Abstract
Background
Prior studies attempting to improve end-of-life care have focused on specific outcomes deemed important to healthcare providers, with disappointing results. Improvement may be best achieved by identifying concerns important to individual patients, communicating the patients' concerns to the treating medical team, and repeating the process frequently until all concerns are addressed. Our objective was to conduct a preliminary evaluation of this innovative patient-centred quality improvement strategy.
Methods
Initial interviews elicited participants' ideas for improvement, which were then fed back to health care providers by the study investigator. A rapid-cycle change model ensured frequent reassessment and continued feedback. The study involved 36 seriously ill, hospitalized patients on teaching general medical inpatient units of a tertiary care hospital. The main outcome measure was participants' ratings of satisfaction within different domains of care on follow-up interviews.
Results
The proportion of participants who rated various aspects of their care as "excellent" or "very good" on initial interview was 72% for overall care, 64% for symptom control, 66% for level of support, and 75% for discussions about life sustaining treatments. Patients and families identified many actionable steps for improvement such as; better control of pain and shortness of breath, better access to physicians and medical information, more help with activities of daily living, improving the patient's environment, and shorter waits for nursing care, diagnosis, and treatment. Following feedback to the clinical team, participants reported improvement in overall care (32%), symptom control (44%), and support (40%). Only a minority had further discussions about life sustaining treatments.
Conclusion
A patient-centred approach using rapid-cycle change was feasible and shows promise for improving the quality of end-of-life care. It should be evaluated on a larger sample in a controlled trial.
Keywords: Patient-centred, end-of-life care, quality improvement
Background
Implicit in the question, "What is a good death?" is the question of how to make deaths better. Prior studies of quality improvement in end-of-life care have focused on specific outcomes deemed to be important from the providers' perspective, such as reducing time to first dose of analgesic, educating patients about pain control, or increasing the completion rate of advance directives [1-6]. The largest of these studies, the SUPPORT trial, was unable to demonstrate a significant change in outcome measures dealing primarily with life-sustaining treatment decisions. The focus of the study dealt with physicians' concerns related to end-of-life care. These concerns formed the basis for discussions between study nurses and patients, while patients' concerns were not specifically addressed. In a SUPPORT substudy focused on pain control, a pain intervention was designed, but very few patients received measurable changes in their pain management, and the study was negative [3].
Improvement in end-of-life care may arguably be best grounded in the perspective of individual patients and their families by focusing on issues considered important to them [7-12]. Almost two decades ago Julia Neuberger, former Chief Executive of the King's Fund, met with seriously ill patients in a Boston hospital to identify their concerns and personally fed back these concerns to the medical team and hospital administration (Neuberger J, Personal communication). This strategy involving rapid feedback of individual patients' preferences through direct discussions with the treating medical team has never been formally studied within end-of-life care.
We set out to test the feasibility and potential impact of such a strategy, so we chose a rapid-cycle improvement design involving frequent reassessment and feedback [13]. Our objective was to determine whether this type of patient-centred improvement strategy, with more direct and intense feedback of patient's preferences, might be associated with better patient satisfaction with end of life care. If the results of this preliminary evaluation were encouraging then a larger controlled study would be warranted.
Methods
Participants and setting
This prospective study was conducted in January, February, and April 2002 on the general medical inpatient teaching units at Toronto's University Health Network (UHN), Western Site. A Palliative care consult service was involved in patient care if requested by the treating medical team and continued to provide usual care during the study period. Eligible participants were required to have an estimated prognosis of less then one year, and needed to be aware of their diagnosis and estimated prognosis. The potential participant's prognosis was estimated by the treating medical team. If the participant was unable to respond appropriately to the interviewer (JP) on two separate occasions, a family member or close friend was interviewed instead.
Sampling and sample size
We approached 63 consecutive potentially eligible participants during the study period. Six did not meet eligibility criteria: one patient's prognosis on further testing was deemed to be greater than one year and five patients were incapable yet did not have family members or close friends to interview. Another 21 refused for the following reasons: too tired (5), too busy dealing with their illness (5), not yet ready to talk about their illness (5), and miscellaneous reasons (6). Therefore, our study sample comprised 36 participants.
Strategies for improvement
We used information from patients and families to stimulate change. First, we encouraged patients and families to reflect on their experience and needs [14,15]. Second, we identified specific aspects of care that the patients and families felt were high priority for improvement. Third, we relayed this information to the treating senior medical resident and nursing team leader. A medical resident (JP) who had recently worked on the unit relayed the information. No specific advice was given to caregivers on how to best address each participant's concern. For example, in one interview we determined that a patient's eyes were sore; we informed the treating team the patient's eyes were sore, not how to treat the sore eyes. Finally, follow-up interviews sought participants' views about changes in their care, as well as any new suggestions for feedback. Health care providers and participants were very receptive to the feedback process.
Data collection
One investigator (JP) interviewed all participants. We used a previously developed patient-centred taxonomy of care as a framework for interviews [7]. The interview guide included questions regarding the participants' satisfaction with overall care, symptom control, support from the healthcare team and discussions about life sustaining treatments. Each domain was addressed by both open-ended questions as well as asking the participant to rate their satisfaction on a scale from "excellent" to "poor", or on follow-up interviews "better", "same", or "worse". Follow-up interviews were conducted approximately every four days and were continued until the patient was discharged, declined further interview, or died. An interpreter was used with non-English speaking participants. All interviews were audiotaped and transcribed. Initial interviews took approximately 30–60 minutes; follow-up interviews approximately 15–30 minutes; and feedback to caregivers took approximately 15–30 minutes.
Analysis of data
We listed the concerns identified by the patients and families at initial interview, then grouped these concerns by theme, using content analysis [16]. We summarized the number of patients rating their care as improved, unchanged, or worse after each cycle of interviews and feedback. The main measure of improvement was patient or family ratings of care.
Research ethics
This study was approved by the UHN Committee for Research on Human Subjects. All participants provided written informed consent.
Results
The baseline demographics of the 36 participants are shown in Table 1.
Table 1.
Characteristics of participants
| Baseline Characteristics | |
| Sex | 19 Male, 17 Female |
| Age | Mean 77, Range 49–99 |
| Interviewee | |
| Patients Only | 12 |
| Patient and Family | 3 |
| Family Only | 21 |
| Language | |
| Patient's First Language Not English | 19 |
| Translator required for interview | 8 |
| Resuscitation Status | |
| DNR | 19 |
| Not Addressed at time of initial interview | 12 |
| Addressed yet no DNR order in chart | 2 |
| Specific request | To ICU for reversible causes = 1 |
| No ACLS = 1 | |
| Full Code | 1 |
| Primary Diagnosis | |
| Cancer | 16 |
| Cardiorespiratory | 11 |
| Neurological | 6 |
| Hepatic Disease | 3 |
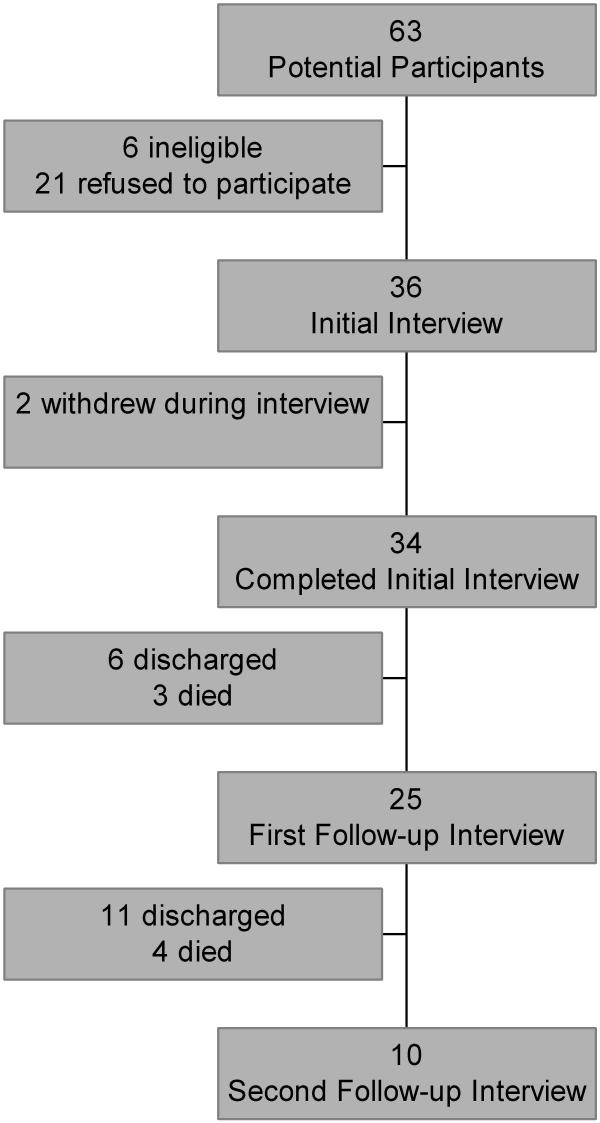
Two participants withdrew because they were too tired to continue with the questioning. Of the remaining 34, 13 patients died in hospital, 20 patients were discharged, and one was alive in hospital at the end of the study period. After the initial interview 6 patients were discharged and 3 died, leaving 25 participants who underwent the first follow-up interview. Eleven patients were discharged and 4 died after the first follow-up interview, leaving 10 participants for the second follow-up interview (See Figure 1).
Figure 1.
Patient follow-up during study period
Initial interview
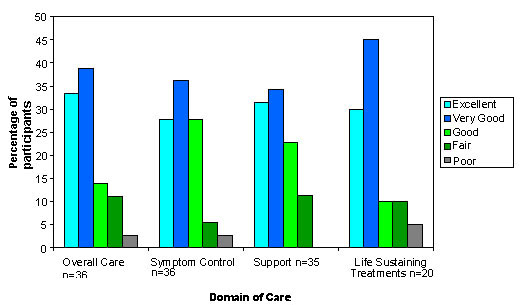
The average number of days from admission to initial interview was 10. Patients were interviewed alone 33% (12 of 36) of the time, families were interviewed alone 58% (21 of 36) and patients and families were interviewed together 8% (3 of 36) of the time. Satisfaction with end-of-life care on the initial interview is shown in Figure 2. The proportion of participants who rated various aspects of their care as "excellent" or "very good" was 72% for overall care, 64% for symptom control, and 66% for level of support. The median response for all domains was "very good". 20 (57%) of the participants reported discussions with their treating team about what treatments they might want if they were to get sicker. Of these, 75% rated their satisfaction with these discussions as "excellent" or "very good". The median response was "very good". Of the 16 individuals who did not have discussions about life sustaining treatment issues at the time of initial interview only 2 requested further discussions as a point for feedback.
Figure 2.
Participants' ratings of satisfaction with care at time of initial interview
The initial concerns or issues raised by the participants and relayed to the health care team are grouped according to theme as shown in Table 2.
Table 2.
Participant priorities for improving care
| Issue | Frequency |
| Alleviate Symptoms | 34 |
| Pain | 9 |
| Shortness of breath | 7 |
| Thirst | 3 |
| Other symptoms (gastrointestinal, cough, agitation oversedation, headache, feeling cold, dry skin, itching) | 15 |
| Reduce Delays | 28 |
| Delays in daily bedside care | 9 |
| Delays in diagnosis or treatment | 8 |
| Delay in transfer from emergency room to ward | 6 |
| Delays in discharge to home | 5 |
| Improve Daily Care | 29 |
| Assist with activities of daily living | 9 |
| Improve hospital environment | 6 |
| Arrange help at home | 4 |
| Personalize medical treatment | 3 |
| Improve food | 3 |
| Ensure staff are aware of special care needs | 2 |
| Coordinate timing of diagnostic tests | 1 |
| Obtain palliative care consultation | 1 |
| Better Access to MDs/Medical information | 11 |
| Improve Therapeutic Alliance | 10 |
| Identify responsible physician | 3 |
| Avoid unprofessional behaviors and comments | 3 |
| Reduce changes in nursing staff | 2 |
| Avoid repetitive questioning | 1 |
| Apologize for medical error | 1 |
| Address Emotions | 9 |
| Fear of abandonment | 7 |
| Loneliness | 2 |
| Discuss Life Sustaining Treatments | 8 |
| Further discussions | 5 |
| Prior discussion should have been done differently | 3 |
| Provide Personal Support | 3 |
| Write letter so family can visit from outside country | 1 |
| Wants to see chaplain | 1 |
| Want someone to speak to my loved one in their native language about their medical condition | 1 |
First follow-up interview
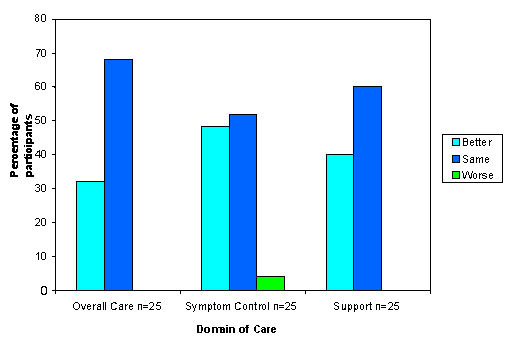
The follow-up data for overall care, symptom control and support are shown in Figure 3. Following feedback to the clinical team, participants reported improvements in overall care (32%), symptom control (44%), and support (40%). One participant rated their initial satisfaction with symptom control as being excellent, yet at follow-up interview felt it was worse because of the new onset of painful oral ulcers.
Figure 3.
Participants' evaluation of the change in care at time of follow-up interview one
Of the 25 participants who had at least one follow-up interview, only six reported subsequent discussions about life sustaining treatments. Five of the six rated their satisfaction with these discussions as the same, and one rated their satisfaction as better. Four of the six had identified the need for further discussions at the time of the initial interview.
Second follow-up interview
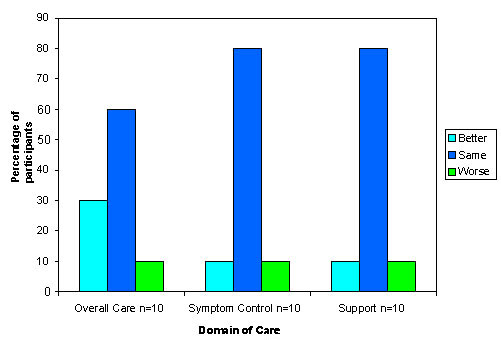
As shown in Figure 4, the responses from the second follow-up interview show less improvement but the trend continues for overall care with 30% of this small sample saying their care had improved, and only 10% saying it had worsened. There was no continued improvement for symptom control and support. Only three participants reported subsequent discussions about life sustaining treatment at second follow-up. Two of the three rated their satisfaction with discussions as the 'same', and one rated their satisfaction as better. Two of the three had identified the need for further discussions at the previous interviews.
Figure 4.
Participants' evaluation of the change in care at time of follow-up interview two
Discussion
To our knowledge, this is the first evaluated attempt in a clinical setting to improve end-of-life care by combining rapid-cycle change and a patient-centred perspective. In our patient population we found plenty of room for improvement with 28% of patients and families reporting their satisfaction with overall care as only "good", "fair", or "poor." Patients and families identified actionable steps for improvement such as, better control of pain and shortness of breath, better access to physicians and medical information, more help with activities of daily living, improvement of the patient's environment and shorter waits for nursing care, diagnosis, and treatment. Our strategy was associated with improvements in ratings of overall care, symptom control, and support. We made little improvement in the area of discussions regarding life sustaining treatments as few patients asked for further discussions on life sustaining treatment issues and such discussions rarely occurred without feedback.
The reported improvement in multiple domains of end-of-life care in our study is in contrast to the disappointing results of previous trials to improve end-of-life care [3,4]. The largest of these studies, the SUPPORT trial failed to change physicians' behaviors or measurable outcomes [4]. One possible reason for the SUPPORT trials' lack of significant change was the untargeted focus on life-sustaining treatments or pain. By contrast, we focused on issues deemed important by individual patients, and found pent up concerns of dying patients and their families that had not been addressed by the standard provider-focused approach to end-of-life care. Few patients identified life sustaining treatment discussions as a priority, and for many of our patients, pain was not the primary symptom of concern. Our approach released patients' concerns into the care process allowing for individually important change to occur. We strived for frequent and complete feedback to our treating physicians. In the SUPPORT study, although complete feedback was the intent, only 34% of study physicians recalled receiving any feedback [3].
The rapid-cycle change model utilized in this study has had prior successes in end-of-life care. In 1997 the Institute for Healthcare Improvement and the Centre to Improve Care of the Dying used a rapid cycle change program to improve important processes of care, such as time from request to first dose of analgesia [1,2]. The flexibility of the rapid cycle change method may be well suited for the complexities of end of life care.
There are several limitations to this study. First, there is no concurrent control group so we do not know what participants' perceptions would have been in the absence of any intervention. In our attempt to initiate change we tested this innovative approach for feasibility and utility. It would have been premature to do a controlled trial, but now that we have defined a clear intervention it would be reasonable to proceed with such a study. Second, our data reflects the experiences of a small group of patients at a single hospital. However, despite the relatively small sample size, we were able to show that our innovative approach led to improvement. Third, the intervention and satisfaction assessments were performed by a single resident physician (JP) known to the healthcare staff. The positive effect of the intervention may reflect the popularity and enthusiasm of the interviewer. However, the interviewer (JP) used a standardized questionnaire to identify participant's concerns and acted simply as a conduit between participants and their caregivers. The changes in care were carried out by many different members of the healthcare team. We believe that the major impact was the feedback of patients' concerns, and the role of the individual interviewer was secondary. Finally, there were a relatively large number of potential participants (33.3%) who refused to participate. The number of refusals did not surprise us, as it is consistent with prior work with severely ill patients [1,2]. Many patients refused because they were too tired, too busy dealing with illness, or not read to talk about their illness. We suspect that these potential participants were not completely satisfied with their care, and would also have responded to a patient-centred quality improvement strategy
Our study tested the utility of an innovative initiative to induce change within end-of-life care. We found that our patient-centred approach using rapid cycle change was feasable and shows promise for improving the quality of end-of-life care. It should be evaluated on a larger sample in a controlled trial
Authors' contributions
JP interviewed all participants and communicated feedback to the treating medical teams. All investigators were involved in development of the quality improvement strategy, in data analysis and manuscript preparation.
Competing interests
None declared.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
DKM is supported by an Ontario Ministry of Health and Long-Term Care Career Scientist award. PAS is supported by a Canadian Institutes of Health Research Distinguished Investigator award. This research project was support by The University Health Network and the University of Toronto Joint Centre for Bioethics. We would like to thank the staff on 8A, 8B and 3A Fell for their assistance in implementing participants' suggestions for improvement.
Contributor Information
Jeff Powis, Email: JeffEPowis@aol.com.
Edward Etchells, Email: edward.etchells@sw.ca.
Douglas K Martin, Email: douglas.martin@utoronto.ca.
Susan K MacRae, Email: sue.macrae@utoronto.ca.
Peter A Singer, Email: peter.singer@utoronto.ca.
References
- Lynn J, Schall M, Milne C, Nolan K, Kabcenell A. Quality in end of life care: insights from two collaboratives. Jt Comm J Qual Improv. 2000;26:254–267. doi: 10.1016/s1070-3241(00)26020-3. [DOI] [PubMed] [Google Scholar]
- Lynn J, Nolan K, Kabcenell A, Weissman D, Milne C, Berwick DM. Reforming care for persons near the end of life: the promise of quality improvement. Ann Intern Med. 2002;137:117–122. doi: 10.7326/0003-4819-137-2-200207160-00010. [DOI] [PubMed] [Google Scholar]
- Desbiens NA, Wu AW, Yasui Y, Lynn J, Alzola C, Wenger NS, Connors A.F.,Jr., Phillips RS, Fulkerson W. Patient empowerment and feedback did not decrease pain in seriously ill hospitalized adults. Pain. 1998;75:237–246. doi: 10.1016/S0304-3959(97)00225-X. [DOI] [PubMed] [Google Scholar]
- A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- Danis M, Southerland LI, Garrett JM, Smith JL, Hielema F, Pickard CG, Egner DM, Patrick DL. A prospective study of advance directives for life-sustaining care. N Engl J Med. 1991;324:882–888. doi: 10.1056/NEJM199103283241304. [DOI] [PubMed] [Google Scholar]
- Schneiderman LJ, Kronick R, Kaplan RM, Anderson JP, Langer RD. Effects of offering advance directives on medical treatments and costs. Ann Intern Med. 1992;117:599–606. doi: 10.7326/0003-4819-117-7-599. [DOI] [PubMed] [Google Scholar]
- Singer PA, Martin DK, Kelner M. Quality end-of-life care: patients' perspectives. JAMA. 1999;281:163–168. doi: 10.1001/jama.281.2.163. [DOI] [PubMed] [Google Scholar]
- Steinhauser KE, Christakis NA, Clipp EC, McNeilly M, McIntyre L, Tulsky JA. Factors considered important at the end of life by patients, family, physicians, and other care providers. JAMA. 2000;284:2476–2482. doi: 10.1001/jama.284.19.2476. [DOI] [PubMed] [Google Scholar]
- Cleary PD, Edgman-Levitan S. Health care quality. Incorporating consumer perspectives. JAMA. 1997;278:1608–1612. doi: 10.1001/jama.278.19.1608. [DOI] [PubMed] [Google Scholar]
- Wensing M, Elwyn G. Methods for incorporating patients' views in health care. BMJ. 2003;326:877–879. doi: 10.1136/bmj.326.7394.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good care of the dying patient. Council on Scientific Affairs, American Medical Association. JAMA. 1996;275:474–478. [PubMed] [Google Scholar]
- Hanson LC, Danis M, Garrett J. What is wrong with end-of-life care? Opinions of bereaved family members. J Am Geriatr Soc. 1997;45:1339–1344. doi: 10.1111/j.1532-5415.1997.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Berwick DM. A primer on leading the improvement of systems. BMJ. 1996;312:619–622. doi: 10.1136/bmj.312.7031.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepucha KR, Belkora JK, Mutchnick S, Esserman LJ. Consultation planning to help breast cancer patients prepare for medical consultations: effect on communication and satisfaction for patients and physicians. J Clin Oncol. 2002;20:2695–2700. doi: 10.1200/JCO.2002.10.068. [DOI] [PubMed] [Google Scholar]
- Greenfield S, Kaplan S, Ware J.E.,Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102:520–528. doi: 10.7326/0003-4819-102-4-520. [DOI] [PubMed] [Google Scholar]
- Manning PK, Cullum-Swan B. Narrative, content and semiotic analysis. In: DenzinNK and LincolnYS, editor. Handbook of Qualitative Research. Thousand Oaks, Sage Publications Inc.; 1994. pp. 463–477. [Google Scholar]