Summary
Background
Posttraumatic Stress Disorder (PTSD) is a major public health concern, especially given the recent wars in Iraq and Afghanistan. Nevertheless, despite a sharp increase in the incidence of psychiatric disorders in returning veterans, empirically based prevention strategies are still lacking. To develop effective prevention and treatment strategies, it is necessary to understand the underlying biological mechanisms contributing to PTSD and other trauma related symptoms.
Methods
The “Marine Resiliency Study II” (MRS-II; October 2011–October 2013) Neurocognition project is an investigation of neurocognitive performance in Marines about to be deployed to Afghanistan. As part of this investigation, 1195 Marines and Navy corpsmen underwent a fear conditioning and extinction paradigm and psychiatric symptom assessment prior to deployment. The current study assesses (1) the effectiveness of the fear potentiated startle paradigm in producing fear learning and extinction and (2) the association of performance in the paradigm with baseline psychiatric symptom classes (healthy: n = 923, PTSD symptoms: n = 42, anxiety symptoms: n = 37, and depression symptoms: n = 12).
Results
Results suggest that the task was effective in producing differential fear learning and fear extinction in this cohort. Further, distinct patterns emerged differentiating the PTSD and anxiety symptom classes from both healthy and depression classes. During fear acquisition, the PTSD symptom group was the only group to show deficient discrimination between the conditioned stimulus (CS+) and safety cue (CS−), exhibiting larger startle responses during the safety cue compared to the healthy group. During extinction learning, the PTSD symptom group showed significantly less reduction in their CS+ responding over time compared to the healthy group, as well as reduced extinction of self-reported anxiety to the CS+ by the end of the extinction session. Conversely, the anxiety symptom group showed normal safety signal discrimination and extinction of conditioned fear, but exhibited increased baseline startle reactivity and potentiated startle to CS+, as well as higher self-reported anxiety to both cues. The depression symptom group showed similar physiological and self-report measures as the healthy group.
Discussion
These data are consistent with the idea that safety signal discrimination is a relatively specific marker of PTSD symptoms compared to general anxiety and depression symptoms. Further research is needed to determine if deficits in fear inhibition vs. exaggerated fear responding are separate biological “domains” across anxiety disorders that may predict differential biological mechanisms and possibly treatment needs. Future longitudinal analyses will examine whether poor learning of safety signals provides a marker of vulnerability to develop PTSD or is specific to symptom state.
Keywords: Extinction, Fear, PTSD, Anxiety, Military, Fear inhibition, Safety signal, Startle
1. Introduction
Posttraumatic Stress Disorder (PTSD) is a major public health concern among current and former military members, including those who have recently experienced combat in Iraq and Afghanistan (Baker et al., 2012). For instance, while most service members remain resilient following deployment, the incidence of psychiatric disorders among active-duty service members has increased by 62% since these wars began in 2001. Specifically, there has been an increase of 656% for PTSD and 226% for anxiety disorders. In addition, the cost to the Department of Defense (DoD) for treating these service members doubled between 2007 and 2012 (Blakeley and Jansen, 2013 Congressional Research Service Report). The Department of Veterans Affairs (VA) and society at large will continue to bear the cost of treating service members with chronic psychiatric issues long after these individuals are discharged from the military. According to a recent report by the Institute of Medicine, DoD prevention efforts are hampered by an insufficient empirical base (National Research Council, 2014). Identifying the underlying biological mechanisms of PTSD from other stress-related disorders is a key step in developing an evidence base on which to design more effective prevention and treatment efforts.
The “Marine Resiliency Study II” (MRS-II; October 2011–October 2013) Neurocognition project is an investigation of neurocognitive performance in Marines about to be deployed to Afghanistan. Similar to the original MRS (Baker et al., 2012), Marines were assessed in a 3.5 h test battery in which clinical assessment, self-report, and biological assays are combined with comprehensive neurocognitive assessments once before deployment and then again 3–6 months after deployment. The purpose of MRS-II is to discriminate between biological markers that predict risk/resiliency for development of combat-stress related disorders and markers associated specifically with symptom state. Here we focus on one aspect of these assessments, measurement of fear conditioning and extinction learning and its association with psychiatric symptom groups prior to deployment.
Increased responses to conditioned fear cues and reduced ability to inhibit these responses are well-known features of PTSD in civilian and combat-veteran populations (for review see VanElzakker et al., 2013). Reduced ability to inhibit fear has recently been suggested to be a potential “biomarker” specific to PTSD, with PTSD subjects exhibiting poor learning of safety signals (cues that predict absence of threat) compared to depressed subjects (Jovanovic and Norrholm, 2011; Jovanovic et al., 2009, 2010). Studies in high trait anxious participants or other anxiety disorders are inconsistent, showing either normal or reduced fear inhibition as measured by safety signal learning (Kindt and Soeter, 2014; Gazendam et al., 2013; Lissek et al., 2009). Reduced inhibition in PTSD patients is thought to reflect disruption of frontal cortical and hippocampal circuits to inhibit amygdala activation and concomitant fear responses (Admon et al., 2013; Acheson et al., 2012a,b). However, increased fear responding to conditioned cues, aversive contexts, or over-generalization of fear responses are shown across multiple anxiety disorders and thus may reflect biological processes that are shared across disorders (McTeague and Lang, 2012; Lissek et al., 2013; Grillon et al., 1998). Results are less clear however for depression, with reports of lower, normal, and higher aversive responding or fear conditioning (McTeague and Lang, 2012; Grillon et al., 2013; Robinson et al., 2012; Jovanovic et al., 2010) depending on the type of conditioned cues and aversive stimuli. Heightened fear responding may be due to increased amygdala, extended amygdala, and/or dorsal anterior cingulate activity in these disorders (Admon, 2013; Grillon, 2008). Understanding the differential patterns of fear conditioning and inhibition between symptom types will help identify specific endophenotypes for further biological interrogation across stress-related disorders (Cuthbert and Kozak, 2013; McTeague and Lang, 2012; Admon et al., 2013). Given that MRS-II is a longitudinal study, we will ultimately be able to determine in future analyses if these putatively differential phenotypes are vulnerability factors or related specifically to symptom state after trauma.
To test the hypothesis that PTSD, depression, and general anxiety symptoms may reflect distinct biological mechanisms and subsequent differential patterns of fear conditioning and inhibition abnormalities, we used a cross-sectional design to directly compare fear conditioning and extinction across participants endorsing symptoms of general anxiety, depression, and PTSD at pre-deployment. We used the fear potentiated startle (FPS) paradigm established by Norrholm et al. (2006), as this paradigm is sensitive to both the reduced fear inhibition (i.e., safety signal learning and extinction) and increased fear conditioning described in PTSD patients (Norrholm et al., 2011). This protocol uses an aversive air-puff as the unconditioned aversive stimulus. Though other fear conditioning paradigms have used aversive electrical shock as the unconditioned stimulus (i.e., Milad et al., 2007), we chose to use air puff for a number of reasons. One, use of an air puff increased the feasibility of testing such a large active duty population in a time-limited manner as it does not require initial “customization” of shock stimuli. Lack of required customization reduced setup time as well as technical difficulty. Two, we anticipated that shock stimuli would be less acceptable to study participants and to local and military institutional review boards given the special population status of active duty military. Third, this protocol uses startle reactivity as the operational measure of conditioned fear, a cross species measure of fear conditioning for translational applications in animal models, and which may be more sensitive to “automatic” or implicit fear learning compared to other measures such as skin conductance (Sevenster et al., 2014; Glover et al., 2011).
2. Methods
2.1. Participants
1195 infantry Marines and Navy Corpsmen enrolled in a longitudinal study of the health effects of deployment to Afghanistan and completed the pre-deployment assessment. Data was collected on two separate infantry battalions, identified with the assistance of Marine Corps leadership, 1–2 mo prior to deployment. The first battalion was deployed from March 2012 to October 2012, and the second battalion from September 2012 to April 2013. At the time of this collection period all Marine infantry were male, thus females did not participate. All data collection occurred on a single day, with the entire testing battery (of which only a portion is being presented here) was completed over the course of approximately 4 h. This study was approved by the institutional review boards of the University of California San Diego, VA San Diego Research Service, and the Naval Health Research Center. Written informed consent was obtained from all participants.
2.2. Fear conditioning and extinction procedure
Apparatus
Startle pulses (108 dB, 40 ms) were delivered using a San Diego Instruments (SDI, San Diego, CA, USA) SR-HLAB Electromyography (EMG) system. Sound levels were measured using continuous tones calibrated with a Quest Sound Level Meter on the A scale, coupled to the headphones with an artificial ear. The air puff was set at 250 psi and delivered via a plastic tube positioned 2.5 cm from the center of the throat. Air-puff onset was controlled by a solenoid system triggered by the same Acer laptop computer that controlled the startle stimuli. Conditioned stimuli were presented via E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA, USA) run on a Dell desktop computer with a 48 cm monitor positioned directly in front of the participant. Presentation of the stimuli by the E-Prime software was triggered by signals from the EMG system to control synchronization of conditioned, startle, and air-puff stimuli.
Eyeblink EMG responses were recorded via Ag/Ag 3 M Red Dot electrodes placed at the orbicularis oculi muscles at the left eye connected to the SDI SR-HLAB EMG system and Acer laptop computer (Acheson et al., 2013, 2012a,b). A reference electrode was placed at the mastoid bone behind the left ear. Before electrode placement, skin was cleaned with an alcohol swab and gently exfoliated with 3M electrode prep tape. All electrode resistances were <10kΩ. EMG data were recorded at a sampling rate of 1 kHz, amplified (0.5 mV electrode input was amplified to 2500 mV signal output), band-pass filtered (100–1000 Hz), rectified, and then smoothed with a 5-point rolling average. Expectancy responses were recorded on a trial-by-trial basis via the participant’s responses on a key pad linked to E-Prime software. Additional self-report responses were recorded at the end of each experimental phase via the same keypad.
Eyeblink data were scored via SR-HLAB EMG Utilities software as previously described (Acheson et al., 2012a,b). In brief, eyeblink responses were examined on a trial by trial basis at a window starting 100 ms before the startle pulse and ending 200 ms after the pulse. Only responses that peaked within 100 ms of pulse onset were scored as a startle response. Trials in which excessive baseline noise or artifact obscured the startle response were removed (2.1% of trials) and replaced with an imputed value based on the average of the immediately preceding and following trials.
Fear conditioning and extinction task
The fear conditioning and extinction protocol consisted of two discrete testing sessions or “phases”: acquisition and extinction. Before the acquisition phase the participants were instructed that one of the colored symbols predicted when the air puff would appear. Each phase began with 6 startle pulses presented in the absence of any other stimuli to stabilize startle responding. The acquisition phase consisted of eight 6-s presentations of the conditioned stimulus (CS+; either a blue or yellow circle or square, balanced across subjects) that was paired with the air puff in 75% contingency, eight 6-s presentations of a non-reinforced conditioned stimulus (CS−; also either a blue or yellow circle or square) that was never paired with the air puff, and 8 presentations of the startle stimulus in the absence of any stimuli (noise alone or “NA” trial) which served as a measure of baseline startle across the phase. The CS+ and air puff co-terminated on reinforced trials. Startle pulses were presented approximately 4 s following CS+ or CS− onset. The stimuli serving as CS+ and CS− (blue or yellow circles or squares) were randomly assigned across participants. Contingency awareness was measured using a numbered keypad to report at each CS+ and CS− trial whether or not they expected to receive the air puff. Participants responded with a “1” if they expected the air puff, “2” if they were unsure, and “3” if they did not expect the air puff. After the acquisition phase, contingency awareness was again assessed via a questionnaire asking participants which stimulus predicted the shock. Self-reported anxiety during the cues was also measured at this time, as was the subjective aversiveness of the air-puff stimuli.
After completing the acquisition phase, participants were asked to sit quietly for 5min before beginning the extinction phase. Before the extinction phase began, the subjects were told to “remember what they learned” in the previous session. The extinction phase consisted of 16 presentations of each stimulus type (CS+, CS−, and NA). No air puffs were presented during this phase. Presentations of startle pulses were delivered and ratings of air-puff expectancy were collected in the same fashion as in the acquisition phase. After this phase, participants again rated their level of anxiety during the cues. After these ratings were made, participants were disconnected from the apparatus and went on to other assessment stations (see Baker et al. (2012) for full details of Marine Resiliency Study assessment battery).
2.3. Assessment of psychiatric symptoms
Posttraumatic Stress Disorder
Post-traumatic stress symptoms were assessed using a structured diagnostic interview, the Clinician Administered PTSD Scale (CAPS; Blake et al., 1995). CAPS total scores can range from 0 to 136 and can be used as a measure of PTS symptom severity. PTSD symptom group membership was defined using the partial PTSD criteria articulated by Stein et al. (1997). Partial PTSD criteria were chosen due to the relative psychological health of an active duty Marine cohort. Criteria for assignment to the PTSD symptom group were the presence of at least 1 B symptom, 2 C symptoms, and 2 D symptoms, with minimum frequency ratings of 1 and minimum intensity ratings of 2. Inter-rater reliability in MRS was high for both the CAPS total score (intraclass correlation coefficient = .99) and for PTSD diagnosis (kappa = .714). All interviews were conducted by study personnel who were trained, certified and supervised by a licensed psychiatrist (D.G.B.; Baker et al., 2012).
Anxiety
Assignment to the anxiety symptoms group was defined as scoring in the Moderate to Severe range (>15) on the Beck Anxiety Inventory (BAI; Beck and Steer, 1993). The BAI is a reliable measure of general anxiety symptoms present within the past week, and discriminates between anxiety vs. depressive symptoms fairly well (Clark et al., 1994).
Depression
Assignment to the depression symptoms group was defined as scoring in the Moderate to Severe range (>19) on the Beck Depression Inventory 2 (BDI-2; Beck et al., 1996). The BDI-2 measures the presence of depressive symptoms within the past 2 weeks.
Trauma history
The Life Events Checklist (LEC; Gray et al., 2004) was used to assess previous trauma history. The LEC evaluates the participant’s lifetime experience of a wide range of traumatic events, including civilian traumas and combat or war-zone exposure, and further assesses whether the event directly happened to the individual, the individual witnessed the event happening to others, or whether the event was learned about second-hand. The LEC score reported here was calculated by summing all of the items scored as “happened to me” and/or “witnessed it”.
2.4. Data analysis
Final sample
Of the original 1195 Marines and Corpsmen who underwent the fear conditioning and extinction protocol, data on 21 were rendered unusable due to technical difficulties during testing. An additional 125 (10.6% of the remaining sample) were excluded from the analysis because they failed to show a CS+ response greater than baseline during the last half of the acquisition phase. This failure to potentiate above baseline suggested that the air puff was ineffective in inducing fear in these subjects that would be sufficient to support learning in these participants. Further, 35 subjects met our cutoffs for more than one symptom group and were excluded from the analysis. This approach was taken to enable comparison of relatively “pure” symptom classes on fear conditioning and extinction phenotypes. See supplemental materials Table S1 for demographic data on these excluded subjects. The remaining 1014 subjects were included in all analyses.
Startle
Startle data for the acquisition and extinction phases were analyzed as previously described in Acheson et al. (2013) by averaging responses to each stimulus type into blocks of two trials. Within each block, the NA averages were subtracted from the CS+ and CS− averages to adjust for changes in baseline startle across the session. Thus, each CS+ and CS− block represented startle above baseline for that block (e.g., (CS+) − (NA)). Thus there were 4 blocks for the CS+ and CS− during the acquisition phase, and 8 blocks for the CS+ and CS− for the extinction phase.
To compare acquisition across symptom groups, the analysis was simplified by averaging the last two blocks of the session across both CS types to create a measure of responding over the last half of the acquisition phase. To assess function of the task, acquisition phase data were initially analyzed within the healthy group only using a repeated-measures ANOVA to assess differences in response to each CS type. To assess differences by symptom group, a 2 (CS type) × 4 (symptom group) mixed ANOVA was conducted on the entire sample. Significant interactions were followed up with alpha-adjusted post hoc tests to assess Cue response differences within each symptom group. To assess symptom group differences in baseline startle, a one-way ANOVA, with appropriate post hoc tests, was conducted on the average NA trial response across the last half of the extinction phase.
Extinction phase data were analyzed by computing a measure of “% conditioned fear”. This score is similar to the “extinction retention index” originated by Milad et al. (2007, 2008) in their studies of fear extinction memory recall, which use a normalization approach to reduce confounds of differences in fear conditioning on measurement of extinction. For each subject, the maximal CS+ response during the acquisition phase is identified. A % conditioned fear is then calculated for each of the 8 extinction blocks using the following equation: 100* (CS+ response on extinction block/maximum response across acquisition blocks). For simplicity of presentation and analysis, these scores were further averaged into 4 extinction blocks consisting of 4 trials each. The first block, Early Extinction, consisted of the first 4 trials of the phase, Mid Extinction 1 trials 5–8, Mid Extinction 2 trials 9–12, and Late Extinction trial 13–16. To assess function of the task, extinction phase data were initially analyzed within the healthy group only using a repeated-measures ANOVA to assess decrease in responding across the phase. To assess differences by symptom group, a 4 (symptom group) × 4 (Extinction Block) mixed ANOVA was conducted on the entire sample. To assess symptom group differences in baseline startle response during the extinction phase, a 4 (symptom group) × 4 (Extinction Block) mixed ANOVA, with appropriate post hoc tests, was conducted on the NA responses averaged into blocks analogous to those above.
Expectancy and self-report
Expectancy responses were re-coded as: expect air puff=1, unsure = 0, do not expect air puff = −1. Expectancy responses over the last half of the acquisition phase (4 trials/stimulus type) were averaged together as with the startle data. ANOVAs were applied to assess both task effectiveness and differences by symptom group in the same manner as with the startle responses.
Expectancy responses during the extinction phase were analyzed by trial, including the last 4 trials of the acquisition phase (20 total trials). Task effectiveness was assessed using a repeated-measures ANOVA on the healthy group only. A 4 (symptom group) × 20 (trial) mixed ANOVA was used to assess differences by symptom group across the entire sample.
To assess task effectiveness on self-reported anxiety, CS type differences on post-phase questionnaires were analyzed using repeated measures ANOVA on the healthy group alone. A 2 (CS type) × 4 (symptom group) mixed ANOVA was used to assess differences across symptom groups. Task effectiveness in assessing change across phase in self-reported anxiety was assessed using a repeated-measures ANOVA in the healthy group only. Differences across phase by symptom group were assessed with 4 (symptom group) × 2 (phase) mixed ANOVA on the entire sample. In all analyses, significant interactions were followed up with two-tailed Tukey post hoc tests.
3. Results
3.1. Demographics
Sample demographics are displayed in Table 1. There were no differences across symptom groups on any demographic variable. Differences between symptom groups did emerge on the LEC [F(3,1010) = 9.03, p < .0001, partial η2 = .03], such that all symptom groups reported more trauma experience relative to healthy controls (ps< .04). However, the symptom groups did not differ from one another. Two subjects were taking psychiatric medication for reasons other than smoking cessation or sleep (1 in the PTSD symptom group and 1 in the anxiety symptom group). Both of those subjects reported taking fluoxetine at unknown dosages. As expected from our selection criteria, the symptom groups had significantly higher scores on their respective assessment measures relative to the other groups (Table 1; omnibus tests F(3,1010) > 129.55, ps< .0001; ps< .05 for comparisons vs. reference group). All symptom groups had higher levels of PTSD, anxiety and depression symptoms compared to controls healthy controls (ps< .05).
Table 1.
Demographics and symptom measures.
| Symptom group |
||||
|---|---|---|---|---|
| Healthy | PTSD | Anxiety | Depression | |
| N | 923 | 42 | 37 | 12 |
| Age (SD) | 22.23 (2.81) | 22.63 (4.08) | 22.4 (3.27) | 21.38 (2.33) |
| Months in the military (SD) | 31.29 (26.18) | 39.5 (43.89) | 32.7 (28.74) | 31 (29.64) |
| Education | ||||
| <H.S. | 3.3% | 2.4% | 2.7% | 8.3% |
| H.S. | 69.3% | 76.2% | 73% | 91.7% |
| Some college | 25% | 21.4% | 21.6% | 0% |
| B.A. | 2.4% | 0% | 2.7% | 0% |
| Post-graduate | 0% | 0% | 0% | 0% |
| Rank | ||||
| Junior enlisted | 71.3% | 76.2% | 78.4% | 91.7% |
| NCO | 27.5% | 23.8% | 18.9% | 8.3% |
| Officer | 1.2% | 0% | 2.7% | 0% |
| Race | ||||
| White | 87.4% | 85.7% | 83.3% | 83.3% |
| African-American | 3.7% | 0% | 0% | 0% |
| Other | 8.9% | 14.3% | 16.2% | 16.6% |
| Ethnicity | ||||
| Not Hispanic or Latino | 75.8% | 64.3% | 67.5% | 75% |
| Hispanic or Latino | 24.2% | 35.7% | 32.4% | 25% |
| Marital status | ||||
| Single, never married | 68.5% | 69% | 75.7% | 75% |
| Married | 29.3% | 28.6% | 21.6% | 25% |
| Divorced | 1.4% | 2.4% | 0% | 0% |
| Separated | 0.9% | 0% | 2.7% | 0% |
| Pathology measures (SD) | ||||
| CAPS total score | 9.66a (9.34) | 43.74 (11.29) | 17.95a (10.91) | 27.83a (12.06) |
| BAI total score | 2.87a (4.03) | 4.4a (5.54) | 20.41 (5.45) | 6.67a (4.92) |
| BDI-2 total score | 3.89a (4.19) | 9.86a (5.43) | 9.65a (5.44) | 24.17 (3.33) |
| LEC score | 4.16 (2.80) | 5.93b (3.60) | 5.54b (3.12) | 5.92b (2.27) |
p < .05 for comparisons vs. category reference group (i.e., PTSD group reference for CAPS score comparisons).
p < .05 vs. healthy.
3.2. Overall task effectiveness
3.2.1. Acquisition
Startle
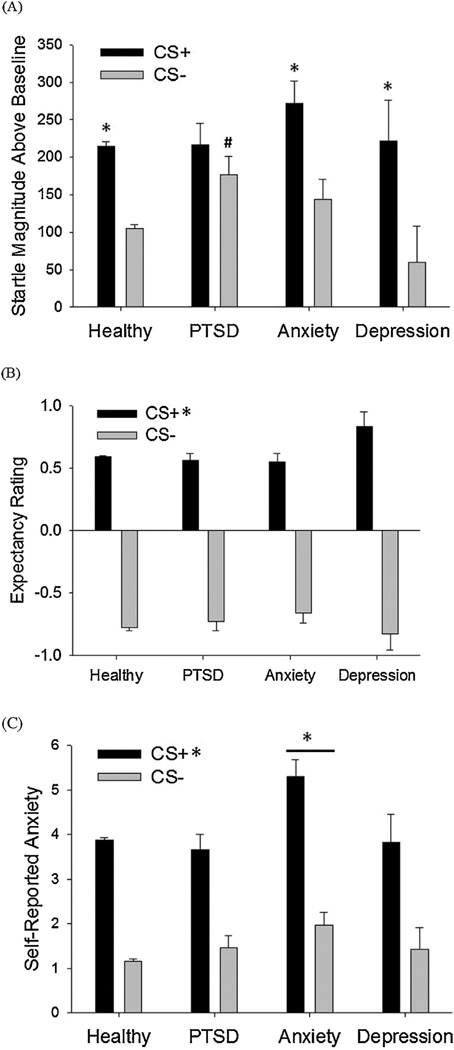
As expected, startle responses during the Acquisition phase showed a significant effect of Cue type, with the CS+ response being elevated relative to the CS−, indicating successful differential fear conditioning [Fig. 1A, F(1,918) =475.14, p < .0001, partial η2 = .34].
Figure 1.
(A) Potentiated startle magnitudes across the last half of the acquisition phase by symptom group. *p < .05 for CS+ vs. CS− comparisons. #p < .05 for PTSD symptoms vs. healthy comparison. (B) Expectancy ratings across the last half of the acquisition phase by symptom groups. *p < .05 for the CS+ vs. CS− main effect. (C) Self-reported anxiety by symptom groups following the acquisition phase. *p < .05 for CS+ vs. CS− main effect and anxiety symptoms vs. healthy comparison.
Expectancy and self-report
For expectancy ratings, participants correctly identified the CS+ as predictive of the shock [Fig. 2A; F(1,913) = 3916.39, p < .0001, partial η2 = .811]. On a 1 (expect air puff) to −1 (do not expect air puff) scale, participants averaged a 0.59 rating for the CS+ and a −0.78 rating for the CS−.
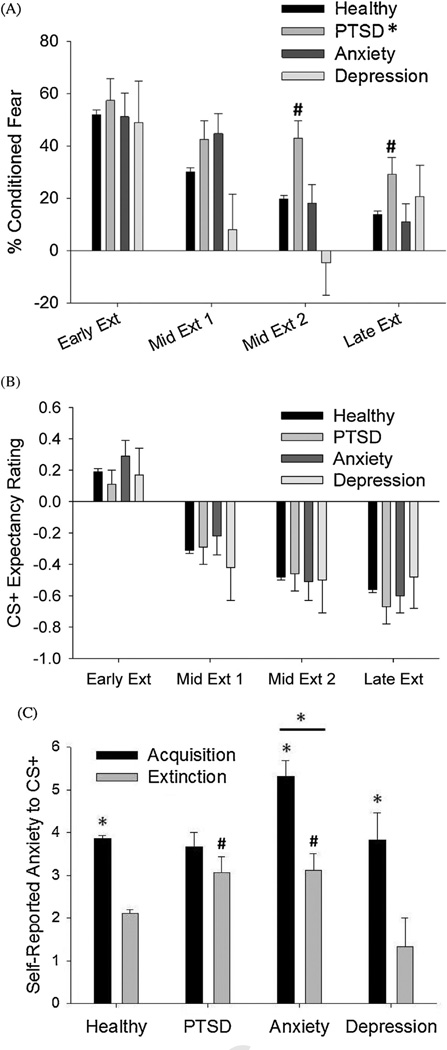
Figure 2.
(A) % acquisition response retained across the extinction phase by symptom group. *p < .05 for PTSD symptoms vs. healthy comparison. #p < .05 for exploratory comparisons vs. healthy controls. (B) CS+ expectancy ratings across the entire extinction phase. Ratings have been combined into 4-trial blocks for clarity. (C) Self-reported anxiety following the acquisition and extinction phases by symptom group. *p < .05 for comparisons across phase and for the anxiety symptoms vs. healthy comparison. #p < .05 for PTSD and anxiety symptoms vs. healthy comparisons within the extinction phase.
On the post-phase questionnaire, 88.9% of participants correctly identified the CS+ as predictive of the air puff. 6.7% of participants were not sure which CS predicted the air puff, and 3.1% misidentified the CS− as predictive of the air puff. Overall, participants assigned the air puff an average aversiveness rating of 2.31 out of 5 (SD = 1.02). Participants rated higher levels of subjective anxiety in the presence of the CS+ relative to the CS−, again indicative of differential fear conditioning [Fig. 3A; F(1,911) = 1298.43, p < .0001, partial η2 = .588].
3.2.2. Extinction
Startle
As expected, percentage of conditioned fear (normalized to the fear levels displayed in the acquisition phase) decreased significantly across the phase, demonstrating successful fear extinction [Fig. 2A; F(3,2751) = 182.87, p < .0001, partial η2 = .166].
Expectancy and self-report
Expectancy ratings to the CS+ decreased significantly across the late acquisition and extinction phases [Fig. 2B; F(19,16682) = 573.56, p < .0001, partial η2 = .395]. From the acquisition to extinction phases, post-phase ratings of anxiety to the CS+ decreased significantly [Fig. 3B; F(1,902) = 529.15,p < .0001, partial η2 = .37].
3.3. Comparison of task performance between psychiatric symptom groups
3.3.1. Acquisition
Baseline startle
There was a significant difference between symptom groups in average baseline startle during the last half of the acquisition phase [F(3,1010) = 3.05, p < .03, partial η2 = .009], such that the anxiety symptom group had a higher magnitude of startle relative to healthy controls (p < .009). No other symptom group differed from healthy controls.
Startle potentiation
When participants meeting criteria for inclusion in a symptom group were examined, a significant symptom group × Cue type interaction emerged [Fig. 1A; F(3,1005) = 3.4, p < .02, partial η2 = .01]. Post hoc tests revealed that responding to the CS+ was significantly higher than responses to the CS− for the healthy, anxious, and depressed symptom groups (ps<.001), but not for the PTSD symptom group (p < .09) suggesting reduced differential fear conditioning in the PTSD symptom group. This deficit in differential conditioning was driven by higher CS− responses in the PTSD symptom group relative to the healthy group (p < .004). In contrast, the anxiety symptom group exhibited a trend for increased CS+ responding (p < 0.06) and no significant differences in CS− responses compared to healthy controls. Maximum CS+ responding was also calculated across the groups, and the anxiety symptom group showed significantly larger maximum CS+ responses compared to the healthy group [supplemental Fig. 1; F(3,1010) = 2.73, p < .05, partial η2 = .008; anxiety symptoms vs. healthy p < .02]
Expectancy and self-report
For expectancy ratings, there was no symptom group × Cue type interaction [Fig. 2A; F(3,1000) = 1.62, ns], nor was there an overall effect of symptom group [F(3,1000) < 1.0, ns]. For self-reported anxiety, there was a significant effect of symptom group [Fig. 3A; F(3,997) = 5.78, p < .001, partial η2 = .017] with anxious subjects reporting higher levels of anxiety in response to both cues (p < .001). There was no symptom group × Cue type interaction [F(3,997) = 1.65, ns].
3.3.2. Extinction
Baseline startle
There was a trend toward differential responding between symptom groups across the extinction phase [F(3,1010) = 2.09, p < .1, partial η2 = .006], again with the anxiety symptom group trending toward higher response relative to healthy controls (p < .1).
Startle potentiation
A significant main effect of symptom group was apparent on %conditioned fear during the extinction phase [F(3,1005) = 3.05, p < .03, partial η2 = .009], such that the PTSD symptom group maintained a higher level of conditioned fear across the entire session compared to the healthy controls (p < .006). There was also a trend for a block × symptom group interaction [Fig. 2A; F(9,3015) = 1.66, p < .1, partial η2 = .005]. Exploratory post hoc analyses at each block showed that the PTSD symptom group maintained a higher level of conditioned fear relative to healthy controls at both the Mid Extinction 2 and Late Extinction blocks (ps < .05). The anxiety symptom group showed a trend toward higher responding relative to controls during Mid Extinction 1 (p < .07), however this trend was not apparent at the later extinction blocks. The depression symptom group did not differ from healthy controls.
Expectancy and self-report
Expectancy ratings to the CS+ did not vary by symptom group across the phase [Fig. 2B; F(45,14505) = 1.33, ns], nor was there a main effect of symptom group [F(3,967)< 1.0, ns]. For self-reported anxiety, there were significant differences in change across phases by symptom group [Fig. 3B; F(3,988) =4.24, p < .01, partial η2 = .013], such that all groups showed significant reductions across phase (ps < .05) with the exception of the PTSD symptom group. The PTSD and anxiety symptom groups had higher responses to the CS+ during the extinction phase relative to the healthy group (ps<.02). In addition, there was a significant main effect of symptom group, with the anxiety symptom group showing higher ratings overall relative to the healthy group [F(3,988) = 5.12, p < .002, partial η2 = .015].
4. Discussion
As expected, the conditioning paradigm was effective in producing conditioned fear learning and subsequent extinction learning in our active-duty Marine and Navy volunteers. Psychiatrically healthy participants acquired differential fear-potentiated startle and self-reported anxiety responses to the CS+ vs. the CS− and showed contingency awareness (expectancy ratings). Across the extinction phase, when the air puff was absent, responses to the CS+ decreased in terms of both potentiated startle and self-reported anxiety. Expectancy ratings showed intact contingency learning across extinction as well. Successful learning in this paradigm enables comparisons to be made in the learning patterns among the various psychiatric symptom groups.
Differential patterns of learning performance emerged between psychiatric symptom groups. The PTSD symptom group was unique in failing to show a differential potentiated startle response to CS+ and CS− at the end of fear acquisition. This failure was due to PTSD symptom group subjects maintaining a relatively high startle response to the CS−. The observation of high startle responses to the CS− is in line with existing research showing that individuals with PTSD have difficulty learning to inhibit startle responses in the presence of a safety signal (Jovanovic et al., 2009, 2010). Though not explicitly termed “safety signal” in the current paradigm, presentation of the CS− effectively signals the absence of the air puff, or safety. Interestingly, the participants in the PTSD symptom group showed intact contingency awareness in the expectancy ratings, as well as intact discrimination learning as assessed by self-reported anxiety. These findings suggest a “disconnect” between the participant’s explicit experience and automatic physiological responses to the safety cue (i.e., potentiated startle).
Across the extinction phase, the PTSD symptom group maintained potentiated startle to the CS+ overall relative to the healthy group. The finding that conditioned fear responses were maintained throughout extinction supports existing research suggesting a disruption in fear extinction learning and recall in PTSD symptom group subjects relative to healthy controls (Norrholm et al., 2011; Milad et al., 2008; Wessa and Flor, 2007; Orr et al., 2000; Peri et al., 2000). This greater maintenance of conditioned fear was also apparent in the self-report of anxiety in response to the CS+, which remained relatively unchanged in the PTSD group after extinction training, unlike the other groups. Again, the PTSD symptom group showed normal explicit learning that the CS+ no longer predicted the US (as evidenced by the expectancy ratings across the extinction session), further supporting a disconnect between explicit contingency awareness and fear expression. Thus the current findings of deficient inhibition of potentiated startle to a safety cue and reduced extinction of physiological and emotional fear responses in the presence of intact contingency awareness supports the theory that PTSD is characterized by a failure to inhibit automatic, physiological fear responses. This failure of inhibition is observed even though the subject is explicitly aware of a lack of threat or danger.
The anxiety symptom group showed significantly higher baseline startle responding and higher CS+ potentiation compared to the healthy group. This group also reported significantly higher anxiety to both CS+ and CS− after acquisition relative to the healthy group. The finding that CS+/− discrimination is normal in participants with high generalized anxiety symptoms is in line with other report that high trait anxiety participants exhibit normal CS+/CS− discrimination (Kindt and Soeter, 2014; Gazendam et al., 2013). The present findings of higher self-reported anxiety to the conditioned cues are also in line with past reports using a similar protocol (Gazendam et al., 2013). During extinction training, the anxiety symptom group successfully extinguished ‘both potentiated startle and US expectancy to the CS+. They also successfully extinguished self-reported anxiety to the CS+, however overall responding remained high compared to the other groups. Taken together, this pattern of results is suggestive of greater explicit anxiety responses during aversive anticipation in this group while fear inhibition and discrimination processes are relatively normal.
The depression symptom group showed response patterns in all measures that were indistinguishable from healthy controls. The normal fear inhibition and potentiated startle in the depression group as assessed by safety signal learning and extinction is in line with previous studies (Jovanovic et al., 2010, 2012). The present results differ however from a recent study in major depression patients in a task which incorporates both predictable and unpredictable aversive stimuli (Grillon et al., 2013). In this task, MDD patients exhibited higher baseline startle reactivity as well as greater potentiation during the cue that was predictive (100% contingency) of an aversive event. The increased startle potentiation was associated with symptom chronicity as well as severity. The different results across this study and the present study are unlikely due to differences in symptom severity (mean BDI 26 vs. 29 for present and previous studies, respectively) or treatment (both studies used unmedicated participants). It is possible that the difference between the Grillon et al. study and the present study are due to differences in the chronicity of symptoms, gender demographics (mixed vs. all male sample respectively) and comorbid anxiety (high vs. relatively low respectively). The lack of significant differences in the present study must also be interpreted with caution given the relatively small sample size in this group (N= 12).
The present results suggest differential performance between PTSD and anxiety symptom groups, with general anxiety symptoms being more associated with exaggerated fear responses and PTSD symptoms being specifically associated with a failure to appropriately inhibit fear responses to safety signals and reduced extinction. This differential pattern of results is suggestive of differences at the neurocircuit level. The higher overall responding in the anxiety symptom group may reflect hyperactivity in emotion-generating limbic circuits, consistent with the neuroimaging evidence for heightened amygdala activation to negative provocation in subjects with generalized anxiety (i.e., Rauch et al., 2003). While PTSD has also been associated with limbic system hyperactivity (Shin et al., 2006), neuroimaging studies have shown more pronounced findings of hypoactivation in structures responsible for inhibition of the limbic system, specifically the medial prefrontal cortex (mPFC) and the rostral and dorsal regions of the anterior cingulate cortex (Etkin and Wager, 2007). Further, Milad et al. (2007, 2008) have demonstrated that individuals with PTSD exhibit reduced ability to recall fear extinction (or fear inhibition) 24 h after initial learning, an ability that is dependent upon mPFC activation. Reduced activity of ventromedial prefrontal cortex is also associated with increased potentiation to CS− and reduced extinction of CS+ (Jovanovic et al., 2013). Thus this pattern of hypoactivation in fear inhibition circuits may be reflected in the current results of relatively normal magnitude of fear responses but poor safety-signal learning and reduced extinction in PTSD symptom groups. The present findings also raise the possibility that this task could identify, via differential patterns of response (exaggerated fear response vs. impaired fear inhibition), those who are neuro-biologically at risk for developing a certain class of pathology post-trauma. Previous research has suggested that impaired fear extinction may be a marker for increased risk of developing PTSD following a trauma (Guthrie and Bryant, 2006; Pole et al., 2009; Lommen et al., 2013). Future studies may examine whether these phenotypes predict differential treatment responses to pharmacological or behavioral therapies.
Some limitations of the current study must be acknowledged. First, the paradigm was not effective in producing fear-potentiated startle in ∼11% of the study participants tested. While this failure resulted in a reduction of sample size, the excluded participants did not appear to differ systematically from the study volunteers as a whole (supplemental Table 1). Second, the study was conducted on a highly screened cohort of active duty Marines and Navy corpsmen, which limited the number of participants displaying psychiatric symptoms of sufficient intensity for inclusion in the symptom groups. Therefore, the number of participants included in the symptom groups is relatively small, particularly the depression group. It is possible that low power may have contributed to the inability to detect significant differences in between the depression and healthy control group. However it is important to note that the present findings of normal fear inhibition and extinction in the depression symptom group replicate previous studies with greater subject numbers (Jovanovic et al., 2009, 2010). Third, the current study did not explicitly examine the effects of trauma or deployment history on fear conditioning and extinction performance, or on psychiatric outcomes. All symptom groups exhibited significantly higher trauma burden severity (i.e., LEC scores) compared to the healthy group, however no differences were detected between PTSD, anxiety and depression symptom groups, suggesting that trauma burden alone is unlikely to explain differences in task performance across the symptom groups. Future analyses will investigate the role of these variables in influencing task performance, as well as their interaction with psychiatric symptoms. Finally, while the symptom groups had significantly higher scores on their respective assessment measures relative to the other groups (Table 1), all symptom groups also differed from healthy controls across all measures. This elevation across symptom measures speaks to the difficulty of achieving “pure” symptom categories given the large amount of overlap in phenomenology among these conditions. However, the current paradigm was effective in discriminating between symptom classes based on severity, and as whole it appears that the current results have captured differences between groups characterized by predominant symptoms unique to anxiety and PTSD. A final limitation is the use of categorical cutoffs for our symptom groups, which are necessarily arbitrary. However, treating our symptom indicators as quantitative is problematic given our largely healthy sample. Future research in other naturalistic samples may wish to examine quantitative relationships between fear learning indices and symptoms of psychopathology.
In sum, the fear conditioning and extinction paradigm appears to function as anticipated in this active-duty Marine/Navy cohort. Further, the current study represents the first direct comparison of fear conditioning and extinction performance across healthy control, PTSD, anxiety, and ‘ depression symptom groups in a fairly homogenous sample. The results point to differential biobehavioral “signatures” associated with distinct symptom groups and may lead toward development of objective markers for classification of psychiatric dysfunction. Future research in this sample will continue to characterize the nature of fear learning abnormalities and examine whether poor learning of safety signals provides a marker of vulnerability to develop PTSD or is specific to symptom state.
Supplementary Material
Acknowledgements
This work received support from the Navy Bureau of Medicine and Surgery N62645-11-C-4037 and the VA Center of Excellence for Stress and Mental Health (DGB, VBR, MAG). Additional support came from a Brain and Behavior Research Foundation (NARSAD) Young Investigator Award (DTA), and NIH MH042228 (MAG and VBR). We would like to acknowledge additional contributions from the MRS administrative core (Anjana Patel, Andrew De La Rosa, and Elin Olsson) as well as the numerous clinician-interviewers and data collection staff who contributed to the project. We also wish to thank the Marine and Navy Corpsmen volunteers who participated in the study.
In the past three years, MAG has received consulting compensation from Abbott, Addex, Cerca, Dart, Lund-beck/Otsuka, Neurocrine, Omeros, Sunovion, Takeda, and Teva, and holds an equity interest in San Diego Instruments. MAG also has research grant support from Intracellular Therapeutics, Johnson & Johnson, NIDA, NIMH, and the U.S. Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center. VBR has received grant funding from Janssen and Omeros.
Footnotes
Conflict of interest
The rest of the authors report no conflicts of interest associated with the current manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2014.09.030.
References
- Acheson DT, Gresack JE, Risbrough VB. Hippocam-pal dysfunction effects on context memory: possible etiology for posttraumatic stress disorder. Neuropharmacology. 2012a;62:674–685. doi: 10.1016/j.neuropharm.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Stein MB, Paulus MP, Geyer MA, Risbrough VB. The effect of pregabalin on sensorimotor gating in ‘low’ gating humans and mice. Neuropharmacology. 2012b;63:480–485. doi: 10.1016/j.neuropharm.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Feifel D, de Wilde S, McKinney R, Lohr J, Risbrough VB. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology (Berl.) 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn. Sci. 2013;7:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM, et al. Predictors of risk and resilience for posttraumatic stress disorder among ground combat Marines: methods of the Marine Resiliency Study. Prev. Chronic Dis. 2012;9:110134. doi: 10.5888/pcd9.110134. http://dx.doi.org/10.5888/pcd9.110134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt Brace and Company; 1993. [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a clinician-administered PTSD scale. J. Trauma. Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Blakeley K, Jansen DJ. Post-traumatic stress disorder and other mental health problems in the military: Oversight issues for congress. Congressional Research Service Report; 2013. [Google Scholar]
- Clark DA, Steer RA, Beck AT. Common and specific dimensions of self-reported anxiety and depression: implications for the cognitive and tripartite models. J. Abnorm. Psychol. 1994;103:645–654. [PubMed] [Google Scholar]
- Cuthbert BN, Kozak MJ. Constructing constructs for psychopathology: the NIMH research domain criteria. J. Abnorm. Psychol. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazendam FJ, Kamphuis JH, Kindt M. Deficient safety learning characterizes high trait anxious individuals. Biol. Psychol. 2013;92:342–352. doi: 10.1016/j.biopsycho.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, Ressler KJ, Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depress. Anxiety. 2011;28:1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Litz BT, Hsu JL, Lombardo TW. Psychometric properties of the Life Events Checklist. Assessment. 2004;11:330–341. doi: 10.1177/1073191104269954. [DOI] [PubMed] [Google Scholar]
- Grillon C. Evoked amygdala responses to negative faces revealed by adaptive MEG beamformers. Brain Res. 2008;1244:103–112. doi: 10.1016/j.brainres.2008.09.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biol. Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Franco-Chaves JA, Mateus CF, Ionescu DF, Zarate CA. Major depression is not associated with blunting of aversive responses: evidence for enhanced anxious anticipation. PLoS ONE. 2013 doi: 10.1371/journal.pone.0070969. http://dx.doi.org/10.1371/journal.pone.0070969. [DOI] [PMC free article] [PubMed]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom. Med. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Front. Behav. Neurosci. 2011;5:1–8. doi: 10.3389/fnbeh.2011.00044. http://dx.doi.org/10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos AM, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress. Anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M. Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology. 2012;62:695–704. doi: 10.1016/j.neuropharm.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M. Fear inhibition in high trait anxiety. PLoS ONE. 2014 doi: 10.1371/journal.pone.0086462. http://dx.doi.org/10.1371/journal.pone.0086462. [DOI] [PMC free article] [PubMed]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, et al. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behav. Res. Ther. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C. Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.025. http://dx.doi.org/10.1016/j.biopsych.2013.07.025. [DOI] [PMC free article] [PubMed]
- Lommen MJ, Engelhard IM, Sijbrandij M, van den Hout MA, Hermans D. Pre-trauma individual differences in extinction learning predict posttraumatic stress. Behav. Res. Ther. 2013;51:63–67. doi: 10.1016/j.brat.2012.11.004. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress. Anxiety. 2012;29:264–281. doi: 10.1002/da.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall of fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Preventing Psychological Disorders in Service Members and their Families: An Assessment of Programs. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol. Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J. Abnorm. Psychol. 2000;109:290–298. [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biol. Psychiatry. 2000;2000(47):512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective prediction of posttraumatic stress disorder symptoms using fear potentiated auditory startle responses. Biol. Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Wright CI. Neuroimaging studies of amygdala function in anxiety disorders. Ann. N. Y Acad. Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am. J. Psychiatry. 2012;169:152–159. doi: 10.1176/appi.ajp.2011.11010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M. Fear conditioning of SCR but not the startle reflex requires conscious discrimination of threat and safety. Front. Behav. Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00032. http://dx.doi.org/10.3389/fnbeh.2014.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann. N. Y Acad. Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Stein MB, Walker JR, Hazen AL, Forde DR. Full and partial posttraumatic stress disorder: Findings from a community survey. Am. J. Psychiatry. 1997;154:1114–1119. doi: 10.1176/ajp.154.8.1114. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren KM, Davis CF, Dubois S, Shin LM. From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2013;13:S1074–S7427. doi: 10.1016/j.nlm.2013.11.014. http://dx.doi.org/10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. Am. J. Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.