Abstract
Objective
To evaluate long-term survival among patients undergoing radiation therapy (RT), followed by surgical resection of retroperitoneal sarcomas (RPS).
Background
Despite a lack of level 1 evidence supporting neoadjuvant RT for RPS, its use has increased substantially over the past decade.
Methods
The 1998–2011 National Cancer Data Base was queried to identify patients who underwent resection of RPS. Subjects were grouped by use of neoadjuvant RT. Perioperative variables and outcomes were compared. Multivariable logistic regression was performed to assess predictors of neoadjuvant RT. Groups were propensity matched using a 2:1 nearest neighbor algorithm and short-term outcomes were compared. Finally, long-term survival was evaluated using the Kaplan-Meier method, with comparisons based on the log-rank test.
Results
A total of 11,324 patients were identified. Neoadjuvant RT was administered to 696 patients (6.1%). During the study period, preoperative RT use increased from 4% to nearly 15%. Male sex, tumor size larger than 5 cm, treatment at an academic/research program, and higher tumor grade all predicted neoadjuvant RT administration. After propensity matching, the only difference in baseline characteristics was the use of neoadjuvant chemotherapy. Although neoadjuvant RT was associated with a higher rate of negative margins (77.5% vs 73.0%; P = 0.014), there was no corresponding improvement in 5-year survival (53.2% vs 54.2%; P = 0.695).
Conclusions
Despite the increasing use of neoadjuvant RT for patients with RPS, the survival benefit associated with this treatment modality remains unclear. Continued investigation is needed to better define the role of RT among patients with RPS.
Keywords: neoadjuvant, preoperative, radiation therapy, radiotherapy, retroperitoneal sarcoma, sarcoma
Retroperitoneal sarcomas (RPSs) require multidisciplinary care, yet they often represent a therapeutic dilemma because of the heterogeneous nature of their biology, behavior, and natural history. Characterized by multiple histologic subtypes—the most common being liposarcoma (40%) and leiomyosarcoma (25%)—median survival ranges from approximately 3 to 12 years for high- and low-grade tumors, respectively.1 After tumor grade, the most important factor impacting survival is extent of resection, as incomplete resection is associated with a 70% higher risk of death.2 However, negative margins are often difficult to achieve, especially in large tumors that encroach on vital organs or other structures, and incomplete resection occurs in up to one-fifth of operations.2,3
There are several hypothetical benefits for the use of neoadjuvant radiation therapy (RT) in patients with RPSs. As compared with adjuvant RT, administration of radiation in the preoperative period may allow the radiation oncologist to better define the target volume so that the RT can be focused on the tumor rather than the adjacent normal structures. If RT is delivered after surgery, the adjacent normal structures frequently fall into the postoperative tumor bed, which can limit the radiation dose to the target and increase the amount of radiation to normal structures. This may be a particular concern for bowel, which can become fixed as a consequence of fibrosis and adhesions, so that the same segment of bowel receives daily radiation during adjuvant RT.3,4 In addition, several studies have suggested that preoperative RT may improve short-term oncologic outcomes (eg, tumor resectability and achievement of negative margins) in retroperitoneal and other soft tissue sarcomas.5 Finally, there is some evidence that preoperative RT may decrease locoregional recurrence.5–8 Unlike extremity sarcoma, mortality among patients with RPSs is often secondary to local recurrence rather than distant metastasis.9 Thus, it is conceivable that improvements in local control may translate to increases in overall survival, although this remains widely debated.5–8
Notably, a randomized controlled trial evaluating the efficacy of neoadjuvant RT in the treatment of RPS has never been completed. In 2006, the American College of Surgeons Oncology Group had to terminate its phase 3 trial Z9031 because of inadequate patient accrual. Eight years later, efforts are underway by the European Organisation for Research and Treatment of Cancer (EORTC) to recruit patients for a similar, multicenter trial (EORTC 62092-22092).10 However, even if accrual is successful, results are still years away.
The National Cancer Data Base (NCDB) is administered by the American College of Surgeons together with the American Cancer Society and at present contains data from more than 1500 cancer institutions including more than 30 million patients.11 The Commission on Cancer estimates that the NCDB captures approximately 70% of all cancer diagnoses in the United States.11 Thus, the NCDB is a particularly valuable tool for evaluating rare malignancies in which highly specialized treatment modalities are offered only at select institutions. The purpose of this study was to utilize the NCDB to evaluate long-term oncologic outcomes associated with neoadjuvant RT for RPSs.
METHODS
The NCDB participant user file for 1998 through 2011 was utilized for this retrospective analysis. Patients were identified as having undergone resection of an RPS first by querying all patients treated at a NCDB participating institution for tumors in the retroperitoneal location with International Classification of Diseases for Oncology, Third Edition (ICD-O-3) topography codes C480 and C494-6. Relevant histologic subtypes were selected on the basis of ICD-O-3 histology codes 8800, 8801, 8802, 8804, 8810, 8830, 8851, 8852, 8854, 8858, 8890, 9120, and 9540 (all of which represent soft-tissue sarcomas). Other inclusion criteria included malignant behavior, primary cancer diagnosis, no distant metastasis, and known status for preoperative RT.
Subjects were then classified by the use of neoadjuvant RT, defined by the NCDB as “radiation therapy given before surgery to the primary site.”12 Baseline characteristics and outcomes between groups were compared using Pearson χ2 test for categorical variables and analysis of variance for continuous variables. Multivariable logistic regression was used to predict factors that were associated with the administration of neoadjuvant RT. To control for confounding in the use of neoadjuvant RT, we developed propensity scores, which we defined as the conditional probability of being treated with RT before major resection. Patients were then matched on these propensity scores, using a 2:1 nearest neighbor algorithm. The following variables were used in our propensity match: patient age, sex, race, Charlson/Deyo comorbidity score, patient census tract education and income levels, tumor size, histologic subtype, histologic grade, treatment facility type (academic or community hospital), and extent of resection. Adjusted medians and proportions between the propensity-matched groups were then compared, and long-term survival among the groups was evaluated using the Kaplan-Meier method with comparisons based on the log-rank test.
Results are reported as median (interquartile range), proportions (%), and odds ratios (95% confidence interval) as applicable. P values less than 0.05 indicate statistical significance, and we controlled for type I error at the level of the comparison. All statistical analyses were performed using R (The R Foundation for Statistical Computing, version 3.0.2, Vienna, Austria).
RESULTS
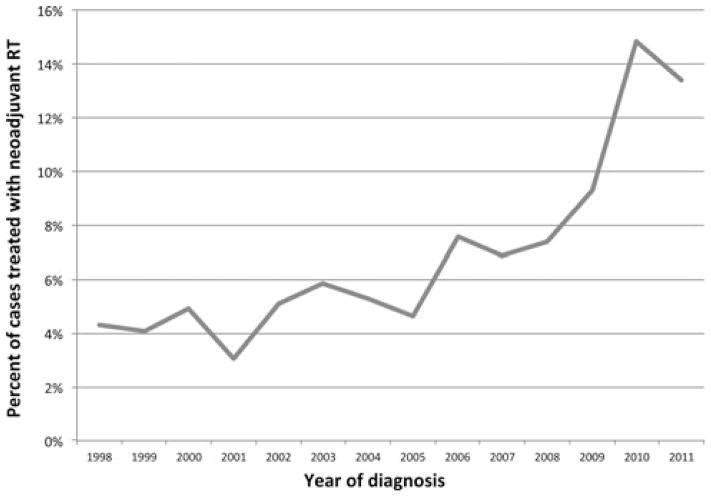
A total of 11,324 patients were identified who had undergone resection of an RPS. Subjects were grouped by neoadjuvant RT (696 patients, 6.1%) versus no neoadjuvant RT (10,628 patients, 93.9%). During the study period, there was an increase in the use of neoadjuvant RT from approximately 4% of patients in 1998 to nearly 15% in 2011. The most pronounced increase in neoadjuvant RT occurred between 2005 and 2010 (Fig. 1).
FIGURE 1.
Use of neoadjuvant radiation for retroperitoneal sarcomas by year.
Baseline characteristics are shown in Table 1. Patients who underwent neoadjuvant RT were slightly younger (58 vs 59; P = 0.005) and more likely to be male sex (55.9% vs 48.8%; P < 0.001). In addition, they were more likely to have been treated at an academic/ research hospital (71.4% vs 46.5%; P < 0.001) than at a community or comprehensive community program and thus lived further from their treatment centers (26 vs 11 miles; P < 0.001). There were no differences between groups with regard to preoperative comorbidities, income, or education.
TABLE 1.
Baseline Patient Characteristics
| Variable | Overall (n = 11,324) | Preoperative RT (n = 696) | No preoperative RT (n = 10,628) | P |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, yr (IQR) | 59 (48–71) | 58 (47–68) | 59 (48–71) | 0.005 |
| Female | 5747 (50.8%) | 307 (44.1%) | 5440 (51.2%) | <0.001 |
| Race | 0.219 | |||
| White | 9526 (85.4%) | 598 (87.7%) | 8928 (85.3%) | |
| Black | 1201 (10.8%) | 63 (9.2%) | 1138 (10.9%) | |
| Other | 425 (3.8%) | 21 (3.1%) | 404 (3.9%) | |
| Charlson Comorbidity Score | 0.476 | |||
| 0 | 5939 (81.3%) | 436 (83.2%) | 5,503 (81.2%) | |
| 1 | 1108 (15.2%) | 73 (13.9%) | 1035 (15.3%) | |
| ≥2 | 256 (3.5%) | 15 (2.9%) | 241 (3.6%) | |
| Education above median | 6461 (60.3%) | 401 (61.9%) | 6060 (60.2%) | 0.426 |
| Income above median | 7397 (69.1%) | 435 (67.1%) | 6962 (69.2%) | 0.292 |
| Distance to cancer center (IQR) | 11.6 (4.7–34.4) | 26 (8–84) | 11 (5–32) | <0.001 |
| Treatment facility | <0.001 | |||
| Community Cancer Program | 827 (7.5%) | 14 (2%) | 813 (7.9%) | |
| Comprehensive Community Cancer Program | 4908 (44.5%) | 183 (26.6%) | 4725 (45.7%) | |
| Academic/Research Program | 5302 (48%) | 491 (71.4%) | 4811 (46.5%) | |
| Insurance | < 0.001 | |||
| Private | 5877 (54.3%) | 357 (55.5%) | 5520 (54.2%) | |
| Medicare | 3850 (35.5%) | 208 (32.3%) | 3642 (35.8%) | |
| Medicaid | 610 (5.6%) | 33 (5.1%) | 577 (5.7%) | |
| Government | 95 (0.9%) | 15 (2.3%) | 80 (0.8%) | |
| Uninsured | 398 (3.7%) | 30 (4.7%) | 368 (3.6%) | |
| Tumor characteristics | ||||
| Tumor size (mm) | 105 (60–175) | 115 (78–170) | 105 (60–180) | 0.856 |
| Tumor size | <0.001 | |||
| <5 cm | 1757 (17.6%) | 50 (8.1%) | 1707 (18.2%) | |
| 5–9.9 cm | 2799 (28.1%) | 182 (29.4%) | 2617 (28%) | |
| 10.0–19.9 cm | 3385 (33.9%) | 292 (47.1%) | 3093 (33.1%) | |
| >20.0 cm | 2033 (20.4%) | 96 (15.5%) | 1937 (20.7%) | |
| Grade | <0.001 | |||
| Well differentiated | 2681 (29.2%) | 90 (15.3%) | 2591 (30.2%) | |
| Moderately differentiated | 1421 (15.5%) | 83 (14.1%) | 1338 (15.6%) | |
| Poorly differentiated | 2954 (32.2%) | 229 (39%) | 2,725 (31.8%) | |
| Undifferentiated/anaplastic | 2111 (23%) | 185 (31.5%) | 1926 (22.4%) | |
| Histology | <0.001 | |||
| Dedifferentiated liposarcoma | 1104 (9.7%) | 40 (5.7%) | 1064 (10%) | |
| Epithelioid sarcoma | 182 (1.6%) | 11 (1.6%) | 171 (1.6%) | |
| Fibrosarcoma NOS | 340 (3%) | 14 (2%) | 326 (3.1%) | |
| Giant cell sarcoma | 487 (4.3%) | 79 (11.4%) | 408 (3.8%) | |
| Hemangiosarcoma | 163 (1.4%) | 5 (0.7%) | 158 (1.5%) | |
| Leiomyosarcoma | 3342 (29.5%) | 162 (23.3%) | 3180 (29.9%) | |
| Liposarcoma (well differentiated) | 1568 (13.8%) | 47 (6.8%) | 1521 (14.3%) | |
| Malignant fibrous histiocytoma | 1309 (11.6%) | 90 (12.9%) | 1219 (11.5%) | |
| MPNST | 290 (2.6%) | 21 (3%) | 269 (2.5%) | |
| Myxoid liposarcoma | 761 (6.7%) | 59 (8.5%) | 702 (6.6%) | |
| Pleomorphic liposarcoma | 294 (2.6%) | 27 (3.9%) | 267 (2.5%) | |
| Sarcoma NOS | 987 (8.7%) | 87 (12.5%) | 900 (8.5%) | |
| Spindle cell sarcoma | 497 (4.4%) | 54 (7.8%) | 443 (4.2%) | |
| Treatment specifics | ||||
| Neoadjuvant RT | 696 (6.1%) | 696 (100%) | 0 (0%) | <0.001 |
| Neoadjuvant chemo | 191 (4.3%) | 95 (25.3%) | 96 (2.4%) | <0.001 |
| Surgery type | 0.001 | |||
| 1: Excision | 8714 (77%) | 536 (77%) | 8178 (76.9%) | |
| 2: Complete resection | 1011 (8.9%) | 38 (5.5%) | 973 (9.2%) | |
| 3: Resection with debulking | 410 (3.6%) | 28 (4%) | 382 (3.6%) | |
| 4: Radical resection | 1189 (10.5%) | 94 (13.5%) | 1095 (10.3%) | |
IQR indicates interquartile range; MPNST, malignant peripheral nerve sheath tumors; NOS, not otherwise specified.
Focusing on tumor characteristics, patients who underwent neoadjuvant RT were more likely to have larger tumors and higher-grade tumors (Table 1). With regard to histologic subtype, neoadjuvant RT was more common among giant cell sarcomas (11.4% vs 3.8%), malignant fibrous histiocytomas (12.9% vs 11.5%), myxoid liposarcomas (8.5% vs 6.6%), pleomorphic liposarcomas (3.9% vs 2.5%), spindle cell sarcomas (7.8% vs 4.2%), and sarcomas not otherwise specified (12.5% vs 8.5%). Neoadjuvant RT was less common among dedifferentiated liposarcomas (5.7% vs 10.0%), leiomyosarcomas (23.3% vs 29.9%), and well-differentiated liposarcomas (6.8% vs. 14.3%). Patients who underwent neoadjuvant RT were also more likely to have received neoadjuvant chemotherapy (25.3% vs. 2.4%; P < 0.001). Using logistic regression, independent predictors of neoadjuvant RT use included male sex, tumor size larger than 5 cm, treatment at an academic/research program, and tumor grade higher than well-differentiated (Table 2).
TABLE 2.
Predictors of RT Use Before Resection
| Predictor | Odds Ratio | Upper 95% | Lower 95% | P |
|---|---|---|---|---|
| Age (per year) | 0.99 | 0.99 | 1.00 | 0.118 |
| Female sex | 0.74 | 0.60 | 0.93 | 0.008 |
| Race (ref = white) | ||||
| Black | 0.91 | 0.63 | 1.32 | 0.628 |
| Other | 0.78 | 0.43 | 1.39 | 0.349 |
| Charlson/Deyo | 0.85 | 0.68 | 1.07 | 0.165 |
| Education above median | 1.28 | 0.98 | 1.68 | 0.070 |
| Income above median | 0.77 | 0.59 | 1.02 | 0.066 |
| Tumor size (ref: <5 cm) | ||||
| 5–9.9 cm | 2.32 | 1.51 | 3.55 | <0.001 |
| 10.0–19.9 cm | 3.02 | 2.00 | 4.55 | <0.001 |
| >20.0 cm | 1.36 | 0.85 | 2.18 | 0.201 |
| Facility type (ref = community) | ||||
| Academic/research program | 2.73 | 2.15 | 3.46 | <0.001 |
| Tumor grade (ref = well differentiated) | ||||
| Moderately differentiated | 1.67 | 1.12 | 2.48 | 0.012 |
| Poorly differentiated | 2.03 | 1.48 | 2.80 | <0.001 |
| Undifferentiated/anaplastic | 2.29 | 1.65 | 3.18 | <0.001 |
After propensity adjustment, all variables except for neoadjuvant chemotherapy were highly similar (Table 3). Propensity-adjusted postoperative treatment specifics and outcomes are shown in Table 4. As expected, neoadjuvant RT was associated with a delay to surgery (median 114 vs 10 days; P < 0.001). Patients who underwent neoadjuvant RT were less likely to go on to receive both adjuvant RT (6.3% vs 32.9%; P < 0.001) and adjuvant chemotherapy (6.1% vs 14.3%; P < 0.001). Neoadjuvant RT was associated with a higher rate of negative margins (77.5% vs 73.0%; P = 0.014). There was no difference in 30-day mortality or readmission. Hospital length of stay was 1 day longer (6 vs 5 days; P = 0.027) in the group receiving neoadjuvant RT.
TABLE 3.
Adjusted Baseline Characteristics After Propensity Matching
| Variable | Preoperative (n = 696) | No preoperative RT (n = 1392) | P |
|---|---|---|---|
| Patient characteristics | |||
| Age, yr (IQR) | 58 (47–68) | 59 (47–68) | 0.394 |
| Female | 307 (44.1%) | 609 (43.8%) | 0.913 |
| Race | 0.464 | ||
| White | 598 (87.7%) | 1,171 (86%) | |
| Black | 63 (9.2%) | 150 (11%) | |
| Other | 21 (3.1%) | 41 (3%) | |
| Charlson Comorbidity score | 0.407 | ||
| 0 | 436 (83.2%) | 880 (84.2%) | |
| 1 | 73 (13.9%) | 146 (14%) | |
| ≥2 | 15 (2.9%) | 19 (1.8%) | |
| Education above median | 401 (61.9%) | 788 (61.2%) | 0.818 |
| Income above median | 435 (67.1%) | 851 (66.1%) | 0.695 |
| Distance to cancer center (IQR) | 26 (8–84) | 17 (6–50) | 0.294 |
| Treatment facility | 0.534 | ||
| Community Cancer Program | 14 (2%) | 22 (1.6%) | |
| Comprehensive Community Cancer Program | 183 (26.6%) | 392 (28.5%) | |
| Academic/Research Program | 491 (71.4%) | 960 (69.9%) | |
| Insurance | 0.418 | ||
| Private | 357 (55.5%) | 725 (54.7%) | |
| Medicare | 208 (32.3%) | 439 (33.1%) | |
| Medicaid | 33 (5.1%) | 89 (6.7%) | |
| Government | 15 (2.3%) | 21 (1.6%) | |
| Uninsured | 30 (4.7%) | 51 (3.8%) | |
| Tumor characteristics | |||
| Tumor size (mm) | 115 (78–170) | 110 (70–160) | 0.721 |
| Tumor size | 0.549 | ||
| <5 cm | 50 (8.1%) | 88 (7.1%) | |
| 5–9.9 cm | 182 (29.4%) | 399 (32.1%) | |
| 10.0–19.9 cm | 292 (47.1%) | 581 (46.7%) | |
| >20.0 cm | 96 (15.5%) | 175 (14.1%) | |
| Grade | 0.996 | ||
| Well differentiated | 90 (15.3%) | 184 (15.7%) | |
| Moderately differentiated | 83 (14.1%) | 164 (14%) | |
| Poorly differentiated | 229 (39%) | 453 (38.6%) | |
| Undifferentiated/anaplastic | 185 (31.5%) | 373 (31.8%) | |
| Histology | 0.955 | ||
| Dedifferentiated liposarcoma | 40 (5.7%) | 74 (5.3%) | |
| Epithelioid sarcoma | 11 (1.6%) | 19 (1.4%) | |
| Fibrosarcoma NOS | 14 (2%) | 43 (3.1%) | |
| Giant cell sarcoma | 79 (11.4%) | 151 (10.8%) | |
| Hemangiosarcoma | 5 (0.7%) | 9 (0.6%) | |
| Leiomyosarcoma | 162 (23.3%) | 305 (21.9%) | |
| Liposarcoma (well differentiated) | 47 (6.8%) | 91 (6.5%) | |
| Malignant fibrous histiocytoma | 90 (12.9%) | 211 (15.2%) | |
| MPNST | 21 (3%) | 45 (3.2%) | |
| Myxoid liposarcoma | 59 (8.5%) | 122 (8.8%) | |
| Pleomorphic liposarcoma | 27 (3.9%) | 58 (4.2%) | |
| Sarcoma NOS | 87 (12.5%) | 166 (11.9%) | |
| Spindle cell sarcoma | 54 (7.8%) | 98 (7%) | |
| Treatment specifics | |||
| Neoadjuvant RT | 696 (100%) | 0 (0%) | <0.001 |
| Neoadjuvant chemo | 95 (25.3%) | 24 (3.9%) | <0.001 |
| Surgery type | 0.991 | ||
| 1: Excision | 536 (77%) | 1080 (77.6%) | |
| 2: Complete resection | 38 (5.5%) | 73 (5.2%) | |
| 3: Resection with debulking | 28 (4%) | 56 (4%) | |
| 4: Radical resection | 94 (13.5%) | 183 (13.1%) | |
IQR indicates interquartile range; MPNST, malignant peripheral nerve sheath tumors; NOS, not otherwise specified.
TABLE 4.
Propensity-Adjusted Treatment Specifics and Outcomes
| Variable | Preoperative RT (n = 696) | No preoperative RT (n = 1392) | P |
|---|---|---|---|
| Treatment characteristics | |||
| Days to definitive surgery (IQR) | 114 (93–145) | 10 (0–36) | <0.001 |
| Adjuvant RT | 44 (6.3%) | 458 (32.9%) | <0.001 |
| Adjuvant chemo | 23 (6.1%) | 88 (14.3%) | <0.001 |
| Surgical endpoints | |||
| Surgical margins | 0.014 | ||
| Negative | 458 (77.5%) | 855 (73%) | |
| Positive margin-microscopic | 78 (13.2%) | 151 (12.9%) | |
| Positive margin-macroscopic | 55 (9.3%) | 166 (14.2%) | |
| Short-term outcomes | |||
| 30-d mortality | 6 (0.9%) | 24 (1.9%) | 0.163 |
| 30-d readmission | 23 (4.6%) | 35 (3.5%) | 0.343 |
| Hospital LOS (IQR) | 6 (3–9) | 5 (1–8) | 0.027 |
IQR indicates interquartile range.
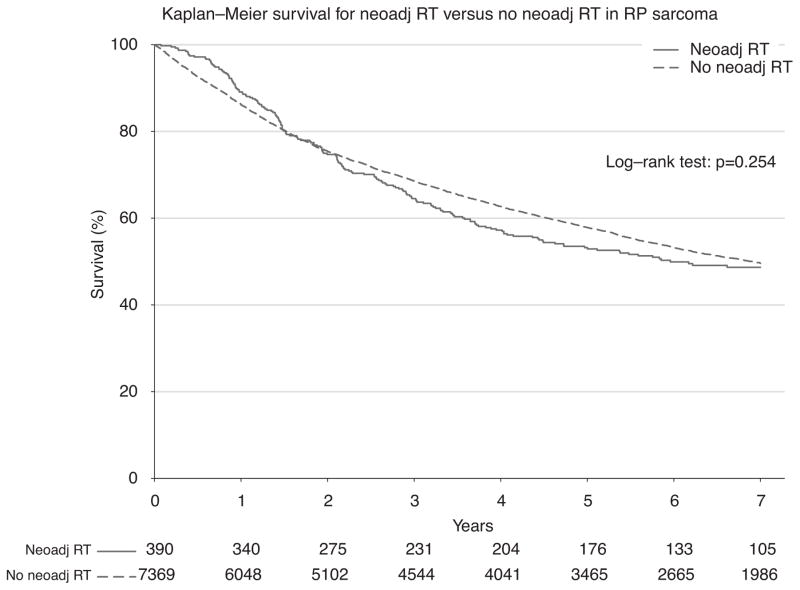
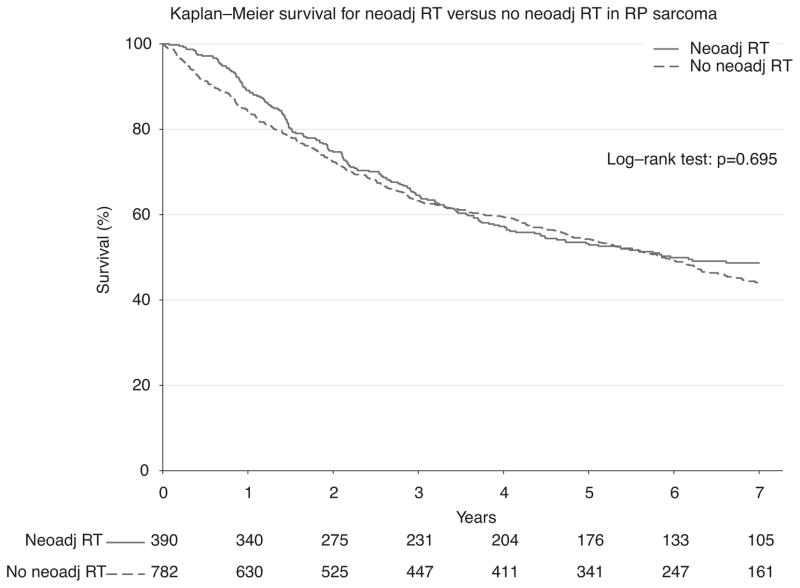
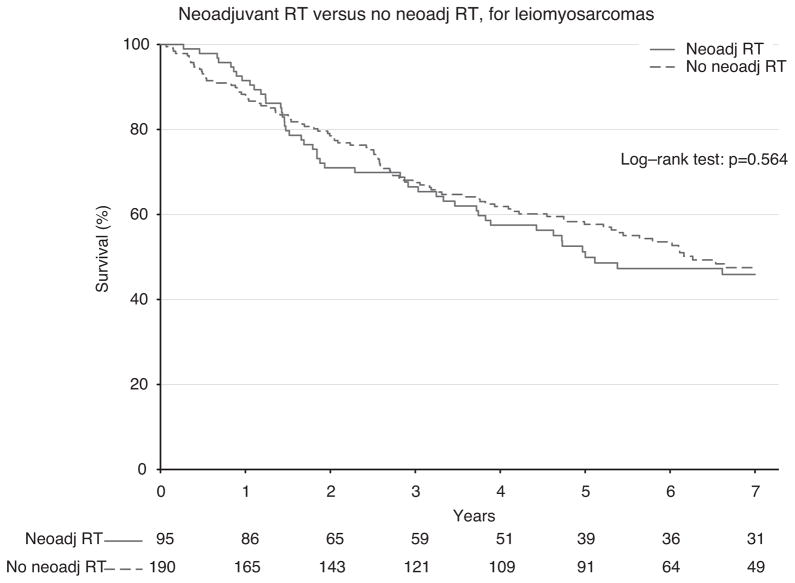
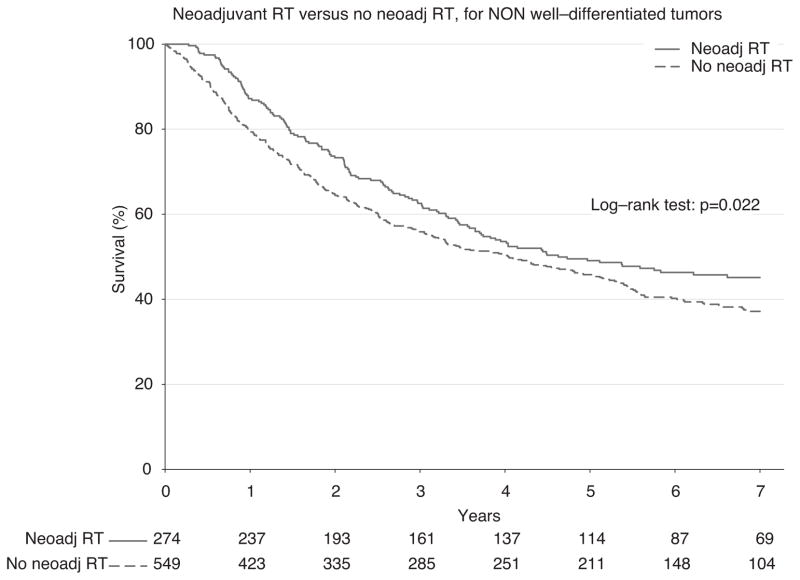
To ensure completeness of survival data, the NCDB reports only vital status for patients after 5 years have elapsed since the time of diagnosis. Thus, long-term survival from the time of diagnosis was evaluated for all patients who were diagnosed between 1998 and 2006. First, survival was estimated among all patients who underwent surgery during this period (n = 7759; Fig. 2). Among all comers, there was no statistically significant difference in 5-year survival among groups treated with or without neoadjuvant RT (53.2% vs 57.8%; P = 0.254). Next, propensity-matched groups were compared to adjust for confounding variables (n = 1172; Fig. 3). Using the Kaplan-Meier method, again there was no observable difference in 5-year survival (53.2% vs 54.2%; P = 0.695) for patients treated with or without neoadjuvant RT (Fig. 3). Because the classification of sarcoma subtype can be a diagnostic challenge, we evaluated survival for leiomyosarcomas alone (n = 285), as these tumors can be stained for specific smooth muscle markers and thus may have greater diagnostic uniformity. Again, there was no difference in survival, and these curves closely mirrored our overall survival plots (Fig. 4). Finally, in an exploratory analysis investigating the effect of neoadjuvant RT among only higher-grade tumors (moderately, poorly, and undifferentiated/anaplastic histology; n = 823), we detected a small but statistically significant 5-year survival advantage (49.1% vs 46.2%; P = 0.022) for patients treated with neoadjuvant RT (Fig. 5).
FIGURE 2.
Kaplan-Meier survival for neoadjuvant RT versus no neoadjuvant RT in RPS among all patients.
FIGURE 3.
Kaplan-Meier survival for neoadjuvant RT versus no neoadjuvant RT in RPS among propensity matched groups.
FIGURE 4.
Kaplan-Meier survival for neoadjuvant RT versus no neoadjuvant RT in leiomyosarcomas among all patients.
FIGURE 5.
Neoadjuvant RT versus no neoadjuvant RT for non–well-differentiated RPS.
DISCUSSION
In 2007, Caudle and colleagues3 published one of the largest series documenting survival data for patients treated with neoadjuvant RT for RPSs. The size of their study sample was 14 subjects, revealing the paucity of information regarding this patient population. In their retrospective review, the authors found that grossly negative surgical margins were achieved in 13 patients, and among this group, only one-third had a local recurrence within a year, with 1-year survival approaching 90%.3 Other larger series out of Case Western Reserve University (n = 19),13 Tom Baker Cancer Center in Canada (n = 25),14 and MD Anderson Cancer Center/University of Toronto (n = 57),7 however, have reported less favorable long-term outcomes, describing 5-year survival of approximately 40%, 74%, and 50%, respectively (n values reflect number of patients undergoing neoadjuvant RT, followed by surgical resection). These equivocal outcomes data—and more importantly—the complete lack of clinical trials or even large retrospective data, make it increasingly difficult to establish whether preoperative RT improves survival, and if so, which patients are likely to derive a benefit (Table 5). This uncertainty is especially important given the potential for rare—albeit clinically meaningful—radiation toxicities, such as gastrointestinal upset/dehydration, intestinal obstruction, ureteral stricturing, leukopenia, and anemia/ bleeding.3,15,16
TABLE 5.
Key Studies to Date Evaluating Neoadjuvant RT for Retroperitoneal Sarcomas
| Authors (Year) | Institution | Study Design | Sample Size* | Key Results |
|---|---|---|---|---|
| Caudle et al (2007)3 | The University of North Carolina at Chapel Hill | Single-institution review | 14 | Low rates of treatment toxicity; 13/14 with gross negative margins, 7/14 with microscopic negative margins; median OS 21 mo |
| Zagar et al (2008)13 | Case Western Reserve University | Single-institution review | 19 | ~40% 5-yr survival for patients treated with preoperative RT |
| White et al (2007)14 | Tom Baker Cancer Center (Calgary, AB) | Single-institution review | 25 | For primary tumors, actuarial 5-yr survival is 90%, DFS is 80%; for recurrent tumors, median disease-free interval is 91 mo |
| Pawlik et al (2006)7 | MD Anderson Cancer Center, University of Toronto (Ontario, Canada) | Review of 2 prospective trials | 57 | 54/57 with gross negative margins; 5-yr survival for all patients 50%; 5-yr survival for patients with R0/R1 resection is 61% |
| McBride et al (2013)8 | Brigham and Women’s Hospital | Single-institution review | 33 | Three-year locoregional recurrence of 37%; OS 64%; multifocal disease a predictor of local recurrence |
| Alford et al (2013)17 | Peter MacCallum Cancer Centre (Victoria, Australia) | Single-institution review | 18 | 100% with gross negative margins; 5-yr local recurrence rate of 22% |
| El-Bared et al (2013)18 | Montreal University Health Centre (Quebec, Canada) | Single-institution review | 21 | Five-year survival is 51%; improved survival associated with lower grade; improved local control associated with R0 resection, smaller tumors, liposarcoma, and primary diagnosis |
| Smith et al (2014)19 | Princess Margaret Hospital, Mount Sinai Hospital (Ontario, Canada) | Multi-institutional phase II prospective trial | 40 | Five-year survival is 70%, 10-yr survival is 64% |
| Roeder et al (2012)16 | University of Heidelberg (Heidelberg, Germany) | Single-institution phase I/II prospective trial | 20 | Preoperative toxicity >grade 2† in 2 patients; severe postoperative complications in 2/13 patients‡ |
Evaluating preoperative RT, followed by surgical resection (if study included other treatment modalities/patient populations or no surgery).
Based on Common Terminology Criteria for Adverse Events version 3.0.
Defined by the need for reintervention or intensive care treatment.
DFS indicates disease free survival; OS, overall survival.
Following the early termination of the American College of Surgeons Oncology Group-Z9031 trial in 2006, 6 years elapsed before the EORTC began recruiting patients for their phase 3 randomized trial of neoadjuvant RT (EORTC 62092-22092).10 In the interim, little survival data have surfaced regarding preoperative RT for RPSs. Published reports in that time have included several additional single-institution reviews,8,13,15,17,18 2 prospective studies evaluating survival only among patients treated with radiation,16,19 and 2 preliminary trials evaluating the safety and efficacy of concomitant chemoradiation therapy20,21 (Table 5). Here, we utilize the NCDB to help better define the impact of neoadjuvant RT for this malignancy and to provide more meaningful survival data for patients treated with preoperative RT. By using a large, nationwide clinical cancer database, we are able to identify more than 11,000 patients who underwent surgery for RPSs, among whom 696 underwent preoperative RT. Thus, this analysis represents by far the largest study to date examining long-term outcomes associated with preoperative RT, followed by surgical resection.
Of note, we first observed that the use of preoperative RT had increased over 3-fold throughout our study period, which is in concordance with our group’s previous research in this area.22 Although only 4% of patients received neoadjuvant RT in 1998, by 2011 the rate was close to 15%. Although during that time there were several studies suggesting that preoperative RT could improve resectability and possibly decrease locoregional recurrence,5–8 there certainly existed no level 1 evidence concluding that neoadjuvant RT improved survival. In this study, we found that preoperative RT was associated with higher rates of negative margins (77.5% vs 73.0%). Notably, this improvement in margin status seemed to be the result of decreased macroscopic (9.3% vs 14.2% positivity rate) rather than microscopic (13.2% vs 12.9% positivity rate) margins. Therefore, it is conceivable that neoadjuvant RT shifted a similar percentage of patients from resections with a grossly positive margins (R2) to resections with positive microscopic margins (R1) as it did R1 resections to complete resections with negative margins (R0). It is less likely that neoadjuvant RT converts an R2 resection into an R0 resection. The NCDB does not provide data on concomitant resection of adjacent organs, which could act as an additional marker for larger resections; however, extent of resection, as measured via the criteria in the NCDB, was similar between groups, as was tumor size.
In survival analysis, we first assessed unadjusted survival (ie, among all 7759 patients with survival data) and found no difference in 5-year survival for patients treated with and without pre-operative RT (53.2% vs 57.8%; P = 0.254). Next, we compared propensity-matched groups to attempt to control for bias in tumor and patients characteristics that may have affected the decision to administer RT. Among this cohort, again we found no difference in overall survival (54.1% vs 54.4% 5-year survival). Finally, we compared survival among patients with higher-grade sarcomas. Here, we identified a small but statistically significant survival advantage for patients treated with neoadjuvant RT (49.1% vs 46.2%; P = 0.022). Although these results are encouraging, they must be interpreted with caution. Patients who underwent preoperative RT had a relative delay to surgery of 104 days compared with patients who did not undergo RT. During this period, it is likely that the majority of patients were restaged radiographically. Patients who were identified as having developed metastatic disease at this time may not have proceeded to surgery, thereby enriching the group that did not receive neoadjuvant RT group for patients at a higher risk for early recurrence. In addition, in this retrospective review, it is possible that other kinds of selection bias may have influenced the decision of the treating physicians to use preoperative RT. Therefore, it is not clear whether preoperative RT does indeed provide a survival advantage for patients with higher-grade sarcomas.
However, our findings do not exclude a potential benefit for RT in the treatment of RPSs. First, it is possible that adjuvant RT can be used in select cases (eg, among higher-grade sarcomas, after local vs more aggressive resection, and after margin-positive resection) to improve outcomes.23–25 The fact that 27.1% (vs 6.3%) of patients who did not receive neoadjuvant RT went on to receive postoperative RT may mask the magnitude of the benefit of preoperative RT. In addition, although we adjust for tumor size and histologic grade, it is impossible to account for more subjective radiographic findings, such as the preoperative assessments of “resectability” or “likelihood of achieving negative margins” that are used by surgeons to select patients for neoadjuvant treatment modalities. Thus, although our data suggest that patients with higher grade sarcomas may be a subgroup that benefits from preoperative RT, it is possible that we have yet to identify a more select subset of patients who derive an even greater survival advantage from RT. Finally, there is some evidence that combined neoadjuvant chemotherapy and RT may be needed to achieve an optimal effect,20,21 and only 25% of patients in our study received both treatment modalities.
Moreover, although the NCDB is the largest clinical cancer database using national standardized coding definitions and data transmission specifications, there are nonetheless other limitations that must be acknowledged. First, because this is a large retrospective analysis, there exists the inherent possibility for selection bias regarding patients chosen to receive neoadjuvant RT and bias at the level of the observer with regard to data collection. Second, our analysis is limited by the variables and outcomes captured through the NCDB. As such, our ability to describe the extent of resection is limited by reporting of margin status and 4 grades of surgery type ranging from “excision” to “radical resection.” Correspondingly, although we can categorize patients by the administration of neoadjuvant RT, we do not have data describing the dose or type of radiation used specifically within the neoadjuvant period. This lack of granularity may be particularly important given the increasing interest in “aggressive” resections24,25 and in delivering escalating doses of neoadjuvant radiotherapy to the area of anticipated close or positive margin.8,15,18,19 Moreover, advances in radiation technology, such as intensity-modulated RT, have improved the ability to focus the high-dose radiation region to the target to minimize treatment toxicity.16,18 Third, the pathologic diagnosis of sarcomas—including histologic subtyping and grading—may not be uniform across centers. However, our subgroup analysis of leiomyosarcomas—a tumor that can specifically be distinguished by its expression of smooth muscle markers—corresponded well with our overall analysis, increasing our confidence that differences in pathologic evaluation were not a major limitation. Finally, we are unable to comment on short-term postoperative outcomes such as surgical site infection, wound complications, other major morbidity, and hospital cost, although many of these data have previously been reported by our group and others.3,22,26,27
CONCLUSIONS
In this study, we demonstrate that long-term survival is similar between patients treated with and without neoadjuvant RT. However, there may be a small (~3%) survival benefit for patients with higher-grade sarcomas, but this remains unclear, and this observation should be tested in a prospective clinical trial. Therefore, our retrospective results provide further clinical equipoise for the ongoing EORTC trial and can be used to encourage centers to endorse participation in this important study. Of note, we found that patients who underwent neoadjuvant RT were more likely to have been treated at an academic/research hospital than at a community or comprehensive community program. One reason for this difference may be that patients at an academic/research hospital may be more likely to be evaluated by a multidisciplinary team that includes a radiation oncologist before surgical resection. It is also possible that radiation oncologists at academic centers may have more experience treating relatively uncommon tumors such as RPSs with radiotherapy. Therefore, they may be more comfortable with delivering RT to a large volume of the abdomen. Regardless, this finding suggests that clinical trials for RPS that include neoadjuvant RT should target academic/research hospitals to increase the likelihood of patient accrual. Continued investigation of the role of RT is certainly needed to better define which patients with RPSs may benefit from neoadjuvant RT.
Acknowledgments
The authors acknowledge Sandra S. Stinnett, Dr. P.H., for her review of the statistical methodology utilized in this study.
Footnotes
Disclosure: Supported by departmental funds (Department of Surgery). The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. The authors declare no conflicts of interest.
References
- 1.Lewis JJ, Leung D, Woodruff JM, et al. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gronchi A, Miceli R, Shurell E, et al. Outcome prediction in primary resected retroperitoneal soft tissue sarcoma: histology-specific overall survival and disease-free survival nomograms built on major sarcoma center data sets. J Clin Oncol. 2013;31:1649–1655. doi: 10.1200/JCO.2012.44.3747. [DOI] [PubMed] [Google Scholar]
- 3.Caudle AS, Tepper JE, Calvo BF, et al. Complications associated with neoadjuvant radiotherapy in the multidisciplinary treatment of retroperitoneal sarcomas. Ann Surg Oncol. 2007;14:577–582. doi: 10.1245/s10434-006-9248-9. [DOI] [PubMed] [Google Scholar]
- 4.Tzeng CW, Fiveash JB, Popple RA, et al. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 5.Pawlik TM, Ahuja N, Herman JM. The role of radiation in retroperitoneal sarcomas: a surgical perspective. Curr Opin Oncol. 2007;19:359–366. doi: 10.1097/CCO.0b013e328122d757. [DOI] [PubMed] [Google Scholar]
- 6.Zlotecki RA, Katz TS, Morris CG, et al. Adjuvant radiation therapy for resectable retroperitoneal soft tissue sarcoma: the University of Florida experience. Am J Clin Oncol. 2005;28:310–316. doi: 10.1097/01.coc.0000158441.96455.31. [DOI] [PubMed] [Google Scholar]
- 7.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate-or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006;13:508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 8.McBride SM, Raut CP, Lapidus M, et al. Locoregional recurrence after preoperative radiation therapy for retroperitoneal sarcoma: adverse impact of multifocal disease and potential implications of dose escalation. Ann Surg Oncol. 2013;20:2140–2147. doi: 10.1245/s10434-013-2868-y. [DOI] [PubMed] [Google Scholar]
- 9.Brennan MF. Lessons learned from the study of soft tissue sarcoma. Int J Surg. 2013;11(suppl 1):S8–S10. doi: 10.1016/S1743-9191(13)60005-9. [DOI] [PubMed] [Google Scholar]
- 10.Bonvalot S, Litiere S, Nzokirantevye A, et al. A phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcomas. Brussels, Belgium: European Organisation for Research and Treatment of Cancer; [Accessed May 1, 2014 ]. http://www.eortc.org/ [Internet] Available at http://www.eortc.be/protoc/details.asp?protocol=62092. [Google Scholar]
- 11.National Cancer Data Base. American College of Surgeons: cancer programs. National Cancer Data Base (NCDB); Available at http://www.facs.org/cancer/ncdb/. Published 2013. [Google Scholar]
- 12.National Cancer Data Base. National Cancer Data Base—Data Dictionary. Chicago, IL: NCDB (American College of Surgeons Commission on Cancer); 2013. [Accessed May 1, 2014]. http://ncdbpuf.facs.org/?q=node/259. [Google Scholar]
- 13.Zagar TM, Shenk RR, Kim JA, et al. Radiation therapy in addition to gross total resection of retroperitoneal sarcoma results in prolonged survival: results from a single institutional study. J Oncol. 2008;2008:824036. doi: 10.1155/2008/824036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White JS, Biberdorf D, DiFrancesco LM, et al. Use of tissue expanders and pre-operative external beam radiotherapy in the treatment of retroperitoneal sarcoma. Ann Surg Oncol. 2007;14:583–590. doi: 10.1245/s10434-006-9139-0. [DOI] [PubMed] [Google Scholar]
- 15.Sargos P, Dejean C, de Figueiredo BH, et al. High-dose pre-operative helical tomotherapy (54 Gy) for retroperitoneal liposarcoma. Radiat Oncol. 2012;7:214. doi: 10.1186/1748-717X-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeder F, Schulz-Ertner D, Nikoghosyan AV, et al. A clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma. BMC Cancer. 2012;12:287. doi: 10.1186/1471-2407-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alford S, Choong P, Chander S, et al. Outcomes of preoperative radiotherapy and resection of retroperitoneal sarcoma. ANZ J Surg. 2013;83:336–341. doi: 10.1111/j.1445-2197.2012.06211.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Bared N, Taussky D, Mehiri S, et al. Preoperative intensity modulated radiation therapy for retroperitoneal sarcoma. Technol Cancer Res Treat. 2013;13:211–216. doi: 10.7785/tcrt.2012.500371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith MJ, Ridgway PF, Catton CN, et al. Combined management of retroperitoneal sarcoma with dose intensification radiotherapy and resection: long-term results of a prospective trial. Radiother Oncol. 2014;110:165–171. doi: 10.1016/j.radonc.2013.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Yoon SS, Duda DG, Karl DL, et al. Phase II study of neoadjuvant bevacizumab and radiotherapy for resectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2011;81:1081–1090. doi: 10.1016/j.ijrobp.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gronchi A, De Paoli A, Dani C, et al. Preoperative chemoradiation therapy for localised retroperitoneal sarcoma: a phase I-II study from the Italian Sarcoma Group. Eur J Cancer. 2014;50:784–792. doi: 10.1016/j.ejca.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 22.Nussbaum D, Speicher P, Gulack B, et al. The effect of neoadjuvant radiation therapy on perioperative outcomes among patients undergoing resection of retroperitoneal sarcomas. Surg Oncol. doi: 10.1016/j.suronc.2014.07.001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoeckle E, Coindre JM, Bonvalot S, et al. Prognostic factors in retroperitoneal sarcoma: a multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Le Pechoux C, Musat E, Baey C, et al. Should adjuvant radiotherapy be administered in addition to front-line aggressive surgery (FAS) in patients with primary retroperitoneal sarcoma? Ann Oncol. 2013;24:832–837. doi: 10.1093/annonc/mds516. [DOI] [PubMed] [Google Scholar]
- 25.Sampath S, Hitchcock YJ, Shrieve DC, et al. Radiotherapy and extent of surgical resection in retroperitoneal soft-tissue sarcoma: multi-institutional analysis of 261 patients. J Surg Oncol. 2010;101:345–350. doi: 10.1002/jso.21474. [DOI] [PubMed] [Google Scholar]
- 26.Bartlett EK, Roses RE, Meise C, et al. Preoperative radiation for retroperitoneal sarcoma is not associated with increased early postoperative morbidity. J Surg Oncol. 2013;24:832–837. doi: 10.1002/jso.23534. [DOI] [PubMed] [Google Scholar]
- 27.Meric F, Milas M, Hunt KK, et al. Impact of neoadjuvant chemotherapy on postoperative morbidity in soft tissue sarcomas. J Clin Oncol. 2000;18:3378–3383. doi: 10.1200/JCO.2000.18.19.3378. [DOI] [PubMed] [Google Scholar]