Abstract
Purpose
The term “chemobrain” is sometimes used to denote deficits in neuropsychological functioning that may occur as a result of cancer treatment. As breast cancer survivors now commonly reach late life, it is not known whether previous exposure to chemotherapy may affect long-term risk for cognitive impairment. To help address this concern, this study tested whether successfully surviving chemotherapy earlier in life was associated with later differences in brain metabolic function as an older adult compared to controls. This question was examined using PET measures of brain glucose metabolism in elderly women cancer survivors.
Methods
Breast cancer survivors (N=10), currently free of recurrent cancer and without a diagnosis of a cognitive disorder, were compared to matched healthy controls (N=10). All subjects were imaged at rest with [18F]fluorodeoxyglucose (FDG). Images were analyzed semi-quantitatively using the Alzheimer's Discrimination Tool (PMOD) and a VOI-based approach derived from co-registered MRIs.
Results
Relative FDG uptake (normalized to global) was significantly lower in the survivors compared to control subjects in bilateral orbital frontal regions, consistent with differences between the groups in cognition and executive function (i.e., TMT-B, MMSE) and despite no significant differences with respect to age, education, intelligence, or working memory. None of the survivors and only one control manifested a global PET score consistent with an Alzheimer's Disease (AD) metabolic pattern.
Conclusion
Breast cancer survivors treated with chemotherapy may manifest long-term changes in brain glucose metabolism indicative of subtle frontal hypometabolism, a finding consistent with results from neuropsychological testing and other imaging modalities.
Keywords: breast cancer, [18F]fluorodeoxyglucose, FDG, brain metabolism, chemobrain
INTRODUCTION
Changes in cognitive function associated with cancer therapy, colloquially referred to as “chemobrain”, have been reported since the 1970s, but are typically observed to resolve after treatment is concluded (Ahles and Saykin 2007; Anderson-Hanley, et al. 2003). Due to the excellent treatment response for breast cancer chemotherapy, many patients are surviving for decades post-therapy, mandating a more thorough understanding of the possible long-term sequelae of treatment (Ahles and Saykin 2007; Weiss 2008). Three recent reviews by Simo, et al. (Simo, et al. 2013), Scherling and Smith (Scherling and Smith 2013) and Koppelmans, et al. (Koppelmans, et al. 2013) have detailed the neuroimaging evidence for long-term effects of chemotherapy on brain structure and function. Simo, et al.,(Simo et al. 2013) concluded that, in a subgroup of patients, there are persistent decreases in grey and white matter volumes with a predominant frontal cortical hypometabolism, impairments that are consistent with the reported executive function deficits (Scherling and Smith 2013) reported in some breast cancer survivors. These conclusions were based primarily on structural and functional MRI data, with only one report by Silverman, et al. (Silverman, et al. 2007) utilizing positron emission tomography (PET)-based assessments of brain metabolism in chemotherapy treated breast cancer long-term survivors. Silverman et al. (2007) imaged breast cancer survivors with and without a history of chemotherapy with [15O]water and [18F]fluorodeoxyglucose (FDG) 5 to 10 years after diagnosis (mean = 7.4 years). The [15O]Water measures of change in cerebral blood flow during a memory activation task showed an abnormal activation pattern in the inferior frontal gyrus (chemotherapy > non-chemotherapy) and the posterior cerebellum. Although there were no differences between the breast cancer groups or between breast cancer patients and a control population in resting glucose metabolism, the uptake of FDG in the left inferior frontal gyrus in the chemotherapy treated survivors was significantly correlated with the Rey-Osterreith Complex Figure (ROCF) Delayed Recall performance. This relationship with the ROCF led the authors to hypothesize that the “increased frontal activation during performance of the memory task may represent a compensatory response to lower resting metabolism found in this region of the brain in treated impaired patients.” (Silverman et al. 2007)
An investigation into the long-term effects of breast cancer has been conducted at our institution with the neuropsychological sequelae being reported by Yamada et al. (Yamada, et al. 2010) and Nguyen, et al.(Nguyen, et al. 2013). Yamada, et al. (Yamada et al. 2010) analyzed the neuropsychological function in 30 long-term elderly cancer survivors (at least 65 years at evaluation and at least 50 years at diagnosis) compared to matched controls. In this analysis, survivors scored significantly worse in executive function tasks as measured by the Trail Making Test, Part B (TMT-B) and Wisconsin Card Sorting Tasks (WCST). Survivors also scored lower on tasks of working memory as measured by the WAIS Digit Span Reverse and Letter-Number Sequencing, suggesting potential dysfunction in frontal-subcortical brain regions. Nguyen, et al. (Nguyen et al. 2013) reported on the final sample of N = 87 subjects subdivided into individuals receiving chemotherapy, local therapy only and non-cancer controls. Significant differences were seen between the groups for the MMSE, Letter-Number Sequencing, WCST and Trail Making Test, Part A with the breast cancer survivors scoring lower even after controlling for age, education and medical comorbidity.
The subjects investigated in the current imaging study were drawn from the breast cancer survivors detailed in the Yamada and Nguyen studies. Based on the reported findings, it was hypothesized that the observed deficits in neuropsychological function would manifest in reductions in relative glucose metabolism, as measured by FDG PET, in brain areas associated with executive processing and working memory.
METHODS
Subjects
The breast cancer participants in the current imaging study were drawn from the survivor cohort from the studies reported by Yamada, et al. (Yamada et al. 2010) and Nguyen, et al. (Nguyen et al. 2013) and healthy controls were drawn from a study examining changes in decision-making across the adult lifespan (Denburg, et al. 2007). A more complete description of the recruitment procedures are discussed in these references. Breast cancer survivor participants (N = 10) included women at least 50 years old at diagnosis, and currently older than 65 years (mean age = 73.7 ± 3.8 years, range 67 – 78) who had received chemotherapy (N = 9) or chemoradiation therapy (N = 1) and survived breast cancer without recurrence for a minimum of ten years (mean = 16.3 ± 2.6 years). The post-surgical chemotherapy employed were the standard multi-agent regimens using cyclophosphamide, methotrexate and 5-fluorouracil or an anthracycline (doxorubicin). Participants were excluded if they possessed a CNS disorder (e.g., multiple sclerosis, Parkinson's disease), had a history of closed head trauma with an extended loss of consciousness, had currently active or unstable metabolic, psychiatric, or cardiovascular disease or a history of cerebrovascular events or substance abuse. These breast cancer (BC) survivors were matched, as a group, to healthy female controls (HC) (N = 10, mean = 75.1 ± 9.9 years, range: 61 - 90 years) based on demographic and neuropsychological (verbal IQ) variables. Participants did not exhibit significant cognitive deficits warranting a clinical dementia diagnosis (mean Mini Mental State Exam (MMSE): BC: 28.5 ± 1.4, HC: 29.6 ± 0.7, range 26 – 30) and all were community-dwelling. Because all subjects were participants in the larger studies described above (Nguyen et al. 2013; Yamada et al. 2010), multiple measures of neuropsychological function were available. However, due to the small number of subjects in this study, the analyses were limited to three representative tests, the “working memory index – total” (WMI-Total), the Rey-Osterrieth Complex Figure (ROCF) (Rey 1941) and the Trail Making Test, Part B (TMT-B) (Spreen and Strauss 1998). WMI-Total was calculated as the sum of the scaled scores for the Digit Symbol, Letter Number Sequencing and Arithmetic tests of the Wechsler Adult Intelligence Scale – Third Edition (WAIS-III) (Wechsler 1997). The ROCF – total delay score was selected because it was the test that demonstrated the most significant cognitive deficit in breast cancer survivors studied by Silverman, et al. (Silverman et al. 2007). The TMT-B was selected because it represents a general test of cognitive performance sensitive to changes in executive function that has been reported to be significantly related to brain image-based parameters (for example, in subjects with mild cognitive impairment (MCI) as reported by Ponto, et al. (Ponto, et al. 2006)).
The sub-sample of participants selected for the imaging study was representative of the entire sample of breast cancer survivors investigated by Yamada, et al. (Yamada et al. 2010) and Nguyen, et al. (Nguyen et al. 2013) with respect to the neuropsychological measures that had been found to differ significantly between survivors and the healthy controls. Minor, non-statistically significant differences did exist between the subjects included in the imaging study and the balance of the chemotherapy-treated subjects. The imaging subjects were non-significantly older (73.3 versus 70.0 years, p = 0.14), less educated (14.0 versus 15.0 years, p = 0.42) with a lower IQ (110.9 versus 117.4, p = 0.21) but better MMSE scores (28.5 versus 27.7, p = 0.23) than the non-imaged chemotherapy-treated survivors. See Table 1. Although none of the subjects was currently undergoing tamoxifen treatment, five had previous tamoxifen exposure and five did not. All participants, survivors and controls, signed a written informed consent document approved by the University of Iowa Institutional Review Board for both the parent study and the imaging substudy.
Table 1.
Selected Neuropsychological Measures for Breast Cancer Survivor, Imaging Subsample, and Healthy Controls Compared to Breast Cancer Survivor Sample and Healthy Controls from Nguyen et al. (2013)
| Measure | Breast Cancer Imaging Subsample (N = 10) Mean ± SD | Healthy Controls Imaging Sample (N = 10) Mean ± SD | Chemotherapy only Breast Cancer Survivors, Sample (N = 27) (results from Nguyen, et al., 2013) Mean ± SD | Healthy Controls (N = 30) (results from Nguyen, et al., 2013) Mean ± SD |
|---|---|---|---|---|
| Age (years) | 73.7 ± 3.8 | 75.1 ± 9.9 | 72.0 ± 4.9 | 72.6 ± 5.5 |
| Education (years) | 14.0 ± 3.3 | 15.7 ± 2.3 | 14.6 ± 2.8 | 14.3 ± 2.2 |
| WASI FSIQ* | 110.9 ± 13.7 | 119.4 ± 11.3 | 114.5 ± 12.1 | 112.9 ± 9.9 |
| MMSE** | 28.5 ± 1.4 | 29.6 ± 0.7*** | 28.0 ± 1.7 | 29.4 ± 0.7 |
| Working Memory Index – Total (WMI-Total)† | 30.7 ± 3.3 | 34.0 ± 5.8 | ||
| Trail Making Test, Part B (sec)# | 100.1 ± 37.4 | 74.9 ± 29†† | 97.0 ± 35.5 | 72.4 ± 26.6 |
| Rey-Osterrieth Complex Figure, total delay score (ROCF) | 16.7 ± 3.7 | 16.1 ± 5.2 | 15.9 ± 5.1 | 15.6 ± 5.6# |
WASI FSIQ = Wechsler Abbreviated Scale of Intelligence, full-scale IQ
MMSE = mini-mental state examination
Statistically significantly different at p = 0.02 controlled for age.
Working Memory Index – total = sum of the scaled scores for digit span, letter-number sequencing and the arithmetic subtest of the WAIS-III. Higher values are more advantageous.
Trail Making Test, Part B = time (in seconds) required to complete the test. Shorter times (i.e., lower numbers) indicate better performance.
Statistically significantly different at p = 0.03 controlled for age.
MRI Acquisition
MRI imaging was conducted on a Siemens 3T TIM Trio scanner. The acquisition collected volumetric T1 weighted scans using a coronal MP-RAGE sequence (TI=900ms, TE=3ms, TR=2530ms, flip angle=10°, matrix=256x256x220, FOV=256x256x220, averages=1, bandwidth=220Hz/pixel).
PET Imaging
Subjects were administered approximately 185 MBq (5 ± 10% mCi) of [18F]fluorodeoxyglucose (FDG) after a minimum of a 6 hour fast and after verifying that the blood glucose level was ≤ 200 mg/dL. Tracer uptake was in a quiet, darkened room with eyes open and ears unplugged. Imaging (10 minute static image), began at approximately 50 minutes post-administration on a Siemens ECAT EXACT HR+ scanner (Siemens Medical Solutions USA, Inc., Knoxville, TN) (50 – 60 minutes post-administration). Images were iteratively reconstructed (iterative reconstruction, 6 iterations/16 subsets, Gaussian 3.0 mm kernel).
Global Image Analysis
In order to assess the individual's imaging-based risk of Alzheimer's disease (AD), FDG images were analyzed using the Alzheimer's Discrimination Tool (PMOD Biomedical Image Quantification, version 3.5, PMOD Technologies, Ltd., Zurich, Switzerland). With this tool, the voxel values are normalized based on a mask representing AD- preserved activity and then compared to a predicted activity calculated from the subject's age and the voxel-dependent age regression parameters. The differences between the normalized measured activity and the predicted voxel values were transformed into t-values and a t-map was produced. The abnormal t-values in a predefined AD mask were summed (AD t-sum) and tested for the significance of the abnormality. The t-sum is converted to a PET score based on the threshold representing a statistically significant risk of AD (i.e., PET score > 1).
Regional Image Analyses
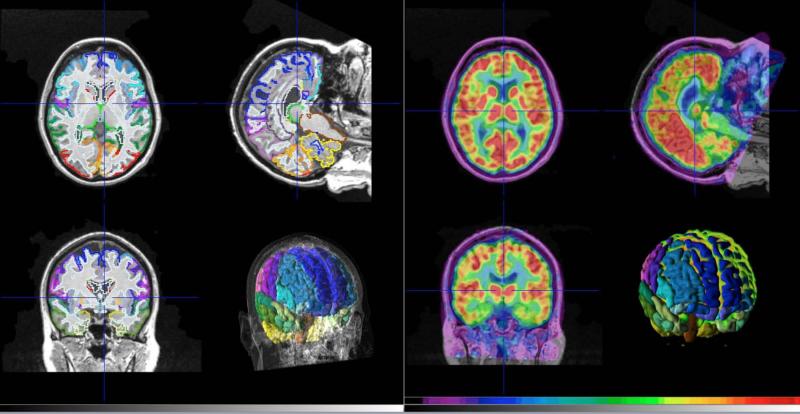
FDG images were regionally analyzed by comparing the relative uptake of FDG, a measure of glucose metabolism, in volumes-of-interest (VOIs) derived from the individual's T1-weighted structural MRI and the maximum probability atlas as implemented by the PMOD Neuro Tool (PMOD Biomedical Image Quantification, version 3.5, PMOD Technologies, Ltd., Zurich, Switzerland). See Figure 1 for an example of the image analyses. All survivor participants had a structural MRI available, however, for half (N = 5) of the control participants, an individual structural MRI was not available. For those subjects, the individual's FDG PET image was normalized to the available PET atlas and the same VOIs were applied. Whether derived from the individual's T1 MRI or from the PET atlas, 78 VOIs were available for each image. VOIs were scaled in standardized uptake values (SUVs) normalized to the global mean value for comparison purposes.
Figure 1.
Example of the structural magnetic resonance imaging with 2D and 3D volumes of interest determined by the maximum probability atlas (Neuro Tool, PMOD) (left panel) and [18F]fluorodeoxyglucose image (scaled to 6.5 standardized uptake value units (11 kBq/cc) maximum) co-registered to the magnetic resonance imaging (right panel) with 3D volumes of interest.
RESULTS
Global Analyses
Consistent with the MMSE (mean: 28.5 ± 1.4 and 29.6 ± 0.7 for survivors and controls, respectively), none of the survivors and only one control had a PET score (as described above) even approaching the AD threshold (mean = 0.36 ± 0.23, range = 0.06 – 0.74 and 0.40 ± 0.3, range = 0.07 – 1.08 for survivors and controls, respectively). The control with the PET score exceeding this threshold was the oldest participant at 90 years with an MMSE = 28. No significant differences existed between survivor and control groups with respect to the neuropsychological measures except for the MMSE (lower) and TMT-B, which was significantly longer (i.e., greater functional impairment) for the survivors compared to the control participants when controlling for age (p = 0.02 and p = 0.03, respectively). See Table 1. No differences were seen in these measures based on tamoxifen exposure except for MMSE (BC with tamoxifen = 27.8 ± 0.5, BC without tamoxifen = 29.2 ± 0.5, HC = 29.6 ± 0.3, p = 0.02).
Regional Analyses
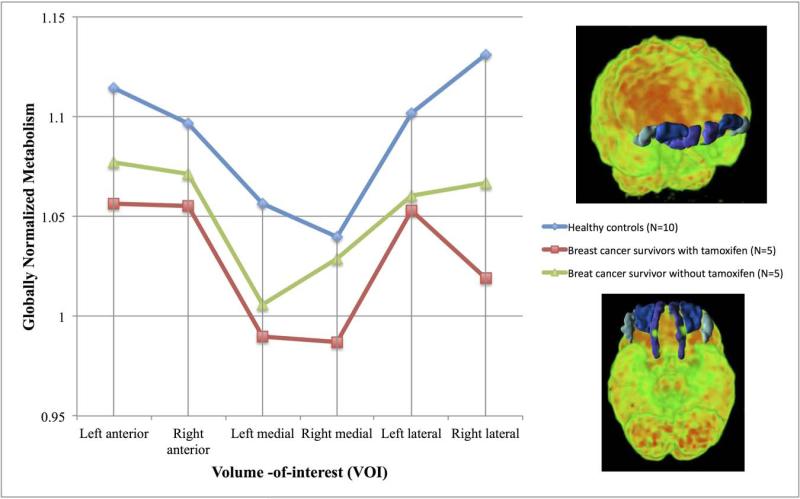
The results of comparisons of globally-normalized standardized uptake values determined from anatomically-based volumes-of-interest between survivors and controls with and without control for participant age are presented in Table 2. Significant differences were observed in the bilateral orbital frontal gyri (i.e., left medial and right lateral orbital gyri) indicative of frontal hypometabolism as well as hypometabolism in the substantia nigra (right) and brainstem. The hypometabolism in the substantia nigra was more profoundly seen in the survivors who were also treated with tamoxifen (p = 0.02). Hypermetabolism was observed in the corpus callosum and the left postcentral gyrus of the parietal lobe. Because of the a priori hypotheses associated with possible frontal lobe dysfunction and the significant differences in individual orbital frontal cortical volumes, the anterior, lateral and medial orbital frontal gyri were investigated as a group. See Figure 2. For the orbital frontal regions, the comparison between BC and HC participants was significant whether controlled for age (MANOVA df = 1,17, p = 0.01) or not (MANOVA df = 1,18, p = 0.02). Likewise, further subdividing the BC group by tamoxifen therapy, also found significant differences when controlled by age (BC with tamoxifen < BC without tamoxifen < HC; MANOVA df = 1,16, p = 0.04).
Table 2.
Volumes-of-interest (VOIs) with Statistically Significant Differences for Globally-normalized Standardized Uptake Values (SUVs) between Breast Cancer Survivors and Healthy Controls with and without Controlling for Participant Age. Bolded values are significant at p < 0.05 uncorrected for the number of comparisons.
| Region | Direction (survivor compared to control) | Parametric comparison (t test)* | Non-parametric comparison (Wilcoxon)** | Controlled for age*** |
|---|---|---|---|---|
| Frontal lobe regions: | ||||
| Left medial orbital gyrus | BC < HC | 0.014 | 0.026 | 0.015 |
| Right lateral orbital gyrus | BC < HC | 0.059 | 0.021 | 0.037 |
| Basal ganglia regions: | ||||
| Right substantia nigra | BC < HC | 0.038 | 0.10 | 0.026 |
| Other regions: | ||||
| Brainstem | BC < HC | 0.022 | 0.045 | 0.020 |
| Left postcentral gyrus (parietal lobe) | HC < BC | 0.033 | 0.021 | 0.026 |
| Corpus callosum | HC < BC | 0.030 | 0.038 | 0.035 |
p value based on two sample t test, uncorrected for the number of comparisons.
p value based on the Wilcoxon rank scores, uncorrected for the number of comparisons.
p value based on regression analysis with age and participant group as parameters.
Figure 2.
Plot of globally normalized metabolism (=SUVVOI/SUVglobal) for anterior, medial and lateral orbital frontal gyri volumes of interest (VOIs) for healthy controls (blue line), breast cancer survivors who received chemotherapy but not tamoxifen (green line) and breast cancer survivors who received chemotherapy and then tamoxifen therapy (red line). The VOIs are illustrated in the 3D volume-rendered [18F]fluorodeoxyglucose images (frontal – upper panel, bottom – lower panel) scaled to 6.5 standardized uptake value units (11 kBq/cc) with the lateral (light blue), medial (purple), and anterior (dark blue) orbital frontal gyri VOIs highlighted for a representative breast cancer subject. Differences between the groups are significant when controlled for age (MANOVA, df = 2,16, p = 0.04).
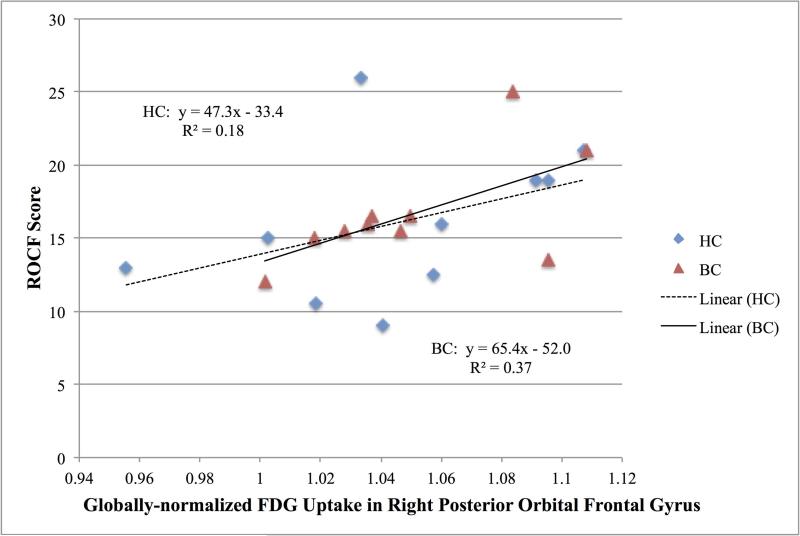
For the combined sample of breast cancer survivors and healthy controls, the relationships between the normalized FDG uptake in the frontal regions and the neuropsychological measures were explored. The ROCF score was significantly correlated with the right posterior orbital gyrus uptake with and without controlling for age (Spearman ρ = 0.54, p = 0.015, controlled for age p = 0.04). See Figure 3. The WMI-total score was significantly positively correlated with the right middle and superior frontal gyri uptake and negatively correlated with the left subcallosal area uptake, but these relationships were not significant when controlling for age (Spearman ρ = 0.45, p = 0.05, controlled for age p = 0.34; Spearman ρ = 0.46, p = 0.04, controlled for age p = 0.07; Spearman ρ = -0.51, p = 0.02, controlled for age p = 0.55, respectively).
Figure 3.
Relationship between globally normalized [18F]fluorodeoxyglucose uptake in the right posterior orbital frontal gyrus and the Rey- Osterrieth Complex Figure (ROCF), total delay score for breast cancer survivors (BC: triangles) and healthy controls (HC: diamonds).
DISCUSSION
The long-term sequelae of cancer chemotherapy, for at least a subset of treated patients, involves both anatomical and functional brain changes. (Koppelmans et al. 2013; Scherling and Smith 2013; Silverman et al. 2007; Simo et al. 2013) The functional changes, determined by neuropsychological testing and functional MRI, predominantly indict alterations in frontal lobe processing. The current investigation using FDG PET imaging found primarily a pattern of relative hypometabolism in orbital frontal regions in the breast cancer survivors when compared to matched healthy control participants. Neuropsychological testing in the parent sample of older breast cancer survivors from which this imaging sub-sample was selected found significantly lower scores in the cognitive domains of executive functioning, working memory, and divided attention, reflecting potential dysfunction in frontal-subcortical brain regions (Yamada et al. 2010). The imaging results supported the neuropsychological findings and further substantiated the existence of the long-term manifestations of “chemobrain.” Although the participant groups were demographically and cognitively matched, minor deficits in frontal and subcortical regions were identified more than a decade after treatment. Reassuringly, there were no major imaging-based pathological findings detected in the survivor group.
The age of this imaging survivor sample represents a particularly relevant demographic with respect to the breast cancer incidence profile. According to the most recent SEER (Surveillance, Epidemiology and End Results) Stat Fact Sheet (http://seer.cancer.gov/statfacts/html/breast.html, accessed 1/09/2014) based on data from the 2006 – 2010 survey interval, 47.4% of breast cancer diagnoses were made between the ages of 45 and 64 years of age with a median age at diagnosis of 61 years. The current survivors were 52 to 63 years at the time of treatment and 67 to 78 years at the time of imaging, therefore, providing insight into the potential cognitive outcomes at a critical cognitive juncture (i.e., elderly transition) for participants exposed to breast cancer treatment at a critical physiological juncture (i.e., menopause).
To investigate the effects after breast cancer chemotherapy approximately a decade post-treatment, de Ruiter, et al. (de Ruiter, et al. 2011) employed BOLD activation during both an executive functioning task and an episodic memory task. “Hyporesponsiveness” was observed in the left dorsolateral prefrontal cortex during the executive task, the parahippocampal gyrus during the memory task and in the bilateral posterior parietal cortex during both tasks. The only region investigated that exhibited greater activation in the chemotherapy compared to the control group was the “anterior portion of the left parietal operculum (the inferior part of the postcentral gyrus).” Whereas the current study with uptake under resting rather than activation conditions found relative hypometabolism in the orbital frontal areas rather than the dorsolateral prefrontal cortex, both studies found relative hypermetabolism in the left postcentral gyrus. Abraham, et al. (Abraham, et al. 2008) reported a pilot investigation using diffusion tensor imaging (DTI) measures of fractional anisotropy (FA) in the corpus callosum of breast cancer survivors 3 – 34 months post-therapy. Lower FA values in the genu, the area of the corpus callosum that connects the frontal lobes, but not the splenum the area that connects the posterior hemispheres. The FA values were positively correlated to the processing speed. Whether disruption in the white matter integrity of the corpus callosum, indicated by lower FA values, would be associated with hypo- or hypermetabolism is unknown but the current investigation provides evidence that increased relative glucose metabolism may be needed when white matter is compromised.
The findings in the current study were in general agreement with the findings of Silverman, et al. (Silverman et al. 2007), the only other FDG PET-based investigation of the long-term effects of chemotherapy in breast cancer currently available in the literature. Relevant similarities and differences between the studies will be explored. First, the time frame of the current investigation examines long-term sequelae of breast cancer treatment at a later time frame from diagnosis (i.e.. 5 to 10 versus greater than 10 years) and in an older age group (i.e., mid 50s versus mid 70s) than the Silverman, et al., study. Secondly, although both studies found frontal lobe hypometabolism, Silverman, et al. isolated this metabolic impairment to the inferior frontal lobe, whereas, in the current investigation the orbital frontal areas were found to be hypometabolic. Third, whereas, Silverman, et al. found the lentiform nucleus to be adversely affected by the combination of tamoxifen and chemotherapy, the current investigation found that this pattern was exhibited by the right substantia nigra instead. Fourth, Silverman, et al. found a 3% decrease in FDG activity in the left inferior frontal gyrus with each standard deviation (SD) change in ROCF performance in the chemotherapy-treated patients only. Whereas, in the current investigation, a change of 1 SD in ROCF (SD = 3.7) was associated with a 5% change in the right posterior orbital gyrus FDG normalized uptake in the survivor group. Therefore, the primary findings of the previous and current FDG PET studies of “chemobrain,” although differing in the age and time post-therapy, have yielded remarkably consistent results implicating persistent effects in the frontal lobes. The minor anatomical differences likely stem from technical differences between the studies, specifically in the brain atlases used for region definition (e.g., 42 versus 78 regions) and the use of standardized volumes of interest versus individual MRI-based anatomical regions.
FDG PET brain imaging may provide insights into potential long-term neurological sequelae of cancer chemotherapy. The potential abnormalities in the frontal and subcortical regions identified in the imaging sub-sample appear to be consistent with the subtle neuropsychological abnormalities in working memory observed in this group of elderly cancer survivors. Whether based on the imaging or the neuropsychological findings, the cognitive impairments observed a decade post-treatment were relatively minor yet potentially exacerbated by tamoxifen therapy, a finding similar to that of Castellon, et al. (Castellon, et al. 2004). The limitations of the current study were related to the small sample sizes relative to the number of comparisons available and the selection of community-dwelling, non-demented subjects only. In addition, the timeframe of treatment in relation to the current investigation made ascertainment of the exact chemotherapy regimen essentially impossible. However, all subjects did receive a standard multi-agent chemotherapy regimen involving cyclophosphamide, methotrexate and 5-fluorouracil (CMF) or an anthracycline (doxorubicin) consistent with the standard-of-care for Stage I through Stage IIIA breast cancer at the time of their treatment. Furthermore, brain metabolism was measured under the resting state rather than during an activation condition. In spite of these limitations, the findings are consistent with recent publications that have found that cognitive functions were generally well-preserved in the majority of chemotherapy-treated breast cancer survivors (Biglia, et al. 2010; Jenkins, et al. 2006; Quesnel, et al. 2009), yet, there is evidence of a persistent “chemobrain” syndrome manifested as frontal hypoactivation.
CONCLUSIONS
FDG PET brain imaging may provide insights into potential long-term neurological sequelae of cancer chemotherapy. The potential abnormalities in the frontal and subcortical regions identified in the comparison between breast cancer survivors and matched healthy controls appear to be consistent with the subtle neuropsychological abnormalities in working memory observed in this group of elderly cancer survivors. Whether based on the imaging or the neuropsychological findings, the cognitive impairments observed a decade post-treatment were relatively minor, potentially exacerbated by tamoxifen therapy and add further support to the concept of “chemobrain” in breast cancer survivors.
ACKNOWLEDGMENTS
This work was supported by The University of Iowa Holden Comprehensive Cancer Center grant 5P30CA086862-09, National Institutes of Health grant R01CA122934 (Schultz, PI), and a DANA Foundation Program in Brain and Immuno-Imaging grant (Denburg, PI). None of the authors have any financial conflicts-of-interest. The authors would like to acknowledge the assistance of Leigh Beglinger, PhD, Karen Ekstam-Smith, RN, and the technical staff (John Richmond, Christine Mundt, Dean Clermont, Julie Riggert), nursing staff (Lea Weldon, Amy Conklin) and chemistry staff (Len Watkins, Kathy Thede-Reynolds, Garrett Stratton) of the PET Imaging Center.
Footnotes
This material, in part, was presented as a poster at the American College of Neuropsychopharmacology meeting, Hollywood, Florida, December 7, 2009.1
Schultz SK, Menda Y, Denburg N, Ponto LB, [18F]Fluorodeoxyglucose (FDG) Positron Emission Tomography (PET) Study of Brain Metabolism among Elderly Women Breast Cancer Survivors, American College of Neuropsychopharmacology, Hollywood FL, December 7, 2009.
References
- Abraham J, Haut MW, Moran MT, Filburn S, Lemiuex S, Kuwabara H. Adjuvant Chemotherapy for Breast Cancer: Effects on Cerebral White Matter Seen in Diffusion Tensor Imaging. Clinical Breast Cancer. 2008;8:88–91. doi: 10.3816/CBC.2008.n.007. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson-Hanley C, Sherman ML, Riggs R, Agocha VB, Compas BE. Neuropsychological effects of treatments for adults with cancer: A meta-analysis and review of the literature. Journal of the International Neuropsychological Society. 2003;9:967–982. doi: 10.1017/S1355617703970019. [DOI] [PubMed] [Google Scholar]
- Biglia N, Moggio G, Peano E, Sgandurra P, Ponzone R, Nappi RE, Sismondi P. Effects of surgical and adjuvant therapies for breast cancer on sexuality, cognitive functions, and body weight. Journal of Sexual Medicine. 2010;7:1891–1900. doi: 10.1111/j.1743-6109.2010.01725.x. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive Performance in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy and Tamoxifen. Journal of Clinical and Experimental Neuropsychology. 2004;26:955–969. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- de Ruiter MB, Reneman L, Boogerd W, Veltman DJ, van Dam FSAM, Nederveen AJ, Boven E, Schagen SB. Cerebral hyporesponsiveness and cognitive impairment 10 years after chemotherapy for breast cancer. Hum Brain Mapp. 2011;32:1206–1219. doi: 10.1002/hbm.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, Wallace RB. The Orbitofrontal Cortex, Real-World Decision Making, and Normal Aging. Annals of the New York Academy of Sciences. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V,VS, Deutsch G, Bloomfield D, Morris R, Allan S, Bishop H, Hodson N, Mitra S, Sadler G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. British Journal of Cancer. 2006;94:828–834. doi: 10.1038/sj.bjc.6603029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Breteler MMB, Boogerd W, Seynaeve C, Schagen SB. Late effects of adjuvant chemotherapy for adult onset non-CNS cancer; cognitive impairment, brain structure and risk of dementia. Critical Reviews in Oncology/Hematology. 2013;88:87–101. doi: 10.1016/j.critrevonc.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Nguyen CM, Yamada TH, Beglinger LJ, Cavanaugh JE, Denburg NL, Schultz SK. Cognitive features 10 or more years after successful breast cancer survival: comparisons across trypes of cancer interventions. Psycho-Oncology. 2013;22:862–868. doi: 10.1002/pon.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponto LLB, Magnotta VA, Moser DJ, Duff KM, Schultz SK. Global Cerebral Blood Flow in Relation to Cognitive Performance and Reserve in Subjects with Mild Memory Deficits. Molecular Imaging and Biology. 2006;8:363–372. doi: 10.1007/s11307-006-0066-z. [DOI] [PubMed] [Google Scholar]
- Quesnel C, Savard J, Ivers H. Cognitive impairments associated with breast cancer treatments: results from a longitudinal study. Breast Cancer Res Treat. 2009;116:113–123. doi: 10.1007/s10549-008-0114-2. [DOI] [PubMed] [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Scherling C, Smith A. Opening up the Window into Chemobrain: A Neuroimaging Review. Sensors. 2013;13:3169–3203. doi: 10.3390/s130303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman D, Dy C, Castellon S, Lai J, Pio B, Abraham L, Waddell K, Petersen L, Phelps M, Ganz P. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Research and Treatment. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Simo M, Rifa-Ros X, Rodriguez-Fornells A, Bruna J. Chemobrain: A systematic review of structural and functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2013;37:1311–1321. doi: 10.1016/j.neubiorev.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford University Press; New York: 1998. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-III (WAIS-III) Psychological Corporation; San Antonio, Texas: 1997. [Google Scholar]
- Weiss B. Chemobrain: A translational challenge for neurotoxicology. NeuroToxicology. 2008;29:891–898. doi: 10.1016/j.neuro.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada TH, Denburg NL, Beglinger LJ, Schultz SK. Neuropsychological Outcomes of Older Breast Cancer Survivors: Cognitive Features Ten or More Years After Chemotherapy. J Neuropsychiatry Clin Neurosci. 2010;22:48–54. doi: 10.1176/appi.neuropsych.22.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]