Abstract
The aim of this study was to compare biological actions between isopropanol and ethanol extracts of Artemisia including antioxidant, anti-inflammatory, and cytoprotective actions. Antioxidant activities were evaluated using 2,2-diphenyl-1-picrylhydrazyl (DPPH) method and confocal microscopy on lipopolysaccharide-induced RGM1 cells, cytoprotection effects evaluated by detecting heme oxygenase-1 (HO-1), Nf-E2 related factor2 (Nrf2) and heat shock protein 70 (HSP70), and anti-inflammatory effects investigated by measuring inflammatory mediators. Water immersion restraint stress was imposed to provoke stress related mucosal damages (SRMD) in rats. Isopropanol extracts of Artemisia showed the higher DPPH radical scavenging activity and lesser LPS-induced reactive oxygen species productions and increased HO-1 expression through increased nuclear translocation of Nrf2 transcription factor compared to ethanol extracts. The increased expression of HSP70 and decreased expression of endothelin-1 were only increased with isopropanol extracts. A concentration-dependent inhibition of LPS-induced COX-2 and iNOS even at a rather lower concentration than ethanol extract was achieved with isopropanol extracts. Cytokine protein array revealed Artemisia extracts significantly attenuated the levels of CXCL-1, CXCL-16, and MCP-1. These orchestrated actions led to significant rescue from SRMD. Conclusively, Artemisia extracts imposed significant antioxidant and anti-inflammatory activity against SRMD and isopropanol extracts were superior to ethanol extracts in these beneficiary actions of Artemisia.
Keywords: artemisia asiatica, isopropanol extracts, HO-1, anti-inflammation, stress related mucosal damages
Introduction
Extracts of the whole herb of Artemisia had been used in traditional oriental medicine to treat various inflammatory diseases and to accelerate their regenerations and additionally utilized as food component featured with its good flavor. Since an ethanol extract of Artemisia was proven to possess anti-oxidative and anti-inflammatory effects in various kinds of experimental model of gastric diseases and to afford significant levels of cytoprotection in experimentally induced gastrointestinal (GI) damages as well as other hepatic and pancreatic damage,(1–4) their formulated pills come to clinic for the treatment of inflammation based GI diseases including gastritis, enterocolitis, and ulcer diseases. The preclinical facts that the ethanol extracts of Artemisia very effectively lessened the severity of dextran sulfate sodium or trinitrozobenzoic acid-induced colitis through either scavenging oxygen free radicals or attenuating cytokine/chemokines involved in gut inflammation as well as significant protection from reflux esophagitis and various irritants-induced gastric damages had led to the successful clinical trials to launch as novel remedy for gastritis,(4–6) DA-9601 as a formulated ethanol extract of Artemisia, of which prescriptions were further extended to use in other Asian countries under same clinical indications.
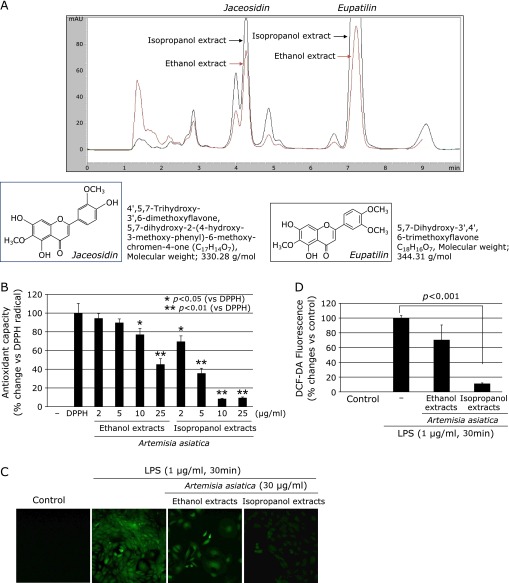
However, in spite of these achievements of Artemisia extracts for clinic, the several limitations were found in clinic, in detail, the possible risk of coagulopathy due to presence of dicoumarol, essential adding step prerequisite for ethanol extraction and the dread risk of GI malignancy due to unlimited cytoprotection, by which the needs for solving this limitation as well as pursuits for higher pharmacological actions of Artemisia extract were put forwarded in clinic after successful marketing. Stimulated with these limitations, isopropanol extraction was tried since dicoumarol addition is not required in case of isopropanol extraction. Preliminarily, additional benefits with isopropanol extraction were found that higher amounts of jaceosidin and eupatilin (Fig. 1A), representative flavonoids contained in Artemisia, on chromatographic analysis with isopropanol extracts and different cellular biology supporting lower risk of GI malignancy because 50–100 µg/ml isopropanol extracts imposed cytotoxicity,(7–9) whereas ethanol extracts did not (Supplemental Fig. 1*). Therefore, there might be unrevealed biological superiority in isopropanol extraction, fortifying higher antioxidative and anti-inflammatory activities prerequisite for the treatment of gastritis than current ethanol extracts of Artemisia.
Fig. 1.
Comparison of antioxidative activities according to extract solvent. (A) Chromatography of ethanol extracts and isopropanol extracts of Artemisia. Peak of eupatilin and jaceosidin was significantly higher in isopropanol extracts compared to ethanol extracts of Artemisia. Molecular structure and chemical name was shown. (B) Higher DPPH radical reduction activity in isopropanol extracts than ethanol extracts of Artemisia. To compare the antioxidant capacity between the ethanol extracts and the isopropanol extracts of Artemisia, DPPH scavenging capability was measured. (C) Confocal imaging of DCF-DA according to extracts. The intracellular accumulation of ROS induced by LPS was measured by using DCF-DA as a probe capable of detecting peroxides. LPS caused increased intracellular accumulation of ROS in RGM1 cells, which was apparently abolished by pretreatment with isopropanol extracts. (D) The data were presented as mean ± SD. for three different experiments performed in triplicate.
Under the object to obtain extracts with higher pharmacological activities, the present study was aimed to either compare antioxidant and anti-inflammation potential between isopropanol extracts and ethanol extracts of Artemisia against gastric inflammation or explore the molecular mechanisms to explain the difference in pharmacological actions using stress related mucosal damages model, in the current study, water immersion restraint stress (WIRS)-induced gastric ulcers.
Materials and Methods
Reagents
All chemical reagents were obtained from Sigma (St. Louis, MO). Both kinds of Artemisia extracts, ethanol or isopropanol extracts, were provided by Jeil Pharmaceutical Co., Ltd (Seoul, Korea). Briefly, for the standardized isopropanol extract of Artemisia, Artemisia were refluxed in isopropanol for 24 h, in a round bottom flask and filtered. Filtrates were evaporated under reduced pressure on water bath to obtain crude (20:1) and subsequently subjected to phytochemical and biological assay. The standardized ethanol extracts of Artemisia were also prepared according to the published procedure.(10) Standardization of Artemisia extracts was done using HPLC fingerprinting with chemical standards (jaceosidin and eupatilin). A HPLC was performed using an Inertsil ODS II (5C18, 4.6 mm ID × 250 mm L) column. The mobile phase consisted of acetic acid-ammonium acetate buffer and acetonitrile (65:35) at a flow rate of 1.0 ml/min. The injection volume was 10 µl. The monitor wave length was set at 350 nm (Fig. 1A). Western blotting detection reagents were obtained from Amersham Biotechnology (Bucks, UK). Primers for RT-PCR were synthesized by Bioneer (Daejeon, Korea). Reverse transcriptase was from Promega (Madison, WI). Antibodies for Nf-E2 related factor2 (Nrf2), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and 70 kDa heat shock protein (HSP70) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and heme oxygenase 1 (HO-1) were from R&D Systems, Inc (Minneapolis, MN).
Cell culture and cytotoxicity assay
The rat gastric mucosal cells, RGM1, were kindly given by Prof. Hirofumi Matsui (University of Tsukuba, Japan) and were maintained at 37°C in a humidified atmosphere containing 5% CO2 and cultured in Dulbecco’s modified Eagle’s medium containing 10% (v/v) fetal bovine serum and 100 U/ml penicillin. Cell cytotoxicity was measured by MTT, [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], assay.
DPPH free radical scavenging assay
The scavenging activity of the extracts was estimated using DPPH (0.1 mM) as the free radical model according to the method adapted from Zhao.(11) An aliquot of 800 µl of samples (2, 5, 10, and 25 µg/ml) and control (100% methanol), respectively, was mixed with 200 µl of DPPH. The mixture was shaken vigorously and left to stand at room temperature for 30 min in the dark. The mixture was measured spectrophotometrically at 515 nm.
Measurement of intracellular ROS accumulation
Accumulation of reactive oxygen species (ROS) in RGM1 cells was monitored using the fluorescence-generating probe 2',7'-dichlorofluorescein diacetate (DCF-DA). Cells were rinsed with HBSS solution and loaded with 10 µM DCF-DA. After 30-min incubation at 37°C, cells were examined under a confocal fluorescence microscope set at 488 nm for excitation and 530 nm for emission (Leica Microsystems, Heidelberg GmbH, Heidelberg, Germany).
Western blot analysis
This assay was performed as previously described. Briefly, treated cells were washed twice with PBS and then lysed in ice-cold cell lysis buffer (Cell Signaling Technology, Beverly, MA) containing 1 mM PMSF. After 1 h of incubation, samples were centrifuged at 12,000 g for 15 min. Supernatants were then collected. Proteins were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes, which were incubated with appropriate antibodies and visualized using an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK). Antibodies used in the current study were HO-1, Nrf2, COX-2, HSP-27, HSP-60, HSP-70, all purchased from Cell Signaling Technology.
RNA isolation and RT-PCR
This assay was performed as previously described. After incubation, media was removed by suction and cells were washed with PBS twice. Trizol (Invitrogen, Carlsbad, CA) was added to plates, which were then incubated for 10 min at 4°C. Trizol was harvested and placed in a 1.5 ml tube, and 100 µl chloroform (Merck, Rahway, NJ) was added and gently mixed. After incubation for 10 min in ice, samples were centrifuged at 10,000 g for 30 min. Supernatants were extracted and mixed with 200 µl isopropanol (Merck), and mixtures were incubated at 4°C for 1 h. After centrifuging at 13,000 g for 30 min, pellets were washed with 70% (v/v) ethanol. After allowing the ethanol to evaporate completely, pellets were dissolved in 40 µl of DEPC-treated water (Invitrogen). cDNA was prepared using reverse transcriptase originating from Murine-Moloney leukemia virus (Promega, Madison, WI), according to the manufacturer’s instructions. PCR was performed over 25 cycles of: 94°C for 20 s, 55°C for 30 s, and 72°C for 45 s. Oligonucleotide primers designed by authors using NCBI/primer-blast. Oligonucleotide primers were purchased from Bioneer (Daejeon, Korea). Oligonucleotide primers were as follows; For HO-1, sense 5'-GAC AGC ATG TCC CAG GAT TT-3', antisense 5'-GGT TCT GCT TGT TTC GCT CT-3', for COX-2, sense 5'-GAA ATG GCT GCA GAG TTG AA-3', antisense 5'-TCA TCT AGT CTG GAG TGG GA-3', for iNOS, sense 5'-TTT TCC CAG GCA ACC AGA CG-3', antisense 5'-GTA GCG GGG CTT CAG AAT GG-3', for IL-6, sense 5'-AAG AGA CTT CCA GCC AGT TG-3', antisense 5'-TGG ATG GTC TTG GTC CTT AG-3', and for GAPDH, sense 5'-GGT GCT GAG TAT GTC GTG GA-3', antisense 5'-TTC AGC TCT GGG ATG ACC TT-3'.
Immunofluorescence staining
RGM1 cells were placed on four-well chamber slides and were treated with extracts for 3 h. Cells were rinsed rapidly with PBS and then fixed for 30 min at room temperature with 4% formaldehyde. After washing the fixed cells with PBS, they were incubated further for 2 h at room temperature in PBS containing 10% BSA and 0.5% Tween-20. The nuclear translocation of Nrf2 was visualized using a rabbit polyclonal antibody. The Nrf2 antibody was added after 1:100 dilution with the blocking buffer, and cells were incubated overnight at 4°C. Afterwards, the incubated cells were washed with PBS and then labeled with diluted (1:1000) Fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Zymed Laboratories, South San Francisco, CA) and incubated for additional 1 h at room temperature. Cells were then rinsed with PBS and stained with propidium iodide for 10 min. After washing with PBS, cells were analyzed under a confocal microscope and photographed (Leica Microsystems Heidelberg GmbH).
Transient transfection
For siRNA transfection, RGM1 cells were seeded in 60-mm dishes and grown to 60 to 70% confluence in growth media. Nrf2 siRNA (Genolution, Seoul, Korea) was transfected into RGM1 cells with lipofectamine RNAi-MAX (Invitrogen) reagent according to the manufacturer’s instructions. After 48 h transfection, cells were treated with isopropanol extracts for additional 6 h, and the cell lysis was carried out with the lysis buffer for Western blot analysis.
Cytokine array
Cytokine array and chemokine array were performed Rat Cytokine and chemokine Antibody Array Kit (2 membrane arrays) with Accessories, for simultaneous detection of 89 Cytokines and chemokine related proteins in 2 samples (Supplemental Table 1*) from RayBiotech (Norcross, CA). After blocking the array membranes for 30 min, the membranes were incubated with 1 ml of serum at room temperature for 2 h. After washing with buffer, we added primary biotin-conjugated antibody to each membrane, for incubation at room temperature for 2 h. After washing with buffer and addition of horseradish peroxidase-conjugated streptavidin to each membrane, we exposed them to detection buffer, using a luminescent image analyzer system (LAS-4000, Fuji Film, Tokyo, Japan). Density was expressed as the % of the detected value from the sample vs the housekeeping gene expression using a Gelpro32 program (MediaCybernetics, Rockville, MD).
Water immersion restraint stress-induced gastritis (WIRS)
A total of 36 Wistar rats were purchased from Charles River (Osaka, Japan) and kept in an animal facility. Animals were handled in an accredited animal facility in accordance with the AAALAC International Animal Care Policies. The animals were deprived of food, but allowed free access to water 24 h before exposure of water immersion restraint stress-induced gastritis (WIRS). Twelve rats in each group were placed in strain cages and immersed in water for 8 h. Animals were killed immediately after the end of the 8 h WIRS. Animals were divided into three groups as follows: normal rats without any intervention except oral administration of normal saline, WIRS group as applying WIRS for 8 h, and Artemisia extracts pretreated group 1 h before WIRS. The stomachs of rats were removed and opened along the greater curvature, and then washed with iced cold PBS solutions. The number and size of either erosions or ulcers were determined under the magnified photographs, after which half of each dissected stomach was spread onto a plastic sheet, fixed in 10% buffered formalin for 4 h, and prepared for paraffin tissue slides and the remaining half was kept in a liquid nitrogen tank for further molecular study. The mucosal homogenates were pooled together.
Statistical analysis
The data were analyzed by one-way ANOVA and the statistical significance between groups was determined by Student’s t test. Statistical significance was accepted when p<0.05.
Results
Higher 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical reduction activity with isopropanol extracts than ethanol extracts of Artemisia
On chromatography, peak column was compared between ethanol and isopropranol extracts of Artemisia (Fig. 1A) and eupatilin and jaceosidin was significantly increasingly peaked in isoprapranol extract compared to ethanol extract. Their molecular structure as well as molecular weights was displayed in Fig. 1A. To compare the antioxidant capacity between the ethanol extracts and the isopropanol extracts of Artemisia, DPPH scavenging capability assay was first measured. The relatively stable organic radical DPPH assay is often used to evaluate the total antioxidant power and the radical scavenging activities of crude extracts of various plants.(12) Fig. 1B shows that the isopropanol extracts of Artemisia scavenged the DPPH radical in a dose-dependent manner. The ethanol extracts of Artemisia also scavenged the DPPH radical, but the scavenging effect was not as significant as that of the isopropanol extracts. At a concentration of 10 µg/ml, the value for isopropanol extracts amounted to 90%, while ethanol extracts showed a far lower antioxidant activity (20%).
LPS-induced intracellular ROS accumulation was significantly scavenged with the isopropanol extracts of Artemisia compared to ethanol extracts
To compare the antioxidant effects of the ethanol extracts and the isopropanol extracts of Artemisia, RGM1 cells were stimulated with lipopolysaccharide (LPS; 1 µg/ml, 30 min) based on previous study that the induction of ROS generation by LPS has been observed in RGM1 cells.(13,14) In this study, the intracellular accumulation of ROS induced by LPS was measured by using DCF-DA as a probe capable of detecting peroxides such as H2O2. As shown in Fig. 1C, LPS caused increased intracellular accumulation of ROS in RGM1 cells, which was abolished by pretreatment with isopropanol extracts. Interestingly, isopropanol extracts (30 µg/ml) elicited the more pronounced antioxidant effect than ethanol extracts (30 µg/ml) (Fig. 1D).
Significant induction of heme oxygenase-1 (HO-1) with isopropanol extracts of Artemisia
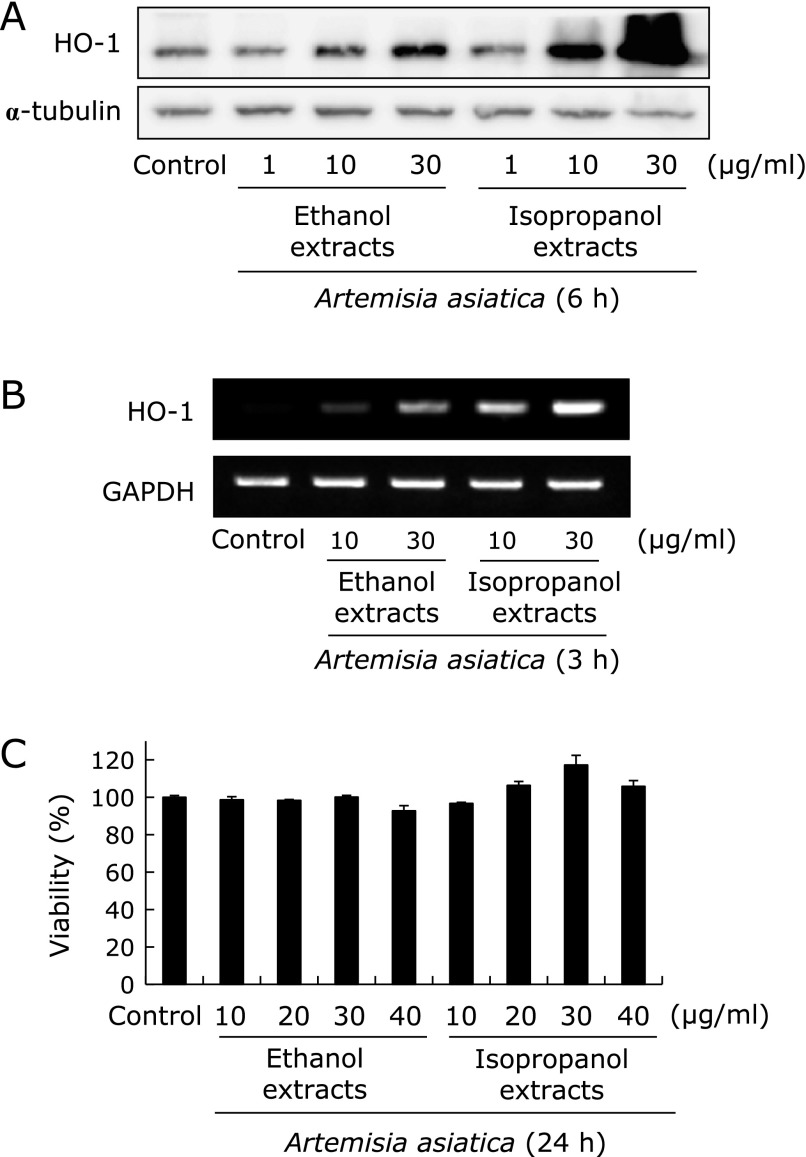
Many natural compounds elicit cell-protection activity and anti-inflammatory activity through Nrf2-mediated up-regulation of HO-1 and glutamate cysteine ligase (GCL).(15,16) As such, we examined the effect of extracts of Artemisia on the expression of HO-1 among phase II antioxidative and antimutagenic enzyme. RGM1 cells were treated with the ethanol extracts and the isopropanol extracts of Artemisia at various concentrations to determine the potential effects on HO-1 expression respectively. As shown in Fig. 2A, although HO-1 protein levels were increased following Artemisia extracts treatment in a dose-dependent manner irrespective of extraction method, isopropanol extracts exerted the more potent effects on HO-1 protein induction than ethanol extracts tested (p<0.001). In agreement with what we observed with HO-1 protein level, the RT-PCR analysis showed same results, statistically significantly increased in 30 µg/ml isopropranol extract than ethanol extract (Fig. 2B, p<0.001). Isopropanol extracts were significantly more potent than ethanol extracts. In this phenomenon, the effect of the Artemisia extracts on RGM1 cell viability was determined by an MTT assay to find difference in real cell viability according to HO-1 expression, hypothesis that HO-1 induction might be compensatory cell adoptive response in isopropanol extracts. Cells cultured with the either ethanol extracts or isopropanol extracts of Artemisia at the concentrations (0, 10, 20, 30, and 40 µg/ml) for 24 h. As seen in Fig. 2C, there was no change in cell viability up to 40 µg/ml concentration, suggesting HO-1 induction is not related with any compensatory cytotoxicity of isopropanol extract of Artemisia.
Fig. 2.
Comparison of HO-1 expression according to extraction solvent. Isopropanol extracts induces HO-1 protein (A) and mRNA expression (B). HO-1 protein and mRNA expression were increased following Artemisia extracts treatment in a dose-dependent manner irrespective of extraction solvent. However, 30 µg/ml isopropanol extract showed significantly increased expression of HO-1. HO-1 protein (A) and mRNA (B) expression were analyzed by Western blotting and RT-PCR, respectively. (C) Cell viability. Artemisia extracts were tested for their cytotoxic activity using the MTT colorimetric assay. The data were presented as mean ± SD. for three different experiments performed in triplicate.
The isopropanol extracts of Artemisia significantly induced Nrf2-mediated up-regulation of HO-1
Nrf2 is a redox-sensitive transcription factor that regulates antioxidative response element (ARE)-driven HO-1 expression.(17) After nuclear translocation, Nrf2 binds to ARE localized in the promoter region of genes encoding cytoprotective phase II detoxifying/antioxidant enzymes, so called phase 2 enzyme, and stimulates their transcriptions. To compare that Artemisia extracts stimulates nuclear translocation of Nrf2, Western blot analysis of nuclear extracts were conducted. Though Artemisia extracts showed the tendency to increase the nuclear translocation of Nrf2, only isopropanol extracts treated cells significantly increased the nuclear Nrf2 expression much more than ethanol extracts (Fig. 3A, p<0.01). To confirm the findings from Western blot, confocal imaging analysis was done. Immunocytochemical analysis confirmed the increased nuclear localization of Nrf2 in RGM1 cells treated with isopropanol extracts (Fig. 3B). Moreover, we examined the HO-1 expression after siRNA knockdown of Nrf2. The isopropanol extracts-induced up-regulation of HO-1 in RGM1 cells was abolished by silencing of Nrf2 expression with specific siRNA, whereas transfection of the cells with the same amount of non-specific control siRNA was not effective (Fig. 3C). These data suggest that isopropanol extracts imposed induction of HO-1 via activation of Nrf2.
Fig. 3.
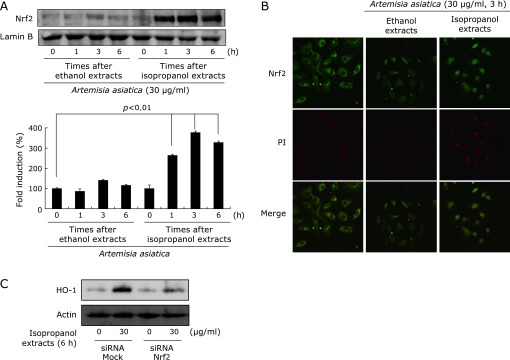
Comparison of Nrf2 activation according to extract solvent. (A) Isopropanol extracts of Artemisia activated nuclear translocation of Nrf2. Nuclear fraction analyzed to determine the nuclear Nrf2 levels by Western blotting. Nuclear translocation of Nrf2 with isopropanol extracts was significantly increased compared to ethanol extracts in RGM1 cells. (B) Confocal imaging of Nrf2. Nrf2 nuclear translocation was examined under confocal microscope after its immunofluorescence staining. Increased nuclear translocation of Nrf2 was seen in isopropanol extracted group compared to ethanol extracts treated group, as evidenced with yellow color. (C) Nrf2 dependence of HO-1 induction. After RGM1 cells were transfected with nonspecific or Nrf2 siRNA, HO-1 protein expression was analyzed by Western blotting. The isopropanol extracts-induced up-regulation of HO-1 was abolished by silencing of Nrf2 expression with specific siRNA.
The isopropanol extracts of Artemisia elicited higher anti-inflammatory effect than the ethanol extracts
To compare the anti-inflammatory effects of the ethanol extracts and the isopropanol extracts of Artemisia, effects on LPS-induced inflammation in RGM1 cells were investigated. As expected, LPS-stimulated RGM1 cells significantly increased expression of the inflammation-associated enzymes, iNOS and COX-2, as determined by Western blot and RT-PCR analyses (Fig. 4A and B, p<0.01) and these increases were significantly inhibited by treatment with Artemisia extracts (p<0.05). Fig. 4A and B showed that incubation with Artemisia extracts in the presence of LPS inhibited COX-2, iNOS, and IL-6 protein and their mRNA expressions in RGM1 cell in a dose-dependent manner. Especially, isopropanol extracts more significantly decreased in the LPS-induced COX-2 and iNOS expression than the ethanol extracts group. The intensity of protein bands was analyzed and showed an average of 50% down-regulation of COX-2 and iNOS proteins, respectively, after treatment with isopropanol extracts compared with the ethanol extracts group. To further clarify the global genes engaged in anti-inflammation relevant to Artemsia extracts treatment, we performed the cytokine/chemokine array after LPS treatment in RGM1 cell lines. Using the cytokine/chemokine antibody array designed for simultaneous detection of 81 cytokines and chemokines proteins (see methods and Supplemental Table 1*), we examined significant spots differed between before and after each Artemisia extract treatment, after which chemokine (C-X-C motif) ligand (CXCL1), chemokine (C-X-C motif) ligand 16 (CXCL16), and monocyte chemotactic peptide (MCP)-1 were identified to be significantly decreased in the isopropanol extracts treated group compared to ethanol extracts treated group (Fig. 4C).
Fig. 4.
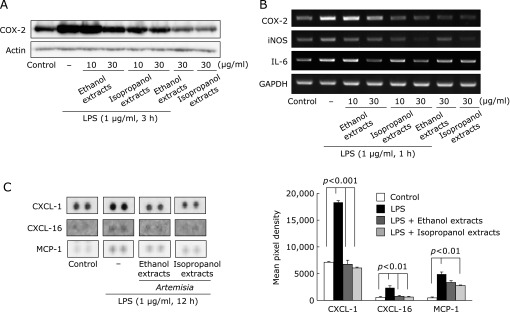
Comparison of inflammatory mediators including iNOS and COX-2 according to extract solvent. (A) Isopropanol extracts reduces COX-2 protein expression. COX-2 protein expression was analyzed by Western blotting. Isopropanol extracts in the presence of LPS inhibited COX-2 protein in RGM1 cell in a dose-dependent manner. (B) Isopropanol extracts of Artemisia reduced more COX-2, iNOS, and IL-6 mRNA expression than ethanol extracts. COX-2, iNOS, and IL-6 mRNA expression were analyzed by RT-PCR. Isopropanol extracts in the presence of LPS inhibited COX-2, iNOS, and IL-6 mRNA expression in RGM1 cell in a dose-dependent manner. (C) Protein array for inflammatory mediators. CXCL-1, CXCL-16, and MCP-1 were inhibited in density in the isopropanol extracts treated group than ethanol extracts treated group. The low panel shows the relative intensity of decreased each cytokine.
The isopropanol extracts of Artemisia imposed significant cytoprotective action through either inducing heat shock protein-70 (HSP-70) expression or repressing endothelin-1 (ET-1) expression
ET-1 is known as strong vasoconstriction factor, by which increased expression of ET-1 was regarded as etiologic genes for cysteamine-induced duodenal ulcer and stress-induced gastroduodenal ulcer.(18,19) In protein array, isopropanol extracts significantly decreased ET-1 expression compared to ethanol extracts under LPS-stimulation (Fig. 5A, p<0.01), inferring cytoprotective characteristics of isopropanol extracts of Artemisia. The HSP70 as a molecular chaperone has been suggested to exert its gastroprotective action by protecting mitochondria and by interfering with the stress-induced apoptotic program.(20) We examined the effect of extracts of Artemisia on the expression of HSP70 protein. Fig. 5B result shows that the treatment with the isopropanol extracts significantly up-regulated HSP70 protein (p<0.01) in a dose dependent manner, while ethanol extracts did not increased the levels of these proteins. Significant induction of HSP70 protein suggests that isopropanol extracts has a protective effect of damaged gastric cell by induction of HSP70.
Fig. 5.
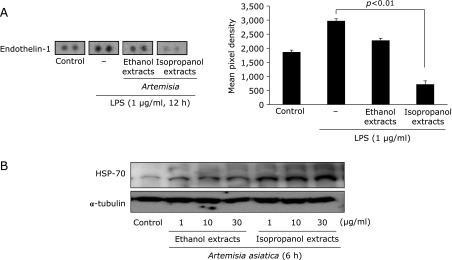
Comparison of endothelin-1 (ET-1) and HSP70 expression according to extract solvent. (A) Isopropanol extracts of Artemisia significantly reduced endothelin-1 protein expression than ethanol extracts. ET-1 is known as strong vasoconstriction factor engaged in gastric mucosal damages as exemplified in stress-induced ulcer, Helicobacter pylori-associated ulcer, and duodenal ulcerogenesis. In protein array, isopropanol extracts significantly decreased ET-1 expression compared to ethanol extracts under LPS-stimulation, inferring cytoprotective characteristics of isopropanol extracts of Artemisia. (B) Isopropanol extracts induced HSP70 protein expression. The HSP70 as a molecular chaperone has been suggested to exert its gastroprotective action by protecting mitochondria and by interfering with the stress-induced apoptotic program. The treatment with the isopropanol extracts of Artemisia provoked significant up-regulation of HSP70 protein, while ethanol extracts did not increased the levels of these proteins.
The isopropanol extracts of Artemisia imposed significant rescuing action from water immersion restraint stress-induced gastric ulceration
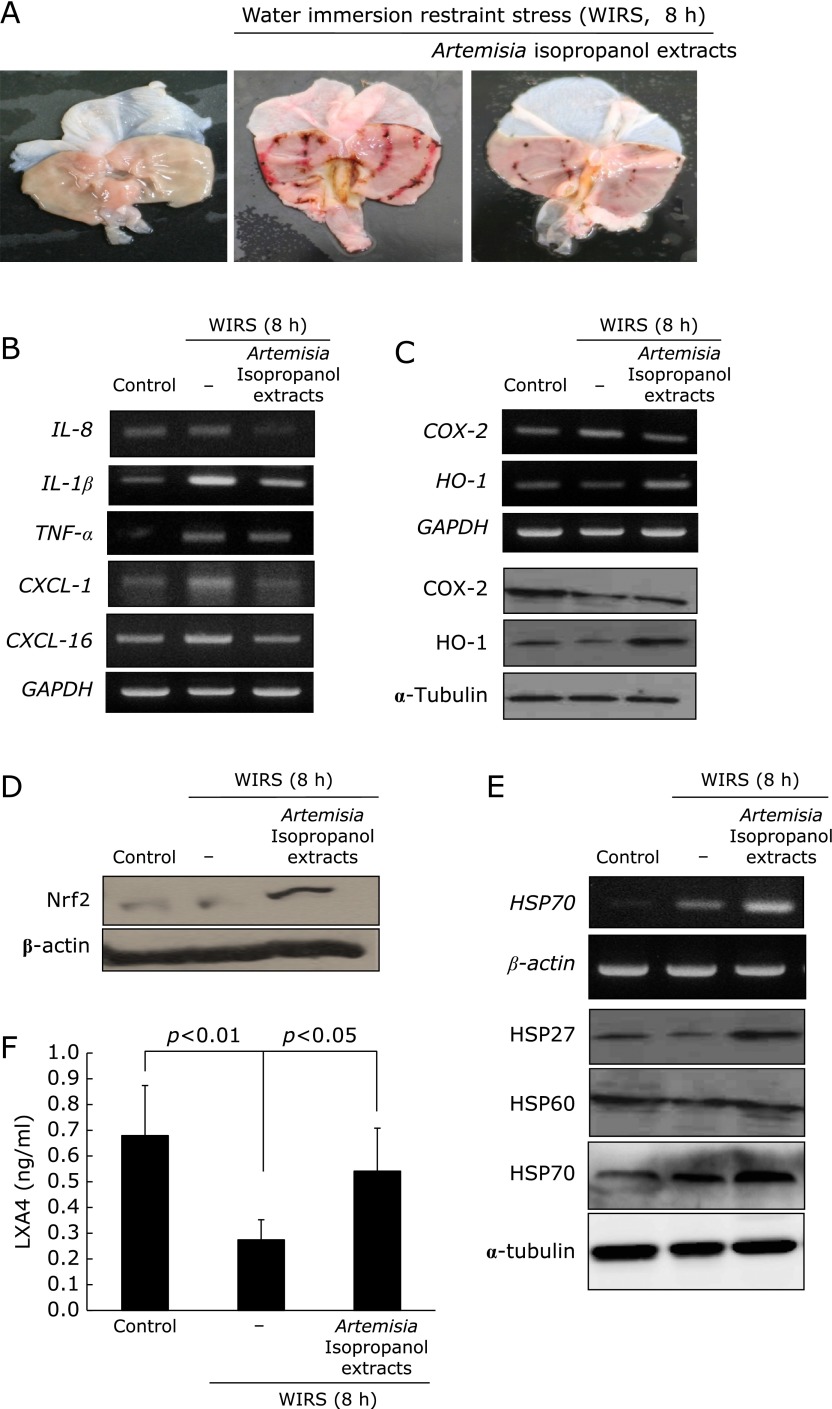
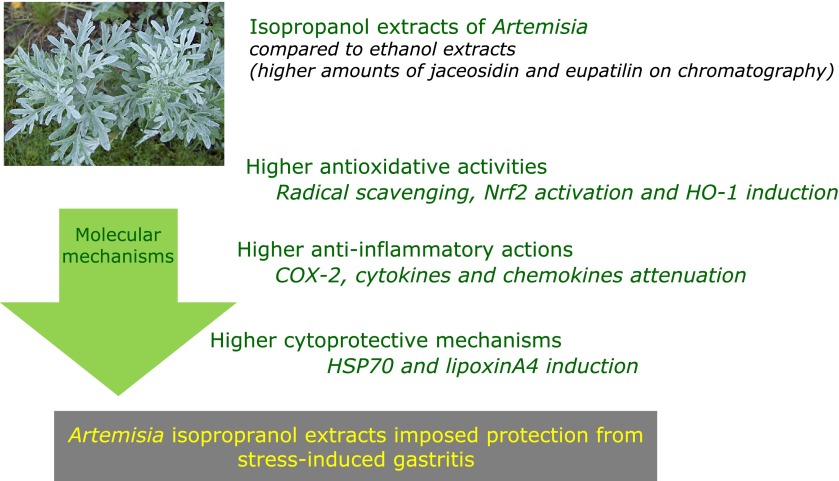
In order to validate these in vitro privileges of Artemisia extract against stress-induced gastric damages, we have imposed restraint stress in SD rats without or with Artemisia extracts (Fig. 6A). Artemisia pretreatment significantly attenuated WIRS-induced gastric damages (p<0.001), in which significantly decreasing expressions of IL-1β, IL-8, CXCL-1, and COX-2 were noted, while HO-1 were significantly increasingly noted (Fig. 6B and C, p<0.05). Using cytoplasmic fractions from gastric mucosal homogenates, Nrf2 were measured according to group as seen in Fig. 6D, significantly increased expression of Nrf2 were noted in group treated with isopropanol extracts of Artemisia. HSP70 is one of core chaperone protein in gastric protection under stress, by which HSP27, HSP60, and HSP70 was measured. As noted in Fig. 6E, HSP27 and HSP70 were apparently increasingly expressed with Artemisia treatment. Finally resolution of stress-induced gastric inflammation can be accelerated with anti-inflammatory mediators and lipoxinA4 has been suggested to be significantly implicated in resolution of inflammation. As observed in Fig. 6F, the levels of LXA4 were significantly decreased with overwhelming stress, but it levels were significantly increased in Artemisia treatment (Fig. 6F, p<0.05). In summary, isopropanol extracts of Artemisia offered three kinds of beneficial actions compared to ethanol extracts, higher antioxidative activities reflected with higher HO-1 and Nrf2 activation, higher anti-inflammatory actions accompanied with attenuated inflammatory cytokines and chemokines, and higher chance of cytoprotection including HSP27 and HSP70 induction, all of these benefits from isopropanol extraction can orchestrate higher clinical efficacy of gastritis treatment, especially stress related mucosal damages (Fig. 7).
Fig. 6.
Water Immersion Restraint Stress (WIRS)-induced gastric damages and rescuing action of Artemisia extracts. (A) Gross morphology (B) RT-PCR for IL-8, IL-1β, TNF-α, CXCL-1, CXCL-16 (C) RT-PCR for COX-2 and HO-1 followed with western blot for COX-2 and HO-1 (D) The western blot for Nrf2 using cytoplasmic fraction (E ) RT-PCR for HSP70 and western blot for HSP27, HSP60, and HSP70. (F) The changes of gastric mucosal levels of lipoxinA4 (LAX4) according to group.
Fig. 7.
Isopropranol extracts of Artemisia could rescue from stress related gastric damages, in which higher antioxidative, anti-inflammatory, and cytoprotective mechanisms were intervened. Rescuing action of Artemisia against stress-induced gastritis Improved biological actions of Artemisia with isopropanol extraction was validated in animal model. Isopropanol extracts of Artemisia offered three kinds of beneficial actions compared to ethanol extracts, higher antioxidative activities reflected with higher HO-1 and Nrf2 activation, higher anti-inflammatory actions accompanied with attenuated inflammatory cytokines, and higher chance of cytoprotection including HSP70 induction as well as decreased endothelin-1, all of these benefits from isopropanol extraction can orchestrate higher clinical efficacy of gastritis treatment.
Discussion
In this study, we found, for the first time, that isopropanol extracts of Artemisia 1) showed significantly higher induction of antioxidative activities, 2) imposed higher anti-inflammatory actions, and 3) exerted higher cytoprotective mechanisms compared to ethanol extracts of Artemisia. Since the ethanol extracts of Artemisia are already available as drug in clinic for the treatment of gastritis and gastric ulcer, we anticipate the isopropanol extracts can yield higher clinical efficacy. However, large scaled clinical evaluation should be followed to confirm this superiority of isopropanol extract of Artemisia. Though Artemisia extracts had been widely used for the treatment of gynecological disorders, including infertility and dysmenorrhea commonly caused by endometriosis,(21) increasing antimicrobial activities,(22) strengthening anti-nociceptive and antipyretic activities,(23) improving penile erection,(24) and treating gastritis, gastric ulcer, pancreatitis, and hepatic fibrosis in either western clinic or oriental clinic,(25–27) we anticipated isopropanol extracts can afford more widespread prescription than current ethanol extracts or other kinds of formula for the above described targets.
Among these clinical targets, Artemisia extracts have shown very excellent therapeutic actions against gastric damages provoked by alcohol, NSAIDs, and stress. In reality, the efficient protective actions of Artemisia extract pretreatment against ethanol-induced gastric damage were shown supported with the following novel mechanisms, antioxidative actions presented with attenuated lipid peroxidation and nitrosative stress, inhibitory actions of GSH depletion, reducing inductions of CYP2E1 after alcohol, decreasing generations of inflammatory cytokines, and the induction of endogenous molecules for cytoprotection.(25) Another example of Artemisia therapeutic efficacy was the rescue from NSAID-induced gastric damages,(28) in which study, cultured feline esophageal epithelial cells were used to investigate the ability of eupatilin, a flavon component obtained from Artemisia, to induce expression of HO-1. Zinc protoporphyrin as an HO-1 inhibitor significantly repressed eupatilin-induced HO-1 activity and endowed the protective effect of eupatilin against indomethacin-induced cell injury, suggesting that HO-1 was partly responsible for the eupatilin-mediated protective action of esophageal epithelial cells against indomethacin via ERKs and PI3K/Akt pathways as well as Nrf2 nuclear translocation. In this study, these protective HO-1 inducing capabilities were significantly enhanced in isopropanol extracts of Artemisia compared to ethanol extracts. On chromatography analysis, eupatilin and jaseosidin amounts were significantly increased in isopropanol extracts (Fig. 1A), leading to conclusion that isopropanol extracts of Artemisia might provide higher protection from NSAID-induced gastric damages than ethanol extracts based on these advantages (Fig. 2 and 3). In addition, our study additionally showed that higher apoptotic activities as well as cell cycle arrest were noted in isopropanol extracts of Artemsia compared to ethanol extracts (Supplemental Fig. 1*). Though Choi et al.(29) and Kim et al.(30) documented ethanol extract of Artemisia exhibited antitumor activity through the induction of cell cycle arrest and differentiation of gastric cancer cells, our data showed lower fear of cancer promotion with isopropanol extract of Artemisia than ethanol extract.
Eupatilin (5,7-Dihydroxy-3',4',6-trimethoxyflavone, C18H16O7, molecular weight; 344.31 g/mol) and jaceosidin [4',5,7-Trihydroxy-3',6-dimethoxyflavone,5,7-dihydroxy-2-(4-hydroxy-3-methoxy-phenyl)-6-methoxy-chromen-4-one, C17H14O7, molecular weight; 330.28 g/mol] has been known to be responsible for antioxidative and anti-inflammatory actions from natural products. Choi et al.(2) studied whether eupatilin has a property of antioxidant activity and protects gastric epithelial cells from hydrogen peroxide-induced damage and found that eupatilin ameliorated H2O2-induced actin disruption in gastric cells. Eupatilin also reduces the expression of such oxidative-responsible genes as HO-1, PLAUR and TNFRSF10A in H2O2-treated cells. However, in our study, higher amounts of eupatilin or other active components obtained through isopropanol extraction might induce HO-1, imposing significant levels of gastroprotection under LPS stimulation. Jaceosidin as flavonoids from Artemisia also has been known to induce G2/M cell cycle arrest, induce apoptosis, and inactivate cdc25,(31,32) inhibit neuroinflammation,(33) alleviate hypersensitivity,(34) impose antioxidative activity,(35) and induce direct anti-inflammation by blocking ERK signaling.(36,37) Even though these flavonoid components from Artemisia might significantly improve ameliorating action against ethanol-induced gastric damages and indomethacin-induced gastropathy in vitro condition, but their efficacies were not be proved clearly in animal disease models, signifying that extracts seems to work beneficially rather than each active components, orchestrating whole protective mechanisms against gastric damages.
ROS are by-products generated by cellular oxidative metabolism and may lead to aging, inflammation, and other chronic diseases,(38) among which ROS are principally involved in the pathogenesis of gastritis and colitis. For example, GI lumen can come under attack by many factors, including Helicobacter pylori and other commensal bacteria, non-steroidal anti-inflammatory drugs, and gastric acid.(39) Since ROS can react with cellular proteins or lipids, transforming them into oxidized forms, or bind with nucleic acids and ROS activate signal transduction for the expression of inflammatory cytokines and chemokines,(40) enhanced antioxidative action with isopropranol extract of Artemisia might provide higher therapeutic effects much better than ethanol extracts. Chronic inflammation has been shown to be associated with a number of human ailments such as chronic atrophic gastritis, inflammatory bowel disease, and cancer.(41) Against these attacks, gastric cells have a battery of cytoprotective enzymes, HO-1 is believed to play a cytoprotective role in a variety of pathological models such as inflammation,(42) catalyzing the rate-limiting step in converting heme into bilirubin, carbon monoxide (CO), and iron. The cytoprotective properties of HO-1 are related to the production of bilirubin to reduce of oxidative stress and production of CO to decrease the production of inflammatory mediators such as NO.(43) In this study, we clearly documented that isopropanol extracts of Artemisia significantly increased HO-1 through Nrf2 activation. In the current study, we measured the changes of LXA4, which have been approved for potent anti-inflammatory and immunomodulatory properties with resolvins. Based on the important roles of LXA4 in resolving inflammation and immunomodulation,(44) Huang et al.(45) investigated the effects of LXA4 on LPS-induced proliferation and found that LXA4 inhibited LPS-induced proliferation through the G0/G1 phase arrest in RAW264.7 macrophages, and the inhibitory effect might depend on NF-κB signaling transduction pathway. Significant attenuated levels of LXA4 were noted with imposing stress, whereas significant preservation of LXA4 was done with Artemisia extracts (Fig. 6F). Recently since significant inflammation resolution was triggered with omega-3 polyunsaturated fatty acids,(46) our experiment additionally signified Artemisia extracts also contributed resolution of inflammation in a same fashion.
In conclusion, our data that simple change of extraction method resulted in enormously improved pharmacological actions that isopropanol extracts of Artemisia either increased antioxidative capability or enhanced anti-inflammatory activities as well as imposing significant cytoprotective actions compared to ethanol extracts. It imparts the high possibility of being efficient therapeutics with the application of modern science. Incorporation of basic science into natural product might facilitate the development of safer and more efficient phytochemicals applicable to inflammation-based diseases than or far beyond the scope of synthetic chemical drug.
Acknowledgments
This work was supported by a grant from the Ministry of Education and Science Technology (2010-0002052), Korea and Asian CORE Program of JSPS, Japan.
Abbreviations
- COX-2
cyclooxygenase-2
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- HO-1
heme oxygenase-1
- HSP70
70 kDa heat shock protein
- iNOS
inducible nitric oxide synthase
- LXA4
lipoxin A4
- Nrf2
Nf-E2 related factor 2
Conflict of Interest
No potential conflicts of interest were disclosed.
Supplementary Material
References
- 1.Seo HJ, Park KK, Han SS, et al. Inhibitory effects of the standardized extract (DA-9601) of Artemisia asiatica Nakai on phorbol ester-induced ornithine decarboxylase activity, papilloma formation, cyclooxygenase-2 expression, inducible nitric oxide synthase expression and nuclear transcription factor kappa B activation in mouse skin. Int J Cancer. 2002;100:456–462. doi: 10.1002/ijc.10489. [DOI] [PubMed] [Google Scholar]
- 2.Choi SC, Choi EJ, Oh HM, et al. DA-9601, a standardized extract of Artemisia asiatica, blocks TNF-alpha-induced IL-8 and CCL20 production by inhibiting p38 kinase and NF-kappaB pathways in human gastric epithelial cells. World J Gastroenterol. 2006;12:4850–4858. doi: 10.3748/wjg.v12.i30.4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee S, Park HH, Son HY, et al. DA-9601 inhibits activation of the human mast cell line HMC-1 through inhibition of NF-kappaB. Cell Biol Toxicol. 2007;23:105–112. doi: 10.1007/s10565-006-0103-3. [DOI] [PubMed] [Google Scholar]
- 4.Oh TY, Lee JS, Ahn BO, et al. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364–371. doi: 10.1136/gut.49.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek YH, Lee KN, Jun DW, et al. Augmenting Effect of DA-9601 on Ghrelin in an Acute Gastric Injury Model. Gut Liver. 2011;5:52–56. doi: 10.5009/gnl.2011.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh TY, Ryu BK, Ko JI, et al. Protective effect of DA-9601, an extract of Artemisiae Herba, against naproxen-induced gastric damage in arthritic rats. Arch Pharm Res. 1997;20:414–419. doi: 10.1007/BF02973932. [DOI] [PubMed] [Google Scholar]
- 7.Moscatelli V, Hnatyszyn O, Acevedo C, Megías J, Alcaraz MJ, Ferraro G. Flavonoids from Artemisia copa with anti-inflammatory activity. Planta Med. 2006;72:72–74. doi: 10.1055/s-2005-873177. [DOI] [PubMed] [Google Scholar]
- 8.Pelzer LE, Guardia T, Osvaldo Juarez A, Guerreiro E. Acute and chronic antiinflammatory effects of plant flavonoids. Farmaco. 1998;53:421–424. doi: 10.1016/s0014-827x(98)00046-9. [DOI] [PubMed] [Google Scholar]
- 9.Ji HY, Kim SY, Kim DK, Jeong JH, Lee HS. Effects of eupatilin and jaceosidin on cytochrome p450 enzyme activities in human liver microsomes. Molecules. 2010;15:6466–6475. doi: 10.3390/molecules15096466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu BK, Ahn BO, Oh TY, Kim SH, Kim WB, Lee EB. Studies on protective effect of DA-9601, Artemisia asiatica extract, on acetaminophen- and CCl4-induced liver damage in rats. Arch Pharm Res. 1998;21:508–513. doi: 10.1007/BF02975366. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J, Liu T, Ma L, et al. Antioxidant and preventive effects of extract from nymphaea Candida flower on in vitro immunological liver injury of rat primary hepatocyte cultures. Evid Based Complement Alternat Med. 2011;2011:497673. doi: 10.1093/ecam/nep003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng JS, Chi CS, Huang SS, Shie PH, Lin TH, Huang GJ. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J Ethnopharmacol. 2011;137:1161–1171. doi: 10.1016/j.jep.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 13.Zhao S, Zhang L, Lian G, et al. Sildenafil attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-κB signaling pathways in N9 microglia. Int Immunopharmacol. 2011;11:468–474. doi: 10.1016/j.intimp.2010.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, Wu C, Zhao S, et al. Demethoxycurcumin, a natural derivative of curcumin attenuates LPS-induced pro-inflammatory responses through down-regulation of intracellular ROS-related MAPK/NF-kappaB signaling pathways in N9 microglia induced by lipopolysaccharide. Int Immunopharmacol. 2010;10:331–338. doi: 10.1016/j.intimp.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Surh YJ, Kundu JK, Na HK. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 16.Kuhn AM, Tzieply N, Schmidt MV, et al. Antioxidant signaling via Nrf2 counteracts lipopolysaccharide-mediated inflammatory responses in foam cell macrophages. Free Radic Biol Med. 2011;50:1382–1391. doi: 10.1016/j.freeradbiomed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 17.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng X, Szabo S, Khomenko T, Jadus MR, Yoshida M, Chen L. Detection of duodenal ulcer-associated genes in rats. Dig Dis Sci. 2008;53:375–384. doi: 10.1007/s10620-007-9890-5. [DOI] [PubMed] [Google Scholar]
- 19.Said SA, El-Mowafy AM. Role of endogenous endothelin-1 in stress-induced gastric mucosal damage and acid secretion in rats. Regul Pept. 1998;73:43–50. doi: 10.1016/s0167-0115(97)01056-2. [DOI] [PubMed] [Google Scholar]
- 20.Rokutan K. Role of heat shock proteins in gastric mucosal protection. J Gastroenterol Hepatol. 2000;15 Suppl:D12–D19. doi: 10.1046/j.1440-1746.2000.02144.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, Jung SH, Yang YI, et al. Artemisia leaf extract induces apoptosis in human endometriotic cells through regulation of the p38 and NFκB pathways. J Ethnopharmacol. 2013;145:767–775. doi: 10.1016/j.jep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Ahameethunisa AR, Hopper W. In vitro antimicrobial activity on clinical microbial strains and antioxidant properties of Artemisia parviflora. Ann Clin Microbiol Antimicrob. 2012;11:30. doi: 10.1186/1476-0711-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habib M, Waheed I. Evaluation of anti-nociceptive, anti-inflammatory and antipyretic activities of Artemisia scoparia hydromethanolic extract. J Ethnopharmacol. 2013;145:18–24. doi: 10.1016/j.jep.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 24.Kim HK, Choi BR, Bak YO, et al. The role of capillarisin from Artemisia capillaris on penile erection. Phytother Res. 2012;26:800–805. doi: 10.1002/ptr.3635. [DOI] [PubMed] [Google Scholar]
- 25.Park SW, Oh TY, Kim YS, et al. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. J Gastroenterol Hepatol. 2008;23:976–984. doi: 10.1111/j.1440-1746.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- 26.Yoo BM, Oh TY, Kim YB, et al. Novel antioxidant ameliorates the fibrosis and inflammation of cerulein-induced chronic pancreatitis in a mouse model. Pancreatology. 2005;5:165–176. doi: 10.1159/000085268. [DOI] [PubMed] [Google Scholar]
- 27.Ahn BO, Ko KH, Oh TY, et al. Efficacy of use of colonoscopy in dextran sulfate sodium induced ulcerative colitis in rats: the evaluation of the effects of antioxidant by colonoscopy. Int J Colorectal Dis. 2001;16:174–181. doi: 10.1007/s003840000282. [DOI] [PubMed] [Google Scholar]
- 28.Song HJ, Shin CY, Oh TY, Min YS, Park ES, Sohn UD. Eupatilin with heme oxygenase-1-inducing ability protects cultured feline esophageal epithelial cells from cell damage caused by indomethacin. Biol Pharm Bull. 2009;32:589–596. doi: 10.1248/bpb.32.589. [DOI] [PubMed] [Google Scholar]
- 29.Choi EJ, Oh HM, Wee H, et al. Eupatilin exhibits a novel anti-tumor activity through the induction of cell cycle arrest and differentiation of gastric carcinoma AGS cells. Differentiation. 2009;77:412–423. doi: 10.1016/j.diff.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Kim MJ, Kim DH, Na HK, Oh TY, Shin CY, Surh Ph D Professor YJ. Eupatilin, a pharmacologically active flavone derived from Artemisia plants, induces apoptosis in human gastric cancer (AGS) cells. J Environ Pathol Toxicol Oncol. 2005;24:261–269. doi: 10.1615/jenvironpatholtoxicoloncol.v24.i4.30. [DOI] [PubMed] [Google Scholar]
- 31.Lee JG, Kim JH, Ahn JH, Lee KT, Baek NI, Choi JH. Jaceosidin, isolated from dietary mugwort (Artemisia princeps), induces G2/M cell cycle arrest by inactivating cdc25C-cdc2 via ATM-Chk1/2 activation. Food Chem Toxicol. 2013;55:214–221. doi: 10.1016/j.fct.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Lv W, Sheng X, Chen T, Xu Q, Xie X. Jaceosidin induces apoptosis in human ovary cancer cells through mitochondrial pathway. J Biomed Biotechnol. 2008;2008:394802. doi: 10.1155/2008/394802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nam Y, Choi M, Hwang H, et al. Natural flavone jaceosidin is a neuroinflammation inhibitor. Phytother Res. 2013;27:404–411. doi: 10.1002/ptr.4737. [DOI] [PubMed] [Google Scholar]
- 34.Yin Y, Sun Y, Gu L, et al. Jaceosidin inhibits contact hypersensitivity in mice via down-regulating IFN-γ/STAT1/T-bet signaling in T cells. Eur J Pharmacol. 2011;651:205–211. doi: 10.1016/j.ejphar.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 35.Kim MJ, Han JM, Jin YY, et al. In vitro antioxidant and anti-inflammatory activities of Jaceosidin from Artemisia princeps Pampanini cv. Sajabal. Arch Pharm Res. 2008;31:429–437. doi: 10.1007/s12272-001-1175-8. [DOI] [PubMed] [Google Scholar]
- 36.Jeong MA, Lee KW, Yoon DY, Lee HJ. Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbol-ester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in cultured human mammary epithelial cells. Ann N Y Acad Sci. 2007;1095:458–466. doi: 10.1196/annals.1397.049. [DOI] [PubMed] [Google Scholar]
- 37.Min SW, Kim NJ, Baek NI, Kim DH. Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps on carrageenan-induced inflammation in mice. J Ethnopharmacol. 2009;125:497–500. doi: 10.1016/j.jep.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Devasagayam TP, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. J Assoc Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 39.Ock CY, Kim EH, Choi DJ, Lee HJ, Hahm KB, Chung MH. 8-Hydroxydeoxyguanosine: not mere biomarker for oxidative stress, but remedy for oxidative stress-implicated gastrointestinal diseases. World J Gastroenterol. 2012;18:302–308. doi: 10.3748/wjg.v18.i4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gloire G, Legrand-Poels S, Piette J. NF-kappaB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72:1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Kundu JK, Surh YJ. Emerging avenues linking inflammation and cancer. Free Radic Biol Med. 2012;52:2013–2037. doi: 10.1016/j.freeradbiomed.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 42.Prawan A, Kundu JK, Surh YJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7:1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Kim YH, Kim EK, Ryu EY, Lee SJ. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalis lipopolysaccharide. Chem Biol Interact. 2010;188:437–445. doi: 10.1016/j.cbi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Filep JG. Biasing the lipoxin A4/formyl peptide receptor 2 pushes inflammatory resolution. Proc Natl Acad Sci U S A. 2013;110:18033–18034. doi: 10.1073/pnas.1317798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang YH, Wang HM, Cai ZY, Xu FY, Zhou XY. Lipoxin A4 inhibits NF-κB activation and cell cycle progression in RAW264.7 cells. Inflammation. 2014;37:1084–1090. doi: 10.1007/s10753-014-9832-2. [DOI] [PubMed] [Google Scholar]
- 46.Gyurko R, Van Dyke TE. The role of polyunsaturated ω-3 fatty acid eicosapentanoic acid-derived resolvin E1 (RvE1) in bone preservation. Crit Rev Immunol. 2014;34:347–357. doi: 10.1615/critrevimmunol.2014009982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.