and for identifying the earliest reasonable point for intervention.
Cognitive impairment is a key feature of MCI and current guidelines recommend that objective cognitive impairment is 1-1.5 standard deviations below normative expectations [11]. Evolving definitions of MCI [12-16] and lack of a universally accepted approach to the objective identification of cognitive impairment in MCI [17] have led to highly variable prevalence rates, and complicate the earliest identification of MCI [18]. As in older cohorts, the prevalence of MCI in younger cohorts varies widely (from 2.4-13.7%) depending on how MCI is operationally defined [19-21].
Hippocampal atrophy is one of the earliest neuropathological changes associated with MCI, and is predictive of further cognitive decline, particularly in amnestic presentations [16, 22, 23]. Medial temporal lobe changes also significantly improve ability to distinguish normal aging from MCI [24], MCI from Alzheimer’s Disease [25] and prediction of future decline [26]. Therefore, with the goal of early detection, we sought to evaluate potential neuroanatomical support (based on hippocampal volume and atrophy estimates) for five different and common sets of neuropsychological criteria for cognitive impairment in MCI in a large cohort of men in their 50s. Our early identification of a cognitive presentation consistent with MCI uses a neuropsychological categorization that differs somewhat from criteria that rely more heavily on standard clinical interviewing and history taking to diagnose MCI [27, 28]. Given the difficulty in early identification of MCI, particularly in younger cohorts, our approach that emphasizes comprehensive neuropsychological testing may be better able to identify cognitive deficits consistent with MCI in adults who are only middle-aged.
Methods
Participants
Participants were enrolled in the MRI arm of the Vietnam Era Twin Study of Aging (VETSA), a longitudinal study of cognitive and brain health beginning in midlife (for overview, see Kremen et al. 2006). Participants were drawn from the Vietnam Era Twin Registry, a nationally distributed sample of male-male twin pairs born between 1939 and 1957 who served in the US military at some time between 1965 and 1975. Although the participants were veterans, the VETSA is not a patient or VA sample and the majority was not exposed to combat during their military service. VETSA participants are a reasonably representative sample of late middle-aged men in the United States [29]. The VETSA MRI study began in year 3 of the primary VETSA project; approximately 90% of those contacted agreed to participate, though ultimately, not all were appropriate to participate in the MRI study due to safety considerations or concerns about claustrophobia. Participants underwent assessments including comprehensive neuropsychological testing and neuroimaging at the University of California, San Diego or Boston University. The study was approved by human subjects protection committees at both participating institutions and all participants provided written informed consent.
Exclusion criteria included conditions that can result in non-MCI-related cognitive deficits including seizure disorder, multiple sclerosis, stroke, HIV/AIDS, schizophrenia, substance dependence, brain cancer, or dementia. Because only 10.75% of the sample endorsed the possibility of a history of traumatic brain injury with loss of consciousness and none were hospitalized for the incident, no one was excluded for history of severe TBI. Following exclusions, the present study was based on data from 460 participants. Mean age was 55.7 years (SD=2.5, range 51-60) and the mean years of education was 13.8 (SD=2.2). All participants were functionally intact as determined by their ability to travel independently (usually flying) to the test sites for evaluations.
MCI Criteria
Participants underwent comprehensive neuropsychological testing. For purposes of defining MCI, six cognitive domains each containing multiple neuropsychological measures were covered. Domains included verbal and visual episodic memory, executive functioning, attention/working memory, language, visual-spatial functioning, and processing speed (see Table 1). The neuropsychological test battery was designed to cover a range of cognitive functions and avoid ceiling effects in a community-dwelling, middle-aged sample. Because there are no specific agreed upon operational criteria for defining what constitutes objective cognitive impairment in MCI, we utilized five different operational definitions drawn from the MCI literature that employed different cut-points for impairment, varied the number of tests which needed to be impaired, or used cognitive domain composite scores [30, 31].
Table 1. Cognitive Domains and Neuropsychological Tests Used in MCI Diagnoses.
| Cognitive Domain | Measure |
|---|---|
| Episodic Memory | California Verbal Learning Test (CVLT; Learning Trials, Delayed Free Recall) |
| Wechsler Memory Scale-III (WMS-III) Logical Memory, Immediate.; |
|
| WMS-III Visual Reproductions (Immediate & Delayed Recall) | |
| Executive Function | Delis-Kaplan Executive Functioning System (DKEFS) Trails Switching |
| DKEFS Category Switching | |
| Stroop Color-Word & Interference | |
| Wechsler Adult Intelligence Scale-III (WAIS-III) Matrix Reasoning |
|
| Attention | WAIS-III Digit Span |
| WAIS-III Spatial Span | |
| WAIS-III Letter-Number Sequencing | |
| DKEFS Visual Scanning | |
| Language | Wechlser Abbreviated Scale of Intelligence (WASI) Vocabulary |
| DKEFS Letter & Category Fluency | |
| Visual-Spatial Ability | Hidden Figures |
| Mental Rotation | |
| WMS-III Visual Reproductions Copy |
Following from Jak et al. (2009), we defined impairment according to three sets of criteria determined by the number of measures below a particular cut-point within a cognitive domain [31]. The “Typical criteria” were defined on the basis of one measure in a domain being greater than 1.5 SD below the mean; it is called “Typical” because it is the most common criterion for impairment, consistent with that used by Petersen [14]. The “Comprehensive criteria” were developed to better approximate a clinical decision-making processes and because the interpretive value of an isolated impaired score is often limited. The comprehensive criteria utilized a less-stringent cut-point of 1 SD, but at least two measures greater than 1 SD below normative expectations within a domain were required for that domain to be considered impaired. To examine a two test per domain analog of the Typical Criteria, the “Conservative criteria” required at least two measures in a domain to be impaired at a cut-point of greater than 1.5 SD below the mean [31]. A one test per domain analog of the comprehensive criteria has been explored in other samples and results in approximately 75% of the sample being classified as MCI and likely represents a high proportion of false-positive diagnoses [31] and was therefore not examined here.
Composite scores were also created based on an approach by Ganguli et al., (2011) in which standard scores (based on published norms for each test) on each measure are transformed into z-scores, and then z-scores are averaged within cognitive domains to create composite scores [30]. The standard deviation of the average of a set of z-scores will actually be less than 1 and so it is incorrect to interpret a composite z-score of −1 as equating to performance that is 1 SD below the mean. To address this problem, we examined cut-points at the 5th (“Composite 5”) and 2.5th (“Composite 2.5”) percentiles, which correspond roughly to 1.65 and 2 SD below the mean. Detailed explanation of these different criteria, and their prevalence rates and heritabilities in the VETSA have been reported elsewhere [32].
Participants were characterized according to these five criteria sets (for summary, see Table 2) to classify individuals as cognitively normal or MCI. Current conceptualization of MCI [11] highlights assessment of cognitive impairment in one or more domains and emphasizes that those with impairments in memory have higher progression rates to Alzheimer’s Disease than those without memory deficits. Past research has also noted differential diagnostic outcomes for amnestic versus non-amnestic presentations and single cognitive domain presentations versus multiple domains [33-37]. Therefore, MCI was further subtyped as amnestic (met criteria for impairment in memory), non-amnestic (met criteria for impairment only in non-memory cognitive domains), single-domain (met criteria for impairment in only one cognitive domain), and multiple-domain MCI (met criteria for impairment in more than one cognitive domain) [30, 31].
Table 2. Summary of MCI Definitions.
| MCI Definition | Cut-point for impairment |
No. impaired measures required per domain |
Amnestic (n) |
Non- Amnestic (n) |
Single Domain (n) |
Multi- Domain (n) |
|---|---|---|---|---|---|---|
| Typical | <1.5 SDs below norm | 1 | 179 | 110 | 135 | 154 |
| Comprehensive | <1 SD below norm | 2 | 125 | 128 | 121 | 132 |
| Conservative | <1.5 SDs below norm | 2 | 59 | 63 | 87 | 35 |
| Composite 5 | 5th percentile | Average of alla | 77 | 31 | 78 | 30 |
| Composite 2.5 | 2.5th percentile | Average of alla | 47 | 14 | 41 | 20 |
Averages computed after transforming scores on individual measures to z-scores.
Prior Level of Cognitive Function
We had the benefit of the availability of the Armed Forces Qualification Test (AFQT) on all participants. The AFQT is 50-minute paper and pencil measure that is administered to all service members prior to military induction as an initial screen for military selection and included measures of word knowledge, arithmetic reasoning, spatial perception and tool recognition. The AFQT score is highly correlated with standard IQ measures [38]. Initial AFQT scores (mean age=19.8 years [SD=1.5]) were obtained from military records and the AFQT was administered again as part of the current study. We were, therefore, able to use AFQT scores to adjust for an empirically-derived level of prior general cognitive ability and ensure that our MCI diagnoses were not simply a proxy for low overall cognitive ability and that the classifications represented a decline from prior levels of functioning. Therefore, any cognitive impairments existed after adjusting for an individual’s overall cognitive ability at age 20. That is, scoring below the cutoffs listed above means that scores fell below that level following adjustment for AFQT performance at age 20 [32]. Because test scores were adjusted for premorbid intellectual functioning and scores could therefore not be compared to standard normative tables, we used the VETSA sample as the normative sample rather than age- and education-based norms from test manuals.
Imaging Methods
MRI images were acquired on Siemens 1.5 Tesla scanners [(n=242 on a Siemens Symphony at the University of California, San Diego; and n=218 on a Siemens Avanto at Massachusetts General Hospital (MGH)]. Sagittal T1-weighted MPRAGE sequences were employed with a TI=1000 ms, TE=3.31 ms, TR=2730 ms, flip angle=7°, slice thickness=1.33 mm, and voxel size=1.3×1.0×1.3 mm. Raw DICOM MRI scans were downloaded to the MGH site, automatically corrected for spatial distortion, and the two acquired T1-weighted images were registered and averaged to improve signal-to-noise. Hippocampal volume segmentation methods [39, 40] were based on the FreeSurfer software package and is a semi-automated, fully 3D whole-brain segmentation procedure using probabilistic atlas and a Bayesian classification rule to neuroanatomically label each voxel [39, 40]. To be more representative of the VETSA sample and yield more accurate measurements, a new atlas was manually derived from 20 unrelated, randomly selected VETSA participants [41]. Hippocampal volumes did not differ across scanning sites. Since statistical analyses should covary for individual differences in head size when assessing volume [Barnes et al 2010], we used the estimated total intracranial volume (eTIV) provided by FreeSurfer [42]. Due to the lack of subarachnoid CSF signal on T1-weighted images, a direct measurement of cranial vault is not possible, therefore, FreeSurfer incorporated a published approach [Buckner] that derives eTIV from the atlas scaling factor based on the transformation of the full brain mask into atlas space [see https://surfer.nmr.mgh.harvard.edu/fswiki/eTIV]. Although not a direct volumetric measurement, eTIV correlates well with other cranial vault measurements that incorporate T2-weighted information, including manual tracings and multi-channel tissue segmentations in controls and older adults [42, 43]. In addition to bilateral hippocampal volumes (HCV; left+right hippocampal volumes) we also calculated a hippocampal occupancy score (HOC; hippocampal volume / (hippocampal volume + inferior lateral ventricle volume) as a way to cross-sectionally estimate hippocampal atrophy [44]. Standard hippocampal volume measurements are adjusted for intracranial volume or overall head size whereas hippocampal occupancy is a measure of the process of expansion of the ventricles resultant from cortical atrophy since hippocampal volume is adjusted for the sum of the hippocampal and temporal horn area. In a prior examination of HOC’s ability to predict conversion from MCI to AD in the Alzheimer’s Disease Neuroimaging Initiative (ADNI), it was shown to perform better (in both discriminative and predictive accuracy) than standard hippocampal volume measure [44], possibly because it is better able to differentiate those with premorbidly small hippocampi from those whose hippocampi have atrophied due to degeneration.
Statistical Analysis
Data were collected as part of a twin study; however, the analyses performed here were not twin analyses. That is, we did not use the twin structure of the data to estimate genetic and environmental influences. When twin data are used to estimate genetic and environmental influences, the unit of analysis is the twin pair. Here the unit of analysis was each individual. Because twins within pairs violates the standard assumption that observations are independent, data were analyzed using a multilevel, mixed linear model (SAS Proc Mixed, SAS version 9.2), which allows for utilization of all available data and adjustment for non-independence of observations (i.e., clustering of twin pairs). No adjustment was made for zygosity, and hippocampal volumes did not differ between monozygotic and dizygotic groups.
Analyses examined the effects of amnestic vs. non-amnestic and single vs. multiple domain MCI on bilateral HCV and on bilateral HOC. To account for the non-normal distribution of the HOC, this variable was log-transformed in all analyses. The statistical model included TIV, age, and scanner as covariates. Results were based on the type III test of fixed effects that control for all other elements of the model.
3. Results
Hippocampal Occupancy (HOC) in Amnestic vs. Non-Amnestic MCI
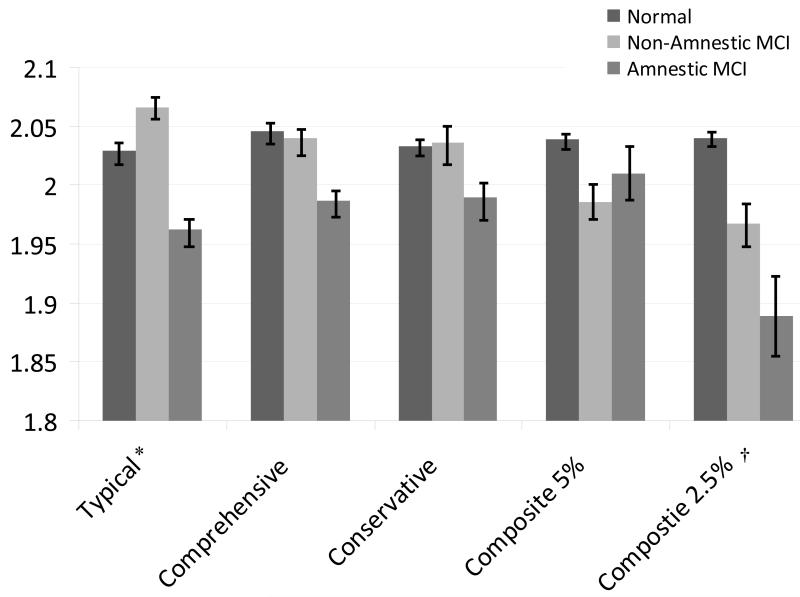
Prevalence rates of MCI varied widely according to the operational criteria applied and have been reported in detail previously (see [32]) but are summarized in Table 2. First, MCI was categorized based on the presence of a memory deficit (normal, non-amnestic, or amnestic). In this framework, when groups were defined using the Typical definition of MCI (1 test, >1.5 SD), we found a significant group effect for HOC (F(2,454)= 4.91, p = .008). Post-hoc tests revealed that HOC distinguished the cognitively normal group from the amnestic MCI group (t(454)=2.03, p=.042, d=.25) and the non-amnestic from the amnestic MCI group (t(454)=3.13, p=.002, d=.38). A significant MCI group effect for HOC was also found when applying the Composite 2.5 definition of MCI (F(2,454)=3.34, p=.036). Post-hoc tests showed that when the Composite 2.5 definition was in effect, HOC differentiated the normal from the amnestic MCI group (t(454)=2.21, p=.042, d=.56). No group effect for HOC was found when MCI was characterized via the Comprehensive (2 tests, >1 SD), the Conservative (2 tests, >1.5 SD) or Composite 5 definitions (all p’s>.12; see Table 3 and Figure 1).
Table 3. Association of Amnestic versus Non-Amnestic MCI with Bilateral Hippocampal Volume and Hippocampal Occupancy Score.
| Type III Test of Fixed Effects |
Post-Hoc Group Comparisons t-value |
||||
|---|---|---|---|---|---|
| MCI Definition | F | p | Normal vs. Non-Amnestic MC |
Normal vs. Amnestic MCI |
Non-Amnestic vs. Amnestic MCI |
| Bilateral Hippocampal Volume | |||||
| Typical | 1.14 | .3210 | -- | -- | -- |
| Comprehensive | 1.66 | .1909 | -- | -- | -- |
| Conservative | 0.72 | .4891 | -- | -- | -- |
| Composite 5 | 1.05 | .3494 | -- | -- | -- |
| Composite 2.5 | 3.17* | .0430 | 2.51* | -0.06 | -1.32 |
| Bilateral Hippocampal Occupancy Score | |||||
| Typical | 4.91** | .0078 | −1.26 | 2.03* | 3.13** |
| Comprehensive | 2.08 | .1265 | -- | -- | -- |
| Conservative | 0.72 | .4868 | -- | -- | -- |
| Composite 5 | 1.21 | .3000 | -- | -- | -- |
| Composite 2.5 | 3.34* | .0362 | 1.73 | 2.04* | 0.96 |
All models include intracranial volume, age, and scanning site as covariates. Degrees of freedom for F-tests = 2, 454. Degrees of freedom for t-tests = 454.
indicates p ≤ .05
indicates p < .01
Figure 1. Bilateral hippocampal occupancy score as a function of MCI definition and status.
* Significant difference between Normal and Amnestic groups and between Non-Amnestic and Amnestic groups
† Significant difference between Normal and Amnestic groups
Error bars represent standard error
Standard Hippocampal Volume (HCV) in Amnestic vs. Non-Amnestic MCI
An examination of standard bilateral hippocampal volume revealed a significant group effect only for the Composite 2.5 definition (F(2,454)=3.17, p =.043). Post-hoc tests indicated that standard hippocampal volume only distinguished the cognitively normal group from the non-amnestic MCI group (t(454)=2.51, p=.013, d=.35). There was no group effect of HCV for the Comprehensive, Typical, Conservative, or Composite 5 definitions (p’s >19; See Table 3).
Hippocampal Occupancy in Single vs. Multi-domain MCI
The sample was also categorized irrespective of memory; MCI was examined based on comparisons of cognitively normal, single domain MCI, and multiple domain MCI. Using this framework, when HOC was used, there was no group effect of HOC for the Comprehensive, Typical, Conservative, or Composite 2.5 or Composite 5 definitions (all p’s >.05).
Standard Hippocampal Volume in Single vs. Multi-domain MCI
When standard hippocampal volume measurement was used, no differences in bilateral hippocampal volumes were found for any MCI definitions (p’s>.10; see Table 4).
Table 4. Association of Any-MCI (Normal, Single Domain, Multi-Domain) with Bilateral Hippocampal Volume and Hippocampal Occupancy Score.
| Type III Test of Fixed Effects |
||
|---|---|---|
| MCI Definition | F | p |
| Bilateral Hippocampal Volume | ||
| Typical | 1.12 | .3259 |
| Comprehensive | 0.88 | .4164 |
| Conservative | 1.17 | .3110 |
| Composite 5 | 0.60 | .5520 |
| Composite 2.5 | 2.32 | .0996 |
| Bilateral Hippocampal Occupancy Score | ||
| Typical | 0.02 | .9770 |
| Comprehensive | 0.96 | .3850 |
| Conservative | 1.41 | .2460 |
| Composite 5 | 2.05 | .1302 |
| Composite 2.5 | 2.97 | .0524 |
All models include intracranial volume, age, and scanning site as covariates. Degrees of freedom for F-tests = 2, 454. Degrees of freedom for t-tests = 454.
Analyses were also conducted adjusting for cardiovascular disease, depression, and apolipoprotein E ε4 allele but this did not substantially alter results.
Conclusion
In this middle-aged cohort, the Typical and Composite 2.5 neuropsychological definitions of MCI and use of hippocampal occupancy measures resulted in the best correspondence of MCI to expected neuroanatomical results. Using the Typical or Composite 2.5 definitions, HOC was significantly reduced in the amnestic MCI group as compared to the cognitively normal group. Use of the Typical definition also resulted in significant differences in HOC between amnestic and non-amnestic MCI participants. The Composite 2.5 MCI definition was the only one that resulted in significant differences in HCV between the normal and non-amnestic groups.
The alignment of these neuroanatomical measures with MCI characterization when using the Typical or Composite 2.5 definitions is noteworthy. Previously, the Comprehensive criteria have been found to be an effective and stable operational definition [31] and correlate with hippocampal volumes in older cohorts [45], but this approach did not show the same level of effectiveness in this younger cohort. While diagnostic grouping based on the Typical or Composite 2.5 definitions were not previously found to relate to hippocampal volumes in older adults, the current study suggests that these operational definitions have utility in a younger sample and may be useful in early identification of MCI. Because the Composite 2.5 definition is more stringent, this approach may be capturing a smaller but higher risk group of individuals with neuropsychological functioning approximately two standard deviations below expectations and measurably smaller hippocampal volumes, but is unlikely to have captured everyone at risk for poor cognitive outcomes over time.
The group differences in hippocampal occupancy suggest that amnestic MCI presentations are associated with medial temporal lobe neuropathological processes even in adults as young as their 50s and is consistent with the specificity of temporal lobe findings in older individuals with amnestic MCI [46]. The Typical and Composite 2.5 definitions did correspond to a neuroimaging biomarker for cognitive decline and are therefore likely useful for researchers seeking to identify individuals who may be at highest risk for poor cognitive outcomes, and thus for intervention. Huey and colleagues (2013) recently found that aMCI was more likely to progress to dementia than were those whose cognitive profiles were predominantly dysexecutive in nature [47] and suggest that the strong relationship between amnestic cognitive presentations and corresponding neuroimaging marker found here would place this group at high risk for future decline.
Hippocampal occupancy, which provides an estimate of degree of hippocampal atrophy (albeit based on a single scan), more readily distinguished MCI groups than did a standard hippocampal volume measurement, except in the most cognitively impaired groups. This is consistent with prior work which found that the Apolipoprotein ε4 allele significantly impacts longitudinal change/decline in hippocampal volumes (volumetric atrophy) in older adults but may not differentiate on the basis of between-group variation in hippocampal volume measured only at one time-point [48]. In general, HOC is a strong predictor of decline in MCI [49] and may hold more utility than a standard hippocampal volume measure, particularly in middle-aged adults.
Some limitations in the current study should be mentioned. Neither subjective cognitive complaints nor informant reports were part of the MCI definitions; diagnostic classifications and results might have differed if such reports were included. However, the use of subjective complaints is potentially of limited utility, particularly in a community-based sample or in samples, such as ours, that have an empirical measure of early adult cognitive functioning (AFQT) [35, 50].
The hippocampus was the only neuroanatomical structure examined in the current study, and a more detailed examination of other neuroanatomy in relation to MCI diagnosis is certainly warranted, particularly to find corresponding neuroanatomical changes that more clearly distinguish normal cognition from non-amnesic presentations. Using an automated segmentation program is also a potential weakness of this study, but a necessity in a large sample. Freesurfer may consistently overestimate hippocampal volume, as compared to manual outlining methods [51], however, Freesurfer also consistently is empirically shown to provide high correspondence to manual outlining volumes with good test/retest reliability and good ability to detect group differences even though individual volume estimates from Freesurfer may differ from manual outlining [52].
MRI images were collected on two different Siemens 1.5 scanners. Although a number of prior studies have demonstrated differences and potential biases in image processing outcomes associated with scanner, field strength, and sequence employed [53, 54] many studies have demonstrated that pooling data to increase sample size can often increase power despite these differences [55] particularly with the appropriate statistical modeling approach [43]. Importantly, the scanner is included as a covariate in these analyses, as a random effect, as supported by our findings in a previous comparison of statistical modeling of pooled MRI data [43].
Follow-up is in progress, but rates of progression to dementia are currently unknown. Therefore, the strategy with the most predictive utility or highest sensitivity or specificity cannot yet be confirmed. Clinical outcomes of participants with MCI should be a focus of continued investigation and future directions include longitudinal assessment of cognitive functioning which may also hold more promise in identifying those at highest risk for progression to AD [56]. Additionally, because hippocampal atrophy is not specific to MCI or dementia, concurrent use of multiple biomarkers have been shown to improve diagnostic accuracy of early MCI [57] as have more detailed examinations of hippocampal subfields [58]; such examinations in larger and younger samples are targets for future investigations.
Strengths of the study include a comprehensive neuropsychological test battery that included cognitive domains that often are overlooked in the assessment of MCI (e.g., non-verbal memory, visual-spatial ability, and processing speed), multiple measures in each domain, and tests selected specifically to avoid ceiling effects in a younger, community-based sample. These factors likely increase the sensitivity to detect mild impairment in a relatively young cohort and reduce measurement error. Importantly, all results were adjusted for actual general cognitive ability at age 20, which increases confidence that the MCI classifications do not simply reflect lifelong low overall cognitive ability. Use of a community-based sample to inform diagnostic procedures is also valuable in identifying the best early detection strategies, particularly in a younger cohort, since few cases in this age-range would be likely to present to memory clinics.
In summary, results provide empirical support for the ability to detect MCI in men as young as their 50’s but highlight that neuropathological correlates differ as a function of altering operational criteria for MCI. The convergence of neuropsychological and neuropathological data, particularly imaging measures that allow an estimate of hippocampal atrophy (HOC) as opposed to standard cross-sectional volume (HCV), supports early identification of MCI. The intersection of neuropsychological and neuropathological data within the groups identified by the Typical and Composite 2.5 criteria offer support for the use of criteria that consider multiple neuropsychological tests in a cognitive domain (e.g., Composite 2.5) or at a higher threshold for impairment if only one measure in a domain is used (Typical), particularly when examining individuals under the age of 60. We found small to medium effect sizes that suggest it is possible to get meaningful predictors in a very young cohort. While meaningful, the strength of the predictors may be too small to be of diagnostic utility in isolation, although it would be unexpected for a single variable to completely differentiate MCI groups. The data nonetheless suggest that HOC and the identified MCI diagnostic criteria are promising contributors to future multivariable approaches to MCI identification.
These results also add to the literature providing empirical information regarding best operational definitions for what constitutes cognitive impairment in MCI, information that can be useful in better identifying MCI, particularly early in its course. Subtle cognitive changes have been shown to be detectable very early on in a pre-clinical dementia stage [56] and the subtle cognitive deficits noted in this young sample do correspond to reduced hippocampal occupancy. While not all individuals with such pathophysiological changes will go on to develop dementia, they still likely represent a higher risk group given the presence of both mild cognitive deficits and evidence of hippocampal atrophy. The group identified here might be the target for future secondary prevention studies aimed at those with subtle impairments. These results contribute to our understanding of the earliest identification of MCI and hold clinical significance because delaying the onset of dementia even by five years can result in a substantial reduction in the overall number of dementia cases [2].
Acknowledgments
This work was supported by National Institute on Aging grants R01 AG018386, AG022381, and AG022982 (to William S. Kremen), R01 AG018384 (to Michael J. Lyons), and the Academy of Finland (to Eero Vuoksimaa). It was also, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. All statements, opinions, or views are solely of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government, the NIA, or the NIH. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible. We also appreciate the time and energy of many staff and students on the VETSA projects.
Source of Funding: Anders M. Dale, PhD receives funding to his laboratory from General Electric Medical Systems as part of a Master Research Agreement with the University of California, San Diego. Dr. Dale is a founder of, holds equity in, and serves on the scientific advisory board for CorTechs Labs, Inc. The terms of this arrangement have been reviewed and approved by the University of California, San Diego, in accordance with its conflict of interest policies. This work was supported by National Institute on Aging grants R01 AG018386, AG022381, and AG022982 (to William S. Kremen), R01 AG018384 (to Michael J. Lyons), and the Academy of Finland (to Eero Vuoksimaa). It was also, in part, the result of work supported with resources of the VA San Diego Center of Excellence for Stress and Mental Health. The Cooperative Studies Program of the Office of Research & Development of the United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was presented, in part, at the Alzheimer’s Association International Conference, July, 2011, Paris, France and at the International Neuropsychological Society annual meeting, February 2013, Waikaloa, HI.
Conflicts of Interest
No other authors have any actual or potential conflicts of interest.
References
- 1.Morris JC. Early-stage and preclinical Alzheimer disease. Alzheimer Disease and Associated Disorders. 2005;19:163–165. doi: 10.1097/01.wad.0000184005.22611.cc. [DOI] [PubMed] [Google Scholar]
- 2.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blennow K, et al. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature Reviews Neurology. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 4.Buchhave P, et al. Cerebrospinal fluid levels of beta-amyloid 1-42, but not of tau, are fully changed already 5 to 10 Years before the onset of Alzheimer dementia. Archives of General Psychiatry. 2012;69:98–106. doi: 10.1001/archgenpsychiatry.2011.155. [DOI] [PubMed] [Google Scholar]
- 5.Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment--a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatrica Scandinavica. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 6.Ward A, et al. Mild cognitive impairment: Disparity of incidence and prevalence estimates. Alzheimer’s and Dementia. 2012;8:14–21. doi: 10.1016/j.jalz.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Anstey KJ, et al. Follow-up of mild cognitive impairment and related disorders over four years in adults in their sixties: The PATH trhough life study. Dementia and Geriatric Cognitive Disorders. 2008;26:226–33. doi: 10.1159/000154646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anstey KJ, et al. Characterizing mild cognitive disorders in the young-old over 8 years: Prevalence, estimated incidence, stability of diagnosis, and impact on ADL’s. Alzheimer’s and Dementia. 2013;9:640–648. doi: 10.1016/j.jalz.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 9.Caselli RJ, et al. The neuropsychology of normal aging and preclinical Alzheimer’s disease. Alzheimer’s and Dementia. 2013 doi: 10.1016/j.jalz.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caselli RJ, Reiman EM. Characterizing the preclinical stages of alzheimer’s disease and the prospect of presymptomatic intervention. Journal of Alzheimer’s Disease. 2013;33:s405–416. doi: 10.3233/JAD-2012-129026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albert M, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:270–79. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manly JJ, et al. Implementing Diagnostic Criteria and Estimating Frequency of Mild Cognitive Impairment in an Urban Community. Archives of Neurology. 2005;62(11):1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, et al. Current Concepts in Mild Cognitive Impairment. Archives of Neurology. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 14.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Archives of Neurology. 2005;62:1160–1163. doi: 10.1001/archneur.62.7.1160. [DOI] [PubMed] [Google Scholar]
- 15.Petersen RC, et al. Mild Cognitive Impairment: Clinical Characterization and Outcome. Archives of Neurology. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 16.Tabert MH, et al. Neuropsychological prediction of conversion to alzheimer disease in patients with mild cognitive impairment. Archives of General Psychiatry. 2006;63(8):916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 17.Tuokko HA, McDowell I. An overview of mild cognitive impairment. In: Tuokko HA, Hultsch DF, editors. Mild Cognitive Impairment: International Perspectives. Taylor and Francis; New York: 2006. pp. 3–28. [Google Scholar]
- 18.Petersen RC, et al. Mild cognitive impairment: a concept in evolution. Journal of Internal Medicine. 2014;275:214–28. doi: 10.1111/joim.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hänninen T, et al. Prevalence of mild cognitive impairment: A population-based study in elderly subjects. Acta Neurologica Scandinavica. 2002;106:148–154. doi: 10.1034/j.1600-0404.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumar R, et al. Prevalence of mild cognitive impairment in 60- to 64-year-old community-dwelling individuals: The Personality and Total Health through Life 60+ Study. Dementia and Geriatric Cognitive Disorders. 2005;19:67–74. doi: 10.1159/000082351. [DOI] [PubMed] [Google Scholar]
- 21.Schroder J, et al. Prevalence of mild cognitive impairment in an elderly community sample. Journal of Neural Transmission. Supplementum. 1998;54:51–59. doi: 10.1007/978-3-7091-7508-8_5. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo A, et al. CIND and MCI in the Italian elderly: Frequency, vascular risk factors, progression to dementia. Neurology. 2007;68(22):1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 23.Palmer K, et al. Mild cognitive impairment in the general population: occurrence and progression to Alzheimer disease. American Journal of Geriatric Psychiatry. 2008;16(7):603–11. doi: 10.1097/JGP.0b013e3181753a64. [DOI] [PubMed] [Google Scholar]
- 24.De Leon MJ, et al. Frequency of hippocampal formation atrophy in normal aging and Alzheimer’s disease. Neurobiology of Aging. 1997;18(1):1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 25.Mosconi L, et al. Early detection of Alzheimer’s disease using neuroimaging. Experimental Gerontology. 2007;42:129–138. doi: 10.1016/j.exger.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Rusinek H, et al. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen RC, et al. Mild cognitive impairment: Clinical characterization and outcome. Archives of Neurology. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 29.National Center for Disease Statistics Prevalence of selected chronic conditions by age, sex, race, and Hispanic origin: United States, 1997-2004. NHIS. 2004 cited 2006 June 9. Available from: http://209.217.72.34/aging/TableViewer/tableView.aspx.
- 30.Ganguli M, et al. Outcomes of mild cognitive impairment by definition: A population study. Archives of Neurology. 2011;68:761–7. doi: 10.1001/archneurol.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jak AJ, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. American Journal of Geriatric Psychiatry. 2009;17:368–75. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kremen WS, et al. Early identification and heritability of mild cognitive impairment. International Journal of Epidemiology. 2014;43(2):600–10. doi: 10.1093/ije/dyt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busse A, et al. Mild cognitive impairment: Long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 34.Espinosa A, et al. A longitudinal follow-up of 550 mild cognitive impairment patients: evidence for large conversion to dementia rates and detection of major risk factors involved. Journal of Alzheimer’s Disease. 2013;34(3):769–80. doi: 10.3233/JAD-122002. [DOI] [PubMed] [Google Scholar]
- 35.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 36.Roberts RO, et al. The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology. 2012;78(5):342–51. doi: 10.1212/WNL.0b013e3182452862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winblad B, et al. Mild cognitive impairment - beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. Journal of Internal Medicine. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 38.Lyons MJ, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychological Science. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischl B, et al. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Fischl B, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 41.Kremen WS, et al. Genetic and environmental influences on the size of specific brain regions in midlife: The VETSA MRI study. NeuroImage. 2010;49:1213–23. doi: 10.1016/j.neuroimage.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckner RL, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size nomalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23(2):724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Fennema-Notestine C, et al. Feasibility of multi-site clinical structural neuroimaging studies of aging using legacy data. Neuroinformatics. 2007;5:235–245. doi: 10.1007/s12021-007-9003-9. [DOI] [PubMed] [Google Scholar]
- 44.Heister D, et al. Predicting MCI outcome with clinically available MRI and CSF biomarkers. Neurology. 2011;77(17):1619–28. doi: 10.1212/WNL.0b013e3182343314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jak AJ, et al. Profile of hippocampal volumes and stroke risk varies by neuropsychological definition of mild cognitive impairment. Journal of the International Neuropsychological Society. 2009;2:1–8. doi: 10.1017/S1355617709090638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serra L, et al. Mild cognitive impairment: Same identity for different entities. Journal of Alzheimer’s Disease. 2012 doi: 10.3233/JAD-2012-121663. epub ahead of print; DOI 10.3233/JAD-2012-121663. [DOI] [PubMed] [Google Scholar]
- 47.Huey ED, et al. Course and etiology of dysexecutive MCI in a community sample. Alzheimer’s and Dementia. 2013;9:632–639. doi: 10.1016/j.jalz.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jak AJ, et al. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dementia and Geriatric Cognitive Disorders. 2007;23:282–89. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henneman WJ, et al. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;17:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenehan ME, Klekociuk SZ, Summers MJ. Absence of a relationship between subjective memory complaint and objective memory impairment in mild cognitive impairment (MCI): is it time to abandon subjective memory complaint as an MCI diagnostic criterion? International Psychogeriatrics. 2012;24(09):1505–1514. doi: 10.1017/S1041610212000695. [DOI] [PubMed] [Google Scholar]
- 51.Morey RA, et al. A comparison of automated segmentation and manual tracing for quantifying hippocampal and amygdala volumes. Neuroimage. 2009;45(3):855–866. doi: 10.1016/j.neuroimage.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen L, et al. Comparison of manual and automated determinatino of hippocampal volumes in MCI and early AD. Brain Imaging and Behavior. 2010;4(1):86–95. doi: 10.1007/s11682-010-9088-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han X, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180–94. doi: 10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 54.Jovicich J, et al. MRI-derived measurements of human subcortical, ventricular and intracranial brain volumes: Reliability effects of scan sessions, acquisition sequences, data analyses, scanner updgrade, scanner vendor, and field strength. Neuroimage. 2009;46(1):177–92. doi: 10.1016/j.neuroimage.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jack CR, Jr., et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging. 2008;27(4):685–91. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sperling RA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s and Dementia. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Leon MJ, et al. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiology of Aging. 2006;27(3):394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Apostolova LG, et al. Subregional hippocampal atrophy predicts Alzheimer’s dementia in the cognitively normal. Neurobiology of Aging. 2010;31(7):1077–1088. doi: 10.1016/j.neurobiolaging.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]