Abstract
A nuanced understanding of HIV-positive status disclosure is urgently needed to inform the implementation of prevention interventions, including TasP and PrEP. To provide such understanding for the high HIV-burden setting of rural KwaZulu-Natal, we conducted a prospective cohort study to characterize determinants and trends in HIV-positive status disclosure. 687 consenting HIV-positive individuals (73.2% female; 60.3% ART initiated) were enrolled. Reports of any incidence of disclosure to either a family member or sexual partner at enrollment and follow-up visits (median 4.4 months post-enrolment) were common (91.0%); however, reports of disclosure specifically to sexual partners were relatively rare (34.1%), especially in women (29.8%). Participants not engaged in a stable partnerships, not ART-imitated, and/or who had disclosed to their family were at risk of non-disclosure to sexual partners. These data highlight both an urgent need to empower HIV-positive individuals, and the significant barriers to targeting sero-discordant couples for HIV prevention in this setting.
Keywords: Status disclosure, rural South Africa, HIV prevention, treatment, anti-retroviral therapy
Introduction
In people living with HIV/AIDS (PLWHA), disclosure of HIV-positive status is positively associated with increased access to key support networks, improvements in mental health, and earlier initiation and better adherence to anti-retroviral therapy (ART) (1–5). Consequently, those PLWHA that disclose their HIV status have been reported to have delayed disease progression compared to those who have not disclosed, and may also be less likely to transmit HIV to their sexual partner(s) (6).
Beyond early treatment initiation, disclosure of HIV-positive status specifically to a sexual partner may have independent HIV prevention benefits, allowing couples to make informed decisions concerning their HIV prevention and reproductive health requirements (7). Moreover, the adoption and therefore success of many current and anticipated preventive measures-including voluntary medical male circumcision (VMMC), pre-exposure prophylaxis (PrEP), and treatment-as-prevention (TasP)-may depend largely on couples discovering HIV sero-discordancy via mutual disclosure.
Despite the potential importance and benefits of disclosure of HIV-positive status, reported incidences and determinants of disclosure have not been extensively reported, especially in hyper-endemic settings (8). Previous studies have highlighted the gender, marital and employment status of the discloser as important disclosure determinants, whilst fear of enacted stigma has frequently been cited as reason for non-disclosure, especially amongst women (2, 8–11). However, whilst these studies are informative, a more nuanced and context-specific understanding of disclosure is urgently required in HIV prevention priority regions in order to inform HIV prevention programs. Indeed, to support disclosure, it is important to recognize that determinants of disclosure may vary according to the characteristics both of the person disclosing and to the person being disclosed to. For example, whether or not a PLWHA has initiated on ART is likely to impact HIV-positive status disclosure characteristics, as the need to take pills and visit clinics may increase the visibility of their HIV status to those closest to them. It is also important to qualify when disclosures occur, such that any potential windows of opportunity for supported disclosure are not lost.
In this prospective cohort study, we aimed to provide evidence to contribute to such a nuanced understanding of the incidence and determinants of disclosure in a HIV prevention priority region in rural KwaZulu-Natal, South Africa.
Methods
Study design and setting
This prospective open-cohort study was conducted between June 2006 and August 2009 at the CAPRISA Vulindlela Clinical Research site, approximately 150km west of Durban in KwaZulu-Natal. Vulindlela is a rural community characterized by limited infrastructure, poor economic opportunity and high HIV prevalence (12). Free access to ART and pre-ART care has been provided since 2004 through the CAPRISA AIDS Treatment (CAT) program, funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention. ART eligibility through CAT followed the South African Department of Health guidelines in place at the time viz. CD4+ T-cell count ≤200 cells/µL or clinical stage IV disease.
Study enrollment
Consenting, HIV-positive adults were enrolled from the Vulindlela CAT program. Eligibility criteria for participants were: age ≥18 years old; confirmed HIV-positive sero-status; ability to self-care; residence in the Vulindlela sub-district with no plans to move during the study period; non-active treatment for tuberculosis; and willingness to adhere to the study follow-up schedule.
In order to understand disclosure characteristics and determinants in participants stratified by treatment status, eligible participants were consecutively enrolled into one of two study arms, one consisting of participants who had not yet been initiated on ART, and the other consisting of participants who had initiated ART; enrolment into these arms was performed in a 1:2 ratio, with a target of 700 individuals.
Study Procedures
Written informed consent was obtained from each study participant in the preferred language of the interviewee (isiZulu or English) prior to enrollment.
Those participants who had not been initiated on ART were enrolled into the study at their first CAT program visit after testing HIV-positive, whilst participants initiated on ART were enrolled into the study at their next scheduled clinic appointment.
Socio-demographic characteristics, clinical characteristics, and HIV-positive status disclosure histories of participants were recorded through structured questionnaires administered by trained study staff at enrollment. HIV-positive status disclosure histories were obtained specifically by asking participants if they had told anyone about their HIV status, and if so, to whom had they disclosed. At follow-up 3–12 months post-enrollment, participants were asked if they had told anyone about their HIV status since their last interview, and if so, to whom they had disclosed. Disclosures of HIV-positive status were categorized into partner disclosure (disclosure to any marital or sexual partner), family disclosure (any disclosure to a sibling, parent, or other relative, including those by marriage) or other disclosure. Clinical data on ART initiation date and CD4+ T-cell count were collected as part of routine clinical assessment for HIV/AIDS care.
The study protocol was approved by the Biomedical Research Ethics Committee (BREC) of the Nelson R. Mandela School of Medicine, Faculty of Health Sciences, University of KwaZulu-Natal in Durban, South Africa.
Data analysis
Descriptive statistics were used to characterize the cohort demographics and clinical features, overall and stratified by ART status and gender. Differences in characteristics between ART naïve and ART experienced groups, as well as between males and females, were tested using either Fisher’s exact test (categorical data) or Wilcoxon Rank Sums test (continuous data). An adjusted logistic regression model was used to determine factors associated with any partner disclosures reported at enrolment. All statistical analyses were performed in SAS (version 9.3; SAS Institute Inc., Cary, NC, USA).
Results
Socio-demographic and clinical characteristics of participants at enrollment
A total of 687 HIV-positive participants aged 28–38 years were enrolled into the cohort, the majority of whom were female (73.2%). Overall, female participants were significantly younger (median 31 years vs. 34 years; p=0.005) and had significantly higher CD4+ cell counts (137 cells/uL versus 206 cells/uL; p<0.001) compared to male participants (Table I). Compared to those who had not initiated ART, those participants who had initiated ART (60.3%) had expectedly been living with an HIV-positive diagnosis significantly longer (median 204 days vs. 56 days; p<0.001), and had significantly lower CD4+ cell counts (median 138 cells/µl vs. 346 cells/µl; p<0.001).
Table 1.
Baseline socio-demographic and clinical characteristics of participants
| Non-ART initiated | ART initiated | ||||||
|---|---|---|---|---|---|---|---|
| Overall (N=687) |
Males (N=52) |
Females (N=221) |
p-value | Males (N=132) |
Females (N=282) |
p-value | |
| Female, % (n) | 73.2 (503) | - | - | - | - | - | - |
| Age, median (IQR) | 32 (28–38) | 34 (29–40) | 30 (25–36) | 0.005 | 34 (30–40) | 33 (29–39) | 0.192 |
| Employment, % (n/N)* | |||||||
| Employed | 15.4(95/616) | 22.7(10/44) | 12.0(22/183) | 0.086 | 22.1(28/127) | 13.4(35/262) | 0.085 |
| Unemployed | 81.5(502/616) | 77.3(34/44) | 83.1(152/183) | 76.4(97/127) | 83.6(219/262) | ||
| Student | 3.1(19/616) | 0.0 (0/44) | 4.9(9/183) | 1.6(2/127) | 3.1(8/262) | ||
| Marital status, % (n/N)* | |||||||
| Married/stable partner | 13.7(59/432) | 20.0(8/40) | 14.3(25/175) | 0.624 | 11.1(7/63) | 12.3(19/154) | 1.000 |
| Casual partner/single | 66.2(286/432) | 62.5(25/40) | 64.6(113/175) | 69.8(44/63) | 67.2(104/154) | ||
| Separated from/death of stable partner | 20.1(87/432) | 17.5(7/40) | 21.1(37/175) | 19.1(12/63) | 20.1(31/154) | ||
| Schooling, n (%)* | |||||||
| No schooling | 14.8(63/425) | 9.3(4/43) | 12.4(21/221) | 0.193 | 11.5(7/61) | 21.0(139/143) | 0.310 |
| Primary or less | 26.6(113/425) | 39.5(17/43) | 27.0(48/221) | 23.2(16/61) | 22.4(32/143) | ||
| Secondary or less | 57.2(243/425) | 48.8(21/43) | 60.1(107/221) | 59.0(36/61) | 55.2(79/143) | ||
| Tertiary education | 1.4(6/262) | 2.3(1/43) | 0.6(1/221) | 3.3(2/61) | 1.4(2/139) | ||
| Days between testing HIV+ and enrollment, median (IQR) | 174 (57–446) | 31 (21–270) | 77 (29–407) | 0.004 | 160 (91–359) | 231 (118–477) | 0.002 |
| Missing | n=3 | n=0 | n=2 | n=0 | n=1 | ||
| CD4+ count (cells/µL) at enrollment, median (IQR) | 187 (110–308) | 282 (214–437) | 363 (257–479) | 0.097 | 115 (61–180) | 148 (96–214) | <0.0001 |
| Missing | n=73 | n=21 | n=42 | n=2 | n=8 | ||
Where data missing, n/N is shown in full.
Consistent with high levels of unemployment in rural KwaZulu-Natal, 81.5% of participants had no formal work and a significant proportion had no secondary education (41.4%). Most participants enrolled reported being single or having a casual partner (66.2%), and only a minority were married or engaged in a stable relationship (13.7%).
Disclosure of HIV-positive status by participants at enrolment
At enrolment, a median of approximately six months after HIV-positive diagnosis, the vast majority of participants (88.9%) had disclosed their HIV-positive status to at least one family member or sexual partner (Table II). A lack of disclosure to any family or sexual partner was significantly more likely in those participants who had not initiated ART (19.0% vs. 5.8%; p<0.001).
Table II.
Disclosure of HIV status by participants
| Non-ART initiated | ART initiated |
p-value between arms# |
|||||
|---|---|---|---|---|---|---|---|
| Males | Females |
p- value* |
Males | Females |
p- value* |
||
| Total disclosures reported at enrollment | N=52 | N=221 | N=132 | N=282 | |||
| Partner only, % (n) | 26.9(14) | 10.9(24) | 0.010 | 15.9(21) | 2.1(6) | <0.001 | <0.001 |
| Family only, % (n) | 36.5(19) | 59.3(131) | 47.7(63) | 69.5(196) | |||
| Both, % (n) | 17.3(9) | 10.9(24) | 29.6(39) | 23.1(65) | |||
| Neither, % (n) | 19.2(10) | 19.0(42) | 6.8(9) | 5.3(15) | |||
| Total disclosure reported at baseline and follow-upa | N=52 | N=221 | N=132 | N=282 | |||
| Partner only, n (%) | 17.3(9) | 9.5(21) | 0.055 | 10.6(14) | 1.8(5) | <0.001 | <0.001 |
| Family only, n (%) | 34.6(18) | 56.6(125) | 44.7(59) | 67.0(189) | |||
| Both, n (%) | 28.9(15) | 8.1(40) | 39.4(52) | 27.7(78) | |||
| Neither, n (%) | 19.2(10) | 15.8(35) | 5.3(7) | 3.6(10) | |||
| Incidence of new disclosure between enrollment and follow-up | N=33 | N=141 | N=118 | N=244 | |||
| To partner/100 person years (events) | 43.5 (8) | 30.9 (19) | 0.418 | 40.6 (21) | 16.0 (19) | 0.0032 | 0.142 |
| To family/100 person years (events) | 81.1 (15) | 94.3 (58) | 0.615 | 92.8 (48) | 81.4 (97) | 0.4579 | 0.614 |
| Time to disclosureb | N=44 | N=197 | N=129 | N=281 | |||
| Within a day, % (n) | 86.4(38) | 78.7(155) | 0.908 | 57.4(74) | 60.9(171) | 0.079 | <0.001 |
| Within a month, % (n) | 13.6(6) | 15.2(30) | 26.4(34) | 17.8(50) | |||
| More than a month, % (n) | 0.0(0) | 6.1(12) | 16.4(21) | 21.4(60) | |||
Adjusting for age by logistic regression;
adjusting for age and gender by logistic regression;
median time to follow-up 4.4 months post-enrolment;
from HIV-positive diagnosis.
Overall, reports of disclosure of HIV-positive status to a member of family were common, especially in those participants who had initiated ART (87.7% vs. 67.0%; p<0.001). Regardless of ART initiation status, female participants were more likely to have disclosed to a family member compared to male participants (82.7% vs. 70.6%; p<0.001).
Despite the majority of participants disclosing their status to one or more family members, less than one third (29.4%) of participants reported that they had disclosed their HIV-positive status to a sexual partner. In both ART naïve and ART experienced participants, there was a very notable difference between female and male disclosure to sexual partners, with disclosure in female participants being significantly less common than in male participants (23.7% vs. 45.1%; p<0.001).
Incidence of new disclosures between enrollment and follow-up
Follow-up occurred a median of 4.4 months (IQR 3.0–6.4) post-enrollment, with no significant differences in follow-up time between ART-experienced and ART-naïve participants overall (p=0.779), or sub-stratified by gender (p=0.434 in ART naïve; p=0.321 in ART experienced). Over follow-up, a total of 218 new disclosures were reported to family members, compared to 67 new disclosures reported to partners (Table II).
The incidence of new disclosures to sexual partners was found to be significantly higher in male participants initiated on ART compared to female participants initiated on ART (40.6 disclosures/100 person years [PY] vs. 16.0 disclosures/100 PY; p=0.003). Significant differences in the incidence of new disclosures to sexual partners were not observed between males and females not initiated on ART (43.5 disclosures/100 PY vs. 30.9 disclosures/100 PY; p=0.418), nor between participants initiated and not initiated on ART (33.8 disclosures/100 PY vs. 23.4 disclosures/100 PY; p=0.142).
The incidence of disclosure to family members remained relatively constant across participants, with no significant differences observed either between male and female participants, or ART initiated and uninitiated participants.
Time to disclosure of HIV-positive status
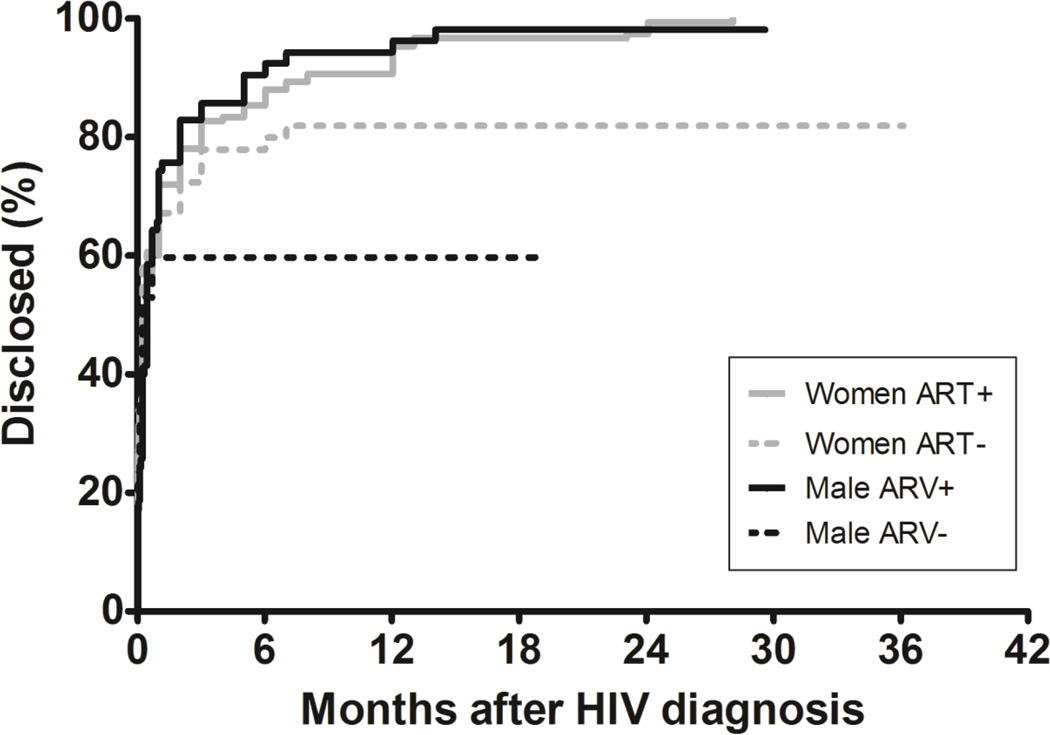
Figure 1 shows a Kaplan-Meir plot highlighting the time to any incidence of disclosure for participants from first HIV positive diagnosis. Overall, the majority (67.3%) of disclosures described by participants either at enrolment or follow-up were reported to have occurred on the day of HIV diagnosis. A further 18.4% of disclosures were reported to have occurred within a month of HIV-positive diagnosis, and 14.3% were reported to have occurred more than a month following HIV-positive diagnosis.
Figure 1.
Kaplan-Meier curve showing time to any reported incidence of disclosure from HIV diagnosis
The proportion of disclosures occurring more than a month post-HIV-positive diagnosis were significantly higher for those participants initiated on ART compared to those who had not been initiated (19.8% vs. 5.0%; p<0.001), suggesting that despite a large proportion of disclosures occurring very close to HIV-positive diagnosis, disclosures do continue to occur over time, and may be catalyzed by ART initiation. Amongst those participants initiated on ART, females were more likely to disclose later (more than a month post-HIV-positive diagnosis) compared to males (21.4% vs. 16.4%; p=0.079).
Predictors of disclosure of HIV-positive status to sexual partner
Given the importance of HIV-positive status disclosure to sexual partners for HIV prevention interventions, we used an adjusted logistic regression model to determine factors associated with any partner disclosures reported at enrolment, in an attempt to better characterize those participants most and least likely to disclose to their sexual partners.
In an initial model (data not shown), we found female participants to be 64% less likely than male participants to disclose to their partners (adjusted odds ratio [aOR]=0.36; confidence interval [CI] 0.22–0.61; p<0.001). As such, we performed all subsequent analyses stratified by gender (Table III).
Table III.
Factors associated with disclosure to sexual partner
| Males (N=184) | Females (N=503) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % disclosing to partner (n/N) |
Unadjusted analysis | Adjusted analysis | % disclosing to partner (n/N) |
Unadjusted | Adjusted | |||||
| Odds ratio (95% CI) |
p- value |
Odds ratio (95% CI) |
p- value |
Odds ratio (95% CI) |
p- value |
Odds ratio (95% CI) |
p- value |
|||
| Age (per 1 year increase) | 1.04 (1.00–1.07) | 0.039 | 1.00 (0.95–1.05) | 0.903 | 0.97 (0.95–0.99) | 0.031 | 0.97 (0.93–0.99) | 0.033 | ||
| Months known HIV+ at enrolment (per 1 month increase) | 1.00 (0.99–1.01) | 0.701 | 0.98 (0.94–1.02) | 0.336 | 1.02 (1.00–1.03) | 0.041 | 1.01 (0.98–1.03) | 0.661 | ||
| Stable partner | ||||||||||
| No | 43.2 (38/88) | 1.00 | 1.00 | 23.5% (67/285) | 1.00 | 1.00 | ||||
| Yes | 80.0 (12/15) | 5.26 (1.39–19.97) | 0.015 | 6.42 (1.22–33.94) | 0.029 | 40.9% (18/44) | 2.25 (1.16–4.36) | 0.016 | 2.41 (1.21–4.80) | 0.013 |
| Employed | ||||||||||
| No | 36.1 (48/133) | 1.00 | 1.00 | 24.2% (94/388) | 1.00 | 1.00 | ||||
| Yes | 73.7 (28/38) | 4.96 (2.22–11.08) | <0.001 | 2.47 (0.88–7.00) | 0.088 | 17.5% (10/57) | 0.67 (0.32–1.37) | 0.269 | 0.84 (0.35–2.04) | 0.696 |
| ART initiated | ||||||||||
| No | 44.2 (23/52) | 1.00 | 1.00 | 21.7% (48/221) | 1.00 | 1.00 | ||||
| Yes | 45.5 (60/132) | 1.05 (0.55–2.00) | 0.881 | 2.22 (0.78–6.28) | 0.133 | 25.2% (71/282) | 1.21 (0.80–1.84) | 0.366 | 2.44 (1.32–4.52) | 0.004 |
| Disclosed to friend | ||||||||||
| No | 45.0 (67/149) | 1.00 | 1.00 | 23.8% (102/428) | 1.00 | 1.00 | ||||
| Yes | 45.7 (16/35) | 1.03 (0.49–2.16) | 0.936 | 1.21 (0.42–3.48) | 0.727 | 22.7% (17/75) | 0.93 (0.52–1.68) | 0.827 | 0.72 (0.34–1.52) | 0.384 |
| Disclosed to family | ||||||||||
| No | 64.8 (35/64) | 1.00 | 1.00 | 34.5% (30/87) | 1.00 | 1.00 | ||||
| Yes | 36.9 (48/130) | 0.32 (0.16–0.62) | <0.001 | 0.33 (0.10–1.08) | 0.067 | 21.4% (89/416) | 0.52 (0.31–0.85) | 0.010 | 0.39 (0.19–0.79) | 0.009 |
For male participants, having a stable partner was significantly associated with an approximately 6-fold increased likelihood of partner disclosure (aOR=6.42; CI 1.22–33.94; p=0.029). Partner disclosure was also more likely if males were employed (aOR=2.47; CI 0.88–7.00; p=0.088), and less likely if males had disclosed to their family (aOR=0.33; CI 0.10=1.08; p=0.067); however, these associations were not statistically significant.
In female participants, initiation of ART (aOR=2.44; CI=1.32–4.52; p=0.004) and having a stable partner (aOR=2.41; CI=1.21–4.80; p=0.004) were both significantly positively associated with partner disclosure. In contrast, older female participants (aOR per year increase=0.93; CI=0.93–0.99; p=0.033) and female participants that had disclosed to their families (aOR=0.39; CI=0.19–0.79; p=0.009) were less significantly less likely to have disclosed to their partners.
Discussion
Despite significant overall levels of disclosure of HIV-positive status, disclosure of status specifically to sexual partners in this setting is very low, particularly in females. Indeed, only approximately one third of participants reported to have disclosed their HIV status to a partner between HIV-positive diagnosis and the end of the study (a period of approximately 10 months).
The high overall levels of disclosure are reassuring, and suggest that the majority of PLWHA in this setting have access to at least some support networks (2). However, low rates of disclosure to sexual partners are concerning, and may impact on the demand and thus the uptake of a number of HIV prevention modalities (including condom use, TasP, PrEP, and VMMC) as many couples may not have an accurate perception of their HIV acquisition risk.
Although participants were not asked specifically about why they choose not to disclose to their sexual partners, those participants who were female, not engaged in a stable partnerships, not initiated on ART, and who had disclosed to their family were all at risk of non-disclosure to their sexual partners.
The low rates of sexual partner disclosure among female participants compared to male participants are suggestive of significant gender-power imbalance within couples in this community, and are consistent with previous qualitative studies that have highlighted fear of enacted stigma and gender-based violence as significant barriers to disclosure (11). Importantly, this gender-disparity in disclosure may augment the effects of non-disclosure in this and similar settings, where women carry a disproportionate burden of HIV infection (13).
Furthermore, although disclosure of HIV-positive status to sexual partners was more common in stable relationships than in casual relationships, reports of such relationships were rare, and even in these relationships only approximately 50% of participants reported disclosing. These data have important implications for the programmatic scale-up of new prevention technologies. Indeed, if PrEP and TasP are to be targeted only at stable sero-discordant couples in an attempt to maximize resource-efficiency (14), the lack of stable couples and non-disclosure of HIV status reported here may represent significant barriers.
Interestingly, those participants who were initiated on ART were more likely to have disclosed to their sexual partners compared to those who had not started treatment. This effect was found to be independent from the length of time participants had been living with an HIV-positive diagnosis, suggesting that it cannot be explained fully by a greater acceptance of their HIV-positive status with time. Indeed, together with our data on time-to-disclosure, these data highlight that ART initiation may act as a catalyst for new disclosures, and that following a ‘first window’ on the day of HIV-positive diagnosis, ART initiation represents a ‘second window’ of opportunity for disclosure. Moreover, support for disclosures during this time-particularly to women-may be an efficient strategy for initiatives aiming to increase overall disclosure levels. Further analysis of the data obtained from this and other studies is needed to confirm this hypothesis (for example, a priority is to explore those participants who initiated ART during the follow-up period, or who recently initiated ART at enrollment), and to understand how it is mediated, although we propose that is may result from a greater need to disclose with the greater visibility imposed by treatment regimens and clinic visits.
A second novel result from our study was the observation of a degree of mutual exclusivity between disclosures to sexual partners and disclosures to family. Indeed, disclosure to sexual partners was overall approximately 65% less likely if participants had disclosed to their family. This pattern could be explained if we consider that participants are largely only disclosing to those from whom they cannot hide their HIV status, for example, those who they live with. Alternatively, enacted stigma on an initial disclosure could deter further disclosures. Future studies should aim to investigate these and other potential hypotheses, as both present significant challenges to supporting disclosures.
The data presented should be interpreted in light of the methodological limitations of the study. Indeed, the time to follow-up was quite variable in an attempt to minimize burden of study participant beyond normal CAT visits for participants. As an adjustment, ‘disclosure incidence’ was calculated, but comparisons of this measure with traditional incidence measures should be cautioned as the nature of disclosure is different to, for example, HIV incidence (disclosure can occur multiple times, but may become less frequent over time as people to disclosure to become limiting). Further, participants were not asked about their number and frequency of current sexual partnerships, thus it is difficult to determine if low reported partner disclosure are simply the result of participants having limited contact with partners. Despite such limitations, the study highlights an urgent need to better understand characteristics and trends in HIV-positive status disclose in order to inform the design both of interventions to support disclosure, and also more broadly of HIV prevention interventions.
Strategic interventions to empower PLWHA –particularly women-to disclose their HIV status to their sexual partners are clearly important both to create demand in couples for HIV prevention technologies, and for their own clinical benefit. Services providing couples’ counseling, testing, and/or treatment initiation may allow for controlled disclosure between sexual partners, minimizing the risks of enacted stigma or gender-based violence (15).
However, whilst important, these interventions may take significant time to implement, and in this setting may be limited by the low rates of stable partnerships. Furthermore, in this and similar high-burden communities, it may be more efficacious as a first step to target prevention interventions such as TasP and PrEP to specific geographies and key high incidence populations such as adolescent girls and young women(16), as opposed to depending on identification of sero-discordant couples via sexual partner disclosure.
Considering the cultural and behavioral context in addition to understanding the local epidemiology will be critical both in ensuring the success of programmatic scale-up of combination HIV prevention, and ultimately in reaching our goals of epidemic control.
Acknowledgements
We pay tribute to the participants in this study; their dedication and commitment made this study possible. We also thank all members of staff of the CAPRISA 060 team for their hard work and contributions to this study. CAPRISA is part of the Comprehensive International Program of Research on AIDS (CIPRA) and is supported by the National Institute of Allergy and infectious Disease (NIAID), National Institutes of Health (NIH) and the US Department of Health and Human Services (DHHS) (grant# 1 U19 AI51794); we are grateful to these contributors for the research infrastructure that made this study possible. We would also like to acknowledge the CAPRISA Fellowship Program, and Columbia University-Southern African Fogarty AIDS International Training and Research Programme Fullbright Clinical Traineeship for direct funding to Rachael Dellar and Benjamin Bearnot, respectively.
Source of Funding
The project described was supported by Award Number D43TW000231 from the Fogarty International Center. Rachael Dellar is supported by the CAPRISA Fellowship Training Program. Benjamin Bearnot was recipient of a Fullbright-Fogarty Clinical Traineeship when he undertook some of this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Fogarty International Center or the National Institutes of Health.
Footnotes
Disclosures
Conflicts of Interest
The authors declare no conflicts of interests.
This work was presented in part at the 2012 International AIDS Society meeting in Washington, D.C., USA (abstract number TUAC0104).
Author contributions
QAK and SSAK conceptualized and designed the project. JAF provided oversight for data collection. BB, LW, QAK, AK and RD contributed to data analysis and interpretation. All authors contributed to either preparation or edits of the final manuscript, and approved revisions.
References
- 1.Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(11):1492–1498. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 2.Wong LH, Rooyen HV, Modiba P, et al. Test and tell: correlates and consequences of testing and disclosure of HIV status in South Africa (HPTN 043 Project Accept) Journal of acquired immune deficiency syndromes. 2009;50(2):215–222. doi: 10.1097/QAI.0b013e3181900172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabilek Z. HIV stigma and disclosure. Can social support help? Positively Aware. 2009;20(5):37–38. [PubMed] [Google Scholar]
- 4.Stutterheim SE, Bos AE, Pryor JB, Brands R, Liebregts M, Schaalma HP. Psychological and social correlates of HIV status disclosure: the significance of stigma visibility. AIDS education and prevention : official publication of the International Society for AIDS Education. 2011;23(4):382–392. doi: 10.1521/aeap.2011.23.4.382. [DOI] [PubMed] [Google Scholar]
- 5.Young SD, Hlavka Z, Modiba P, et al. HIV-related stigma, social norms, and HIV testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043) Journal of acquired immune deficiency syndromes. 2010;55(5):620–624. doi: 10.1097/QAI.0b013e3181fc6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. The New England journal of medicine. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons JT, Schrimshaw EW, Bimbi DS, Wolitski RJ, Gomez CA, Halkitis PN. Consistent, inconsistent, and non-disclosure to casual sexual partners among HIV-seropositive gay and bisexual men. Aids. 2005;19(Suppl 1):S87–S97. doi: 10.1097/01.aids.0000167355.87041.63. [DOI] [PubMed] [Google Scholar]
- 8.Obermeyer CM, Baijal P, Pegurri E. Facilitating HIV disclosure across diverse settings: a review. American journal of public health. 2011;101(6):1011–1023. doi: 10.2105/AJPH.2010.300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bulletin of the World Health Organization. 2004;82(4):299–307. [PMC free article] [PubMed] [Google Scholar]
- 10.Ndiaye C, Boileau C, Zunzunegui MV, et al. Gender-related factors influencing HIV serostatus disclosure in patients receiving HAART in West Africa. World health & population. 2008;10(3):43–54. [PubMed] [Google Scholar]
- 11.WHO Department of Gender, Women and Health. Gender dimensions of HIV status disclosure to sexual partners: Rates, barriers and outcomes for women: A review paper. [cited Sep 20 2014];2003 Available at: http://whqlibdoc.who.int/publications/2004/9241590734.pdf?ua=1.
- 12.Karim QA, Kharsany AB, Frohlich JA, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. International journal of epidemiology. 2011;40(4):922–930. doi: 10.1093/ije/dyq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. Joint United Nations Programme on HIV/AIDS. Global Report: UNAIDS report on the global AIDS epidemic. [cited 2014 Aug 14];2013 Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. [Google Scholar]
- 14.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: a modelling study. PLoS medicine. 2011;8(11):e1001123. doi: 10.1371/journal.pmed.1001123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organisation (WHO) Guidance on couples HIV testing and counselling - including antiretroviral therapy for treatment and prevention in serodiscordant couples: Recommendations for a public health approach. [cited Sep 20 2014];2012 Available at: http://apps.who.int/iris/bitstream/10665/44646/1/9789241501972_eng.pdf?ua=1. [PubMed]
- 16.Barnabas RV, Celum C. Bending the curve: maximising impact with focused HIV prevention. Lancet. 2014;384(9939):216–217. doi: 10.1016/S0140-6736(14)61182-X. [DOI] [PubMed] [Google Scholar]