Abstract
Background
To clarify the role of genetic and environmental risk factors in alcohol use disorders (AUDs), we performed a meta-analysis of twin and adoption studies and explored the impact of sex, assessment method (interview v. hospital/population records), and study design (twin v. adoption study) on heritability estimates.
Method
The literature was searched for all unique twin and adoption studies of AUD and identified 12 twin and five adoption studies. The data were then reconstructed and analyzed using ordinal data full information maximum likelihood in the OpenMx program. Heterogeneity was tested with likelihood ratio tests by equating the parameters across studies.
Results
There was no evidence for heterogeneity by study design, sex or assessment method. The best-fit estimate of the heritability of AUD was 0.49 [95% confidence interval (CI) 0.43–0.53], and the proportion of shared environmental variance was 0.10 (95% CI 0.03–0.16). Estimates of unique environmental proportions of variance differed significantly across studies.
Conclusions
AUD is approximately 50% heritable. The multiple genetically informative studies of this syndrome have produced consistent results that support the validity of this heritability estimate, especially given the different potential methodological weaknesses of twin and adoption designs, and of assessments of AUD based on personal interviews v. official records. We also found evidence for modest shared environmental effects suggesting that environmental factors also contribute to the familial aggregation of AUDs.
Keywords: Adoption study, alcohol use disorder, alcoholism, environment, genetics, meta-analysis, twin study
Introduction
Alcohol use disorder (AUD) is among the most common of psychiatric syndromes (Grant, 1997) and is often accompanied by significant psychosocial dys-function, a range of medical co-morbidities and substantially increased mortality (Secretary of Health and Human Services, 1997). It has been known since classical times that AUD is familial (Bynum, 1984) and this has been verified by more modern family studies (Cotton, 1979). Since 1960, a series of twin (Kaij, 1960; McGue et al. 1992; Reed et al. 1996; Heath et al. 1997; Prescott et al. 1999; True et al. 1999; Knopik et al. 2004; Magnusson et al. 2012) and adoption (Goodwin et al. 1973, 1977; Bohman et al. 1981; Cloninger et al. 1981; Cadoret et al. 1987; Sigvardsson et al. 1996) studies have attempted to determine the relative roles of genetic and environmental factors in the etiology of AUD. While these studies have been reviewed qualitatively several times (e.g. Heath, 1995; Prescott & Kendler, 1995; Dick & Bierut, 2006), we are unaware of any quantitative meta-analysis.
In addition to providing a more reliable estimate of the heritability of AUD, such an analysis could address four additional important questions. First, several early twin and adoption studies suggested that there were quantitative sex effects in the genetic factors for AUD – that genetic effects were stronger in males than in females (Goodwin et al. 1973; Bohman et al. 1981; McGue et al. 1992). The studies that found differential genetic effects as a function of sex, however, often relied on small samples, especially of females. Most recent, larger studies have been unable to detect genetic differences between the sexes. Accordingly, a quantitative meta-analysis of a now larger body of studies would more accurately evaluate this hypothesis.
Relatedly, five twin studies presented results on resemblance for AUDs in opposite-sex dizygotic (DZ) twin pairs and thus were able to test for the presence of qualitative sex effects for AUDs – that the genetic risk factors were not entirely the same in males and females. This is detected largely by comparing the magnitude of the correlation in same- v. opposite-sex DZ pairs. One study found evidence for such an effect for AUDs (Prescott et al. 1999), two did not (Heath et al. 1997; Magnusson et al. 2012), and two did not test for it (Caldwell & Gottesman, 1991; Knopik et al. 2004). Because qualitative sex effects can only be detected reliably with large samples (Prescott & Gottesman, 1993), meta-analytical methods provide an ideal procedure to evaluate evidence for this effect.
Second, some twin studies suggest that shared environmental risk factors contribute to the familial aggregation of AUDs (McGue et al. 1992; Kendler et al. 1997) while others do not (Heath et al. 1997; Prescott et al. 1999). The magnitude of the shared environmental variance, however, is typically small (but see Kaij, 1960 for an exception). In the presence of substantial genetic influences, quite large samples of twins are needed to detect shared environmental effects (Neale et al. 1994). A large meta-analysis would be much better powered to identify shared environmental effects for AUDs than an individual study, if indeed they are present.
Third, these studies utilized two different methods of assessment. A number of the twin and adoption studies utilized personal interviews, an approach that requires cooperation and relies critically on the accuracy of retrospective reporting of symptoms generally seen as socially undesirable (e.g. Cadoret et al. 1987; Reed et al. 1996; Prescott et al. 1999; True et al. 1999). Other studies utilized registry information, particularly from medical (Reed et al. 1996) and temperance board records (Cloninger et al. 1981; Kendler et al. 1997). This approach typically provides contemporaneously recorded information and requires no cooperation, but might have limited sensitivity and specificity in the detection of AUD as they require affected individuals to be detected with alcohol-related problems by the medical or legal system. While both official registrations – typically from medical and/or legal records – and clinical diagnoses are commonly used assessments of AUD, it is possible that the cases diagnosed by different assessment methods could reflect partly independent latent genetic factors. If the reliability of AUD diagnoses differed with these two methods, it could be reflected in additive genetic, shared and individual specific environmental variance components, because unreliability increases specific environmental variance thereby decreasing the proportion of variance due to other components.
Finally, twin and adoption studies are quite different in their assumptions and potential biases. The estimation of genetic effects in these two kinds of studies derives from quite distinct kinds of relationships: monozygotic (MZ) v. DZ twins, and biological and adoptive relatives, respectively. Specifically, twin studies leverage the differential genetic relatedness between MZ and DZ twins with the major assumption that the rearing environments to which they are exposed are equally correlated. By contrast, adoption studies leverage the genetic or environmental similarity between biological or adoptive relatives with the major methodological concern being assortative placement – whether adoptees are placed randomly within adoptive homes. While there are many types of adoption designs, the literature on AUD has focused on the relationship between biological parents and their adopted away offspring. Previous research in other behavioral domains has suggested that estimates of genetic factors from adoption studies are smaller than the same estimates in twin studies (Loehlin, 1992; Rhee & Waldman, 2002). Would the estimated heritability of AUD from these two methods be similar?
In this paper, we perform a quantitative meta-analysis of all available twin and adoption studies of AUD and address these and other questions of interest.
Method
We searched PubMed and Google Scholar® for all twin and adoption studies of AUD. In addition, we searched the bibliographies of all of the relevant studies to further add samples to our analysis. To ensure completeness, we followed up by contacting leading researchers in the AUD genetics field. Studies were included in our analyses if they: (i) examined AUD, here defined as a syndrome including heavy alcohol use with significant adverse social, psychological and/or medical consequences, a definition broadly similar to the DSM-IV concepts of alcohol abuse or dependence (APA, 1994); and (ii) presented sufficient data to permit re-analysis. In practice, across the various studies, AUD was defined by clinical interview, hospital record diagnosis or temperance board registration. If multiple studies were published using the same data, we used the report with the largest sample size. Because these studies utilized a variety of different analytic approaches, it was necessary to reconstruct the data for each study. The features of the available twin and adoption studies are listed in Tables 1 and 2, respectively. Importantly, because we focused exclusively on AUD, twin studies examining only drinking frequency or quantity were omitted.
Table 1.
Twin studies of alcohol abuse and dependence
| Author (year) | Ascertainment | Assessment | Diagnostic measure | Country | N | Sex | Data |
|---|---|---|---|---|---|---|---|
| Included | |||||||
| Kaij (1960) | Twin registry | Temperance board | Registration | Sweden | 1216 | M | Tetrachoric |
| Gurling et al. (1984) | Clinical | Interview | ICDa | England | 735 | M&F | Contingency tables |
| Allgulander et al. (1991) | Twin registry | Hospital discharge | ICDb | Sweden | 12 884 | M&F | Tetrachoric |
| Romanov et al. (1991) | Twin registry | Hospital discharge | ICD-8c | Finland | 5 340 | M&Fd | Contingency tables |
| Caldwell & Gottesman (1991) e | Clinical | Interview | DSM-III | USA | 154 | M&F | Odds ratios |
| McGue et al. (1992) | Clinical | Interview | DSM-III | USA | 258 | M&F | Tetrachoric |
| Reed et al. (1996) | Twin registry | Registry | ICD-8f | USA | 26 974 | M | Tetrachoric |
| Heath et al. (1997) | Twin registry | Interview | DSM III-R | Australia | 5 889 | M&F | Tetrachoric |
| Kendler et al. (1997) | Twin registry | Temperance board | Registration | Sweden | 7 790 | M | Tetrachoric |
| True et al. (1999) | Twin registry | Interview | DSM III-R | USA | 2 364 | M | Tetrachoric |
| Prescott et al. (1999) | Twin registry | Interview | DSM-IV | USA | 9 259 | M | Tetrachoric |
| Magnusson et al. (2012) | Twin registry | Interview/questionnaire | DSM-IV | Sweden | 24119 | M&F | Tetrachoric |
| Excluded | Reason for exclusion | ||||||
| Hrubec & Omenn (1981) | VA medical records | VA medical records | USA | 15 924 | M | Included in Reed et al. (1996) | |
| Pickens et al. (1991) | Clinical | DSM-III | USA | 338 | M&F | Included in McGue et al. (1992) | |
| Kendler et al. (1992) | Population | DSM III-R | USA | 2 060 | F | Included in Prescott et al. (1999) | |
| Prescott et al. (1999) | Population | DSM-IV | USA | 7 032 | M | Included in Prescott et al. (1999) |
Alcoholic addiction, alcoholic psychosis, habitual excessive use of alcohol.
Alcoholism.
Alcoholic liver disease, alcoholic psychosis, alcohol intoxication, alcoholism.
Male and female twin groups were combined in the analysis.
Unpublished.
Code 303.
Table 2.
Adoption studies of alcohol abuse and dependence
| Author (year) | Ascertainment | Assessment | Diagnostic measure | Country | N | Relationship | Data |
|---|---|---|---|---|---|---|---|
| Included | |||||||
| Cloninger et al. (1981) | Stockholm adoption registry | Temperance board | Registration | Sweden | 1724 | Biological mother and father with son | Contingency tables |
| Sigvardsson et al. (1996) | Gothenburg adoption registry | Temperance board | Registration | Sweden | 2474 | Biological mother and father with son and daughter | Contingency tables |
| Cadoret et al. (1987) | Iowa children's and family services adoption records | Telephone interview | DSM-III | USA | 320 | Biological parent with son | Contingency tables |
| Goodwin et al. (1973) | Copenhagen adoption study | Clinical interview | Blind coding of interviews | Denmark | 108 | Biological parent with son | Contingency tables |
| Bohman et al. (1981) | Stockholm adoption registry | Temperance board | Registration | Sweden | 1826 | Biological father with daughter | Contingency tables |
| Goodwin et al. (1977) | Copenhagen adoption study | Clinical interview | Blind coding of interviews | Denmark | 96 | Biological parent with daughter | Contingency tables |
Most of the original twin studies used a model-fitting approach whereby non-significant parameters were sequentially dropped from the model. In such cases, it is standard practice to present reduced models with only the significant parameters. Accordingly, the model parameters that are reported in the original articles are typically not the full statistical model that would be required to reconstruct the data for meta-analysis, but the most parsimonious model for the specific study. However, most studies reported the tetrachoric correlations or odds ratios (ORs) and the prevalence rates, which allowed us to reconstruct the raw data with equivalent population parameters. Tests of heterogeneity within the twin models relied upon this recreated data (effectively resulting in a mega-analysis). Among the adoption studies, some presented ORs while others presented the percent affected in the sample. To put everything on the same metric and to make the adoption studies comparable to the twin studies, we generated raw data from contingency tables from the adoption studies and then computed tetrachoric correlations.
In the twin analysis, heritabilities were estimated using standard structural equation modeling techniques that compare the tetrachoric correlations between multiple groups of twins separated by sex and zygosity (Neale & Cardon, 1992). To assess qualitative genetic sex differences a parameter was included that allowed the correlation between the genetic factors of opposite-sex DZ twins to be smaller than the same-sex DZ correlation (Neale & Cardon, 1992). If significant, this would indicate that at least partially different genetic factors culminate in AUD in males and females. Heritability in adoption studies was estimated by comparing the resemblance between biological parents and their adopted-away offspring, modeled as half the additive genetic variance. This allowed us to analyze the adoption and twin studies jointly within the SEM framework.
No measure comparable to shared environment from twin studies was available from the adoption studies, so in comparing twin and adoption studies we focused solely on the estimates of heritability.
Likelihood ratio tests were used to test for the significance of each of the variance components. Importantly, because twin studies focus on variance, which must be a positive quantity, the likelihood ratio tests for significance is not distributed as a χ2 with, for example, 1 df. Instead, the null distribution is a 50:50 mixture of 0 and χ2 with 1 df (Wu & Neale, 2012). The presented hypothesis tests take this finding into account.
To test for heterogeneity across samples for the various parameters of interest, we used a likelihood ratio test to compare the full model to the restricted model. Twice the difference between the log-likelihoods of the two models is, under certain regularity conditions, distributed as χ2 with degrees of freedom equal to the difference in the number of parameters in the two models. Thus, to test for heterogeneity in, e.g. the genetic parameter, we compared the model where all of the studies had unique genetic parameters to a model where all of the genetic parameters across the studies were equated. This is a multiple degrees-of-freedom test. To test for heterogeneity across sexes, assessment methods, or study design, we relied on a 1 df likelihood ratio test. For example, to test for genetic heterogeneity across sexes, we compared a model with one genetic parameter for males and one for females, to a restricted model where the genetic parameters were equated across sex. All modeling was performed in OpenMx (Boker et al. 2011, 2012).
Results
Twin studies
Additive genetic effects
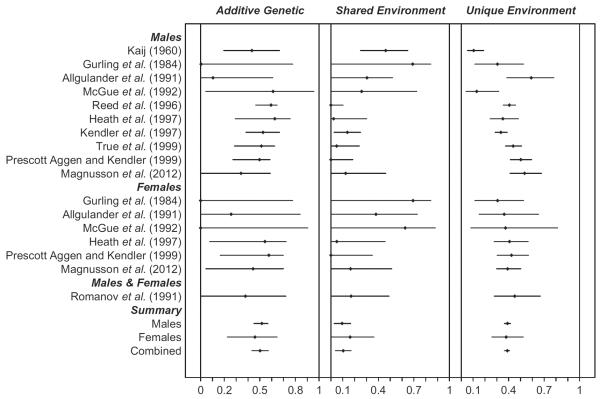
The combined estimate of the heritability of AUDs in the twin studies was 0.51 [95% confidence interval (CI) 0.45–0.56]. We first examined whether there was heterogeneity in the heritability estimate as a function of sex – that is testing for quantitative sex effects. As can be seen in Fig. 1 and Table 3, the genetic effects on AUD can be equated across samples both within men (χ2 = 11.63, df = 11, p = 0.39) and women (χ2 = 4.58, df = 7, p = 0.71), and across both men and women (χ2 = 0.47, df = 1, p = 0.49) without a reduction in the model fit, consistent with minimal heterogeneity. When examined separately, the heritability of AUD was estimated at 0.52 in males (95% CI 0.45–0.57) and 0.44 in females (95% CI 0.25–0.61). In addition, we found no evidence for qualitative sex differences in genetic effects for AUDs. Specifically, the ratio of the correlation between opposite-sex and same-sex twin pairs did not differ significantly from unity (rg-mf = 1, 95% CI 0.42–1.00, χ2 = 1.79, df = 4, p = 0.77), albeit with very large CIs, which suggests that the same genes contribute to AUD risk in both males and females.
Fig. 1.
Forest plot of genetic and environmental variance components for alcohol use disorders in twin studies by sex.
Table 3.
Parameter estimates and confidence intervals (CD for twin studies of alcohol use disorders
| Heritability |
Shared environment |
Unique environment |
|||||
|---|---|---|---|---|---|---|---|
| Relationship | Study | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI |
| All twins | 0.510 | 0.448–0.556 | 0.095a | 0.033–0.158 | 0.393 | 0.375–0.415 | |
| Male twins | All males | 0.516 | 0.447–0.567 | 0.083a | 0.015–0.149 | 0.391a | 0.367–0.417 |
| Kaij (1960) | 0.402 | 0.153–0.646 | 0.481 | 0.261–0.673 | 0.118 | 0.060–0.210 | |
| Gurling et al. (1984) | 0.004 | 0.000–0.777 | 0.690 | 0.003–0.843 | 0.306 | 0.115–0.526 | |
| Allgulander et al. (1991) | 0.161 | 0.000–0.630 | 0.263 | 0.000–0.508 | 0.576 | 0.368–0.777 | |
| Caldwell & Gottesman (1991), Romanov et al. (1991) | 0.772 | 0.242–0.937 | 0.000 | 0.000–0.456 | 0.228 | 0.063–0.560 | |
| McGue et al. (1992) | 0.585 | 0.021–0.950 | 0.275 | 0.000–0.734 | 0.140 | 0.047–0.328 | |
| Reed et al. (1996) | 0.597 | 0.463–0.648 | 0.000 | 0.000–0.107 | 0.403 | 0.352–0.458 | |
| Heath et al. (1997) | 0.675 | 0.379–0.779 | 0.002 | 0.000–0.246 | 0.323 | 0.221–0.450 | |
| Kendler et al. (1997) | 0.529 | 0.384–0.671 | 0.133 | 0.019–0.245 | 0.338 | 0.289–0.391 | |
| True et al. (1999) | 0.510 | 0.277–0.621 | 0.044 | 0.000–0.240 | 0.446 | 0.379–0.519 | |
| Prescott et al. (1999) | 0.489 | 0.294–0.578 | 0.000 | 0.000–0.156 | 0.511 | 0.422–0.609 | |
| Knopik et al. (2004) | 0.599 | 0.288–0.694 | 0.000 | 0.000–0.260 | 0.401 | 0.306–0.511 | |
| Magnusson et al. (2012) | 0.335 | 0.000–0.573 | 0.104 | 0.000–0.431 | 0.561 | 0.427–0.714 | |
| Female twins | All females | 0.441 | 0.246–0.605 | 0.158 | 0.000–0.327 | 0.400 | 0.347–0.455 |
| Gurling et al. (1984) | 0.000 | 0.000–0.777 | 0.693 | 0.003–0.843 | 0.307 | 0.115–0.526 | |
| Allgulander et al. (1991) | 0.229 | 0.000–0.818 | 0.383 | 0.000–0.720 | 0.388 | 0.173–0.672 | |
| Caldwell & Gottesman (1991) | 0.091 | 0.000–0.876 | 0.652 | 0.000–0.868 | 0.257 | 0.069–0.523 | |
| McGue et al. (1992) | 0.000 | 0.000–0.904 | 0.626 | 0.000–0.881 | 0.374 | 0.081–0.814 | |
| Heath et al. (1997) | 0.603 | 0.112–0.738 | 0.007 | 0.000–0.435 | 0.390 | 0.262–0.553 | |
| Prescott et al. (1999) | 0.591 | 0.189–0.706 | 0.001 | 0.000–0.343 | 0.409 | 0.294–0.545 | |
| Knopik et al. (2004) | 0.529 | 0.130–0.649 | 0.015 | 0.000–0.357 | 0.456 | 0.351–0.578 | |
| Magnusson et al. (2012) | 0.404 | 0.024–0.705 | 0.205 | 0.000–0.523 | 0.390 | 0.289–0.511 | |
| Male and female twins | Romanov et al. (1991) | 0.385 | 0.000–0.721 | 0.165 | 0.000–0.491 | 0.449 | 0.279–0.668 |
Parameter has significant heterogeneity.
The second contrast tested whether the heritability estimate differed as a function of the assessment method. Specifically, several studies used population registries or hospital records to infer AUD while others relied on clinical interviews. There was no statistically significant difference between the assessment methods (χ2 = 0.31, df = 1, p = 0.58). Accordingly, there is no evidence of heterogeneity in the heritability estimate due to sex or assessment method. The likelihood ratio tests results are presented in Table 4.
Table 4.
Model comparison results for twin studies
| Name | Number of estimated parameters | −2LL | AIC | χ 2 | df | p |
|---|---|---|---|---|---|---|
| Full model | 89 | 68 205.34 | −238 084.7 | – | – | – |
| Equal A – males | 78 | 68 216.96 | −238 095.0 | 11.626773 | 11 | 0.39 |
| Equal C – males | 78 | 68 227.78 | −238 084.2 | 22.444837 | 11 | 0.02 |
| Equal E – males | 78 | 68 260.04 | −238 052.0 | 54.699729 | 11 | 0.00 |
| Equal A – females | 82 | 68 209.92 | −238 094.1 | 4.585781 | 7 | 0.71 |
| Equal C – females | 82 | 68 213.57 | −238 090.4 | 8.237681 | 7 | 0.31 |
| Equal E – females | 82 | 68 208.44 | −238 095.6 | 3.107698 | 7 | 0.87 |
| Equal rg-mf | 85 | 68 207.13 | −238 090.9 | 1.791517 | 4 | 0.77 |
| Equal A – all | 70 | 68 220.26 | −238107.7 | 14.923547 | 19 | 0.73 |
| Equal C – all | 69 | 68 236.65 | −238 093.3 | 31.314067 | 20 | 0.05 |
| Equal E – all | 70 | 68 263.25 | −238 064.7 | 57.914781 | 19 | 0.00 |
AIC, Akaike's Information Criterion.
Shared environmental effects
A forest plot of the shared environmental variance components is presented in the middle panels of Fig. 1 and in Table 3. The combined estimate of the shared environmental variance proportion for AUDs across all studies was modest 0.10 (95% CI 0.03–0.16) but statistically significant (p < 0.01). Heterogeneity in the common environmental variance component was also tested as a function of both sex and assessment technique. Across all studies, the estimate of shared environmental effects on AUDs in males was modest (0.083, 95% CI 0.01–0.15) and statistically heterogeneous (χ2 = 22.44, df = 11, p = 0.02). As is evident in the figure, this heterogeneity derived largely from a single study: Kaij (1960). When this study was removed, the heterogeneity decreased substantially and became non-significant (χ2 = 9.97, df = 10, p = 0.44). However, when this study was excluded from our analysis, the shared environment effect in males was no longer statistically significant (0.05, 95% CI 0.00–0.12, χ2 = 1.92, df = 1, p = 0.17). In females, the estimate of shared environmental effects was relatively large and marginally statistically significant (c2 = 0.16, 95% CI 0.00–0.33, χ2 = 3.06, df = 1, p = 0.08), and there was no heterogeneity across samples (χ2 = 8.24, df = 7, p = 0.31). When the common environmental parameters were equated across sex, excluding the Kaij outlier, there were no significant differences between the sexes (χ2 = 1.46, df = 1, p = 0.22).
Consistent with the heritability estimates, the comparison of estimates of shared environmental effects in studies using hospital discharge or registration records v. clinical interviews was not statistically significant (χ2 = 0.27, df = 1, p = 0.60). The heterogeneity contrast was unaffected by the inclusion or exclusion of the Kaij study.
Unique environmental effects
The combined estimate of the unique environment proportion of variance is 0.39 (95% CI 0.38–0.42). There was strong heterogeneity in the estimates of the non-shared environment in males (χ2 = 54.70, df = 11, p < 0.001). Given that the Kaij sample had the smallest non-shared parameter estimate, the heterogeneity analysis was re-run with a fixed effect for the Kaij study parameter. While the heterogeneity in the unique environmental parameter was considerably reduced, it was still significant (χ2 = 31.30, df = 10, p < 0.001) and therefore not a function of a single study. In contrast, in females there was no evidence of heterogeneity (χ2 = 3.11, df = 7, p = 0.87). While there was heterogeneity within the male unique environmental estimate, when the estimates of the unique environmental parameter across sexes was constrained to equality, there were no significant differences between the sexes (χ2 = 0.04, df = 1, p = 0.83). Thus, there are no significant differences in estimates of the unique environmental variance for males and females.
When comparing the unique environmental estimate as a function of assessment method, excluding the Kaij outlier study, there was no significant heterogeneity in the parameter estimates (χ2 = 0.27, df = 1, p = 0.60). Follow-up tests were conducted to test for heterogeneity within each assessment group. Significant heterogeneity was found for studies using interview-based assessments (χ2 = 24.68, df = 14, p = 0.04), but not for record-based assessments (χ2 = 7.08, df = 4, p = 0.13). Taken in concert with the previous findings, the heterogeneity within the interview-based assessment method is primarily a function of heterogeneity in the male unique environmental parameters.
Adoption studies
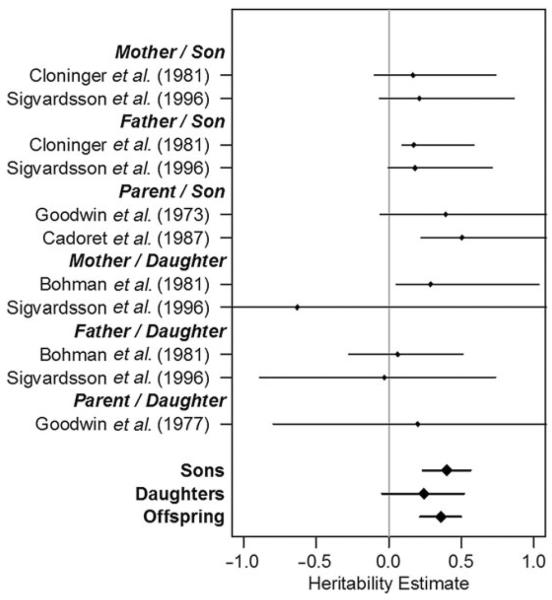
A forest plot of the heritability estimates for the adoption studies is presented in Fig. 2. Very few of the adoption studies individually produced significant estimates of the heritability of AUDs. However, when analyzed together, the estimate of heritability in males was 40.0% and statistically significant (95% CI 0.23–0.56, χ2 = 22.15, df = 1, p < 0.001). In females, the estimated heritability of AUDs was modest (0.241) but not statistically significant (95% CI 0.00–0.52, χ2 = 2.75, df = 1, p = 0.05). When the sexes were combined, no significant differences were seen between them, and the heritability was estimated at 0.36 with relatively wide CIs (0.22–0.50). The model fitting results for the adoption studies are presented in Table 5 and the parameter estimates are presented in Table 6.
Fig. 2.
Forest plot of heritability of alcohol use disorders from adoption studies by relationship.
Table 5.
Model comparison results for adoption studies
| Parameters equated | Estimated parameters | −2LL | AIC | χ 2 | df | p |
|---|---|---|---|---|---|---|
| Full model | 33 | 8 114.85 | −17371.2 | |||
| Sons equated | 28 | 8118.46 | −17377.5 | 3.61 | 5 | 0.61 |
| Daughters equated | 29 | 8117.73 | −17376.3 | 2.88 | 4 | 0.58 |
| All offspring equated | 23 | 8 122.26 | −17383.7 | 7.42 | 10 | 0.69 |
AIC, Akaike's Information Criterion.
Table 6.
Parameter estimates and 95% confidence intervals (CI) for adoption studiesa
| Relationship | Study | Heritability estimate | 95% (CI) |
|---|---|---|---|
| Summary | All offspring | 0.356 | 0.216 to 0.499 |
| Male offspring | 0.400 | 0.235 to 0.562 | |
| Female offspring | 0.241 | −0.045 to 0.517 | |
| Mother/son | Cloninger et al. (1981) | 0.166 | −0.100 to 0.738 |
| Sigvardsson et al. (1996) | 0.210 | −0.062 to 0.865 | |
| Father/son | Cloninger et al. (1981) | 0.172 | 0.092 to 0.588 |
| Sigvardsson et al. (1996) | 0.181 | −0.005 to 0.711 | |
| Parent/son | Cadoret et al. (1987) | 0.504 | 0.222 to 1.555 |
| Goodwin et al. (1973) | 0.392 | −0.061 to 1.426 | |
| Mother/daughter | Bohman et al. (1981) | 0.287 | 0.053 to 1.034 |
| Sigvardsson et al. (1996) | −0.630 | −1.800 to 1.249 | |
| Father/daughter | Bohman et al. (1981) | 0.062 | −0.276 to 0.513 |
| Sigvardsson et al. (1996) | −0.031 | −0.889 to 0.736 | |
| Parent/daughter | Goodwin et al. (1977) | 0.200 | −0.796 to 1.388 |
The heritability estimate for the adoption studies was estimated by doubling the correlation between the biological parents and offspring (in the same way that the genetic correlation between dizygotic twins is half the genetic correlation of monozygotic twins). Accordingly, the theoretical limits of the confidence intervals is −2 and 2 rather than −1 and 1.
Combined twin and adoption study analyses
Our final analyses tested for heterogeneity across twin and adoption studies. Specifically, two models were estimated that simultaneously evaluated the twin and adoption data. In the first model, two heritability coefficients were estimated: one each for twin and adoption studies. As indicated above, these parameters can be equated across sexes in both types of study. In the second model, we constrained these parameters to equality. When the two models are compared using a using a likelihood ratio test, the heterogeneity in the heritability estimates of the twin and adoption studies was not statistical significant (χ2 = 3.51, df = 1, p = 0.06). The overall best estimate of the heritability of AUDs, derived from both twin and adoption studies, was 0.49 with relatively tight CIs (0.43–0.53).
Discussion
The goal of this study was to perform a quantitative meta-analysis of twin and adoption studies of AUD with a specific focus on five questions, which we now review in turn.
First, our best estimate for the heritability of AUDs, based on 13 twin and five adoption studies was 0.49 (95% CI 0.47–0.54). Importantly, while the diagnosis of AUD used in the various studies included in this meta-analysis may have substantial levels of clinical and etiological heterogeneity, the fact that we do not find statistical evidence of differential genetic or environmental heterogeneity as a function of ascertainment or diagnosis implies that the underlying construct of AUD is fairly robust to the specific measure of AUD that is used in any given study. Furthermore, the heterogeneity between AUD in twin and adoption studies was not statistically significant at conventional levels of significance (p = 0.06). While heterogeneity in the parameter estimate is trending towards significance, given that over 1 00 000 observations were included in the analysis, there is ample power to detect even relatively small differences across samples. Further, we found no evidence for genetic qualitative sex effects for AUD. That is, this result predicts that the same genetic factors increase risk for AUDs in males and females. As such, we find no evidence for any type of genetic sex limitation.
It is of interest to compare these findings with those of two other disorders where twin studies have been subject to similar meta-analyses. In major depression, six twin studies were examined and produced an aggregate heritability estimate of 0.37 (95% CI 0.31– 0.42) (Sullivan et al. 2000). In schizophrenia, 12 twin studies were available and the meta-analytic heritability estimate was 0.81 (95% CI 0.73–0.90) (Sullivan et al. 2003). The estimated heritability of AUDs was in between that found for major depression and schizophrenia, and outside the 95% CIs of both disorders. These results imply that the heritability of psychiatric disorders do genuinely differ from one another (Kendler, 2001).
Second, several early twin and adoption studies suggested that genetic factors were more important in the etiology of AUDs in men than women (Goodwin et al. 1973; Bohman et al. 1981; McGue et al. 1992) while more recent studies sometimes reached different conclusions (Heath et al. 1997; Prescott et al. 1999). Our results provide strong evidence that despite large and consistent differences in the prevalence of AUDs in the two sexes (Kessler et al. 1994; Hasin et al. 2007), genetic influences on AUD are similar in magnitude in men and women. The primary reason early twin and adoption studies were unable to detect genetic factors in AUD in females was due to very small sample sizes and relatively low prevalence rates in the female samples. When we aggregated the data across studies, the estimated heritability of AUDs was indistinguishable in the two sexes.
Third, prior studies have produced contradictory evidence about the presence of shared environmental effects for AUDs. This is partly a problem of statistical power as in the presence of substantial heritable influences, quite large twin samples are needed to detect reliably modest shared environmental effects (Neale et al. 1994). Our meta-analysis suggests the presence of modest, though statistically significant, shared environmental influence on risk for AUDs. Our twin findings predict that the correlation in liability for AUDs in siblings should be equal to 0.49/2 + 0.10 = 0.35 of which approximately one third comes from shared environmental and two-thirds from genetic sources of resemblance.
Fourth, the twin studies that we examined used two quite different methods of ascertainment and assessment. One set of studies assessed AUDs via personal interviews, which require subjects to both cooperate and accurately report their AUD symptoms. Another set of studies used registry information, which may be less sensitive but eliminates problems of cooperation and selective recall. Thus, there is considerable heterogeneity in the diagnostic assessments of AUD. In neither the genetic nor the common environmental variance components did the method of assessment induce heterogeneity in the parameter estimates. Accordingly, we can conclude that the method of assessment does not significantly affect estimates of the genetic or common environmental variance components.
Finally, human researchers are provided with two major `experiments of nature' to disentangle genetic and environmental effects: twin and adoption studies. Only rarely are the results of these two methods – which have different strengths and potential limitations – quantitatively compared. For example, in a meta-analytic analysis of the genetics of antisocial behavior, Rhee and Waldman find that heritability estimates are lower in adoption than in twin studies (Rhee & Waldman, 2002). We are fortunate in the field of AUD to have a reasonable number both of high quality twin and high quality adoption studies. Our results show that while heritability estimates are modestly lower for AUD when derived from adoption than from twin studies, the estimates are statistically homogeneous. That is, within the power of the available studies, we obtain the same estimate of the heritability of AUDs from these two main genetic epidemiologic methods. Given the differences in their approach and their distinct potential methodological limitations, we should be considerably more confident in our conclusions about the heritability of AUD given that we obtain similar results from both methods than we could having results from only one of them.
Limitations
These results should be interpreted in light of four potential methodological limitations. First, obtaining parameter estimates from clinically ascertained twin studies required an estimated population prevalence of AUD. We utilized the prevalences contained in each report. If these were inaccurate some bias might have been introduced into the current estimates.
Second, estimates of shared environmental effects can be biased upward by assortative mating for AUDs. This is because when spouses have correlated liabilities for AUD, the correlation between the genetic factors of DZ twins and siblings would be higher than 0.5, which is assumed in the model. The spousal correlation for AUD was estimated at equal to +0.12 as found in one prior large-scale general population study (Maes et al. 1998). If this spousal resemblance results entirely from assortative mating (spouses choosing one another, directly or indirectly, on the basis of their genetic risk for AUD) rather than spousal interaction (spouses mutually influencing each other's drinking and hence liability to AUD), then inflation of the shared environmental variance component would be ~0.06, a little over half of the estimated shared environmental variance component from the meta-analysis. Accordingly, some proportion of our estimate of shared environmental effects for AUD may be a result of assortative mating.
Third, estimates of latent genetic factors in the Classical Twin Design (CTD) formally assume additive genetic effects. The meager proportions of variance that are accounted for by individual genetic variants have led some to conclude that gene–environment interactions or non-additive genetic factors (such as epistasis or dominance) may play pivotal roles in the etiology of AUD. With the CTD it is generally not possible to disentangle the effects of non-additive genetic factors from additive genetic factors (Martin et al. 1978; Neale et al. 1994) due to the lack of power to disentangle these highly collinear genetic factors. Furthermore, when the DZ correlation is greater than half the MZ correlation, most researchers do not even test for dominance as the correlations are more consistent with the shared environment playing a role in the development of the phenotype. More recent evidence, however, suggests that non-additive genetic variation is absorbed into the additive genetic factor (Keller et al. 2010), suggesting that the genetic variance component should be interpreted as a broad genetic factor. This interpretation is consistent with our interpretation of the current results.
Fourth, the possible effects of year of birth and age at assessment were not accounted for in this study. Secular trends in the use and availability of alcohol may have contributed to or obscured sample heterogeneity. The one outlier study by Kaij was also the first, and its unusual estimates may have been partly due to unusual population prevalence or other characteristics of that sample.
Conclusions
There is substantial consistency in the estimates of genetic and shared environmental variance in liability to AUD. In particular, we found no significant differences between the estimates derived from twin studies and adoption studies, from studies relying on personal interviews v. official records for the assessment of AUD or from studies of males v. females. Furthermore by including a large number of studies into the analysis, we were able to detect significant shared environmental effects that account for ~10% of the variance in AUD. These findings should enhance our confidence in the validity of our heritability estimates for AUD and provide a firm footing for efforts now well underway to try to localize on the human genome the specific genetic variants that contribute to the genetic risk for AUD.
Acknowledgements
This work was supported in part by NIH grants AA011408, AA017828, AA018333, DA026119 and DA018673.
Footnotes
Declaration of Interest None.
References
- Allgulander C, Nowak J, Rice JP. Psychopathology and treatment of 30,344 twins in Sweden. II. Heritability estimates of psychiatric diagnosis and treatment in 12,884 twin pairs. Acta Psychiatrica Scandinavica. 1991;83:12–15. doi: 10.1111/j.1600-0447.1991.tb05504.x. [DOI] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders. 4th edn American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bohman M, Sigvardsson S, Cloninger CR. Maternal inheritance of alcohol abuse. Cross-fostering analysis of adopted women. Archives of General Psychiatry. 1981;38:965–969. doi: 10.1001/archpsyc.1981.01780340017001. [DOI] [PubMed] [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, Spies J, Estabrook R, Kenny S, Bates T, Mehta P, Fox J. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Estabrook R, Bates TC, Mehta P, von Oertzen T, Gore RJ, Hunter MD, Hackett DC, Karch J, Brandmaier A. OpenMx 1.2 User Guide. 2012. [Google Scholar]
- Bynum WF. Alcoholism and degeneration in 19th century European medicine and psychiatry. British Journal of Addiction. 1984;79:59–70. doi: 10.1111/j.1360-0443.1984.tb00248.x. [DOI] [PubMed] [Google Scholar]
- Cadoret RJ, Troughton E, O'Gorman TW. Genetic and environmental factors in alcohol abuse and antisocial personality. Journal of Studies on Alcohol. 1987;48:1–8. doi: 10.15288/jsa.1987.48.1. [DOI] [PubMed] [Google Scholar]
- Caldwell CB, Gottesman I. Sex differences in the risk for alcoholism: a twin study. Paper presented at the 21st Annual Meeting of the Behavior Genetics Association; St Louis, MO, USA. 7 June 1991; 1991. [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Archives of General Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Cotton NS. The familial incidence of alcoholism: a review. Journal of Studies on Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. available from: PM:376949. [DOI] [PubMed] [Google Scholar]
- Dick DM, Bierut LJ. The genetics of alcohol dependence. Current Psychiatry Reports. 2006;8:151–157. doi: 10.1007/s11920-006-0015-1. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Hermansen L, Guze SB, Winokur G. Alcohol problems in adoptees raised apart from alcoholic biological parents. Archives of General Psychiatry. 1973;28:238–243. doi: 10.1001/archpsyc.1973.01750320068011. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Schulsinger F, Knop J, Mednick S, Guze SB. Alcoholism and depression in adopted-out daughters of alcoholics. Archives of General Psychiatry. 1977;34:751–755. doi: 10.1001/archpsyc.1977.01770190013001. [DOI] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiologic Survey. Journal of Studies on Alcohol. 1997;58:464–473. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Gurling HM, Oppenheim BE, Murray RM. Depression, criminality and psychopathology associated with alcoholism: evidence from a twin study. Acta Geneticae Medicae Gemellologiae (Roma) 1984;33:333–339. doi: 10.1017/s0001566000007376. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heath AC. Genetic influences on alcoholism risk: a review of adoption and twin studies. Alcohol Health and Research World. 1995;19:166–171. [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PA, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: consistency of findings in women and men. Psychological Medicine. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Hrubec Z, Omenn GS. Evidence of genetic predisposition to alcoholic cirrhosis and psychosis: twin concordances for alcoholism and its biological end points by zygosity among male veterans. Alcoholism: Clinical and Experimental Research. 1981;5:207–215. doi: 10.1111/j.1530-0277.1981.tb04890.x. [DOI] [PubMed] [Google Scholar]
- Kaij L. Alcoholism in Twins: Studies on Etiology and Sequels of Abuse of Alcohol. Almqvist and Wiksell International; Stockholm: 1960. [Google Scholar]
- Keller MC, Medland SE, Duncan LE. Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behavior Genetics. 2010;40:377–393. doi: 10.1007/s10519-009-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS. Twin studies of psychiatric illness: an update. Archives of General Psychiatry. 2001;58:1005–1014. doi: 10.1001/archpsyc.58.11.1005. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. A population-based twin study of alcoholism in women. Journal of the American Medical Association. 1992;268:1877–1882. [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Neale MC, Pedersen NL. Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Archives of General Psychiatry. 1997;54:178–184. doi: 10.1001/archpsyc.1997.01830140090015. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Zhao S, Nelson CB, Hughes M, Eshleman S, Wittchen HU, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Knopik VS, Heath AC, Madden PA, Bucholz KK, Slutske WS, Nelson EC, Statham D, Whitfield JB, Martin NG. Genetic effects on alcohol dependence risk: re-evaluating the importance of psychiatric and other heritable risk factors. Psychological Medicine. 2004;34:1519–1530. doi: 10.1017/s0033291704002922. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. Genes and Environment in Personality Development. Sage Publications; Newbury Park, CA: 1992. [Google Scholar]
- Maes HH, Neale MC, Kendler KS, Hewitt JK, Silberg JL, Foley DL, Meyer JM, Rutter M, Simonoff E, Pickles A, Eaves LJ. Assortative mating for major psychiatric diagnoses in two population-based samples. Psychological Medicine. 1998;28:1389–1401. doi: 10.1017/s0033291798007326. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Lundholm C, Goransson M, Copeland W, Heilig M, Pedersen NL. Familial influence and childhood trauma in female alcoholism. Psychological Medicine. 2012;42:381–389. doi: 10.1017/S0033291711001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NG, Eaves LJ, Kearsey MJ, Davies P. The power of the classical twin study. Heredity. 1978;40:97–116. doi: 10.1038/hdy.1978.10. [DOI] [PubMed] [Google Scholar]
- McGue M, Pickens RW, Svikis DS. Sex and age effects on the inheritance of alcohol problems: a twin study. Journal of Abnormal Psychology. 1992;101:3–17. doi: 10.1037//0021-843x.101.1.3. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Kluwer Academic Publishers B.V.; Dordrecht, The Netherlands: 1992. [Google Scholar]
- Neale MC, Eaves LJ, Kendler KS. The power of the classical twin study to resolve variation in threshold traits. Behavior Genetics. 1994;24:239–258. doi: 10.1007/BF01067191. [DOI] [PubMed] [Google Scholar]
- Pickens RW, Svikis DS, McGue M, Lykken DT, Heston LL, Clayton PJ. Heterogeneity in the inheritance of alcoholism. A study of male and female twins. Archives of General Psychiatry. 1991;48:19–28. doi: 10.1001/archpsyc.1991.01810250021002. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex differences in the sources of genetic liability to alcohol abuse and dependence in a population-based sample of U.S. twins. Alcohol Clinical Experimental Research. 1999;23:1136–1144. doi: 10.1111/j.1530-0277.1999.tb04270.x. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Gottesman I. Power limitations in detecting heterogeneity of genetic effects: the case of sex differences in alcoholism. Presented at the Annual Meeting of the Society for Research on Psychopathology; Chicago. Oct, 1993. 1993. [Google Scholar]
- Prescott CA, Kendler KS. Gender and genetic vulnerability to alcoholism. In: Hunt WA, Zakhari S, editors. In Stress, Gender, and Alcohol Seeking Behavior. National Institutes of Health; Bethesda, MD: 1995. pp. 23–46. NIH Pub. No. 95-3893. [Google Scholar]
- Reed T, Page WF, Viken RJ, Christian JC. Genetic predisposition to organ-specific endpoints of alcoholism. Alcohol Clinical Experimental Research. 1996;20:1528–1533. doi: 10.1111/j.1530-0277.1996.tb01695.x. [DOI] [PubMed] [Google Scholar]
- Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: a meta- analysis of twin and adoption studies. Psychological Bulletin. 2002;128:490–529. [PubMed] [Google Scholar]
- Romanov K, Kaprio J, Rose RJ, Koskenvuo M. Genetics of alcoholism: effects of migration on concordance rates among male twins. Alcohol and Alcohol Supplement. 1991;1:137–140. [PubMed] [Google Scholar]
- Secretary of Health and Human Services . Alcohol and Health: Ninth Special Report to the U.S. Congress from the Secretary of Health and Human Services. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, NIAAA; 1997. [Google Scholar]
- Sigvardsson S, Bohman M, Cloninger CR. Replication of the Stockholm Adoption Study of alcoholism. Confirmatory cross-fostering analysis. Archives of General Psychiatry. 1996;53:681–687. doi: 10.1001/archpsyc.1996.01830080033007. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Archives of General Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, Eisen SA, Lyons MJ, Goldberg J, Tsuang M. Common genetic vulnerability for nicotine and alcohol dependence in men. Archives of General Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- Wu H, Neale MC. Adjusted confidence intervals for a bounded parameter. Behavior Genetics. 2012;42:886–898. doi: 10.1007/s10519-012-9560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]