Abstract
Cerebral microbleeds (CMB) are small hemosiderin deposits indicative of prior cerebral microscopic hemorrhage and previously thought to be clinically silent. Recent population based cross-sectional studies and prospective longitudinal cohort studies have revealed association between CMB and cognitive dysfunction. In the general population, CMB are associated with age, hypertension, and cerebral amyloid angiopathy. In the chronic kidney disease (CKD) population, diminished estimated glomerular filtration rate (eGFR) has been found to be an independent risk factor for CMB, raising the possibility that a uremic milieu may predispose to microbleeds. In the end-stage renal disease (ESRD) population on hemodialysis, the incidence of microbleeds is significantly higher compared to a control group without history of CKD or stroke. We present an ESRD patient on chronic hemodialysis with a history of gradual cognitive decline and progressive cerebral microbleeds. Through this case and literature review we illustrate the need to develop detection and prediction models to treat this frequent development in ESRD patients.
Introduction
Cognitive impairment, including dementia as well as structural brain abnormalities, are highly prevalent among patients with end stage renal disease (ESRD) on hemodialysis and are associated with poor outcomes 1-3. Although the exact pathogenesis of cognitive impairment and dementia in ESRD is unknown, cerebrovascular disease is thought to have a prominent role given that the vascular beds of the brain and kidney have similar hemodynamic features4. Previously named ‘multi-infarct dementia’ and ‘post-stroke dementia’, vascular cognitive impairment comprises a spectrum of disorders and is related to all forms of cerebrovascular diseases. More recently, “mixed cerebrovascular disease” has been proposed as a conceptual definition that incorporates both overt ischemic or hemorrhagic stroke, along with subclinical abnormalities such as white matter disease (or leukoaraiosis) and cerebral microbleeds 5, 6.
The term ‘cerebral microbleeds’ (CMB) refers to focal areas of signal loss in brain parenchyma measuring ≤10 mm on gradient-echo or susceptibility-weighted magnetic resonance imaging (MRI)7. Thus, CMB appear to be direct evidence of microvascular blood leakage. Presence and number of CMB have been shown to be directly and independently associated with cognitive dysfunction in different populations and prospective longitudinal cohort studies, with or without preexisting cerebrovascular disease8-12. In the general population, CMB are associated with age, hypertension and cerebral amyloid angiopathy. CMB are present in approximately 17% of the population between age 60 and 69, and nearly 36% by age 80 and above13. In the population with chronic kidney disease (CKD), diminished estimated glomerular filtration rate (eGFR) has been found to be an independent risk factor for CMB, raising the possibility that a uremic milieu may predispose to microbleeds14. In the ESRD population on hemodialysis, the incidence of microbleeds is significantly higher compared to a control group without history of CKD or stroke15.
The purpose of this case report is to present an ESRD patient on chronic hemodialysis with a history of gradual cognitive decline and progressive structural brain abnormalities. We explore this case within the framework of mixed cerebrovascular disease and the ongoing hemodynamic demands of maintenance hemodialysis.
Case Report
The patient is a 67 year old female with a past medical history significant for insulin-dependent type II diabetes mellitus, hypertension, and presumed hypertensive nephrosclerosis resulting in ESRD. She was started on maintenance hemodialysis three times weekly in a dialysis center beginning 2006. Prior to renal replacement therapy, the patient had worked as an administrative assistant in marketing and maintained an independent lifestyle. The patient started to show signs of cognitive impairment, first described as worsening short term memory and attention deficit in 2006, two years after dialysis initiation. She was evaluated by neurology in 2008 and was initially diagnosed with mild cognitive impairment; MMSE (MMSE or Folstein test)16 at that time was 29 /30. Otherwise, she had no speech difficulty, focal weakness, or numbness. There was no family history of stroke, psychiatric illness, or cognitive dysfunction. Over the next few years, she had progressive memory difficulties, including recalling names and keeping track of medical appointments. By 2012, she had MMSE 26/30.
Radiographic studies were performed between May 2010 and October 2012, including brain MRI with gradient-echo and susceptibility-weighted imaging. MRI scans with T2*-weighted sequence were performed on the same 3T field-strength magnet to ascertain consistency over time. The microbleeds were counted based on 2 mm axial susceptibility weighted imaging and displayed in these figures as thick-slab minimum intensity projection (MIP) images. These images revealed a stable chronic hemorrhagic subcortical infarct extending to the medial aspect of the left thalamus and interval development of a hemorrhagic subcortical infarct involving the left precuneus as well as a smalldeep infarct in the left pons. There were also T2/FLAIR hyperintense white matter abnormalities, moderate for her age group. In addition to the evidence of mixed cerebrovascular disease with moderately severe white matter disease, there were diffuse, progressive microhemorrhagic changes, most within the size range of microbleeds (≤10mm diameter) (Figure 1). Over the more than two-year interval, additional CMB lesions developed in the pons, left cingulate gyrus and a larger chronic hemorrhagic area in the left parietal lobe (Figure 1).
Figure 1.
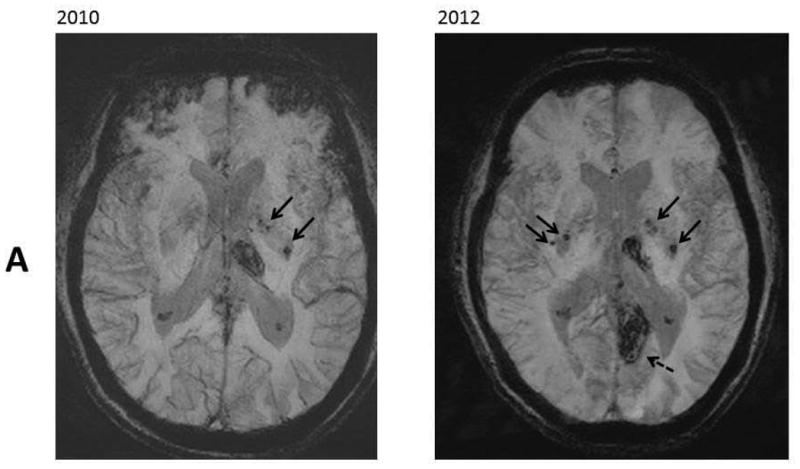
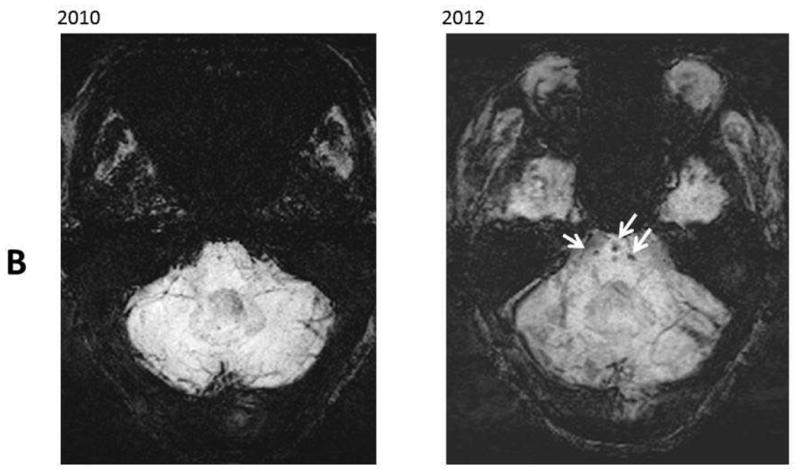
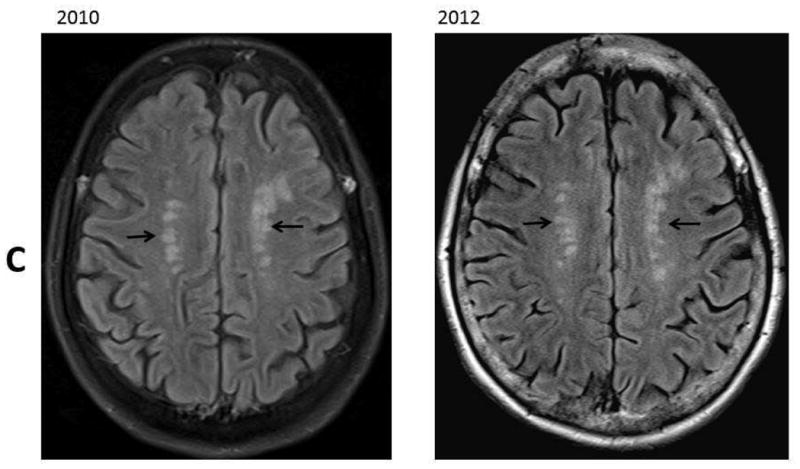
Resentative MR images in 2010 and 2012. Panel A: SWI images at level of lateral ventricles; B: SWI images at level of pons; C: FLAIR images at the level of the centrum semiovale. These images revealed increased number of microbleeds in the basal ganglia (Panel A, black arrows) and in the pons (Panel B, white arrows). There was a stable chronic hemorrhagic infarct involving the left thalamus and an interval development of a hemorrhagic infarct involving the left precuneus (Panel A, dash arrow). There were stable moderate chronic white matter signal abnormalities involving the centrum semiovale (Panel C, black arrows).
The MRI images were reviewed by the same neuroradiologist and the white matter changes appeared stable over the follow-up period. The infarcts were relatively small and sparing the cortex on high resolution 3D T1-weighted images. The number of CMB lesions increased from 5 at the baseline to approximately 12 on the follow-up study. Approximately two third of the lesions were deep or in the infratentorial region, and the remaining lesions were lobar.
During this period while on hemodialysis, the patient exhibited volume-dependent pattern of hypertension with labile pre-dialysis blood pressures up to 200/120 mmHg, even with multiple antihypertensive medications. The dialysis prescription included a standard protocol of heparin infusion (2000 unit bolus at the beginning of dialysis followed by 500 units per hour maintenance afterwards) during hemodialysis treatment. She also received erythropoietin to maintain a hemoglobin goal of 9-11 g/dl. The patient was not prescribed antiplatelet agents. Peritoneal dialysis (PD) was attempted in 2012 but subsequently discontinued due to her progressive memory difficulties and cognitive decline, which were incompatible with continuing home-based renal replacement therapy. She was switched back on maintenance hemodialysis after one episode with PD-associated peritonitis.
Discussion
Cognitive impairment is common among patients with ESRD, and associated with poor outcome. The prevalence rates are three time higher than the age-matched general population in United States17. Cognitive impairment has been associated with high mortality, frequent hospitalization, dialysis withdrawal, and disability. Thus, it has caused significant burden to the family or caregivers of the patient, and the entire healthcare system. However, the signs and diagnosis of early stage cognitive impairment has often been missed by nephrologists and primary care physicians. This is largely due to inconsistency in definition and diagnostic criteria of cognitive impairment or dementia as well as lack of standardized screening protocol and tools. The MMSE is perhaps the most widely used cognitive screening test. A score of less than 24 has been used to define dementia in the general population18. However, the test focuses mainly on memory but not executive function assessment, and executive dysfunction appears to be more common among patients with dementia from vascular causes and among dialysis patients 19, 20. These drawbacks limit its use as a screening tool for early detection of dementia in the ESRD population, as in our case. Newer screening tests have been developed and are available with focus on different domains of cognitive function. However, none of these tests have been validated specifically in the dialysis population.
The underlying mechanisms of cognitive impairment among ESRD patients remains poorly understood. Comparing to the general population, the factors which are unique to patients with ESRD and dialysis (e.g. uremic solutes, hypertension and hemodynamic instability, electrolytes and volume shift during dialysis, and anemia) may contribute to the increased risk of cognitive impairment in this population. Moreover, cerebrovascular disease is thought to have a prominent role in development and progression of cognitive impairment among ESRD patients. Based on the similarity of the juxtamedullary afferent arterioles in the kidney to the perforating arteries in the brain, they are thought to be evolutionally developed to maintain the perfusion of vital tissues such as nephrons and the brain directly from large arteries to deliver blood to tissue21; this phenomenon is often referred as brain-kidney cross talk. Diminished eGFR has been found to be an independent risk factor for CMB 14 and the incidence is significantly higher in ESRD patients compared to a control group without kidney disease 15. Recent population-based cross-sectional studies and prospective longitudinal cohort studies have also revealed close association between CMB and cognitive dysfunction8-12. A potential causal relationship is suggested by the observation that occurrence of CMB at baseline is associated with frontal-executive impairment at long-term follow up22.
Non-contrast brain MRI scans with a T2*-weighted sequence, such as gradient echo (GRE) or susceptibility weighted imaging (SWI), have emerged as important tools to detect microbleeds23, 24. The SWI sequence is highly sensitive and can display both magnitude and phase mappings to increase specificity. The microbleeds would likely be occult if there were only routine T1-weighted and T2-weighted sequences and may not be visualized on computed tomography (CT).
The number and location of cerebral microbleeds also seems to be relevant, with larger numbers of lobar microbleeds correlate with poorer neuropsychological testing. A lobar distribution of CMB is considered to indicate cerebral amyloid angiopathy25, whereas deeper parenchymal and infratentorial microbleeds are thought to relate to hypertensive vasculopathy9, 26. Lobar microbleeds are more robustly associated with motor and executive performance but not memory domains in dementia9, 27. The exact mechanism by which the CMB might cause cognitive function remains unknown. It has been suggested that CMB may induce inflammation and generate reactive oxygen species, resulting in structural injury6.
The pathological substrate of CMB is a subject of considerable interest. Initial observations suggested that CMB were due to tears of small arteries28. Later studies demonstrated a capillary source for cerebral microscopic hemorrhages, some of which may be of sufficient size to be demonstrable radiographically as CMB 29, 30. More recent observations have suggested that CMB may also be indicative of hemorrhagic microinfarctions31. A variant on the latter theme suggests that some CMB result from ischemic injury but may not actually be hemorrhagic32. At present, all these etiologies need to be considered as potentially in play, and incorporated into treatment strategies33.
In the healthy population, the prevalence of CMB increases with age and male gender34;systolic blood pressure and smoking are more likely associated with microbleeds in a deep or infratentorial region13. Among CKD patients, the prevalence of CMB is higher as the CKD stages advance. Diminished eGFR has been found to be an independent risk factor for CMB14. However, there are only limiteddata regarding prevalence and risk factors in the dialysis population. Increased systolic blood pressure, pulse pressure, and presence of hypertension and diabetes were associated with higher prevalence of CMB in a small study of Japanese dialysis patients35.
The case presented in this paper illustrates gradual decline in cognitive functions and neuroradiologic evidence of progressive mixed cerebrovascular disease including CMB two years after the initiation of hemodialysis. The MRI with T2*-weighted sequence showed stable white matter changes over the follow-up period, interval development of infarcts, and progression of CMB lesions.. It is tempting to attribute the patient's cognitive decline to this CMB progression. However, it must be noted that other progressive brain changes were present, including new infarcts in precuneus and pons. These findings illustrate the complexities of mixed cerebrovascular disease, in which ischemic and hemorrhagic changes coexist and progress. Given this complexity, it remains unclear to what extent the CMB lesions in fact produced the patient's cognitive decline.
In this case, the location of the CMB lesions seemed to be consistent with hypertensive vasculopathy and her history of volume-dependent labile high blood pressures. Note that blood pressure variability has been shown to independently predict CMB progression in deep and infratentorial regions after a median follow-up of 14 months36. In the hemodialysis setting, rapid changes in blood pressure, microembolization and dialysis disequilibrium have also been proposed, in addition to microbleeds, as mechanisms of brain injury37.
Due to largely unknown mechanism behind cognitive dysfunction especially in the dialysis population, there is no standard strategy about its prevention and management. A meta-analysis of hypertension trials showed that treatment of hypertension was associated with 13% decreased risk of dementia. However, the individual trials had mixed results for the preventive benefits of antihypertensive treatment on cognitive decline38, and these trials were not designed for patients on dialysis. Several uncontrolled observational studies of patients with ESRD have demonstrated that treatment with recombinant erythropoietin for severe anemia was associated with improvement in cognitive function39-41. However, a large randomized trial of anemia correction using erythropoietin in CKD or ESRD found increased risk for stroke, a major risk factor for dementia42. Similarly, there are conflicting data about using frequent hemodialysis and thus higher Kt/V for cognitive dysfunction prevention and treatment. Several risk factors associated with ESRD and dialysis such as retention of uremic solutes and hemodynamic instability during dialysis may be modified by more frequent hemodialysis and higher Kt/V. This speculation was favored by early observational studies43, 44. However, the recent Frequent Hemodialysis Network trials failed to achieve any benefits. The trials compared in-center 3-times-per-week versus in-center 6-times-per-week hemodialysis, and home or in-center 3-times-per-week versus home 6-times-per-week hemodialysis. These randomized trials showed at four-month and one-year follow-up, no benefits of frequent hemodialysis for the primary and secondary cognitive outcomes 45.
In conclusion, this case illustrates the increased risk of cognitive dysfunction in patients of ESRD on hemodialysis. In the ESRD population on hemodialysis, the incidence of CMB is significantly higher compared to the general population, and CMB have been shown to be directly and independently associated with cognitive dysfunction. Currently, there is no standard strategy for screening, prevention, and management of cognitive decline in this population. MRI with T2*-weighted sequence may serve as a noninvasive biomarker for mixed cerebrovascular disease, but it has not been widely used. Further work is needed to develop a screening protocol for early detection of cognitive dysfunction and occurrence of CMB. Longitudinal studies are required to define the causal relationship between CMB and progression of cognitive dysfunction. Risk factors for development of CMB and dementia in dialysis patients need to be identified in order to individualize the prevention and treatment strategy, in particular those factors that are unique to the dialysis population such as dialysis modality, dialysis dose, intradialytic hemodynamic changes, and medications given during dialysis including erythropoietin analogs, heparin, and intravenous iron.
Acknowledgments
Funding Source: The study was supported by a mentoring award from the National Institute of Diabetes, Digestive and Kidney Disease of the National Institutes of Health K24 DK091419, a philanthropist grant from Mr. Harold Simmons, and NIH NS20989 (MF).
Footnotes
Potential Conflict of Interest: KKZ has received honoraria from Abbott, Amgen, DaVita, Fresenius, Genzyme/Sanofi and Shire. MF has received research support and honoraria from Boehringer-Ingelheim and Otsuka Pharmaceutical Company.
References
- 1.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, Shaffi K, Weiner DE, Sarnak MJ. Anatomic brain disease in hemodialysis patients: a cross-sectional study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:271–278. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurella M, Luan J, Yaffe K, Chertow GM. Validation of the Kidney Disease Quality of Life (KDQOL) cognitive function subscale. Kidney international. 2004;66:2361–2367. doi: 10.1111/j.1523-1755.2004.66024.x. [DOI] [PubMed] [Google Scholar]
- 3.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 4.Mogi M, Horiuchi M. Clinical Interaction between Brain and Kidney in Small Vessel Disease. Cardiology research and practice. 2011;2011:306189. doi: 10.4061/2011/306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher M. The challenge of mixed cerebrovascular disease. Annals of the New York Academy of Sciences. 2010;1207:18–22. doi: 10.1111/j.1749-6632.2010.05758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher M, Vasilevko V, Cribbs DH. Mixed Cerebrovascular Disease and the Future of Stroke Prevention. Translational stroke research. 2012;3:39–51. doi: 10.1007/s12975-012-0185-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Offenbacher H, Fazekas F, Schmidt R, Koch M, Fazekas G, Kapeller P. MR of cerebral abnormalities concomitant with primary intracerebral hematomas. AJNR American journal of neuroradiology. 1996;17:573–578. [PMC free article] [PubMed] [Google Scholar]
- 8.Liem MK, Lesnik Oberstein SA, Haan J, van der Neut IL, Ferrari MD, van Buchem MA, Middelkoop HA, van der Grond J. MRI correlates of cognitive decline in CADASIL: a 7-year follow-up study. Neurology. 2009;72:143–148. doi: 10.1212/01.wnl.0000339038.65508.96. [DOI] [PubMed] [Google Scholar]
- 9.Poels MM, Ikram MA, van der Lugt A, Hofman A, Niessen WJ, Krestin GP, Breteler MM, Vernooij MW. Cerebral microbleeds are associated with worse cognitive function: the Rotterdam Scan Study. Neurology. 2012;78:326–333. doi: 10.1212/WNL.0b013e3182452928. [DOI] [PubMed] [Google Scholar]
- 10.Takashima Y, Mori T, Hashimoto M, Kinukawa N, Uchino A, Yuzuriha T, Yao H. Clinical correlating factors and cognitive function in community-dwelling healthy subjects with cerebral microbleeds. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2011;20:105–110. doi: 10.1016/j.jstrokecerebrovasdis.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.van Es AC, van der Grond J, de Craen AJ, Westendorp RG, Bollen EL, Blauw GJ, Greenberg SM, van Buchem MA, Group PS. Cerebral microbleeds and cognitive functioning in the PROSPER study. Neurology. 2011;77:1446–1452. doi: 10.1212/WNL.0b013e318232ab1d. [DOI] [PubMed] [Google Scholar]
- 12.van Norden AG, van den Berg HA, de Laat KF, Gons RA, van Dijk EJ, de Leeuw FE. Frontal and temporal microbleeds are related to cognitive function: the Radboud University Nijmegen Diffusion Tensor and Magnetic Resonance Cohort (RUN DMC) Study. Stroke; a journal of cerebral circulation. 2011;42:3382–3386. doi: 10.1161/STROKEAHA.111.629634. [DOI] [PubMed] [Google Scholar]
- 13.Poels MM, Vernooij MW, Ikram MA, Hofman A, Krestin GP, van der Lugt A, Breteler MM. Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke; a journal of cerebral circulation. 2010;41:S103–106. doi: 10.1161/STROKEAHA.110.595181. [DOI] [PubMed] [Google Scholar]
- 14.Shima H, Ishimura E, Naganuma T, Yamazaki T, Kobayashi I, Shidara K, Mori K, Takemoto Y, Shoji T, Inaba M, Okamura M, Nakatani T, Nishizawa Y. Cerebral microbleeds in predialysis patients with chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25:1554–1559. doi: 10.1093/ndt/gfp694. [DOI] [PubMed] [Google Scholar]
- 15.Yokoyama S, Hirano H, Uomizu K, Kajiya Y, Tajitsu K, Kusumoto K. High incidence of microbleeds in hemodialysis patients detected by T2*-weighted gradient-echo magnetic resonance imaging. Neurologia medico-chirurgica. 2005;45:556–560. doi: 10.2176/nmc.45.556. discussion 560. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Collins AJ, Kasiske B, Herzog C, Chavers B, Foley R, Gilbertson D, Grimm R, Liu J, Louis T, Manning W, McBean M, Murray A, St Peter W, Xue J, Fan Q, Guo H, Li Q, Li S, Qiu Y, Li S, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zhang R, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Berrini D, Constantini E, Everson S, Eggers P, Agodoa L. Excerpts from the United States Renal Data System 2006 Annual Data Report. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007;49:A6–7. S1–296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Holsinger T, Deveau J, Boustani M, Williams JW., Jr Does this patient have dementia? JAMA : the journal of the American Medical Association. 2007;297:2391–2404. doi: 10.1001/jama.297.21.2391. [DOI] [PubMed] [Google Scholar]
- 19.Kurella Tamura M, Larive B, Unruh ML, Stokes JB, Nissenson A, Mehta RL, Chertow GM Frequent Hemodialysis Network Trial G. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira AA, Weiner DE, Scott T, Chandra P, Bluestein R, Griffith J, Sarnak MJ. Subcortical cognitive impairment in dialysis patients. Hemodialysis international International Symposium on Home Hemodialysis. 2007;11:309–314. doi: 10.1111/j.1542-4758.2007.00185.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertension research : official journal of the Japanese Society of Hypertension. 2009;32:115–121. doi: 10.1038/hr.2008.27. [DOI] [PubMed] [Google Scholar]
- 22.Gregoire SM, Smith K, Jager HR, Benjamin M, Kallis C, Brown MM, Cipolotti L, Werring DJ. Cerebral microbleeds and long-term cognitive outcome: longitudinal cohort study of stroke clinic patients. Cerebrovascular diseases. 2012;33:430–435. doi: 10.1159/000336237. [DOI] [PubMed] [Google Scholar]
- 23.Charidimou A, Werring DJ. Cerebral microbleeds and cognition in cerebrovascular disease: an update. Journal of the neurological sciences. 2012;322:50–55. doi: 10.1016/j.jns.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 24.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM Microbleed Study G. Cerebral microbleeds: a guide to detection and interpretation. Lancet neurology. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SH, Kim SM, Kim N, Yoon BW, Roh JK. Cortico-subcortical distribution of microbleeds is different between hypertension and cerebral amyloid angiopathy. Journal of the neurological sciences. 2007;258:111–114. doi: 10.1016/j.jns.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Qiu C, Cotch MF, Sigurdsson S, Jonsson PV, Jonsdottir MK, Sveinbjrnsdottir S, Eiriksdottir G, Klein R, Harris TB, van Buchem MA, Gudnason V, Launer LJ. Cerebral microbleeds, retinopathy, and dementia: the AGES-Reykjavik Study. Neurology. 2010;75:2221–2228. doi: 10.1212/WNL.0b013e3182020349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roob G, Lechner A, Schmidt R, Flooh E, Hartung HP, Fazekas F. Frequency and location of microbleeds in patients with primary intracerebral hemorrhage. Stroke; a journal of cerebral circulation. 2000;31:2665–2669. doi: 10.1161/01.str.31.11.2665. [DOI] [PubMed] [Google Scholar]
- 28.Fazekas F, Kleinert R, Roob G, Kleinert G, Kapeller P, Schmidt R, Hartung HP. Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR American journal of neuroradiology. 1999;20:637–642. [PMC free article] [PubMed] [Google Scholar]
- 29.Cullen KM, Kocsi Z, Stone J. Pericapillary haem-rich deposits: evidence for microhaemorrhages in aging human cerebral cortex. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:1656–1667. doi: 10.1038/sj.jcbfm.9600155. [DOI] [PubMed] [Google Scholar]
- 30.Fisher M, French S, Ji P, Kim RC. Cerebral microbleeds in the elderly: a pathological analysis. Stroke; a journal of cerebral circulation. 2010;41:2782–2785. doi: 10.1161/STROKEAHA.110.593657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanskanen M, Makela M, Myllykangas L, Rastas S, Sulkava R, Paetau A. Intracerebral hemorrhage in the oldest old: a population-based study (vantaa 85+) Frontiers in neurology. 2012;3:103. doi: 10.3389/fneur.2012.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janaway BM, Simpson JE, Hoggard N, Highley JR, Forster G, Drew D, Gebril OH, Matthews FE, Brayne C, Wharton SB, Ince PG on behalf of the MRCCF, Ageing Neuropathology S. Brain haemosiderin in older people: pathological evidence for an ischaemic origin of MRI microbleeds. Neuropathology and applied neurobiology. 2013 doi: 10.1111/nan.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher MJ. Brain regulation of thrombosis and hemostasis: from theory to practice. Stroke; a journal of cerebral circulation. 2013;44:3275–3285. doi: 10.1161/STROKEAHA.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke; a journal of cerebral circulation. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 35.Naganuma T, Takemoto Y, Yamasaki T, Shima H, Shoji T, Ishimura E, Nishizawa Y, Morino M, Okamura M, Nakatani T. Factors associated with silent cerebral microbleeds in hemodialysis patients. Clinical nephrology. 2011;75:346–355. doi: 10.5414/cnp75346. [DOI] [PubMed] [Google Scholar]
- 36.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, Wu J, Zeng J, Yao C, Huang Y Group CS. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke; a journal of cerebral circulation. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 37.Madero M, Sarnak MJ. Does hemodialysis hurt the brain? Seminars in dialysis. 2011;24:266–268. doi: 10.1111/j.1525-139X.2011.00857.x. [DOI] [PubMed] [Google Scholar]
- 38.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C investigators H. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet neurology. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
- 39.Grimm G, Stockenhuber F, Schneeweiss B, Madl C, Zeitlhofer J, Schneider B. Improvement of brain function in hemodialysis patients treated with erythropoietin. Kidney international. 1990;38:480–486. doi: 10.1038/ki.1990.229. [DOI] [PubMed] [Google Scholar]
- 40.Marsh JT, Brown WS, Wolcott D, Carr CR, Harper R, Schweitzer SV, Nissenson AR. rHuEPO treatment improves brain and cognitive function of anemic dialysis patients. Kidney international. 1991;39:155–163. doi: 10.1038/ki.1991.20. [DOI] [PubMed] [Google Scholar]
- 41.Pickett JL, Theberge DC, Brown WS, Schweitzer SU, Nissenson AR. Normalizing hematocrit in dialysis patients improves brain function. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1999;33:1122–1130. doi: 10.1016/S0272-6386(99)70150-2. [DOI] [PubMed] [Google Scholar]
- 42.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R Investigators T. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. The New England journal of medicine. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 43.Teschan PE. Electroencephalographic and other neurophysiological abnormalities in uremia. Kidney international Supplement. 1975:210–216. [PubMed] [Google Scholar]
- 44.Teschan PE, Bourne JR, Reed RB, Ward JW. Electrophysiological and neurobehavioral responses to therapy: the National Cooperative Dialysis Study. Kidney international Supplement. 1983:S58–65. [PubMed] [Google Scholar]
- 45.Kurella Tamura M, Unruh ML, Nissenson AR, Larive B, Eggers PW, Gassman J, Mehta RL, Kliger AS, Stokes JB Frequent Hemodialysis Network Trial G. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2013;61:228–237. doi: 10.1053/j.ajkd.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


