Abstract
Background
Headache is one of the most common symptoms following traumatic head injury. The mechanisms underlying the emergence of such post-traumatic headache (PTH) remain unknown but may be related to injury of deep cranial tissues or damage to central pain processing pathways, as a result of brain injury.
Methods
A mild closed head injury in mice combined with the administration of cranial or hindpaw formalin tests were used to examine post-traumatic changes in the nociceptive processing from deep cranial tissues or the hindpaw. Histological analysis was used to examine post-traumatic pro-inflammatory changes in the calvarial periosteum, a deep cranial tissue.
Results
At 48 hours after head injury, mice demonstrated enhanced nociceptive responses following injection of formalin into the calvarial periosteum, a deep cranial tissue, but no facilitation of the nociceptive responses following injection of formalin into an extracranial tissue, the hindpaw. Mice also showed an increase in the number of activated periosteal mast cells 48 hours following mild head trauma, suggesting an inflammatory response.
Conclusion
Our study demonstrates that mild closed head injury is associated with enhanced processing of nociceptive information emanating from trigeminal-innervated deep cranial tissues, but not from non-cranial tissues. Based on these finding as well as the demonstration of head injury-evoked degranulation of calvarial periosteal mast cells, we propose that inflammatory-evoked enhancement of peripheral cranial nociception, rather than changes in supraspinal pain mechanisms play a role in the initial emergence of PTH. Peripheral targeting of nociceptors that innervate the calvaria may be used to ameliorate PTH pain.
1. Introduction
Headache is one of the most common symptoms following minor blunt trauma to the head (Keidel and Ramadan, 2006; Nampiaparampil, 2008). Recent studies suggest that post-traumatic headache (PTH) can develop within one week of the head injury, in as many as 50% of these cases, and can persist well beyond a year after the injury (Hoffman et al., 2011; Lucas et al., 2013). The nosological features of PTH are of unclear diagnostic significance (Scher and Monteith, 2014), but in about half of individuals with PTH, the headaches have migraineous features, while in up to 40%, the PTH resembles tension-type headaches (Lucas et al., 2013).
Despite its high prevalence, very little is known about the mechanisms underlying the pathophysiology of PTH. The relationship of PTH to closed head trauma and its resemblance to primary headaches strongly suggests the involvement of nociceptive sensory afferents that innervate deep cranial tissues. The development of cranial cutaneous pain hypersensitivity in PTH (Defrin et al., 2010) may be due to enhanced nociceptive drive from injured deep cranial tissues and the ensuing central sensitization at the levels of the trigeminal or upper cervical dorsal horn. The finding of altered trigeminal and extra-trigeminal pain processing in those with PTH (Defrin et al., 2010) suggests however that the trauma may have also injured supraspinal pain modulating pathways in the brain, which may also contribute to PTH.
The lack of broad consensus on an animal model of closed head injury greatly impedes our ability to study the peripheral sensory mechanisms underlying PTH. Specifically, the prevailing models of concussion involve a craniotomy, an experimental approach, which disrupts the deep cranial tissues (Cole et al., 2011) and thus undermines the study of these peripheral sensory systems and their possible contribution to the emergence of PTH.
In the present work, we employed a mouse model of mild closed head injury that does not involve a craniotomy, and is induced by a weight drop apparatus. We used a mild trauma model because of the higher prevalence of post-traumatic headache in patients suffering mild trauma compared to those with moderate or severe head traumas (Theeler et al., 2013). We used this model to test our hypothesis that mild closed head injury gives rise to acute changes in nociceptive processing arising from deep cranial tissues, in particular the calvarial periosteum. To test our hypothesis, we examined whether the head injury enhances nocifensive behaviors in response to the direct application of dilute formalin to the calvarial periosteum. In a parallel series of animals, we examined the possibility that mild head injury also affects supraspinal pain processing by studying the nocifensive behaviors following injection of dilute formalin into the hindpaw. Finally, we tested whether mild closed head injury triggers a significant acute inflammatory response within the calvarial periosteum, using a cellular marker of inflammation, namely the activation of mast cells (MCs).
2. Methods
2.1 Animals
The study employed Male ICR mice (Harlan) weighing 30–40 g. The mice were kept five per cage under a constant 12 hours light/dark cycle, at room temperature (22±2°C). Food and water were provided ad libitum. Each mouse was used for only one experiment. The Institutional Animal Care and Use Committees of the Sackler Faculty of Medicine and the Beth Israel Deaconess Medical Center approved the experimental protocols. All behavioral tests were conducted in accordance with the International Association for the Study of Pain (IASP) guidelines for animal experiments (Zimmermann, 1983). Efforts were made to minimize the number of animals used for the study. All experimental manipulations were conducted during the light phase of the cycle.
2.2 Experimental mild closed head injury
Experimental mild closed head injury was induced using the concussive head trauma device described previously (Milman et al., 2005; Zohar et al., 2003). Briefly, mice were deeply anesthetized with 3% isoflurane and placed under a weight-drop concussive head trauma instrument. The device consists of a metal tube (inner diameter 13 mm), placed vertically over the mouse head. To induce head injury, a 30 g metal weight was dropped from the top of the tube (80 cm) to strike the head at the temporal right side between the corner of the eye and the ear. A sponge was placed under the animals to support the head while allowing some anterior-posterior motion without any rotational head movement at the moment of the impact. Immediately after the impact, mice were placed in their cages for recovery. Sham treated animals were anesthetized but not subjected to the weight drop. For the formalin tests and tissue harvesting, animals were used 48 hours following the head trauma or sham procedures.
2.3 Nociceptive tests
To examine whether mild closed head injury affects trigeminal nociceptive processing from deep cranial tissues, we employed an episodic pain model, based on a modified mouse formalin test, in which a diluted solution of formalin is injected into a cranial tissue innervated by the trigeminal nerve (Ahn et al., 2008). To determine whether head trauma also affects extracranial (and extra-trigeminal) nociceptive processing, we employed a modified version of the mouse hindpaw formalin test. Both tests were performed in a quiet room. Animals were habituated to the testing chamber (a clear 30 × 30 × 20 cm Plexiglas box with a mirror placed at a 45 degrees angle) for 15 min prior to the day of testing and then again or 15 min prior to the formalin injection. All tests were evaluated in a blinded manner.
For the cranial formalin test, the animal was taken out the observation box and lightly restrained by grasping the scruff of the neck close to the base of the skull between the thumb and forefinger. A 27-gauge needle fitted to a 10μl Hamilton syringe was then advanced through the skin and the underlying galea aponeurotica so that the needle was positioned to deliver formalin solution (10 ul, 1% in 0.9% sterile saline) into the calvarial periosteum around Bregma. The dose chosen was based on a preliminary study in which it produced less than a maximal response, thus minimizing a potential ceiling effect (Ahn et al., 2008). In vehicle control experiments, only saline was injected. Following the administration of formalin or vehicle, the mouse was placed back into the viewing chamber and nociceptive behaviors were recorded for 60 min using a digital video recorder. Formalin-related cranial nociceptive responses were considered as behaviors in which the animal spent time rubbing or wiping the injected area with its forepaws, or scratching the area with its hindpaws. These behaviors were distinct from the normal, mostly forepaws related grooming behaviors, which typically start at a lower body level and continues uninterrupted to the face (Vos et al., 1994).
For the hindpaw formalin test, mice received an intraplantar injection of 10 μl of a 1% formalin solution, or 0.9% saline into the right hindpaw using a 27 g needle. Nociceptive behaviors (i.e. the time the animal spent flinching, biting or licking the injected paw) were video-recorded as with the cranial test for 60 min.
2.4 Tissue preparation and histology
Animals subjected to head trauma or a sham procedure were anaesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and perfused transcardially with 20 ml of phosphate buffered saline (PBS) followed by perfusion with 20 ml of a 4% paraformaldehyde/PBS buffer. The heads were post-fixed overnight in the same fixative solution and then transferred to PBS. The calvarial periosteum was exposed and carefully removed bilaterally using a small periosteal elevator. The periosteal tissue was then mounted on a slide and stained with 1% toluidine blue to visualize degranulated mast cells (MCs). The tissue density of MCs was determined using a bright-field Illumination, under × 200 magnification (Zeiss Axioimager), by counting the number of toluidine blue stained cells. The average density of MCs was based on MC counts from 10 different randomly chosen visual fields. Because the toluidine blue staining method allows visualization of extruded MC granules (a sign of degranulation and a marker of ongoing inflammation), we used this method to investigate the degree of such MC response. Cells were considered degranulated if there was an extensive dispersion of more than 15 extruded vesicles localized near the cell, or when there was an extensive loss of granule staining, giving the cell a ‘ghostly’ look (Levy et al., 2007). MC counts and degranulation levels were conducted in a blinded fashion.
2.5 Data analyses
Data are presented as the means ± S.E.M. For the purpose of analyzing the behavioral data, the time spent in nociceptive behaviors was divided into 5-minute blocks. Statistical differences between formalin and vehicle injections were analyzed using one-way analysis of variance (ANOVA). Changes in formalin-evoked behaviors between head injured and sham animals as well as differences in MC density and degranulation levels were analyzed using unpaired t-test. For all tests, the level of significance was set at p<0.05.
3. Results
3.1 Cranial formalin test
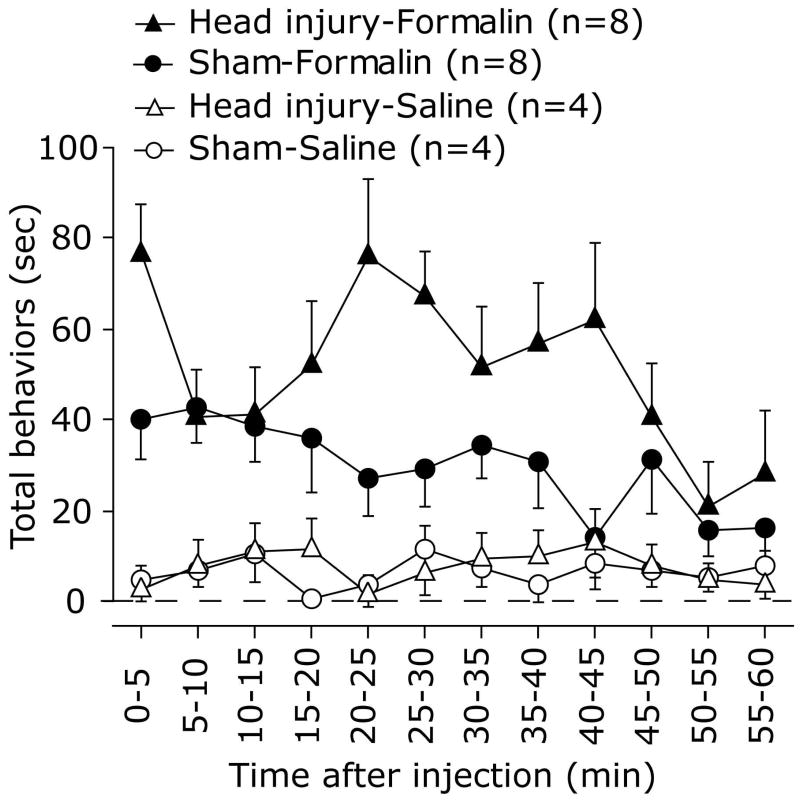
Following injection of formalin into the calvarial periosteum, both head injured and sham animals demonstrated nociceptive behaviors towards the injected area, which included wiping and grooming of the area by the forepaws and scratching with the hindpaws. As Fig. 1 depicts, the time spent performing these nociceptive behaviors at 0-60 min following the formalin injection was much higher than that observed in the vehicle injected groups in both the head injured (F1,10=41.2, p<0.0001) and sham (F1,10 =6.821, p<0.01) groups.
Figure 1.
Time course of the behavioral nociceptive responses following injection of 1% formalin solution or 0.9% saline into the calvarial periosteum of head injured and sham injured animals 48 hours post trauma. Note the monophasic behavioral response of the sham animals and the biphasic response in head injured animals.
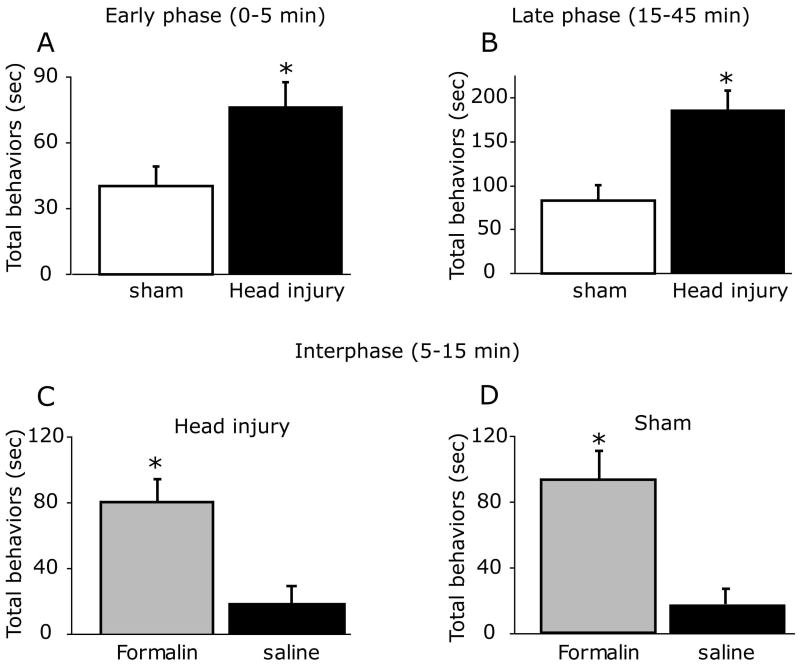
Previous studies have shown that administration of a similar low dose of diluted formalin to the hindpaw or orofacial regions of naïve animals resulted in the development of a biphasic behavioral response with a quiescent interphase (Clavelou et al., 1995; Fischer et al., 2013). As Fig. 1 demonstrates, unlike the behavioral responses seen following hindpaw or orofacial formalin injections, cranial injection of formalin to sham animals gave rise to only a single phase of nociceptive behavior that peaked at 5-10 minutes and remained elevated for up to 45 min. Unlike the sham animals, head injured mice developed a biphasic response with an acute response at 0-5 min and a second phase at 15-45 min that peaked at 20-25 minutes. When compared to sham animals, mice subjected to closed head injury demonstrated an increase in the behavioral nociceptive responses to formalin injection at both the early time point (0-5 min, p< 0.05, Figure 2 A) and the later phase (15-45 min, p<0.01, Figure 2B). In the head injured animals, formalin-evoked nociceptive behaviors during the interphase (5-15 min) were higher than that observed following vehicle injections (p<0.05, Figure 2C), indicating a lack of a quiescent phase. During the intermediate phase (5-15 min) there was no difference in the behavioral responses between the head injured and sham groups.
Figure 2.
Cumulative duration of nociceptive behaviors following periosteal injection of formalin recorded at 0-5 min (early response), 5-15 min (interphase) and 15-45 min (late response). Note that head injury was associated with overall increases in the nociceptive behavioral response at both the early (A) and late phases (B). In both the head injured (C) and sham (D) groups, the behavioral responses to formalin were higher than those observed following saline injection indicating the lack of quiescent interphase. * p<0.05, unpaired t-test.
3.2 Hindpaw formalin test
As Fig. 3A demonstrates, hindpaw injection of formalin elicited nociceptive behavioral responses that were greater than that observed following saline injections in both the sham (F1,11=15.69, p<0.001) and head injured (F1,11=7.02, p<0.05) groups. However, unlike the cranial formalin test, there was no difference in the nociceptive behavioral responses to hindpaw injections between the head injured and sham groups in both the acute (0-5 min, Fig. 3B) and delayed (15-45 min, Fig. 3C) phases.
Figure 3.
Behavioral nociceptors response in the hindpaw formalin test 48 hours following head injury or sham procedure. (A) Time course of nociceptive behaviors following formalin or saline injections. Cumulative durations of nociceptive behaviors at the early (B) and the late (C) phases of the formalin test. Note that head injury did not affect the behavioral nociceptive response to the extracranial formalin test.
3.3 Changes in calvarial periosteal MCs following closed head injury
To examine whether mild head injury promotes an acute inflammatory reaction within the calvarial periosteum we analyzed changes in periosteal MCs. In both the sham and injured mice, MCs were distributed throughout the calvarial periosteum overlying the frontal and parietal cranial bones. Overall, there was no difference in the density of periosteal MCs between the sham and injured animals (Fig. 5A). Nonetheless, when compared to sham treated animals, the percentage of periosteal MCs showing signs of an active degranulation was higher in mice subjected to a head trauma with increasing numbers of degranulated MCs in the trauma site as well as in the contralateral side (both p<0.05, Fig. 5B).
Figure 5.
Acute effect of head injury on the density of calvarial periosteal MCs and their degranulation level. While head trauma did not lead to changes in the density of periosteal MCs (A), a unilateral head trauma promoted a bilateral increase in the number of MCs showing sign of degranulation (B), a marker of an inflammatory response. * p<0.05, unpaired t-test.
4. Discussion
The main finding of this study is that mild closed head injury in mice is associated with a selective enhancement of deep cranial trigeminal nociceptive processing, which is not detected in extracranial tissues (i.e. the hindpaw). In addition, our study demonstrates that a unilateral mild closed head injury gives rise to a bilateral acute inflammatory response within the calvarial periosteum, namely degranulation of periosteal MCs. We propose that the development of increased cranial nociception following mild traumatic head injury, and hence PTH, is related to increased sensitivity of the trigeminal nociceptive system that innervates deep cranial tissues, including the calvarial periosteum, rather than enhancement of pain processing in supraspinal nociceptive pathways. We further suggest that acute inflammatory response within the calvarial periosteum could play role in mediating the acute trigeminal hyper-nociception seen following head trauma.
4.1 Cranial formalin injection evokes distinct behavioral nociceptive responses in naïve mice
Injection of 1% formalin to the calvarial periosteum in animals exposed to the sham closed head injury procedure gave rise to a pattern of nociceptive behaviors that was distinct in its time course from those reported following injection of formalin to the hindpaw (Fischer et al., 2013; Tjolsen et al., 1992) or the orofacial region (Clavelou et al., 1995), in that it lacked the quiescence interphase. In our experiments, formalin was injected into the calvarial periosteum. This deep cranial tissue receives sensory nociceptive innervation by extracranial trigeminal afferents that innervate the scalp as well as by some intracranial meningeal nociceptive afferents that send collaterals that exit the skull (Kosaras et al., 2009; Schueler et al., 2013; Zhao and Levy, 2014). The different behavioral response seen following the cranial formalin injection could reflect differences in the way that trigeminal primary afferents that innervate the calvarial periosteum directly respond to formalin or to its inflammatory sequelae. Alternatively, it may reflect different processing of such nociceptive input by the central nociceptive pathways that receive input from deep cranial tissues.
A recent report provided evidence to suggest that the quiescence interphase of the formalin test is mediated by an active-hyperpolarizing inhibitory response of the affected nociceptors (Fischer et al., 2013). This data points to the possibility that the lack or reduced interphase response following cranial periosteal formalin injections are due to an inherently reduced formalin-evoked hyperpolarizing response in nociceptors that innervate the calvarial periosteum.
4.2 Mild closed head injury enhances the nociceptive responses to cranial formalin injection
Mild closed head injury influenced the behavioral nociceptive responses to cranial formalin injection in two noticeable ways. In mice subjected to trauma there was a development of a biphasic response similar that seen in the non-cranial formalin tests but with the exception that the interphase was not quiescent - during this phase the magnitude of the nociceptive behavior interphase was higher than that observed following vehicle injections. In addition, head injured mice displayed nociceptive responses at the early and late phases that were higher than those observed in the sham animals during the same time course. The early behavioral response to formalin (i.e. the first phase) is considered to be mediated by the activation of chemosensitive primary afferent nociceptive neurons that innervate the injected tissues (Fischer et al., 2013). The mechanisms underlying the generation of the more prolonged late (i.e. second) phase are also considered to have a peripheral origin (Fischer et al., 2013; McCall et al., 1996), although the contribution of activated dorsal horn neurons was also suggested (Tjolsen et al., 1992). The reestablishment of the two behavioral nociceptive phases in head injured animals could be due to an increased responsiveness of the periosteal afferents to formalin, potentially resulting from increased expression of the major sensing molecule that mediates the nociceptive response to low dose of formalin, namely the TRPA1 channel (Fischer et al., 2013; McNamara et al., 2007). The finding of a reduced nociceptive behavior during the interphase in head injured animals could point to an enhancement of the cellular mechanisms that mediates the inhibition phase in nociceptors, however, not to the same extent as seen in nociceptors innervating non-cranial tissues.
4.2 Mild closed head injury does not enhance the nociceptive responses following hindpaw formalin injection
It has been shown that some chronic post-traumatic headache patients exhibit deficits in thermal nociception in both the head and hand (Defrin et al., 2010). Such deficits were suggested to reflect central damage to the pain system, likely supraspinal pain processing pathways, which could play a role in mediating PTH. We postulated that if mild head trauma leads to similar changes in supraspinal pain processing in mice, as a result of brain injury, then it could promote changes in the nociceptive responses to injection of formalin to the hind paw. Our data however indicates that mice subjected to mild closed head injury did not show any changes in the behavioral nociceptive response to injection of a dilute formalin solution into the hindpaw. We therefore propose that following mild head injury, enhancement of peripheral cranial nociception, rather than changes in supraspinal pain processing, play a role in the initial emergence of post-traumatic headache.
4.3 Unilateral mild closed head injury promotes bilateral pro-inflammatory changes in the calvarial periosteum
Our data suggest that mild closed head injury can promote an acute pro-inflammatory response within the cranial periosteum, as judged by the increased numbers of periosteal MCs showing sign of activation (i.e. degranulation). The mechanism underlying this response is not known at present but could possibly be attributed to the mechanical trauma that the calvarial periosteum sustains, the ensuing activation of mechanosensitive periosteal afferents (Zhao and Levy, 2014) and release of factors capable of degranulating MCs (Levy, 2009). Such mechanisms could explain the responses of MCs ipsilateral to the trauma site but not the contralateral effect. A bilateral acute response of the intracranial meninges following the mechanical trauma to the skull (Roth et al., 2014) could serve as a potential underlying mechanism, in particular via the activation of mechanosensitive dural afferents. This event could subsequently lead to a bilateral neurogenic inflammation process in the periosteum, since the calvarial periosteum receives about 30% of its sensory innervation from collaterals of intracranial dural afferents (Zhao and Levy, 2014). Given that MC degranulation can increase the excitability of trigeminal nociceptors (Levy et al., 2007), we speculate that the acute degranulation of periosteal MCs following the head injury was responsible, at least in part to the enhanced nociceptive behavioral response of head injured mice to the cranial formalin injection.
Figure 4.

Unilateral closed head injury evokes an acute bilateral activation of calvarial periosteal MCs. (A and B) Representative, toluidine blue-stained whole-mounts of calvarial periosteal tissues taken 48 hours following a sham head injury showing MCs. Note in high magnification (B) the normal appearance of most of the MCs, showing packed granules with non or very little degranulation. (C and D) Toluidine blue - stained calvarial periosteal wholemount taken 48 hours following closed head injury. Note in D the appearance of many toluidine blue-stained granules outside the MCs indicating an active MC degranulation. Scale bar = 100 μm in A and C and 20 μm in B and D.
What's already known about this topic?
The mechanisms underlying the emergence of headache following traumatic head injury are not well understood.
Clinical data suggest the involvement of local injury to deep cranial tissues with the ensuing peripheral sensitization of cranial nociceptors and possibly damage to central supraspinal nociceptive pathways.
What does this study add?
Using a mouse model of mild closed head injury we found an acute enhancement in the nociceptive processing following noxious stimulation of the calvarial periosteum, a deep cranial tissue but not as a result of noxious stimulation of the hind paw, an extracranial tissue, suggesting that head trauma promotes as acute sensitization of deep cranial nociceptors, rather than damage to central pain system.
Using histology we found an evidence for an acute inflammatory reaction within the calvarial periosteum following a mild closed head trauma, suggesting a peripheral inflammatory mechanism as one mechanism underlying the sensitization of deep cranial nociceptors and the ensuing emergence of post-traumatic headache.
Acknowledgments
Source of funding: Supported by NIH grants NS077882 and AA020305 and the National Headache Foundation to Dr. Levy.
Footnotes
Conflict of interest: None to declare.
Author contribution: T.B. performed behavioral experiments, tissue processing, conducted statistical analyses and prepared figures. R.D. participated in the design of the study and provided feedback on the paper. A.H.A. conducted preliminary behavioral studies and contributed to manuscript drafting. J.Z. carried out histological studies. C.G.P contributed to the study design and manuscript drafting. D.L. conceived the studies, supervised them, performed data analysis, prepared figures and wrote the manuscript.
References
- Ahn AH, Martin E, Basbaum AI. 2008 Neuoroscience meeting Planner. Washington DC: Society for Neuroscience; 2008. A behavioral model of episodic cranial pain in mice conditions a place aversion; p. 267.215. [Google Scholar]
- Clavelou P, Dallel R, Orliaguet T, Woda A, Raboisson P. The orofacial formalin test in rats: effects of different formalin concentrations. Pain. 1995;62:295–301. doi: 10.1016/0304-3959(94)00273-H. [DOI] [PubMed] [Google Scholar]
- Cole JT, Yarnell A, Kean WS, Gold E, Lewis B, Ren M, McMullen DC, Jacobowitz DM, Pollard HB, O'Neill JT, et al. Craniotomy: true sham for traumatic brain injury, or a sham of a sham? Journal of neurotrauma. 2011;28:359–369. doi: 10.1089/neu.2010.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrin R, Gruener H, Schreiber S, Pick CG. Quantitative somatosensory testing of subjects with chronic post-traumatic headache: implications on its mechanisms. Eur J Pain. 2010;14:924–931. doi: 10.1016/j.ejpain.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Fischer M, Carli G, Raboisson P, Reeh P. The interphase of the formalin test. Pain. 2014;155:511–521. doi: 10.1016/j.pain.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Hoffman JM, Lucas S, Dikmen S, Braden CA, Brown AW, Brunner R, Diaz-Arrastia R, Walker WC, Watanabe TK, Bell KR. Natural history of headache after traumatic brain injury. Journal of neurotrauma. 2011;28:1719–1725. doi: 10.1089/neu.2011.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keidel M, Ramadan N. Acute post traumatic headache. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. Philadelphia: Lippincott, Willimas & Wilkins; 2006. pp. 863–872. [Google Scholar]
- Kosaras B, Jakubowski M, Kainz V, Burstein R. Sensory innervation of the calvarial bones of the mouse. The Journal of comparative neurology. 2009;515:331–348. doi: 10.1002/cne.22049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. Migraine pain, meningeal inflammation, and mast cells. Current pain and headache reports. 2009;13:237–240. doi: 10.1007/s11916-009-0040-y. [DOI] [PubMed] [Google Scholar]
- Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain. 2007;130:166–176. doi: 10.1016/j.pain.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas S, Hoffman JM, Bell KR, Dikmen S. A prospective study of prevalence and characterization of headache following mild traumatic brain injury. Cephalalgia. 2014;34:93–102. doi: 10.1177/0333102413499645. [DOI] [PubMed] [Google Scholar]
- McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neuroscience letters. 1996;208:45–48. doi: 10.1016/0304-3940(96)12552-0. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman A, Rosenberg A, Weizman R, Pick CG. Mild traumatic brain injury induces persistent cognitive deficits and behavioral disturbances in mice. Journal of neurotrauma. 2005;22:1003–1010. doi: 10.1089/neu.2005.22.1003. [DOI] [PubMed] [Google Scholar]
- Nampiaparampil DE. Prevalence of chronic pain after traumatic brain injury: a systematic review. JAMA. 2008;300:711–719. doi: 10.1001/jama.300.6.711. [DOI] [PubMed] [Google Scholar]
- Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher AI, Monteith TS. Epidemiology and classification of post-traumatic headache: what do we know and how do we move forward? Comment on Lucas et al., “Prevalence and characterization of headache following mild TBI”. Cephalalgia. 2014;34:83–85. doi: 10.1177/0333102413499644. [DOI] [PubMed] [Google Scholar]
- Schueler M, Messlinger K, Dux M, Neuhuber WL, De Col R. Extracranial projections of meningeal afferents and their impact on meningeal nociception and headache. Pain. 2013;154:1622–1631. doi: 10.1016/j.pain.2013.04.040. [DOI] [PubMed] [Google Scholar]
- Theeler B, Lucas S, Riechers RG, 2nd, Ruff RL. Post-traumatic headaches in civilians and military personnel: a comparative, clinical review. Headache. 2013;53:881–900. doi: 10.1111/head.12123. [DOI] [PubMed] [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. J Neurosci. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Levy D. The sensory innervation of the calvarial periosteum is nociceptive and contributes to headache-like behavior. Pain. 2014;155:1392–1400. doi: 10.1016/j.pain.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zohar O, Schreiber S, Getslev V, Schwartz JP, Mullins PG, Pick CG. Closed-head minimal traumatic brain injury produces long-term cognitive deficits in mice. Neuroscience. 2003;118:949–955. doi: 10.1016/s0306-4522(03)00048-4. [DOI] [PubMed] [Google Scholar]