Abstract
Objectives
To evaluate the association of obesity with a novel biomarker of subclinical myocardial injury, cardiac troponin T measured with a new high sensitivity assay (hs-cTnT), among adults without clinical cardiovascular disease (CVD).
Background
Laboratory evidence suggests a relationship between obesity andmyocardial injury that may play a role in the development of heart failure (HF), but there is limited clinical data regarding this association.
Methods
We evaluated 9,507 participants in the Atherosclerosis Risk in Communities Study without baseline CVD (Visit 4, 1996-1999). We assessed the cross sectional association of body-mass index (BMI) with high (≥14 ng/L) and measurable (≥3 ng/L) hs-cTnT levels after multivariable regression. We further evaluated the independent and combined associations of BMI and hs-cTnT with incident HF.
Results
Higher BMI was independently associated with a positive, linear increase in the likelihood of high hs-cTnT, with severe obesity (BMI >35 kg/m2) associated with an odds ratio of 2.20 (95% CI: 1.59-3.06) for high hs-cTnT after adjustment. Over 12 years of follow-up, there were 869 incident HF events. Obesity and hs-cTnT were both independently associated with incident HF, and individuals with severe obesity and high hs-cTnT had a greater than 9-fold higher risk of incident HF (HR 9.20 [95% CI: 5.67-14.93]) than individuals with normal weight and undetectable hs-cTnT.
Conclusions
Among individuals without CVD, higher BMI has an independent, linear association with subclinical myocardial injury, as assessed by hs-cTnT levels. Obesity and hscTnT provide independent and complementary prognostic information regarding the risk of incident HF.
Keywords: obesity, heart failure, epidemiology, troponin
Introduction
Obesity is a known risk factor for the development of heart failure (HF)(1, 2), but the mechanisms underlying the relationship between obesity and HF are incompletely understood(3). Conditions closely linked to obesity, such as hypertension (HTN) and diabetes mellitus (DM), only partially explain the association between obesity and incident HF(4). Obesity is independently associated with abnormalities of myocardial contractile function and relaxation and abnormal cardiac remodeling(5, 6), changes which precede clinical HF(1). Laboratory studies suggest that myocardial injury, related to the endocrine and inflammatory effects of adipose tissue, may be one pathway by which obesity leads to myocardial dysfunction and subsequent HF(5, 7, 8).
A novel biomarker of subclinical myocardial injury that may provide further insight into the relationship between obesity and HF is cardiac troponin T measured via a new high sensitivity assay (hs-cTnT)(9). Novel high sensitivity assays can detect troponin in the circulation at levels far below the detection limits of conventional assays used in clinical practice. Previous studies among asymptomatic individuals have found that minute elevations in troponin detected with these high sensitivity assays are robust predictors of future HF and mortality, and to a lesser extent, incident coronary heart disease (CHD)(10, 11). Despite increasing evidence regarding the adverse effects of excess adiposity on the myocardium, the relationship between obesity and hs-cTnT among asymptomatic individuals, and the implications of this relationship for the development of HF, has not yet been investigated.
The objective of this study was to test the hypothesis that obesity is independently associated with subclinical myocardial injury, as assessed by hs-cTnT, in a population-based study of individuals free of clinical cardiovascular disease (CVD) at baseline. We further evaluated the independent and combined associations of obesity and hs-cTnT with incident HF, to assess whether these variables provided complementary prognostic information regarding HF risk.
Methods
The Atherosclerosis Risk in Communities (ARIC) Study is a prospective, population-based cohort of 15,792 individuals enrolled from four U.S. communities: Washington County, Maryland; Jackson, Mississippi; Forsyth County, North Carolina; and the suburbs of Minneapolis, Minnesota. The study protocol has been described previously(12). Participants were recruited between 1987 and 1989, and examined at baseline and at three subsequent visits, at approximately 3 year intervals. A fifth study visit is presently ongoing. ARIC Visit 4 (1996-1998), at which hs-cTnT measurements were available for all participants, was the baseline for this analysis.
Of the 11,492 participants who attended ARIC Visit 4, we excluded individuals with a history of self-reported CVD at Visit 1 or a CVD event (including prior hospitalization related to HF, validated non-fatal myocardial infarction or coronary revascularization, or silent myocardial infarction by ECG criteria) at or prior to Visit 4 (N=1,572), a small number of individuals not of black or white race (N=31), and those participants with body-mass index (BMI) < 18.5 kg/m2 (N=74). We also excluded participants missing data on prior CVD (N=214), hs-cTnT (N=242) or anthropometric measurements (N =16), for a final study population of 9,507 individuals. All participants provided informed consent, and the study protocol was approved by the institutional review boards associated with each ARIC field center.
Information on BMI and all covariates of interest were obtained by history, physical and laboratory examination at Visit 4. BMI was calculated from measured height and weight and categorized as normal weight (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese (30-34.9 kg/m2) or severely obese (≥ 35 kg/m2). Smoking status was categorized as current, former or never smoker and self-reported alcohol intake was calculated in grams per week. HTN was defined as having a prior physician diagnosis of HTN, using anti-hypertensive medications, or having a systolic blood pressure of ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg on examination. Individuals were classified as having DM if they had a self-reported history of DM, used hypoglycemic medications, had a fasting blood glucose of ≥ 126 mg/dl or had a non-fasting blood glucose of ≥ 200 mg/dl. Total cholesterol (TC), high density lipoprotein cholesterol (HDL-C) and triglycerides were measured using enzymatic assays, and LDL-C was calculated using the Friedewald equation (TC – HDL-C – [triglycerides/5]) for participants with triglycerides ≤ 400 mg/dl.
Hs-cTnT was measured in 2010 from thawed plasma samples initially obtained at Visit 4 and stored at −80 degrees Celsius, using the Elecsys Troponin T high-sensitivity assay (Roche Diagnostics, Indianapolis, IN) on an automated Cobas e411 analyzer. The primary outcome in cross-sectional analyses was high hs-cTnT levels, defined as ≥14 ng/L, corresponding to the 99th percentile for hs-cTnT in a healthy reference population as provided by the assay manufacturer, a cutpoint used in prior analyses(11, 13). The secondary outcome was measurable hs-cTnT, which reflected levels of hs-cTnT greater than the assay measurement limit of 3 ng/L. The between assay coefficients of variation for control materials with mean hs-cTnT concentrations of 2,378 ng/L and 29 ng/L were 2.6% and 6.9%, respectively.
In prospective analyses, the outcome of interest was incident HF, defined as the first hospitalization or death related to HF occurring after Visit 4, with follow-up through January 2009. Participants were called on a yearly basis to obtain information regarding hospitalizations, and vital records were examined for all deaths. Hospitalizations and deaths due to incident HF were defined by HF discharge codes (ICD-9 code 428 for hospitalizations and deaths early during follow-up and ICD-10 code I50 for later deaths). HF events after 2004 were additionally adjudicated by an expert panel.
Statistical analysis
Differences in demographic, clinical and laboratory characteristics among individuals in different BMI categories were assessed using the chi-squared test for categorical variables and analysis of variance for continuous variables.
In cross-sectional analyses, we evaluated the association of BMI with elevated hs-cTnT levels at Visit 4. We used multivariable logistic regression to assess the relationship of higher BMI categories with high (≥14 ng/L) and measurable (≥3 ng/L) hs-cTnT levels, with normal weight (18.5-24.9 kg/m2) as the reference. Using logistic regression, we also evaluated the continuous association of BMI (per kg/m2) with high hs-cTnT levels using restricted cubic splines, centered at the median BMI. Model 1 was adjusted for age; Model 2 was adjusted for the variable in Model 1 plus sex, race, and smoking status; Model 3 was adjusted for all variables in Model 2 plus DM, HTN, LDL-C, HDL-C, triglycerides, alcohol intake, N-terminal pro-brain natriuretic peptide (NT-proBNP) and estimated GFR. We conducted additional analyses stratified by age (older or younger than 65 years), gender and race, and tested for interactions of these demographic variables with BMI on the outcome of high hs-cTnT.
In prospective analyses, we examined the associations of obesity and hs-cTnT with incident HF. We used Cox proportional hazards models to estimate the adjusted hazard ratios and corresponding 95% confidence intervals for the prospective association between BMI categories at baseline and risk of HF after adjustment for baseline risk factors, utilizing the same modeling approach used for the cross-sectional analyses. To examine the combined effects of BMI and hs-cTnT on incident HF, we conducted analyses stratified by hs-cTnT levels at baseline (undetectable (<3 ng/L), measurable (3-13 ng/L) and high (≥14 ng/L)). We tested for statistical interactions on the multiplicative scale between BMI category and hs-cTnT level on the outcome of incident HF. For analyses modeling hs-cTnT as a continuous variable, spline models were constructed with the value of 1.5 ng/L imputed for those with undetectable hs-cTnT as has been done in prior analyses (10). We performed sensitivity analyses limited to those individuals with adjudicated HF events. We conducted additional sensitivity analyses substituting waist circumference (WC), in quartiles, for BMI as the metric for adiposity.
Statistical analyses were performed with Stata version 10.1. All reported p values are 2-sided.
Results
Individuals in higher BMI categories were slightly younger, and more likely to be female and African-American (Table 1). Mean alcohol intake and the proportion of current smokers was lower in higher BMI categories. As expected, higher BMI category was also associated with a significantly higher prevalence of HTN and DM, as well as with higher triglycerides and lower HDL-C. Severely obese individuals had a slightly higher mean estimated GFR. Higher BMI was also associated with lower levels of NT-proBNP.
Table 1. Baseline Characteristics of the ARIC Study Population at Visit 4 (1996-1999), Stratified by BMI Category.
| Normal Weight N=2,448 |
Overweight N=3,800 |
Obese N=2,118 |
Severely Obese N=1,141 |
p value | |
|---|---|---|---|---|---|
| Mean Age, in years (SE) | 62.9 (0.1) | 62.7 (0.1) | 62.4 (0.1) | 61.6 (0.2) | <0.0001 |
| Female – % | 64.6 | 51.0 | 55.0 | 74.2 | 0.003 |
| African American – % | 13.5 | 20.2 | 27.2 | 33.4 | <0.001 |
| Current Smokers – % | 21.5 | 14.1 | 9.8 | 8.7 | <0.001 |
| Mean alcohol Intake, in grams/week | 39.2 (1.7) | 36.9 (1.4) | 28.8 (1.8) | 15.8 (1.5) | <0.0001 |
| Mean systolic blood pressure, in mmHg (SE) |
123.1 (0.4) | 126.8 (0.3) | 129.5 (0.4) | 132.4 (0.5) | <0.0001 |
| Hypertension – % | 31.5 | 42.1 | 51.7 | 62.7 | <0.001 |
| Diabetes – % | 6.0 | 12.8 | 20.4 | 29.6 | <0.001 |
| Mean LDL-C, in mg/dl (SE) |
119.4 (0.7) | 124.4 (0.5) | 125.3 (0.8) | 122.6 (1.0) | <0.0001 |
| Mean HDL-C, in mg/dl (SE) |
57.8 (0.4) | 49.4 (0.3) | 46.7 (0.3) | 46.5 (0.4) | <0.0001 |
| Median triglycerides, in mg/dl (IQR) |
103 (75-143) |
123 (90-174) |
134 (98-190) |
132 (98-184) |
<0.0001 |
| Median NT-proBNP, in pg ml (IQR) |
80.6 (42.7-139.3) |
58.0 (29.1-111.0) |
52.8 (25.1-104.1) |
59.6 (30.8-117) |
<0.01 |
| Mean eGFR, in ml/min/ 1.73 m2 (SE) |
82.8 (0.4) | 82.5 (0.3) | 82.4 (0.4) | 84.7 (0.6) | 0.0001 |
Measurable hs-cTnT was found in the majority of study participants (65.9%) (Figure 1). The prevalence of measurable hs-cTnT was higher among individuals with severe obesity than those with normal weight (69.9 vs 60.4%, p < 0.001). The prevalence of high hs-cTnT levels increased with higher BMI category, being found in 4.5% of individuals with normal weight, 6.7% of individuals with overweight, 9.0% of those with obesity and 9.5% of those with severe obesity (p for trend <0.001).
Figure 1. Distribution of Cardiac Troponin T Detected with a Highly Sensitive Assay (hs-cTnT) in the ARIC Population without Clinical CVD, Stratified by BMI Category.

Hs-cTnT levels are reported in ng/L
Higher BMI categories were associated with an increased likelihood of having high hscTnT levels, even after adjustment for traditional HF risk factors (Table 2). After adjusting for all HF risk factors, severe obesity was associated with an odds ratio of 2.20 (95% CI: 1.59-3.06) for high hs-cTnT, compared with normal BMI (Model 3). An independent, linear relationship between BMI and high hs-cTnT levels was seen when BMI was modeled continuously using a restricted cubic spline (Figure 2). Each 5 kg/m2 higher increment in BMI was independently associated with a 26% higher odds of high hs-cTnT levels. Significant positive associations were also seen between obesity and measurable hs-cTnT levels (Table 2).
Table 2. Adjusted Odds Ratios for the Cross-Sectional Association of Body Mass Index Categories with Elevated Cardiac Troponin T Detected with a Highly Sensitive Assay (hs-cTnT) in Adults without Clinical CVD.
| Normal Weight (BMI 18.5- 24.9 kg/m2) N=2,448 |
Overweight (BMI 25- 29.9 kg/m2) N=3,800 |
Obese (BMI 30- 34.9 kg/m2) N=2,118 |
Severely Obese (BMI ≥ 35 kg/m2) N=1,141 |
|
|---|---|---|---|---|
|
Odds Ratios (95% CI) for High
hs-cTnT (≥14 ng/L)* |
||||
| Model 1 | 1.0 (Ref) | 1.56 (1.24-1.96) |
2.24 (1.76-2.87) |
2.69 (2.03-3.56) |
| Model 2 | 1.0 (Ref) | 1.28 (1.01-1.63) |
1.89 (1.47-2.44) |
3.14 (2.33-4.22) |
| Model 3 | 1.0 (Ref) | 1.17 (0.91-1.52) |
1.60 (1.21-2.12) |
2.20 (1.59-3.06) |
|
Odds Ratios (95% CI) for
Measurable hs-cTnT (≥3 ng/L) † |
||||
| Model 1 | 1.0 (Ref) | 1.32 (1.19-1.48) |
1.67 (1.47-1.90) |
1.79 (1.52-2.10) |
| Model 2 | 1.0 (Ref) | 1.04 (0.93-1.17) |
1.37 (1.19-1.57) |
1.86 (1.57-2.21) |
| Model 3 | 1.0 (Ref) | 1.03 (0.91-1.17) |
1.29 (1.11-1.49) |
1.66 (1.39-1.99) |
Model 1 Adjusted for age
Model 2 Adjusted for Model 1 variable plus sex, race, and smoking status
Model 3 Adjusted for all variables in Model 2 variables plus DM, HTN, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Reference for high hs-cTnT = not high hs-cTnT
Reference for measurable hs-cTnT = undetectable hs-cTnT
Figure 2. Cross-Sectional Association of BMI with High hs-cTnT Levels in Restricted Cubic Spline Model.
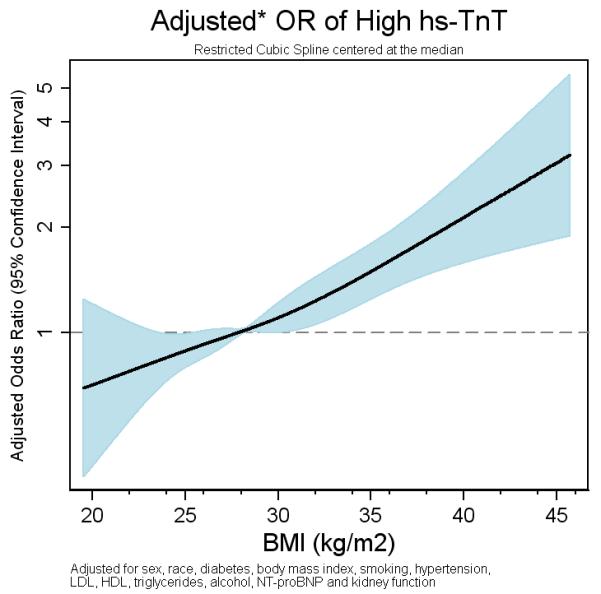
Adjusted for age, sex, race, smoking status, DM, HTN, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Over a median of 12.1 years of follow-up, 869 incident HF events occurred within the study sample. Higher BMI category at baseline was associated with a progressive increase in the risk of incident HF, with severe obesity associated with a hazard ratio of 3.39 (95% CI: 2.74-4.19) in age-adjusted analysis (Figure 3). After adjustment for established HF risk factors and other confounders, higher baseline BMI continued to be significantly associated with incident HF (HR for severe obesity 2.39 [95% CI: 1.89-3.01]). After multivariable adjustment, each 5 kg/m2 higher baseline BMI was independently associated with a 32% higher risk of incident HF.
Figure 3. Adjusted Hazard Ratios for Incident HF Associated With Higher BMI Categories.
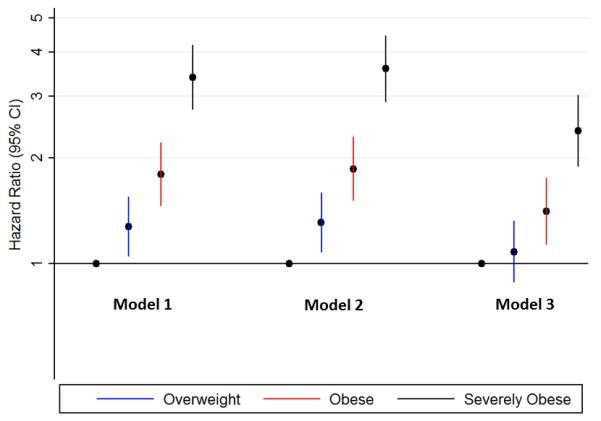
Model 1: Adjusted for age
Model 2: Adjusted for Model 1 variable plus sex, race, and smoking status
Model 3: Adjusted for all variables in Model 2 variables plus DM, HTN, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Figure 4 displays the results of Cox regression analyses on the outcome of incident HF with stratification by both BMI category and hs-cTnT level (as undetectable, measurable or high hs-cTnT). At each level of hs-cTnT, higher BMI was significantly associated with an increased risk of incident HF (Supplementary Table 1). Similarly, within each BMI category, greater values of hs-cTnT were associated with increased HF risk. BMI and hs-cTnT provided complementary prognostic information, with individuals with severe obesity and high-hs-cTnT levels having a greater than 9 fold higher risk of incident HF than individuals with normal weight and undetectable hs-cTnT (HR 9.20; 95% CI: 5.67-14.93). While the combined presence of obesity and high hs-cTnT was associated with markedly increased risk, no statistically significant interaction was seen between BMI and hs-cTnT levels on the outcome of incident HF (p for interaction = 0.10).
Figure 4. Combined Associations of BMI Categories and Hs-cTnT Levels with Risk of Incident HF.
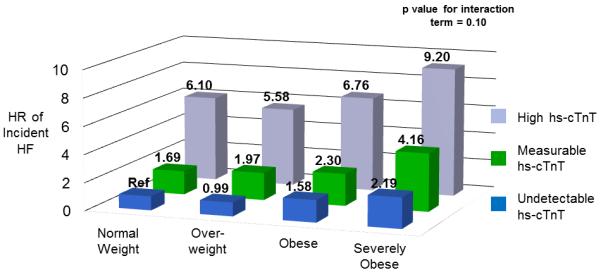
Adjusted for age, sex, race, smoking status, DM, HTN, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Analogous results were seen when using WC rather than BMI as the measure for adiposity. In the full regression model, the top quartile of WC was associated with an odds ratio of 2.08 (95% CI: 1.53-2.82) for high hs-cTnT, compared to the bottom quartile. Furthermore, individuals in the top quartile of WC with high hs-cTnT had a hazard ratio of 8.33 (95% CI: 5.37-12.92) for incident HF relative to those in the lowest waist circumference quartile with undetectable hs-cTnT. Interestingly, when WC was added to models assessing the association between BMI and high hs-cTnT, the association for BMI became nonsignificant (p=0.19) while that for WC remained significant (p<0.001), suggesting that abdominal obesity may be most relevant to the development of myocardial injury.
Positive associations between obesity and hs-cTnT, and between hs-cTnT and HF, were also seen when hs-cTnT was modeled as a continuous variable (Supplementary Figures 1 and 2). Analyses limited to those individuals with adjudicated HF events (N=427 events) demonstrated similar findings. Adjustments for baseline use of anti-hypertensive and cholesterol-lowering medications did not alter the results appreciably. The results were also generally similar across subgroups by age, race and sex, with no statistically significant interactions of these variables with hs-cTnT, although the association between BMI and hs-cTnT tended to be weaker among women compared with men (p for interaction = 0.24), and among African-Americans compared with white participants (p for interaction = 0.30).
Discussion
In this population-based study of 9,507 men and women without CVD at baseline, we found an independent association between higher BMI and subclinical myocardial injury, as reflected by high hs-cTnT levels, that persisted after adjustment for traditional cardiovascular risk factors. This association had significant clinical implications, as obesity and hs-cTnT were both independently associated with an increased risk of hospitalization or death for incident HF over 12 years of follow-up. Even after adjustment for HF risk factors, individuals with severe obesity and high hs-cTnT levels had a greater than 9-fold higher risk of incident HF than individuals with normal weight and undetectable hs-cTnT levels.
Previous studies have demonstrated an association between obesity and incident HF. However, the mechanisms underlying the relationship between obesity and HF have not yet been fully elucidated, with the association being only partially explained by traditional risk factors. Within the Framingham Heart Study, obesity was associated with a 2-fold increased risk of incident HF among men and women after adjustment for HTN, DM, prior myocardial infarction and other risk factors(4). Similar findings have been seen in other cohorts(14-16). Past work from our group has also demonstrated the utility of elevated hs-cTnT, beyond traditional risk factors, for predicting future HF. The present analysis extends prior research by revealing an independent, linear association between BMI and high hs-cTnT levels, and demonstrating the prognostic implications of elevations in both BMI and hs-cTnT for incident HF risk. Our findings also suggest that abdominal obesity may be most important for the development of myocardial injury. Notably, the relationship between BMI and hs-cTnT contrasts with the known inverse relationship between obesity and levels of NT-proBNP, another marker of future HF risk.
This study also adds to the growing body of literature examining the correlates and consequences of hs-cTnT levels among individuals in ambulatory populations(10, 11, 13, 17).. Hs-cTnT levels are strongly associated with incident cardiovascular events, and the available evidence suggests that the association between hs-cTnT and cardiovascular risk is not primarily mediated by macrovascular coronary atherosclerosis. In multiple studies, hs-cTnT is more strongly associated with the risk of incident HF than with CHD events(10, 11). In addition, past research has not shown an independent association between hs-cTnT and coronary artery calcium, a surrogate for subclinical atherosclerosis(13). In contrast, hs-cTnT has been independently associated with abnormalities of myocardial structure and function that commonly precede the development of HF(13).
While there are limited past studies regarding the relationship between obesity and hscTnT(18), there is increasing laboratory evidence linking obesity to subclinical myocardial injury. Obesity is associated with abnormal cardiac remodeling and with impaired myocardial contractile function and relaxation, independent of coronary heart disease and traditional obesity-associated risk factors such as HTN and DM(5, 19). Studies of animal models of obesity suggest that chronic myocardial injury may contribute to the myocardial dysfunction associated with excess adiposity. Rodents genetically predisposed to obesity demonstrate increased rates of myocyte DNA damage, myocardial oxidative injury, myocyte apotosis, myocardial fibrosis, and impaired contractile function compared to leaner, wild-type rats(20-22).. It has been suggested that myocardial injury and resultant apoptosis may play a role in the progression from compensatory ventricular remodeling to clinical HF(23). With the emergence of hs-cTnT as a novel marker of subclinical myocardial damage, evaluations of the relationship between obesity and myocardial injury can now be extended from animal models to clinical studies.
Clinical Implications
This analysis highlights the prognostic valueof obesity and elevated hs-cTnT for HF risk. Obesity was independently associated with incident HF at each level of hs-cTnT, and higher hscTnT levels were similarly associated with increased HF risk within each BMI category. Those individuals with severe obesity and high hs-cTnT had the greatest risk of HF. These findings suggest that in addition to its relationship with subclinical myocardial injury, obesity likely contributes to HF risk via additional mechanisms. Furthermore, obesity and hs-cTnT levels each provide independent and complementary prognostic information, beyond traditional risk factors, regarding HF risk. Individuals without clinical CVD and with high BMI and high hs-cTnT levels appear to represent a group at markedly increased risk for the development of HF.
Limitations
This analysis has certain limitations. Although the study participants were rigorously evaluated for risk factors and there was extensive adjustment for covariates associated with HF, there remains the possibility of residual confounding in this observational analysis. The diagnosis of incident HF was based on hospital discharge and death certificate codes, which may have resulted in some misclassification. This analysis does not account for the potential impact of medical therapies during the follow-up period. Additionally, cardiac imaging was not available to determine the structural and functional myocardial abnormalities leading to HF among individuals with obesity and elevated hs-cTnT. Nonetheless, this is an analysis within a large, biracial and extremely well characterized prospective cohort with long-term follow-up for incident HF events. The large number of HF events provided power to stratify by both BMI category and hs-cTnT levels, in order to fully examine the contributions of both of these variables to incident HF risk. We were also able to exclude individuals with baseline clinical CVD using data on prior adjudicated events.
In conclusion, in this analysis of men and women without baseline CVD, we found an independent, linear association between BMI and subclinical myocardial injury, as indexed by high hs-cTnT levels. Additionally, higher BMI and hs-cTnT levels were both independently associated with incident HF, with individuals with both severe obesity and high hs-cTnT having markedly increased risk. Further investigation is needed to elucidate the mechanisms linking obesity to elevated hs-cTnT, and to understand the interplay of obesity and subclinical myocardial injury in the development of HF.
Supplementary Material
Cross-Sectional Association of BMI with Continuous hs-cTnT Levels in Linear Spline Model
Adjusted for age, sex, race, smoking status, diabetes mellitus, hypertension, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Prospective Association of Continuous hs-cTnT with Incident HF in Restricted Cubic Spline Model
Adjusted for age, sex, race, smoking status, diabetes mellitus, hypertension, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions. Roche provided the reagents to conduct the hs-cTnT assays.
The views expressed in this article are those of the authors and do not represent the views of the Department of Veterans Affairs.
This work was supported by the Robert E. Meyerhoff Professorship, the PJ Schafer Memorial Fund and an Investigator Research Supplement from the National Heart, Lung, and Blood Institute awarded to Dr. Ndumele, and by a National Heart, Lung, and Blood Institute grant (5K23HL096893) awarded to Dr. Nambi.
Abbreviations
- LDL-C
low density lipoprotein- cholesterol
- ICD-9
International Classification of Diseases-9.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
Drs Ballantyne, Nambi and Hoogeveen along with Roche and Baylor College of Medicine have filed a provisional patent (patent #61721475) entitled “Biomarkers to Improve Prediction of Heart Failure Risk”. The other authors have no relevant disclosures.
References
- 1.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 2.Lavie CJ, Alpert MA, Arena R, Mehra MR, Milani RV, Ventura HO. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013;1:93–102. doi: 10.1016/j.jchf.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 4.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–13. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 5.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zalesin KC, Franklin BA, Miller WM, Peterson ED, McCullough PA. Impact of obesity on cardiovascular disease. Med Clin North Am. 2011;95:919–37. doi: 10.1016/j.mcna.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Choi EY, Yan RT, Fernandes VR, et al. High-sensitivity C-reactive protein as an independent predictor of progressive myocardial functional deterioration: the multiethnic study of atherosclerosis. Am Heart J. 2012;164:251–8. doi: 10.1016/j.ahj.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–61. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 10.deFilippi CR, de Lemos JA, Christenson RH, et al. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saunders JT, Nambi V, de Lemos JA, et al. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–76. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives The ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 13.de Lemos JA, Drazner MH, Omland T, et al. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–12. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605–12. doi: 10.1016/s0002-9343(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 15.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 16.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med. 2001;249:253–61. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 17.Rubin J, Matsushita K, Ballantyne CM, Hoogeveen R, Coresh J, Selvin E. Chronic hyperglycemia and subclinical myocardial injury. J Am Coll Cardiol. 2012;59:484–9. doi: 10.1016/j.jacc.2011.10.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pervanidou P, Akalestos A, Bastaki D, Apostolakou F, Papassotiriou I, Chrousos G. Increased circulating High-Sensitivity Troponin T concentrations in children and adolescents with obesity and the metabolic syndrome: A marker for early cardiac damage? Metabolism. 2012 doi: 10.1016/j.metabol.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Turkbey EB, McClelland RL, Kronmal RA, et al. The impact of obesity on the left ventricle: the Multi-Ethnic Study of Atherosclerosis (MESA) JACC Cardiovasc Imaging. 2010;3:266–74. doi: 10.1016/j.jcmg.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch LA, Gao D, Chen L, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–24. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 21.Zhou YT, Grayburn P, Karim A, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A. 2000;97:1784–9. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent HK, Powers SK, Dirks AJ, Scarpace PJ. Mechanism for obesity-induced increase in myocardial lipid peroxidation. Int J Obes Relat Metab Disord. 2001;25:378–88. doi: 10.1038/sj.ijo.0801536. [DOI] [PubMed] [Google Scholar]
- 23.Garg S, Narula J, Chandrashekhar Y. Apoptosis and heart failure: clinical relevance and therapeutic target. J Mol Cell Cardiol. 2005;38:73–9. doi: 10.1016/j.yjmcc.2004.11.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cross-Sectional Association of BMI with Continuous hs-cTnT Levels in Linear Spline Model
Adjusted for age, sex, race, smoking status, diabetes mellitus, hypertension, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR
Prospective Association of Continuous hs-cTnT with Incident HF in Restricted Cubic Spline Model
Adjusted for age, sex, race, smoking status, diabetes mellitus, hypertension, LDL-C, HDL-C, triglycerides, alcohol intake, NT-proBNP and estimated GFR


