Abstract
Rationale and Objectives
To investigate the diagnostic accuracy of ultrasound histogram features in the quantitative assessment of radiation-induced parotid gland injury and to identify potential imaging biomarkers for radiation-induced xerostomia (dry mouth)—the most common and debilitating side effect after head-and-neck radiotherapy (RT).
Materials and Methods
Thirty-four patients, who have developed xerostomia after RT for head-and-neck cancer, were enrolled. Radiation-induced xerostomia was defined by the Radiation Therapy Oncology Group/European Organization for Research and Treatment of Cancer morbidity scale. Ultrasound scans were performed on each patient’s parotids bilaterally. The 34 patients were stratified into the acute-toxicity groups (16 patients, ≤3 months after treatment) and the late-toxicity group (18 patients, >3 months after treatment). A separate control group of 13 healthy volunteers underwent similar ultrasound scans of their parotid glands. Six sonographic features were derived from the echo-intensity histograms to assess acute and late toxicity of the parotid glands. The quantitative assessments were compared to a radiologist’s clinical evaluations. The diagnostic accuracy of these ultrasonic histogram features was evaluated with the receiver operating characteristic (ROC) curve.
Results
With an area under the ROC curve greater than 0.90, several histogram features demonstrated excellent diagnostic accuracy for evaluation of acute and late toxicity of parotid glands. Significant differences (P < .05) in all six sonographic features were demonstrated between the control, acute-toxicity, and late-toxicity groups. However, subjective radiologic evaluation cannot distinguish between acute and late toxicity of parotid glands.
Conclusions
We demonstrated that ultrasound histogram features could be used to measure acute and late toxicity of the parotid glands after head-and-neck cancer RT, which may be developed into a low-cost imaging method for xerostomia monitoring and assessment.
Keywords: Xerostomia, ultrasound, parotid gland, radiation toxicity, sonographic features
Xerostomia (dry mouth) is a common, often permanent, and debilitating morbidity of radiotherapy (RT) for head-and-neck malignancies (1,2). Patients with severe xerostomia have thick secretions, difficulty in swallowing and speaking, and are at high risk for oral infection and dental caries (3). This symptom burden impairs the quality of life (QoL) of many head-and-neck cancer survivors for months, even years, after treatment (4). It is well established that the main cause of RT-induced xerostomia is irradiation of parotid glands—the major salivary glands producing ~60% of total saliva (1). Recent clinical studies indicate that intensity-modulated radiotherapy (IMRT) provides a significant advantage in sparing the parotid glands and reducing xerostomia. However, even with the new technology, 17%–30% of patients treated with IMRT still develop permanent xerostomia. There is substantial heterogeneity in parotid gland injury after radiation (5–7).
In the clinic, radiation-induced xerostomia is assessed using patient-based or physician-based grading systems. For example, the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) established a morbidity scale to evaluate post-RT salivary glands. Specifically, salivary gland toxicity was divided into two categories: acute (≤3 months after RT) toxicity and late (>3 months after RT) toxicity. Physicians assign a score of grade 1 (slight dryness) to grade 4 (necrosis or fibrosis) for acute or late salivary toxicity (8). Such subjective measures of radiation toxicity are prone to intraobserver and interobserver variability. In recent years, many groups have been investigating imaging technologies to evaluate parotid gland injury induced by radiation. Studies using computed tomography (CT), magnetic resonance imaging (MRI), MR sialography, and single-photon emission computed tomography scintigraphy have shown some degree of success in assessing the severity of parotid gland injury and documenting normal tissue response to RT (9–17). However, the high cost, the technical complexity, and the need for dedicated imaging expertise (CT, MRI, or nuclear medicine) preclude their use in routine clinical assessment of xerostomia.
The concept of ultrasound imaging to evaluate parotid gland injury is especially attractive because ultrasound is safe, portable, widely available, easy to use, and cost effective. In particular, because parotid glands are superficial structures wrapping around the mandible, they are readily amenable to ultrasound examination. Ultrasound, therefore, is the standard imaging modality in the assessment of salivary gland diseases such as neoplasms, Sjogren syndrome, sialadenitis, and sialolothiasis. However, there is limited information in the literature about evaluation of radiation-induced parotid gland injury or xerostomia using ultrasound (9). Previously, we have proposed an ultrasound technology based on quantitative analysis of echo-intensity histogram to assess RT-associated parotid gland injury (18). A family of sonographic features was derived from the echo histogram to quantify the echogenicity and heterogeneity of parotid glands, which is used to assess the morphologic and architectural integrity of post-RT parotids. In a pilot study of 12 patients, we demonstrated the clinical feasibility of using these echo histogram features in evaluating parotid gland toxicity after RT (18).
Another appealing factor of ultrasound histogram evaluation of RT-related parotid gland toxicity is that it could eliminate variations in subjective radiologic interpretations of ultrasound images. To further explore this ultrasound technology in the evaluation of RT-induced parotid gland injury, we embarked on this clinical study. The primary objective was to determine the diagnostic accuracy of echo-intensity histogram parameters in the assessment of RT-induced parotid gland injury. In addition, we compared the quantitative ultrasound examination with radiologists’ evaluation of acute and late toxicities of RT to parotid glands. Special emphasis was placed on acute toxicity for patients within 3 months of cancer treatment. We want to emphasize the importance of developing safe and easy ultrasound technology to detect acute toxicity because early detection of parotid gland injury could enable early interventions to minimize long-term morbidity.
MATERIALS AND METHODS
Study Population
The study was approved by our institutional review board and in compliance with the Health Insurance Portability and Accountability Act. The eligibility criteria for post-RT patients included 1) patients aged ≥18 years; 2) biopsy-confirmed histologic diagnosis of squamous cell carcinoma of oropharynx, hypopharynx, larynx, or patients with unknown primary tumor with unilateral metastases to neck lymph nodes; 3) radiation volume ≥80% of major salivary glands (parotids) and ≥27 Gy delivered to parotid glands; 4) no salivary gland malignancy; 5) no salivary gland disease, for example, Sjogren syndrome; and 6) clinically confirmed xerostomia based on the RTOG/EORTC acute- and late-toxicity scoring scheme. We have also enrolled a normal control group, and the eligibility criteria for healthy volunteers included 1) participants aged ≥18 years; 2) no prior RTor surgery to head and neck for any reason; 3) no prior malignancies or chemotherapy; 4) no salivary gland malignancy; and 5) no salivary gland disease, for example, Sjogren syndrome.
We stratified our post-RT patients into the acute-toxicity and late-toxicity groups. In general, radiation toxicity is divided into two categories: acute (early) and chronic (late) toxicity (19). Acute toxicity is defined as toxicity occurring within the first 3 months of treatment completion, whereas late toxicity is defined as toxicity occurring beyond 3 months after treatment. RT-induced salivary injury is a complex process and evolves through phases (8). During the early course of RT (often 4–6 weeks), most patients may experience acute salivary gland swelling and pain. A reduction in salivary function can begin within 1 week of RT and usually persists afterward (20). For some patients, salivary function gradually recovers within 1–2 years after RT. And for others, acute salivary toxicity may progress to chronic radiation-induced sialadenitis and fibrosis.
Ultrasound Imaging Protocol
As described in previous reports, we established a standardized protocol for ultrasound scanning to facilitate quantitative evaluation of parotid glands (18,21). In brief, ultrasound studies were performed using a clinical scanner (SonixTouch; Ultrasonix, British Columbia, Canada) with a linear array transducer (L14-5/38 probe, 128 elements). All ultrasound B-mode images were acquired with the same settings: 10-MHz center frequency, 1.00-cm focal length, 3-cm depth, 72% gain, 31 frames per second, and 80-dB dynamic range. The standard B-mode image consists of 256 levels in gray scale.
Each participant underwent one ultrasound study of the bilateral parotid glands. All participants were scanned in the upright seated position, and longitudinal (vertical) ultrasound scans were performed on the bilateral parotids. During the ultrasound scan, a thin layer of ultrasound gel was used to ensure good coupling between the face and the ultrasound probe. The probe was placed perpendicular to the scan surface with minimal pressure applied to the face.
Echo-intensity Histogram Analysis of the Parotid Gland Ultrasound Image
The sonographic features were derived from the echo-intensity histograms to quantitatively characterize the integrity of the parotid glands. The echo histogram presents a graphical distribution of the pixel intensities within the region of interest. All parotid glands were contoured by a radiologist (S.T.). In a previous study, we have shown that interobserver and intraobserver agreement was excellent in contouring parotids (18). The echo-intensity histograms and sonographic features used to assess radiation damage to the parotid glands were generated by in-house signal processing software written in MatLab (Mathworks, Natick, MA).
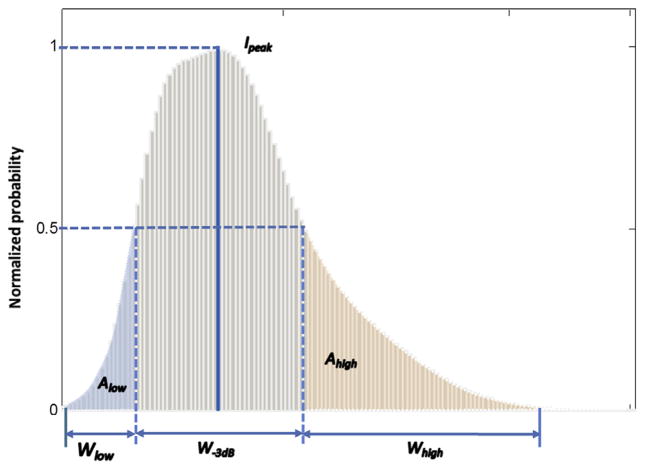
As described in the previous article (18), six sonographic features were computed from the histogram to provide additional quantification of the echogenicity and heterogeneity of the parotid glands (Fig 1). These can be summarized briefly as follows: The Ipeak is the peak intensity value of the histogram. W-3 dB is the −3 dB intensity width of the histogram. Wlow and Whigh capture the width of the low-intensity (<50% Ipeak) and the high-intensity (>50% Ipeak) portions of the histogram, respectively. Alow and Ahigh characterize the area under the low-intensity and high-intensity portions of the curve. These sonographic features provide quantitative measures of the echogenicity (Ipeak), homogeneity (W-3 dB), and heterogeneity (Wlow, Whigh, Alow, and Ahigh) of parotid glands. All histograms are normalized to the peak intensity Ipeak. In other words, all probability distribution is divided by the maximum probability value at Ipeak.
Figure 1.
Echo-intensity histogram: histogram parameters quantify the intensity distribution of the parotid gland.
Subjective Radiologic Evaluation
An experienced radiologist (S.T. with over 10 years’ experience), blinded to the ultrasound histogram findings, retrospectively evaluated all the ultrasound images and classified the echogenicity of the glands as hypoechoic (lower intensity), isoechoic, or hyperechoic (higher intensity) to adjacent musculature. The echotexture heterogeneity was assessed and classified as mild, moderate, or severe heterogeneity.
Statistical Analysis
Radiologic evaluations of the echogenicity and heterogeneity were compared using the chi-squared test among various groups. Analysis of variance, Kruskal–Wallis test, or Wilcoxon rank sum test were used to compare each of the six sono-graphic features among various groups.
The predictive discriminatory powers on patient’s toxicity status of the six sonographic features were further analyzed with receiver operating characteristic (ROC) analysis (22). The ability of these sonographic features to predict toxicity status was determined using ROC curves and measuring the area under the curve (AUC). Whether the AUCs of ROC curves were different from 0.5, which means no ability to predict toxicity, was tested with chi-squared tests. An AUC value between 0.8 and 0.9 indicates good accuracy, and an AUC between 0.9 and 1 indicates excellent accuracy in a diagnostic test. The diagnostic accuracy of a sonographic feature was measured by calculating its sensitivity and specificity. The cutoff value in the ROC curves was estimated while maximizing both sensitivity and specificity. The significance levels were set at .05 for all tests. The SAS statistical package V9.3 (SAS Institute, Inc., Cary, NC) was used for all data analysis.
RESULTS
A total of 47 subjects, consisting of 34 post-RT patients and 13 healthy volunteers, were included in this study. The control group consisted of 13 healthy volunteers (age, 51 ± 11 years). All enrolled patients had received IMRT for head-and-neck malignancies and were clinically diagnosed with grade 1 or grade 2 salivary gland toxicity. The 34 post-RT patients were further stratified into two groups. 1) Acute-toxicity group: sixteen patients received RT for head-and-neck malignancies within 3 months (age, 62 ± 7 years; follow-up time, 1.59 ± 0.79 months). For the acute-toxicity group, the mean dose to the primary tumor was 68.1 ± 3.7 Gy, and the mean dose to the parotid glands was 40.0 ± 14.8 Gy. All patients experienced RTOG grade 1 or 2 acute salivary gland toxicity. 2) Late-toxicity group: eighteen patients received RT for head-and-neck malignancies more than 3 months before imaging (age, 61 ± 7 years; follow-up time, 20.14 ± 10.36 months). All patients in this group experienced grade 1 or 2 late salivary gland toxicity. For the late-toxicity group, the median radiation dose to the primary tumor was 67.8 ± 3.8 Gy, and the mean dose to the parotid glands was 36.3 ± 11.3 Gy. The patient and treatment characteristics—age, follow-up time, gender, primary tumor site, histology, stage, and chemotherapy—are summarized in Table 1 for both acute- and late-toxicity groups.
TABLE 1.
Patient and Treatment Characteristics
| Covariate | Level | Group, n (%) or Mean (±Standard Deviation)
|
|
|---|---|---|---|
| Acute Toxicity Group (N = 16) | Late Toxicity Group (N = 18) | ||
| Age | 62.3 (±7.7) | 60.7 (±7.3) | |
| Gender | Female | 2 (12.5) | 4 (22.2) |
| Male | 14 (87.5) | 14 (77.8) | |
| Primary tumor site | Head (orbit) | 0 (0) | 1 (5.6) |
| Larynx | 1 (6.3) | 3 (16.7) | |
| Nasal cavity | 1 (6.3) | 0 (0) | |
| Oral cavity | 1 (6.3) | 2 (11.1) | |
| Pharynx | 3 (18.8) | 1 (5.6) | |
| Sinus | 2 (12.5) | 0 (0) | |
| Tongue | 4 (25.0) | 6 (33.3) | |
| Tonsil | 3 (18.8) | 4 (22.2) | |
| Unknown | 0 (0) | 1 (5.6) | |
| Vocal cord | 1 (6.3) | 0 (0) | |
| Histology | Adenocarcinoma | 2 (12.5) | 1 (5.6) |
| Squamous cell carcinoma | 13 (81.3) | 17 (94.4) | |
| Undifferentiated carcinoma | 1 (6.3) | 0 (0) | |
| T stage | T1 | 2 (12.5) | 3 (16.7) |
| T2 | 6 (37.5) | 5 (27.8) | |
| T3 | 2 (12.5) | 2 (11.1) | |
| T4 | 6 (37.5) | 6 (33.3) | |
| Tx | 0 (0) | 2 (11.1) | |
| N stage | N0 | 3 (18.8) | 1 (5.6) |
| N1 | 1 (6.3) | 2 (11.1) | |
| N2 | 11 (68.8) | 11 (61.1) | |
| N3 | 1 (6.3) | 3 (16.7) | |
| Nx | 0 (0) | 1 (5.6) | |
| M stage | M0 | 14 (87.5) | 18 (100) |
| Mx | 2 (12.5) | 0 (0) | |
| Concurrent chemotherapy | No | 4 (25) | 1 (5.6) |
| Yes | 12 (75) | 17 (94.4) | |
Ultrasound Images and Histograms: Individual Subjects
Three representative cases were selected, one from each group, for presentation as shown in Figure 2.
Figure 2.
Parotid ultrasound images and histograms of three cases: (a) normal case of a 40-year-old healthy volunteer; (b) acute-toxicity case of a 58-year-old patient with laryngeal cancer, 1 month after radiotherapy (RT); (c) late-toxicity case of a 60-year-old laryngeal cancer patient, 18 months after RT. The dashed yellow lines delineate the parotid glands. Homogeneous texture was observed in the normal parotid gland (a) and heterogeneous textures in parotid glands with acute toxicity (b) and late toxicity (c). (Color version of figure is available online.)
Normal case: This case of a 40-year-old healthy volunteer shows a normal parotid gland that appears homogeneous and has increased echogenicity relative to adjacent muscle on the ultrasound image. As described earlier, with the B-mode echo intensity ranges between 0 and 255, the histogram reveals a Gaussian (symmetric) distribution centered at the peak intensity of 79. The narrow 3-dB band-width (W-3 dB = 39) indicates relatively homogenous distribution. The area under the low-intensity portion of the curve (ie, below Alow) is 5.4, and the area under the high-intensity portion of the curve (ie, above Ahigh) is 8.3; whereas the width of the low-intensity portion (Wlow) is 39, and the width of the high-intensity portion (Whigh) is 89.
Case with acute toxicity: This case is a 58-year-old patient who had completed RT for his laryngeal cancer 1 month before imaging. He experienced mild xerostomia and was clinically diagnosed with RTOG grade 1 acute salivary gland toxicity. On the ultrasound image, the post-RT looks less homogeneous with multiple hypoechoic areas which may be due to patches of inflammatory infiltrate. The histogram reveals a non-Gaussian distribution with a peak intensity of 30. Compared to the normal parotid gland, the Ipeak shifted to the lower intensity which may be mainly due to inflammatory response (darker area in the ultrasound image). The widened 3-dB bandwidth (W-3 dB = 43) indicates relatively heterogeneous distribution. The area under the low-intensity portion of the curve (ie, below Alow) is 1.9, and the area under the high-intensity portion of the curve (ie, above Ahigh) is 13.0; whereas the width of the low-intensity portion (Wlow) is 18, and the width of the high-intensity portion (Whigh) is 179.
Case with late toxicity: This case is a 60-year-old patient who had completed RT for laryngeal cancer 18 months before imaging. The patient experienced moderate xerostomia and was clinically diagnosed with RTOG grade 2 late toxicity of the salivary gland. On the ultrasound image, the parotid gland exhibits heterogeneous echotexture, iso-echoic relative to the adjacent muscle, with multiple hyper-echoic lines and spots interleaved with hypoechoic areas. The histogram reveals a non-Gaussian distribution with a peak intensity of 65. The further widened 3-dB bandwidth (W-3 dB = 75) indicates heterogeneous distribution, which may be due to the existence of inflammatory and fibrotic areas. The area under the low-intensity portion of the curve (ie, below Alow) is 4.1, and the area under the high-intensity portion of the curve (ie, above Ahigh) is 7.5; whereas the width of the low-intensity portion (Wlow) is 33, and the width of the high-intensity portion (Whigh) is 135.
Ultrasound Images and Histograms: Average for Three Groups
Echo histograms were generated for 26 parotid glands from the normal control group, 32 from the acute-toxicity group, and 36 from the late-toxicity group. The means and standard deviations of the six features from the histograms of the normal, acute-toxicity, and late-toxicity groups along with P values are listed in Table 2. All six sonographic features show significant difference among control, acute-, and late-toxicity groups; between the control and the acute-toxicity groups; between the control and the late-toxicity groups except for Wlow (P value = .404); between the control and the irradiated groups (including acute- and late-toxicity groups); and between the acute- and the late-toxicity groups. Figure 3 shows that the average parotid gland echo histograms of the acute-toxicity group and the late-toxicity group are different from that of the control group.
TABLE 2.
Ultrasound Histogram Features (Mean ± Standard Deviation) of the Control, Acute-, and Late-Toxicity Groups
| Group/Parameter | Ipeak | W-3 dB | Alow | Ahigh | Wlow | Whigh |
|---|---|---|---|---|---|---|
| Comparing three groups | ||||||
| Control (N = 26) | 77.7 ± 4.6 | 37.9 ± 6.8 | 4.9 ± 0.7 | 7.3 ± 1.7 | 33.3 ± 5.7 | 75.2 ± 17.5 |
| Acute toxicity (N = 32) | 36.1 ± 10.4 | 45.5 ± 10.6 | 2.9 ± 1.0 | 13.9 ± 4.0 | 18.6 ± 5.7 | 158.5 ± 29.8 |
| Late toxicity (N = 36) | 61.1 ± 15.3 | 60.5 ± 15.7 | 4.1 ± 1.3 | 16.7 ± 4.5 | 31.58 ± 9.29 | 137.0 ± 26.0 |
| P value* | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Comparing control and irradiated (combined acute and late toxicity) groups | ||||||
| Control (N = 26) | 77.7 ± 4.6 | 37.9 ± 6.8 | 4.9 ± 0.7 | 7.3 ± 1.7 | 33.3 ± 5.7 | 75.2 ± 17.5 |
| Toxicity (N = 68) | 49.4 ± 18.2 | 53.4 ± 15.4 | 3.5 ± 1.3 | 15.4 ± 4.4 | 25.5 ± 10.1 | 147.1 ± 29.7 |
| P value* | <.001 | <.001 | <.001 | <.001 | <.001 | <.001 |
| Comparing control and acute-toxicity group | ||||||
| P value* | <.001 | .008 | <.001 | <.001 | <.001 | <.001 |
| Comparing control and late-toxicity group | ||||||
| P value* | <.001 | <.001 | .012 | <.001 | 0.404 | <.001 |
| Comparing acute- and late-toxicity groups | ||||||
| P value* | <.001 | <.001 | <.001 | .009 | <.001 | .002 |
P≤0.05 means statistically significant.
P value is calculated by analysis of variance for Alow, Wlow, and Whigh; Kruskal–Wallis test or Wilcoxon rank sum test for Ipeak, W-3 dB, and Ahigh.
Figure 3.
Average histograms of the control, acute-toxicity, and late-toxicity groups.
Subject Radiologic Evaluations of the B-mode Images of the Parotid Glands
Subjective radiologic evaluation of the echogenicity and heterogeneity is listed in Table 3. Normal parotid glands have high echo intensity (isoechoic 38% or hyperechoic 62% to adjacent musculature); whereas the majority of post-RT glands are hypoechoic to adjacent musculature (50% among acute toxicity group and 33% among late toxicity group) or isoechoic (34% among acute-toxicity group and 47 % among late-toxicity group). The majority of normal parotid glands look homogeneous (uniform); whereas the post-RT groups show a trend toward increased heterogeneity (nonuniform). The majority of normal parotid glands are mildly heterogenous (92%), whereas the majority of post-RT groups are moderately heterogenous (47% among acute-toxicity group and 33% among late-toxicity group) or severely heterogenous (44% among acute-toxicity group and 39% among late-toxicity group). Echogenicity and heterogeneity are significantly different among control, acute-, and late-toxicity groups; between the control and the acute-toxicity groups; between the control and the late-toxicity groups; and between the control and irradiated groups (including acute- and late-toxicity groups; all P values <.001). Echogenicity and heterogeneity are similar between the acute-toxicity group and the late-toxicity group (P value, .375 for echogenicity and .144 for heterogeneity). Therefore, subjective radiologic evaluation cannot distinguish between the acute- and late-toxicity groups.
TABLE 3.
Radiologic Evaluation of B-mode Images of the Control, Acute-, and Late-Toxicity Groups
| Group | Echogenicity, n (%)
|
Heterogeneity, n (%)
|
||||||
|---|---|---|---|---|---|---|---|---|
| Hypoechoic | Isoechoic | Hyperechoic | P Value* | Mild | Moderate | Severe | P Value* | |
| Comparing three groups | <.001 | <.001 | ||||||
| Control (N = 26) | 0 (0) | 10 (38.5) | 16 (61.5) | 24 (92.3) | 2 (7.7) | 0 (0) | ||
| Acute toxicity (N = 32) | 16 (50) | 11 (34.4) | 5 (15.6) | 3 (9.4) | 15 (46.9) | 14 (43.8) | ||
| Late toxicity (N = 36) | 12 (33.3) | 17 (47.2) | 7 (19.4) | 10 (27.8) | 12 (33.3) | 14 (38.9) | ||
| Comparing control and toxicity groups | <.001 | <.001 | ||||||
| Control (N = 26) | 0 (0) | 10 (38.5) | 16 (61.5) | 24 (92.3) | 2 (7.7) | 0 (0) | ||
| Toxicity (N = 68) | 28 (41.2) | 28 (41.2) | 12 (17.7) | 13 (19.1) | 27 (39.7) | 28 (41.2) | ||
| Comparing control and acute-toxicity Groups | <.001 | <.001 | ||||||
| Comparing control and late-toxicity Groups | <.001 | <.001 | ||||||
| Comparing acute- and late-toxicity groups | .375 | .144 | ||||||
P≤0.05 means statistically significant.
P value is calculated by chi-squared test.
Efficiency of Histogram Features: ROC Curves
The ROC analyses to evaluate diagnostic accuracy of toxicity for the histogram parameters are summarized in Table 4. All histogram parameters, except for Ipeak and W-3 dB, have AUCs greater than 0.94 and, therefore, have excellent diagnostic accuracy in classifying the acute-toxicity group and the control group. All histogram parameters except for Alow and Wlow can differentiate the late-toxicity group from the control group. All histogram parameters are useful for correctly classifying patients with acute toxicity and patients with late toxicity; among them, Ipeak, Ahigh, and Whigh have excellent diagnostic accuracy. Overall, all parameters have abilities to predict patient toxicity (acute or late toxicity) versus normal controls.
TABLE 4.
Receiver Operating Characteristic (ROC) Analysis of Histogram Features for the Control, Acute-, and Late-Toxicity Groups
| Parameter* | Area Under ROC Curve | Cutoff Value† | Sensitivity (%)† | Specificity (%)† | P Value‡ |
|---|---|---|---|---|---|
| Probability modeled is group = “Acute Toxicity Group” compared to “Control Group” | |||||
| Ipeak | 1.00 | — | — | — | — |
| W-3 dB | 0.70 | 49.00 | 40.63 | 96.15 | .003 |
| Alow | 0.94 | 3.86 | 84.38 | 88.46 | <.001 |
| Ahigh | 0.98 | 10.61 | 87.50 | 100.00 | <.001 |
| Wlow | 0.97 | 26.00 | 93.75 | 92.31 | <.001 |
| Whigh | 0.99 | 110.00 | 96.88 | 100.00 | <.001 |
| Probability modeled is group = “Late Toxicity Group” compared to “Control Group” | |||||
| Ipeak | 0.86 | 74.00 | 77.78 | 80.77 | <.001 |
| W-3 dB | 0.95 | 45.00 | 97.22 | 84.62 | <.001 |
| Alow | 0.68 | 4.33 | 69.44 | 73.08 | .012 |
| Ahigh | 0.99 | 10.77 | 91.67 | 100.00 | <.001 |
| Wlow | 0.61 | 27.00 | 36.11 | 92.31 | .143 |
| Whigh | 0.99 | 107.00 | 94.44 | 100.00 | <.001 |
| Probability modeled is group = “Acute Toxicity Group” compared to “Late Toxicity Group” | |||||
| Ipeak | 0.90 | 44.00 | 87.50 | 80.56 | <.001 |
| W-3 dB | 0.79 | 43.00 | 50.00 | 97.22 | <.001 |
| Alow | 0.77 | 3.26 | 71.88 | 75.00 | <.001 |
| Ahigh | 0.68 | 13.94 | 62.50 | 75.00 | .005 |
| Wlow | 0.90 | 23.00 | 81.25 | 83.33 | <.001 |
| Whigh | 0.72 | 138.00 | 84.38 | 58.33 | <.001 |
| Probability modeled is group = “Toxicity Group” compared to “Control Group” | |||||
| Ipeak | 0.92 | 66.00 | 76.47 | 100.00 | <.001 |
| W-3 dB | 0.83 | 45.00 | 75.00 | 84.62 | <.001 |
| Alow | 0.80 | 3.89 | 66.18 | 88.46 | <.001 |
| Ahigh | 0.98 | 10.61 | 89.71 | 100.00 | <.001 |
| Wlow | 0.78 | 27.00 | 63.24 | 92.31 | <.001 |
| Whigh | 0.99 | 107.00 | 95.59 | 100.00 | <.001 |
P≤0.05 means statistically significant.
Logistic regression model was used with each histogram parameter.
Cutoff value is the value of each parameter as maximizing sum of the specificity and sensitivity.
P value is to examine if the area under the ROC curve (AUC) is different from 0.5 (AUC = 0.5 means the parameter has no ability to predict toxicity).
DISCUSSION
In this study, we have investigated the diagnostic efficiency of sonographic features based on echo histograms to characterize parotid gland injury after RT. These sonographic features provide quantitative measures of the echo-intensity distributions of the parotid glands, echogenicity (Ipeak), homogeneity (W-3 dB), and heterogeneity (Wlow vs. Whigh or Alow vs. Ahigh) of the parotid glands. Significant differences were observed in these six histogram features between the control and acute-toxicity groups, between the control and late-toxicity groups, and between the acute- and late-toxicity groups (Fig 4). Through the ROC method, we further demonstrated the ability of histogram parameters in differentiating radiation-induced acute toxicity and late toxicity in parotid glands. Among the sonographic features, Wlow, Whigh, Alow, and Ahigh have excellent (AUC ≥ 0.90) diagnostic accuracy in differentiating parotid glands with acute toxicity from normal glands; Whigh and Ahigh have excellent diagnostic accuracy in differentiating parotid glands with late toxicity from normal glands; and Ipeak and Wlow have excellent diagnostic accuracy in differentiating acute from late toxicity.
Figure 4.

Scattergrams of histogram features demonstrate the separation between the control (red triangles), acute-toxicity (green stars), and late-toxicity groups (blue sterns): (a) histogram features: Ahigh, Alow, and Ipeak and (b) histogram features: Whigh, Wlow, and W-3 dB. (Color version of figure is available online.)
The ultrasound studies that have demonstrated changes in the sonographic appearance of parotid glands exposed to radiation are mostly qualitative, or at best, semiquantitative (23,24). In addition, previous studies were either on acute toxicity (24) or late toxicity (23). In our earlier studies (18,21), we demonstrated that histogram analysis could differentiate post-RT parotid glands with late toxicity from normal glands. In this study, we demonstrated significant differences in sonographic features between parotid glands with acute and late toxicities and normal parotid glands. Homogeneous echotexture was observed in normal parotid glands, whereas heterogeneous texture was observed in the parotid glands of both acute- and late-toxicity groups. For the acute-toxicity group, the heterogeneous texture of post-RT parotid glands may be due to the presence of inflammatory infiltrates, which appear as hypoechoic (dark) areas (25–28). For the late-toxicity group, the heterogeneous echotexture may be due to the presence of fibrosis, which appears as hyperechoic (bright) lines or spots (25–28). As shown in our study, physicians’ subjective evaluations based on echogenicity and homogeneity of gray scale ultrasound images of the parotids were unable to distinguish between the acute-toxicity and late-toxicity groups. The echogenicity evaluation of the parotid gland is based on the comparison with the adjacent musculature. However, the adjacent muscles often receive the same dose of radiation as the parotid gland and may suffer from radiation-induced acute and late injury. For instance, the adjacent muscles may develop radiation-induced fibrosis and, therefore, may appear hyperechoic on the ultrasound scan.
The use of RT to treat head-and-neck cancer often involves radiation exposure of the parotid glands. As a result, many patients will develop xerostomia during the course of RT, a few weeks, months, or years after therapy. Xerostomia is commonly assessed through physicians’ physical examination, patient-reported outcomes, and QoL instruments based on the symptoms (eg, altered taste or sensation of dryness) (29). The lack of objective evaluation or validated matrix for the assessment of parotid gland injury has slowed knowledge and treatment development and evaluation of xerostomia (20). We report our ultrasound study of parotid gland injury to highlight these problems and emphasize the importance of early recognition to minimize morbidity. Early detection of parotid gland injury would benefit patients by guiding physicians in modifying treatment regimen or providing early intervention.
One limitation of the sonographic histogram method is that the histogram of the images depends on many factors including time gain compensation and the nonlinear processing of the images (30). To overcome this limitation, we performed a pilot study to determine the optimal preset. All patients were scanned with this preset to facilitate relative comparison among patients. Another approach to overcome this limitation is through rigorous calibration procedures. For example, if two different settings are used for two patients, the settings need to be recorded. A correction map needs to be generated to normalize one image to the other.
Prospective validation of these ultrasound histogram features as convenient, quantitative imaging biomarkers of parotid injury is warranted. The pattern of toxicities changes with new RT treatment regimen alone or in combination with surgery and chemotherapy compared to conventional RT. Therefore, safe and cost-effective imaging methods to define and monitor new radiation-related toxicities are, thus, important in RT. The ongoing longitudinal study will enlarge the patient database and elucidate the trajectory of toxicity development using quantitative ultrasound. In addition, the predictive utility of the imaging method is continuously being refined and validated via patient-reported symptoms such as dry mouth and pain. These ultrasound parameters may provide a useful matrix of parotid gland injury secondary to radiation. The ultrasound technology could be further developed into a low-cost imaging technique for parotid gland monitoring and assessment.
Acknowledgments
Funding Sources: This study was funded by National Institutes of Health (CA114313), National Institute of Biomedical Imaging and Bioengineering (K23EB013221), U.S. Department of Defense (W81XWH-13-1-0269) and the Georgia Cancer Coalition.
References
- 1.Eisbruch A, Rhodus N, Rosenthal D, et al. How should we measure and report radiotherapy-induced xerostomia? Semin Radiat Oncol. 2003;13(3):226–234. doi: 10.1016/S1053-4296(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 2.Meirovitz A, Murdoch-Kinch CA, Schipper M, et al. Grading xerostomia by physicians or by patients after intensity-modulated radiotherapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;66(2):445–453. doi: 10.1016/j.ijrobp.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Lin A, Kim HM, Terrell JE, et al. Quality of life after parotid-sparing IMRT for head-and-neck cancer: a prospective longitudinal study. Int J Radiat Oncol. 2003;57(1):61–70. doi: 10.1016/s0360-3016(03)00361-4. [DOI] [PubMed] [Google Scholar]
- 4.Bjordal K, Kaasa S, Mastekaasa A. Quality-of-life in patients treated for head and neck-cancer—a follow-up-study 7 to 11 years after radiotherapy. Int J Radiat Oncol Biol Phys. 1994;28(4):847–856. doi: 10.1016/0360-3016(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 5.Chao KSC, Ozyigit G, Blanco AI, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59(1):43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Lee N, Xia P, Quivey JM, et al. Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys. 2002;53(1):12–22. doi: 10.1016/s0360-3016(02)02724-4. [DOI] [PubMed] [Google Scholar]
- 7.de Arruda FF, Puri DR, Zhung J, et al. Intensity-modulated radiation therapy for the treatment of oropharyngeal carcinoma: the Memorial Sloan-Kettering Cancer Center experience. Int J Radiat Oncol Biol Phys. 2006;64(2):363–373. doi: 10.1016/j.ijrobp.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation-Therapy Oncology Group (RTOG) and the European-Organization-for-Research-and-Treatment-of-Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31(5):1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 9.Cheng SCH, Wu VWC, Kwong DLW, et al. Assessment of post-radiotherapy salivary glands. Br J Radiol. 2011;84(1001):393–402. doi: 10.1259/bjr/66754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houweling AC, Schakel T, van den Berg CAT, et al. MRI to quantify early radiation-induced changes in the salivary glands. Radiother Oncol. 2011;100(3):386–389. doi: 10.1016/j.radonc.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Ying M, Wu VWC, Kwong DLW. Comparison of sonographic appearance of normal and postradiotherapy parotid glands: a preliminary study. Ultrasound Med Biol. 2007;33(8):1244–1250. doi: 10.1016/j.ultrasmedbio.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Gritzmann N, Rettenbacher T, Hollerweger A, et al. Sonography of the salivary glands. Eur Radiol. 2003;13(5):964–975. doi: 10.1007/s00330-002-1586-9. [DOI] [PubMed] [Google Scholar]
- 13.Astreinidou E, Raaymakers CPJ, Roesink JM, et al. 3D MR sialography protocol for postradiotherapy follow-up of the salivary duct system. J Magn Reson Imaging. 2006;24(3):556–562. doi: 10.1002/jmri.20659. [DOI] [PubMed] [Google Scholar]
- 14.Wada A, Uchida N, Yokokawa M, et al. Radiation-induced xerostomia: objective evaluation of salivary gland injury using MR sialography. Am J Neuroradiol. 2009;30(1):53–58. doi: 10.3174/ajnr.A1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nomayr A, Lell M, Sweeney R, et al. MRI appearance of radiation-induced changes of normal cervical tissues. Eur Radiol. 2001;11(9):1807–1817. doi: 10.1007/s003300000728. [DOI] [PubMed] [Google Scholar]
- 16.Bialek EJ, Jakubowski W, Zajkowski P, et al. US of the major salivary glands: anatomy and spatial relationships, pathologic conditions, and pitfalls. Radiographics. 2006;26(3):745–764. doi: 10.1148/rg.263055024. [DOI] [PubMed] [Google Scholar]
- 17.Buus S, Grau C, Munk OL, et al. Individual radiation response of parotid glands investigated by dynamic C-11-methionine PET. Radiother Oncol. 2006;78(3):262–269. doi: 10.1016/j.radonc.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Yang X, Tridandapani S, Beitler JJ, et al. Ultrasound histogram assessment of parotid gland injury following head-and-neck radiotherapy: a feasibility study. Ultrasound Med Biol. 2012;38(9):1514–1521. doi: 10.1016/j.ultrasmedbio.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bentzen SA, Saunders MI, Dische S, et al. Radiotherapy-related early morbidity in head and neck cancer: quantitative clinical radiobiology as deduced from the CHART trial. Radiother Oncol. 2001;60(2):123–135. doi: 10.1016/s0167-8140(01)00358-9. [DOI] [PubMed] [Google Scholar]
- 20.Deasy JO, Moiseenko V, Marks L, et al. Radiotherapy dose-volume effects on salivary gland function. Int J Radiat Oncol Biol Phys. 2010;76(3):S58–S63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang X, Tridandapani S, Beitler J, et al. Ultrasound GLCM texture analysis of radiation-induced parotid-gland injury in head-and-neck cancer radiotherapy: an in vivo study of late toxicity. Medical Physics. 2012;39(9):5732. doi: 10.1118/1.4747526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8(4):283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 23.Cheng SC, Ying MT, Kwong DL, et al. Sonographic appearance of parotid glands in patients treated with intensity-modulated radiotherapy or conventional radiotherapy for nasopharyngeal carcinoma. Ultrasound Med Biol. 2011;37(2):220–230. doi: 10.1016/j.ultrasmedbio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Imanimoghaddam M, Rahrooh M, Tafakhori Z, et al. Changes of parotid and submandibular glands caused by radiotherapy—an ultrasound evaluation. Dentomaxillofac Radiol. 2012 doi: 10.1259/dmfr/17113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grehn AL, Gustafsson H, Franzen L, et al. Ultrastructural morphometry of parotid acinar cells following fractionated irradiation. Oral Oncol. 1997;33(1):23–28. doi: 10.1016/s0964-1955(96)00040-1. [DOI] [PubMed] [Google Scholar]
- 26.Henriksson R, Frojd O, Gustafsson H, et al. Increase in mast cells and hyaluronic acid correlates to radiation-induced damage and loss of serous acinar cells in salivary glands: the parotid and submandibular glands differ in radiation sensitivity. Br J Cancer. 1994;69(2):320–326. doi: 10.1038/bjc.1994.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konings AWT, Faber H, Cotteleer F, et al. Secondary radiation damage as the main cause for unexpected volume effects: a histopathologic study of the parotid gland. Int J Radiat Oncol Biol Phys. 2006;64(1):98–105. doi: 10.1016/j.ijrobp.2005.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Nagler RM. The enigmatic mechanism of irradiation-induced damage to the major salivary glands. Oral Dis. 2002;8(3):141–146. doi: 10.1034/j.1601-0825.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- 29.Henson BS, Inglehart MR, Eisbruch A, et al. Preserved salivary output and xerostomia-related quality of life in head and neck cancer patients receiving parotid-sparing radiotherapy. Oral Oncology. 2001;37(1):84–93. doi: 10.1016/s1368-8375(00)00063-4. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Tridandapani S, Beitler JJ, et al. Ultrasonic Nakagami-parameter characterization of parotid-gland injury following head-and-neck radiotherapy: a feasibility study of late toxicity. Med Phys. 2014;41(2):022903. doi: 10.1118/1.4862507. [DOI] [PMC free article] [PubMed] [Google Scholar]