Abstract
Recent studies suggest a relationship between intestinal microbiota and metabolic syndromes; however, the underlying mechanism remains unclear. To clarify this issue, we assessed the effects of bacterial cell wall components on adiponectin, leptin and resistin secretion from rat visceral adipocytes in vitro. We also measured the relative population of Firmicutes and Bacteroidetes in fecal microbiota and the amount of fecal mucin as an intestinal barrier function, when mice were fed a high-fat diet. In the present study, we demonstrated that bacterial cell wall components affect the secretion of adipokines, depending on the presence of antigens from gram-positive or gram-negative bacteria. Lipopolysaccharide markedly inhibited adiponectin, leptin, and resistin secretion, whereas peptidoglycan increased adiponectin secretion and decreased resistin secretion in vitro. In vivo experiments showed that the high-fat diet increased the population of Firmicutes and decreased that of Bacteroidetes. In contrast, the high-fat diet downregulated the stool output and fecal mucin content. These results demonstrate that bacterial cell wall components affect the onset of metabolic syndromes by mediating the secretion of adipokines from visceral adipose tissue. Furthermore, we believe that metabolic endotoxemia is not due to the increasing dominance of gram-negative bacteria, Bacteroidetes, but due to the depression of intestinal barrier function.
Keywords: gut microbiota, visceral adipocyte, adipokine secretion, lipopolysaccharide, peptidoglycan
Introduction
There is accumulating evidence supporting the notion that intestinal microbiota plays a significant role in the maintenance of health.(1–3) Recent studies, both epidemiological and clinical, suggest a relationship between intestinal microbiota and the onset of obesity and metabolic syndromes.(4) Cani et al.(5) showed that bacterial lipopolysaccharide (LPS) is a triggering factor for the onset of insulin resistance, obesity, diabetes, and metabolic endotoxemia. However, the underlying cellular and molecular mechanisms remain unclear.
It was reported that the plasma concentration of adiponectin strongly correlates with conditions associated with metabolic syndromes, such as diabetes, hypertension, and atherosclerosis.(6) A negative correlation was observed between plasma adiponectin levels and visceral obesity.(7) Moreover, low adiponectin concentrations are associated with a high incidence of some tumors, including colorectal, gastric, and prostate.(7–10) Adiponectin is secreted by adipose tissue, predominantly by visceral adipose tissue, and the regulatory mechanism is unknown despite the importance of adiponectin in metabolism. Leptin was discovered in association with its role in satiety and energy balance, and it was assumed that leptin is an anti-obesity factor that functions via a feedback loop from adipocytes to the hypothalamus. Resistin, a small cysteine-rich protein, is a major factor associated with insulin resistance in obesity. Resistin induces insulin resistance in the liver, muscle, and fat tissue. In addition, a recent study showed that resistin downregulates glucose-induced insulin release.(11) Adiponectin, leptin, and resistin are produced and secreted by adipose tissue and are termed adipokines.
In this study, we analyzed the effects of bacterial cell wall components on the secretion of adipokines (adiponectin, leptin, and resistin) using a recently established rat visceral adipocyte culture system. This system that we established using stromal vascular cells (SVCs) isolated from mesenteric adipose tissue is a powerful tool for evaluating adipocyte properties such as differentiation and adipokine secretion.(11) The culture system does not contain any synthetic compounds generally used to promote adipogenesis, such as indomethacin, dexamethasone, or peroxisome proliferator-activated receptor-γ agonists. Therefore, we can study adipocyte function directly without the influence of any artificial agent.
Bacteria form symbiotic relationships with many species, including humans, and they live inside the human digestive system. The progress of molecular biology has paved the way for analyzing intestinal microbiota without any cultivating bacteria. Another advantage of the genomic analysis tool is the ability to not only detect an increase or decrease in the size of a population of a single strain but also to analyze microbiota at all taxonomic levels: kingdom, phylum, class, order, family, genus, and species. It has been reported that 80–90% of bacterial phylotypes are members of 2 phyla, namely, Bacteroidetes (including genera Bacteroides and Prevotella) and Firmicutes (including genera Clostridium, Enterococcus, Lactobacillus, and Ruminococcus). Therefore, relative proportions of Firmicutes and Bacteroidetes are considered good indicators of major changes in the composition of intestinal microbiota. Many phylum-level analysis using Firmicutes- or Bacteroidetes-specific primers have shown associations between obesity and a reduced proportion of the phylum Bacteroidetes, accompanied by an increased proportion of Firmicutes.(12–14) Therefore, we investigated whether the proportion of Bacteroidetes, gram-negative bacteria, in mouse feces is increased during a high-fat diet. Furthermore, we measured the fecal mucin content to determine the intestinal barrier function.
Here we show that adipokine secretion from visceral adipocytes was regulated depending on the presence of cell wall components from gram-positive or gram-negative bacteria. In vitro experiments demonstrate that cell wall components derived from intestinal microbiota affect the pathogenesis of metabolic syndromes by mediating the secretion of adipokines, such as adiponectin, leptin, and resistin, from visceral adipose tissue. Futhermore, our results suggest that metabolic endotoxemia is not due to the increasing dominance of gram-negative bacteria but by the depression of intestinal barrier function.
Materials and Methods
Materials
Collagenase (type II) was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Dulbecco’s modified Eagle’s medium nutrient mixture F-12 (DMEM/F12; 1:1) and newborn calf serum (NCS) were purchased from Invitrogen Corporation, Japan. Penicillin, streptomycin, pantothenic acid, biotin, ascorbic acid, octanoic acid, triiodothyronine, and nicotinamide were purchased from Sigma–Aldrich (St. Louis, MO). LPS from Escherichia coli, lipoteichoic acid (LTA) from Staphylococcus aureus, and N-acetylmuramyl-l-alanyl-d-isoglutamine hydrate (MDP) were also purchased from Sigma–Aldrich. In addition, peptidoglycan (PGN) from S. aureus was purchased from Fluka Corporation (Milwaukee, WI). Glass-bottom dishes were purchased from Iwaki Co., Ltd (Tokyo, Japan).
Cell culture
Rat mesenteric preadipocytes were isolated from Sprague–Dawley rats and cultured according to the method developed in our laboratory.(15) Briefly, the mesenteric SVC fraction was prepared from rat mesenteric adipose tissue using collagenase digestion. SVCs (0.5 × 105 cells/cm2) were seeded in 24-well plastic culture plates and cultured in DMEM/F12 (1:1) containing 10% NCS, 100 units/ml penicillin, 100 µg/ml streptomycin, 17 µM pantothenic acid, 33 nM biotin, 100 µM ascorbic acid, 1 µM octanoic acid, 50 nM triiodothyronine, and 2.5 mM nicotinamide. The cells were cultured in a humidified atmosphere containing 5% CO2 in air, and the medium was changed every other day until the end of the experiments.
Stimulation with bacterial cell wall components
On day 6 after the start of culturing, bacterial cell wall components were added to the culture medium, and they were cultured for another 4 days. Following which, the culture supernatants were collected and stored at –80°C until further analysis. Bacterial cell wall components included LPS (100 ng/ml) from E. coli, lipoteichoic acid from S. aureus (100 ng/ml), MDP (100 ng/ml) and PGN (100 ng/ml) from S. aureus.
Quantification of adipokines secreted by visceral adipocytes
The concentration of adiponectin in the culture supernatant was determined using a mouse/rat adiponectin ELISA kit (Otsuka Pharmaceutical Co., Ltd., Tokyo, Japan). Concentrations of leptin and resistin in the culture supernatant were determined using a rat leptin ELISA kit and rat resistin ELISA kit (BioVentor Laboratory Medicine, Inc., Brno, Czech Republic), respectively.
Immunohistological staining for cellular adiponectin
Rat visceral adipocytes were precultured on glass-bottom dishes for 6 days and stimulated using LPS (100 ng/ml) from E. coli for 4 days. Cells in the culture were fixed in 4% formaldehyde, blocked with 2% bovine serum albumin, stained with a biotin-conjugated antiadiponectin antibody for 1 h at room temperature, and then incubated with Quantum Dot 655-conjugated streptavidin (Chemicon International Inc., Billerica, MA) under the same conditions. Further, cell nuclei were stained with H33258 (Wako Pure Chem., Ind., Ltd., Osaka, Japan) and examined under a fluorescence microscope.
Feeding of mice
The animals were maintained in accordance with the Hokkaido University guidelines for the care and use of laboratory animals. The cages were placed in a room with controlled temperature (21–23°C) and lighting (light from 08:00 to 20:00 h). All animals had free access to tap water and a solid laboratory diet (CE-2, Japan Clea, Tokyo, Japan) during the feeding duration before tissue collection. Four-week-old mice (Sankyo Labo Service Corporation Inc., Tokyo, Japan) were housed at 1 mouse per cage in a controlled environment (12-h light cycle). After 1 week of acclimatization, mice were randomly distributed into 2 groups (n = 5) and thereafter were fed experimental diets with free access to drinking water. The 2 experimental diets were a standard diet (CE2) and a high-fat diet (High Fat Diet 32).
Fecal samples
After 55 days, mice were placed in clean cages with bedding for 24 h. All feces from each cage were collected, weighed, air-dried, ground in a mortar, and stored at −20°C until analysis.
DNA extraction from fecal samples and quantitative PCR
One hundred mg of ground fecal samples were weighted before DNA extraction. DNA was extracted using QIAamp DNA Stool Mini Kit (Qiagen, Venlo, Netherlands) according to the instruction manual, and the DNA concentration was determined using NanoDrop. Phylum-level analysis was performed on 3 phyla of intestinal bacteria: Bacteroidetes, Firmicutes, and all bacteria combined using a slight modification of the method described by Guo et al.(16) The relative population of Bacteroidetes and Firmicutes to all bacteria were quantified on the basis of amplification of genomic DNA coding 16S ribosomal RNA (rRNA) using PCR with group-specific primers.(16) For the analysis of Firmicutes, the primers Firm934F (5'-GGAGYATGTGGTTTAATTCGAAGCA) and Firm1060R (5'-AGCTGACGACAACCATGCAC) were used. For the analysis of Bacteroidetes, we used Bact934F (5'-GGARCATGTGGTTTAATTCGATGAT) and Bact1060R (5'-AGCTGACGACAACCATGCAG). For the analysis of all bacteria, foward (5'-ACTCCTACGGGAGGCAGCAGT) and reverse (5'-AGTATTACCGCGGCTGCTGGCAC) were used. Real-time PCR was conducted using LightCycler 480 (Roche Applied Science, Manheim German) according to the SYBR Green I Master protocol.
Fecal mucin analysis
Fecal mucin content was determined using a fluorimetric assay that discriminates O-linked glycoproteins (mucins) from N-linked glycoproteins.(17–19)
Statistical analysis
The data were expressed as mean ± SD (in vitro experiments: 4 wells/data point, in vivo experiments: 5 mice/data point). A statistical analysis was conducted using Student’s t test.
Results
Lipopolysaccharide inhibits adiponectin secretion
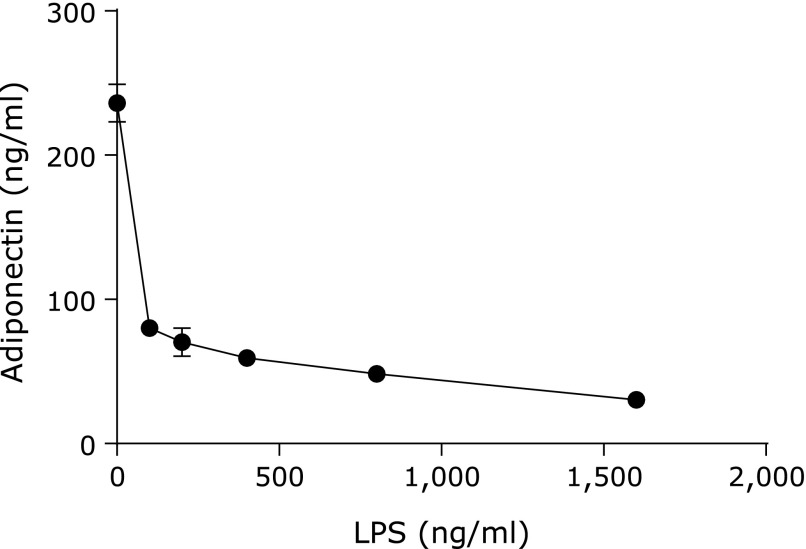
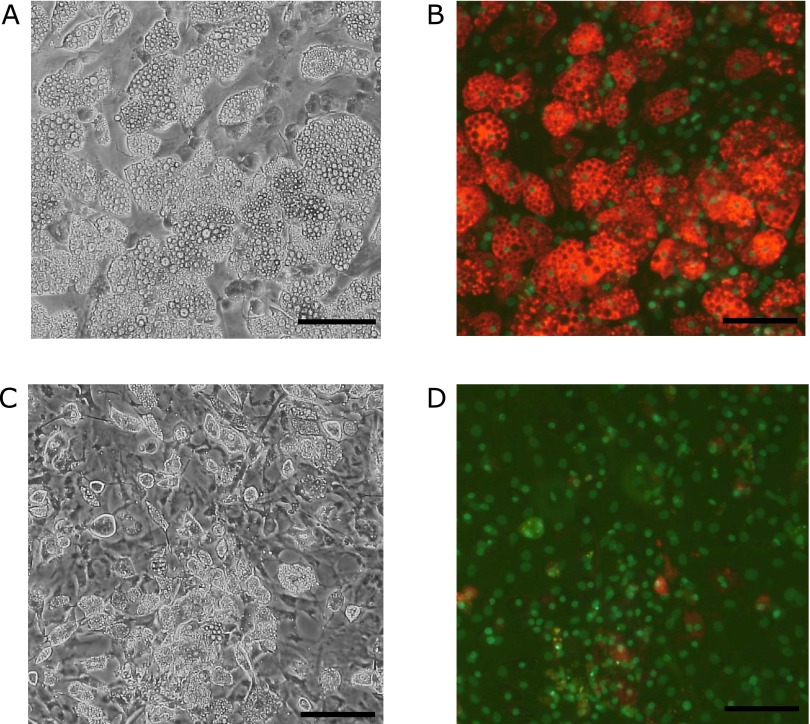
We first tested whether bacterial antigens of gram-negative bacteria affect adiponectin secretion. LPS markedly inhibited adiponectin secretion from visceral adipocytes in a dose-dependent manner (range: 100–1,600 ng/ml) (Fig. 1). In addition, in the immunohistochemical staining for cellular adiponectin, we found that the stimulation with LPS caused a disappearance of adiponectin from the cytosol of adipocytes (Fig. 2). Cell nuclei stained with H33258 showed that these cells were alive even after the addition of LPS to the culture.
Fig. 1.
Effects of LPS concentration on adiponectin secretion. Adiponectin concentration in culture supernatants after stimulation with LPS was determined using an adiponectin ELISA kit (n = 4) as described in Materials and Methods, sections 2.3 and 2.4.
Fig. 2.
Immunohistochemical staining for cellular adiponectin. Adiponectin was stained in visceral adipocytes as described in Materials and Methods, section 2.6. Red: adiponectin, green: nuclei (H33258 stain). A, B: control; C, D: 100 ng/ml LPS was added. The scale bar is 100 µm.
Cell wall components of gram-positive bacteria stimulate adiponectin secretion
Stimulation with cell wall components from gram-positive bacteria, such as LTA, MDP, and PGN, significantly enhanced adiponectin secretion, but the rate of increases was pretty low (Fig. 3A). Under the same culture conditions, LPS stimulation markedly inhibited adiponectin secretion (Fig. 3A); this result was consistent with those stated above (Fig. 1).
Fig. 3.
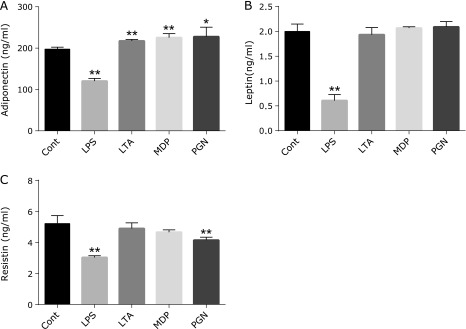
Effects of bacterial cell wall components on adipokine secretion. One hundred ng/ml LPS, lipoteichoic acid (LTA), N-acetylmuramyl-l-alanyl-d-isoglutamine hydrate (MDP), or peptidoglycan (PGN) was added to the visceral adipocyte culture on day 6 after the start of culturing. After further incubation for 48 h, the adiponectin, leptin, and resistin concentrations in the culture supernatant were measured using ELISA kits. A: adiponectin (n = 4), B: leptin (n = 4), C: resistin (n = 4). *p<0.05, **p<0.01 vs control.
Effects of bacterial cell wall components on leptin and resistin secretion
We further tested the adipocyte secretion of other adipokines, leptin, and resistin, after stimulation with bacterial cell wall components. LPS stimulation inhibited the secretion from adipocytes of not only adiponectin but also of leptin and resistin (Fig. 3B and C). Cell wall components of gram-positive bacteria had no effect on leptin secretion (Fig. 3B). PGN reduced resistin secretion, whereas LTA and MDP at the concentration of 100 ng/ml had no effect on resistin secretion by the cultured cells.
Body weight, Firmicutes/Bacteroidetes ratio, stool output, and fecal mucin content
Mice fed with the high-fat diet for 8 weeks showed significantly elevated body weight compared with those fed with the standard diet (Fig. 4A). The high-fat diet decreased stool output and fecal mucin content significantly compared with the standard diet (Fig. 4B and D). Moreover, the analysis of composition of intestinal microbiota revealed that the population of Firmicutes increased and that of Bacteroidetes decreased under the influence of the high-fat diet (Fig. 4C).
Fig. 4.
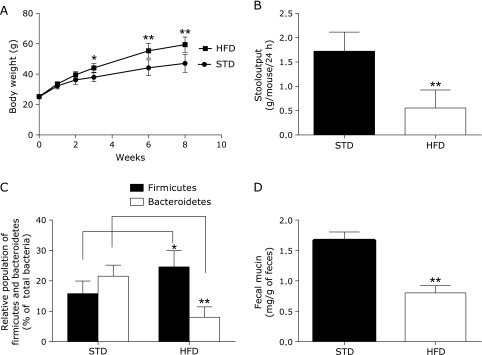
A: Time course of body weight in control mice and mice fed with high-fat diet (with induced obesity). B: Stool output from control mice and mice with high-fat diet-induced obesity. C: Relative population of Firmicutes and Bacteroidetes phyla to all bacteria. D: Fecal mucin contents of control mice and mice with high-fat diet-induced obesity. Fecal mucin content was determined using a fluorimetric assay that discriminates O-linked glycoproteins (mucins) from N-linked glycoproteins. STD: standard diet (n = 5); HFD: high-fat diet group (n = 5). *p<0.05, **p<0.01 vs STD.
Discussion
The present study showed a clear difference in adipokine secretion from visceral adipocytes between the cells stimulated with LPS (the main cell wall component of gram-negative bacteria) and those stimulated with PGN (the main cell wall component of gram-positive bacteria). Adiponectin secretion from visceral adipocytes was downregulated by the addition of LPS, whereas it was significantly upregulated by that of PGN, LTA, MDP, but the rate of increase was pretty low. There are several possible mechanisms behind these findings, including the possibilities that cell wall components directly affect adipocytes or that the influence of those components is mediated by other cells present in the culture. For example, tumor necrosis factor α (TNF-α) is known to suppress the expression and secretion of adiponectin from adipocytes.(20–22) Our visceral adipocyte culture system contains a number of macrophages, and it is possible that the suppression of adiponectin secretion was due to TNF-α secreted by the macrophages. To address this question, we quantified TNF-α in our culture medium after LPS was added, and we found that TNF-α levels were very low (data not shown), indicating that other suppression mechanisms may be involved.
Immunohistological staining for cellular adiponectin showed that almost all adiponectin pooled in the cytosol of adipocytes disappeared by stimulation with LPS (100 ng/ml) for 4 days (Fig. 2). However, even after 4 days of stimulation with the same concentration of LPS, adiponectin secreted into the culture medium was still detectable (Fig. 1). We supposed that the synthesis of adiponectin was stopped by the addition of LPS, but adiponectin secretion may be accelerated. Only adiponectin pooled in the cytosol was secreted into the medium, and this adiponectin may be detected.
Our data show that stimulation with LPS also suppressed leptin secretion. Originally, leptin was discovered in association with its role in satiety and energy balance and was assumed to be an antiobesity factor that functions via a feedback loop from adipocytes to the hypothalamus. The absorption of LPS into intestinal capillaries may be one of the key factors leading to the development of obesity. Our results showed that stimulation with peptidoglycan increased adiponectin secretion and decreased resistin secretion. Altogether, the translocation of bacterial cell wall components from gram-positive bacteria, including Lactobacillus and Bifidobacterium, into intestinal capillaries may help to prevent the medical conditions associated with metabolic syndromes such as diabetes, hyperglycemia, hypertension, and atherosclerosis via the modulation of adipokine secretion from visceral adipose tissue. Because there is a strong evidence of a correlation between low serum levels of adiponectin and many types of cancer, such as colorectal and prostate cancer.(7–10) Our results suggest that bacterial components may control the incidence of some cancers via adiponectin secretion from visceral adipocytes.
Our in vivo experiments showed that the high-fat diet increased the population of Firmicutes and decreased that of Bacteroidetes (Fig. 4C). In contrast, the high-fat diet decreased stool output and fecal mucin content significantly (Fig. 4B and D). These results suggest that the metabolic endotoxemia,(5,23–25) induced by the high-fat diet, is not due to the increasing dominance of gram-negative bacteria (Bacteroidetes) but due to the depression of intestinal barrier function. We concluded that the disturbance of the gut microbiota induced by the high-fat diet may be a consequence of the depression of intestinal barrier function. Because endogenous LPS is continually supplied to the gut as a result of the death of gram-negative bacteria, the depression of intestinal barrier function causes a direct absorption of LPS into intestinal capillaries and allows for bacterial translocation.
In summary, we present that cell wall components derived from the enteric microbiota affect and possibly control the pathogenesis of metabolic syndromes via the regulation of adipokine secretion from visceral adipose tissue. However, our results suggest that metabolic endotoxemia is not due to the increasing dominance of gram-negative bacteria, Bacteroidetes, but by the depression of intestinal barrier function. Some papers showed that serum adiponectin level was reduced, but serum reptin level was elevated when the mice were fed with HFD.(26–28) There is discrepancy of leptin-behavior between in vitro experiment and in vivo experiment. Further studies are required to elucidate the cellular and molecular mechanisms and to verify the proposed concept.
Acknowledgments
This work was supported by a grant-in-aid for Frontier Technology Research from the Northern Advancement Center for Science and Technology (NOASTEC) of Japan and a grant-in-aid for Knowledge Cluster Phase II, Sapporo Bio-S from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
- DMEM
Dulbecco’s modified Eagle’s medium
- HFD
high-fat diet
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- MDP
N-acetylmuramyl-l-alanyl-d-isoglutamine hydrate
- NCS
newborn calf serum
- PGN
peptidoglycan
- PPAR
peroxisome proliferator-activated receptor
- STD
standard diet
- SVC
stromal vascular cell
- TNF
tumor necrosis factor
- VAC
visceral adipocyte
Conflict of Interest
This study was funded by collaborative research Cosmo Bio Co., Ltd., Primary Cell Division and Department of Cell Biological Science, Faculty of Advanced Life Science, Graduate School of Life Science, Hokkaido University.
Sayori Yamaguchi, Kyoko Shimizu and Toshio Taira are employees of Cosmo Bio Co., Ltd., Primary Cell Division. Risa Taira is student of Graduate School of Life Science, Hokkaido University. Kiminori Nakamura and Tokiyoshi Ayabe are employees of Hokkaido University.
References
- 1.Mitsuoka T. The effect of nutrition on intestinal flora. Nahrung. 1984;28:619–625. doi: 10.1002/food.19840280616. [DOI] [PubMed] [Google Scholar]
- 2.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 3.Membrez M, Blancher F, Jaquet M, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J. 2008;22:2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 4.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;22:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 6.Nishida M, Funahashi T, Shimomura I. Pathophysiological significance of adiponectin. Med Mol Morphol. 2007;40:55–67. doi: 10.1007/s00795-007-0366-7. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzawa Y, Shimomura I, Kihara S, Funahashi T. Importance of adipocytokines in obesity-related diseases. Horm Res. 2003;60:56–59. doi: 10.1159/000074502. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–5704. [PubMed] [Google Scholar]
- 9.Goktas S, Yilmaz MI, Caglar K, Sonmez A, Kilic S, Bedir S. Prostate cancer and adiponectin. Urology. 2005;65:1168–1172. doi: 10.1016/j.urology.2004.12.053. [DOI] [PubMed] [Google Scholar]
- 10.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]
- 11.Nakata M, Okada T, Ozawa K, Yada T. Resistin induced insulin resistance in pancreatic islets to impair glucose-induced insulin release. Biochem Biophys Res Commun. 2007;353:1046–1051. doi: 10.1016/j.bbrc.2006.12.134. [DOI] [PubMed] [Google Scholar]
- 12.Gauffin Cano P, Santacruz A, Moya Á, Sanz Y. Bacteroides uniformis CECT 7771 ameriolates metabolic and immmunological dysfunction in mice with high-fat-diet induced obesity. PLoS ONE. 2012;7:e41079. doi: 10.1371/journal.pone.0041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ecburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimizu K, Sakai M, Ando M, et al. Newly developed primary culture of rat visceral adipocytes and their in vitro characteristics. Cell Biol Int. 2006;30:381–388. doi: 10.1016/j.cellbi.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in feces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol. 2008;47:367–373. doi: 10.1111/j.1472-765X.2008.02408.x. [DOI] [PubMed] [Google Scholar]
- 17.Bovee-Oundenhoven IM, Termont DS, Heidt PJ, van der Meer R. Increasing the intestinal resistance of rats to the invasive pathogen Salmonella enteritis: additive effects of dietary lactulose and calcium. Gut. 1997;40:497–504. doi: 10.1136/gut.40.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crowther RS, Wetmore RF. Fluorometric assay of O-linked glycoproteins by reaction with 2-cyanoacetamide. Anal Biochem. 1987;163:170–174. doi: 10.1016/0003-2697(87)90108-4. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki Y, Tomotake H, Tsujimoto K, Sasaki M, Kato N. Consumption of a resistant protein, sericin, elevates fecal immunoglobulin A, mucin, and cecal organoic acids in rats fed a high-fat diet. J Nutr. 2011;141:1975–1981. doi: 10.3945/jn.111.144246. [DOI] [PubMed] [Google Scholar]
- 20.Lumeng CL, Bodzin JL, Saltiel AR. Obesity induces a phenotype switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda N, Takahashi M, Funahashi T, et al. PPAR gamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 22.Lim JY, Kim WH, Park SI. GO6976 prevents TNF-alpha-induced suppression of adiponectin expression in 3T3-L1 adipocytes: putative involvement of protein kinase C. FEBS Lett. 2008;582:3473–3478. doi: 10.1016/j.febslet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Cani PD, Neyrinck AM, Fava F, et al. Selective increase of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 24.Griffiths EA, Duffy LC, Schanbacher FL, et al. In vivo effects of bifidobacteira and lactoferrin on gut endotoxin concentration and mucosal immunity in Balb/c mice. Dig Dis Sci. 2004;94:579–589. doi: 10.1023/b:ddas.0000026302.92898.ae. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Xiao G, Yao Y, Guo S, Lu K, Sheng Z. The role of bifidobacteria in gut barrier function after thermal injury in rats. J Trauma. 2006;61:650–657. doi: 10.1097/01.ta.0000196574.70614.27. [DOI] [PubMed] [Google Scholar]
- 26.Gu M, Zhang Y, Fan S, Ding X, Ji G, Huang C. Extracts of Rhizoma Polygonati Odorati prevent high-fat diet-induced metabolic disorders in C57BL/6 mice. PLoS ONE. 2013;8:e81724. doi: 10.1371/journal.pone.0081724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Choi HN, Kang MJ, Choe E, Auh JH, Kim JI. Chamnamul [Pimpinella brachycarpa (Kom.) Nakai] ameliorates hyperglycemia and improves antioxidant status in mice fed a high-fat, high-sucrose diet. Nutr Res Pract. 2013;7:446–452. doi: 10.4162/nrp.2013.7.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu T, Tang Q, Gao Z, et al. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE. 2013;8:e77585. doi: 10.1371/journal.pone.0077585. [DOI] [PMC free article] [PubMed] [Google Scholar]