Abstract
Cyclooxygenase-2 (COX-2) has been shown to play an important role in colon carcinogenesis. Moreover, one of the components of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, NADPH oxidase 1 (NOX1), dominantly expressed in the colon, is implicated in the pathogenesis of colon cancer. We have reported that sesamol, one of the lignans in sesame seeds, suppressed COX-2 gene transcriptional activity in human colon cancer cells, and also suppressed intestinal polyp formation in Apc-mutant mice. In the present study, we investigated the involvement of NADPH oxidase in the inhibition of COX-2 transcriptional activity by sesamol. We found that several NADPH oxidase inhibitors, such as apocynin, showed suppressive effects on COX-2 transcriptional activity. Moreover, sesamol significantly suppressed NOX1 mRNA levels in a dose-dependent manner. In addition, we demonstrated that knockdown of NOX1 successfully suppressed COX-2 transcriptional activity. These results suggest that inhibition of NADPH oxidase, especially NOX1, may be involved in the mechanism of the suppression of COX-2 transcriptional activity by sesamol.
Keywords: cyclooxygenase-2, NADPH oxidase, sesame, sesamol, colon cancer
Introduction
It is well known that prostaglandins (PGs) are involved in the development of colorectal cancer.(1) In colorectal cancer tissue, cyclooxygenase-2 (COX-2) is over-expressed compared to that in normal colonic mucosal tissue, and accumulated data have demonstrated that selective inhibitors against COX-2 suppress colon carcinogenesis.(1,2)
The sesame plant (Sesamun indicum, Linn.) is known to possess multiple biological functions, such as inhibition of inflammation and carcinogenesis.(3–5) In a previous study, we investigated effects of five sesame seeds constituents (ferulic acid, sesamin, sesamol, sesamolin and syringic acid) on the transcriptional activity of COX-2, and found that one constituent, sesamol, suppresses basal COX-2 transcriptional activity.(6) Moreover, treatment of Apc-mutant Min mice, a mouse model for human familial adenomatous polyposis, with 500 ppm sesamol in diet tended to reduce the total number of intestinal polyps developed, along with suppression of COX-2 mRNA in the polyp parts, compared to that of the untreated mice. Thus, sesamol may possess a remarkable potential to suppress COX-2 expression as a natural compound. However, the mechanism that inhibits COX-2 transcriptional activity by sesamol was not clarified in the previous study. Thus, this study aimed to elucidate the molecular mechanisms responsible for the inhibition of COX-2 transcriptional activity by sesamol.
COX-2 is induced by many stimuli, such as growth factors, mitogens and pro-inflammatory cytokines.(7) Using intestinal polyp samples of Min mice from the previous sesamol study, we performed a preliminary experiment, and obtained a tendency of reduced nicotinamide adenine dinucleotide phosphate oxidase 1 (NOX1) mRNA reduction by sesamol treatment. NOX1 is a component of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and the enzyme produces superoxides and H2O2.(8,9) NOX1 is overexpressed in human colon cancers with a correlation of activating mutations in K-ras.(10) NOX1 has been reported to stimulate mitogenesis, cell transformation and tumorigenesis when ectopically forced to express in NIH3T3 fibroblasts and DU-145 prostate epithelial cells.(11,12) Regarding sesamol, it is only reported to be able to reduce the synthesis of the coenzyme, NADPH.(13)
Therefore, we investigated the relationship between sesamol and NADPH oxidase in this study, and suggest that NOX1 is the one of the molecules responsible for the inhibition of COX-2 transcriptional activity by sesamol.
Materials and Methods
Chemicals
Apocynin and sesamol was purchased from Sigma-Aldrich (St. Louis, MO). 3-Ethoxy-4-hydroxy benzaldehyde was purchased from Tokyo Chemical Industry (Tokyo, Japan). Vanillin was purchased from Nacalai Tesque (Kyoto, Japan).
Cell culture
Details of DLD-1/COX-2-B2-βGal-BSD cells have been reported in a previous paper.(14) The cells were maintained in DMEM medium supplemented with 5% heat-inactivated fetal bovine serum (FBS; Hyclone Laboratories Inc., Logan, UT) and antibiotics (100 µg/ml streptomycin and 100 U/ml penicillin) at 37°C in 5% CO2.
Mouse tissue sample
The mouse intestinal tissue were obtained from the previous experiment reported previously.(6) In brief, six-week-old male Min mice, Apc-mutant mice, were treated with 500 ppm sesamol for 8 weeks. At the end of the experiment, all small intestinal polyps in the proximal segment were selected under a stereoscopic microscope, and the remaining intestinal mucosa (non-polyp part) was removed by scraping, and then both stored at −80°C.
Measurements of cell viability
Cell viability in each culture was determined by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells at a density of 2.0 × 104 cells per well were seeded in 96-well tissue culture plates and treated with NADPH oxidase inhibitors for 48 h. After treatment, the cells were further incubated in a medium containing 0.5 mg/ml of MTT for 1 h. The MTT formazan produced by living cells was dissolved in dimethyl sulfoxide, and absorbance at 595 nm was measured on a microplate reader (Bio-Rad Laboratories, CA).
Reporter gene assay for COX-2 promoter-dependent transcriptional activity
DLD-1/COX-2-B2-βGal-BSD cells were seeded at a density of 2.0 × 104 cells per 96-well tissue culture plate and precultured for 24 h. After 48 h treatment with the NADPH oxidase inhibitors, the total β-galactosidase activities of the cells in each well were determined by colorimetric assay using o-nitrophenyl-β-d-galactopyranoside (ONPG), as described previously.(10,11) The background β-galactosidase activity of DLD-1 cells was determined in non-treated DLD-1/B2-βGal-BSD cells, and the value was set as 0. Basal β-galactosidase activity of non-treated DLD-1/COX-2-B2-βGal-BSD cells was set as 100%. The percentage of β-galactosidase activity of each treatment was calculated from triplicate wells. The viable cell number was assessed by the MTT assay. All assays, including MTT assay, were carried out in triplicate and each experiment repeated at least three times.
Quantitative real-time polymerase chain reaction analysis
DLD-1/COX-2-B2-βGal-BSD cells were seeded at a density of 2.0 × 105 cells in 24-well plates, and incubated with 50 and 100 µM sesamol for 48 h. Total RNA was isolated using TRIzol Reagent (Invitrogen, Grand Island, NY), treated with DNase (Invitrogen) and 1 µg aliquots in a final volume of 20 µl were used for synthesis of cDNA using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Carlsbad, CA). Real-time polymerase chain reaction (PCR) was carried out using Fast Start Universal SYBR Green Mix (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions. Primers for human NOX1 (5’-CCA CTG TAG GCG CCC TAA GTT and 5’-ATG ACC GGT GCA AGG ATC C), NOX2 (5’-GCC CAA AGG TGT CCA AGC T and 5’-TCC CCA ACG ATG CGG ATA T), p22phox (5’-ACC GCC GTG GTG AAG CT and 5’-ACC GAG AGC AGG AGA TGC A), GAPDH (5'-CCA CCC ATG GCA AAT TCC and 5'-TGG GAT TTC CAT TGA TGA CAA) were employed. Primers for mice NOX1 (5'-TCC CTT TGC TTC CTT CTT GA and 5'-CCA GCC AGT GAG GAA GAG TC), NOX2 (5'-GGG GTG TTG AAG GTC TCA AA and 5'-TGT TAC CAA CTG GGA CGA CA), p22phox (5'-CGT GGC TAC TGC TGG ACG TT and 5'-TGG ACC CCT TTT TCC TCT TT), GAPDH (5'-TTG TCT CCT GCG ACT TCA and 5'-CAC CAC CCT GTT GCT GTA) were employed. To assess the specificity of each primer set, amplicons generated from the PCR reaction were analyzed for melting curves.
Design of short-interfering RNA
The siRNA duplex was designed to target the coding sequences of homo sapiens NOX1 mRNA. A scramble siRNA was used as a control. siRNA molecules were synthesized by Sigma Genosys siRNA Service (Sigma-Aldrich). The designed NOX1-siRNA and the control-siRNA sequences were as follows: NOX1-siRNA (siRNA-1): sense, 5'-GUU GUU UGG UUA GGG CUG ATT-3', and antisense, 5'-UCA GCC CUA ACC AAA CAA CTT-3'. NOX1-siRNA (siRNA-2): sense, 5'-GAA ACU GGG UGG UUA ACC ATT-3', and antisense strand RNA, 5'-UGG UUA ACC ACC CAG UUU CTT-3'.
siRNA transfection
The transfections were performed by using the X-tremeGENE siRNA transfection reagent (Roche Diagnostics) according to the manufacturer’s protocol. Briefly, 5.0 × 105 cells were added to each well of a six-well culture plate containing complete DMEM with 5% FBS and incubated overnight for cell attachment. Then the unattached cells were removed by washing and fresh DMEM medium was added. The transfection reagent was mixed with NOX1-siRNA or scrambled siRNA (80 pM) and incubated for 15 min at room temperature before adding them to two plates (one of the 6-well plates for western blot analysis and the other for COX-2 reporter gene assay in which the cells were re-seeded in 96-well plates from the 6-well plates). Control cells were also grown under the same conditions. The NOX1-siRNA-treated cells and controls cells were harvested after 6 days for western blot analysis and COX-2 reporter gene assay. During the experiment, the medium was replaced by fresh medium once every 3 days.
Western blot analysis
NOX1 protein levels were analyzed by western blot. After sesamol, NOX1-siRNA and scramble-siRNA treatment, cells were lysed in 300 µl lysis buffer [0.0625 M Tris–HCl (pH 6.8), 20% 2-mercaptoethanol, 10% glycerol, 5% sodium dodecyl sulfate]. Samples were separated on sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Millipore, MA). Antibodies for NOX1 (Abcam, Cambridge, UK) and β-actin (Sigma-Aldrich) were used at a dilution of 1:1,000 and 1:4,000, respectively. Peroxidase-conjugated secondary antibody for anti-rabbit IgG was obtained from GE Healthcare (Buckinghamshire, UK). Blots were developed with ECL western blotting detection reagents (GE Healthcare).
Statistical analysis
All the results are expressed as mean ± SD values, with statistical analysis using the Student’s t test, except for the COX-2 promoter activity investigation and mRNA examination in the human cell line. The Bonferroni z test was used for statistical analyses of the COX-2 transcriptional activity and of mRNA levels. Differences were considered to be statistically significant at p<0.05.
Results
Suppression of COX-2 transcriptional activity in human colon cancer cells by NADPH oxidase inhibitors
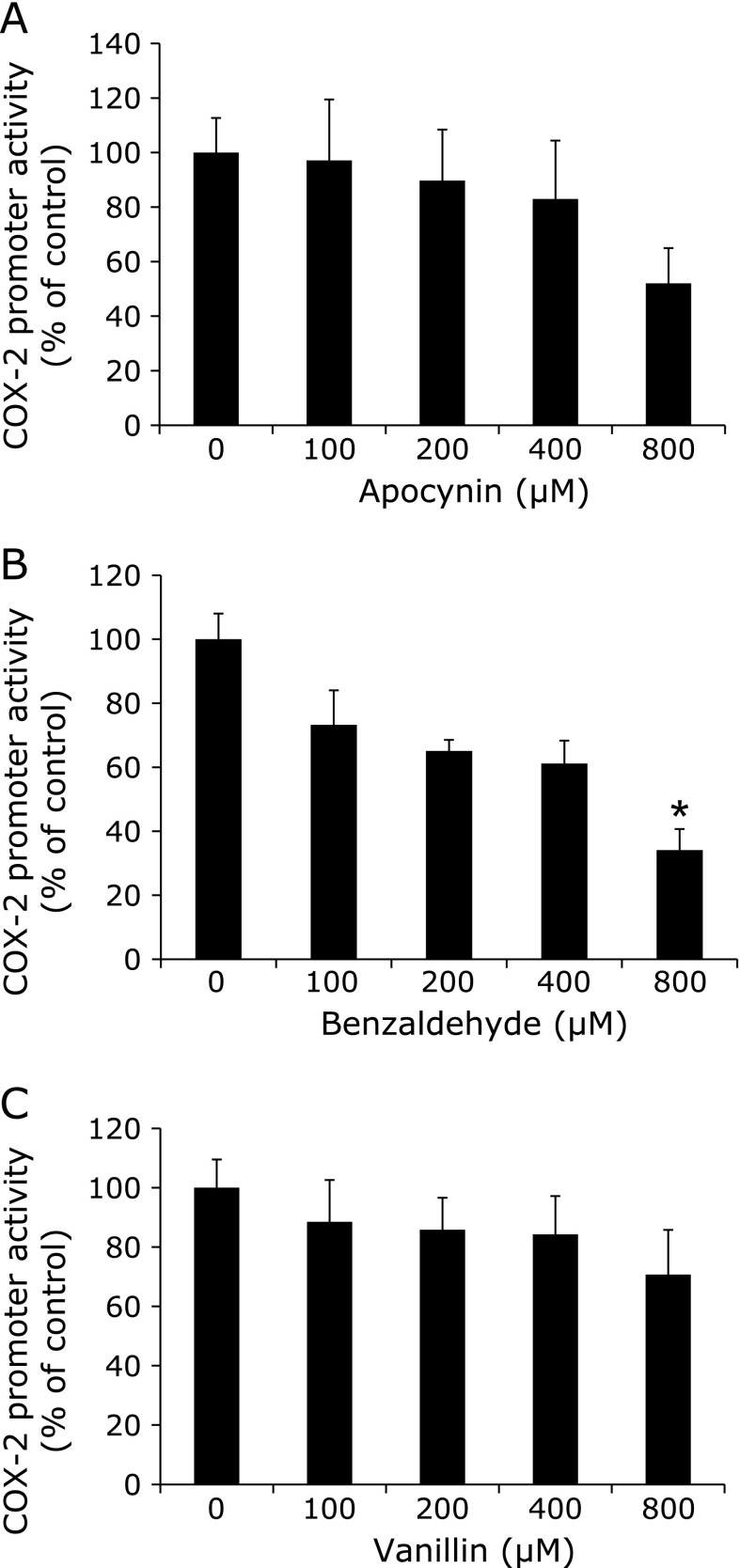
To clarify the involvement of NADPH oxidase function on COX-2 transcription, three NADPH oxidase inhibitors, apocynin, benzaldehyde and vanillin were tested at various concentrations up to 800 µM with regard to their effects on COX-2 transcriptional activity (Fig. 1). Among the NADPH oxidase inhibitors, benzaldehyde at 800 µM decreased COX-2 transcriptional activities to less than 40% of the untreated control value (Fig. 1B). The other NADPH oxidase inhibitors tended to suppress COX-2 transcriptional activities. During the experiment, those NADPH oxidase inhibitors at the used concentrations did not show remarkable inhibition of cell viability assessed by MTT assay.
Fig. 1.
Effects of NADPH oxidase inhibitors on reporter gene activity in DLD-1/COX-2-B2-βGal-BSD cells. DLD-1/COX-2-B2-βGal-BSD cells were seeded in 96-well multi-well plates at a density of 2.0 × 104 cell/well and cultured in medium containing the indicated NADPH oxidase inhibitors, apocynin (A), benzaldehyde (B) and vanillin (C), at concentrations up to 800 µM for 48 h. After 48 h, the COX-2 transcriptional activity was evaluated by β-galactosidase activity and was normalized for viable cell numbers assessed by MTT assay. The columns indicate the values of the mean percentages of triplicate wells of transcriptional activity of DLD-1/COX-2-B2-βGal-BSD cells. The data are representative of more than three independent experiments. Data are mean ± SD (n = 3). *p<0.01 vs 0 µM.
Decrease of NADPH oxidase mRNA and protein levels in human colon cancer cells by sesamol
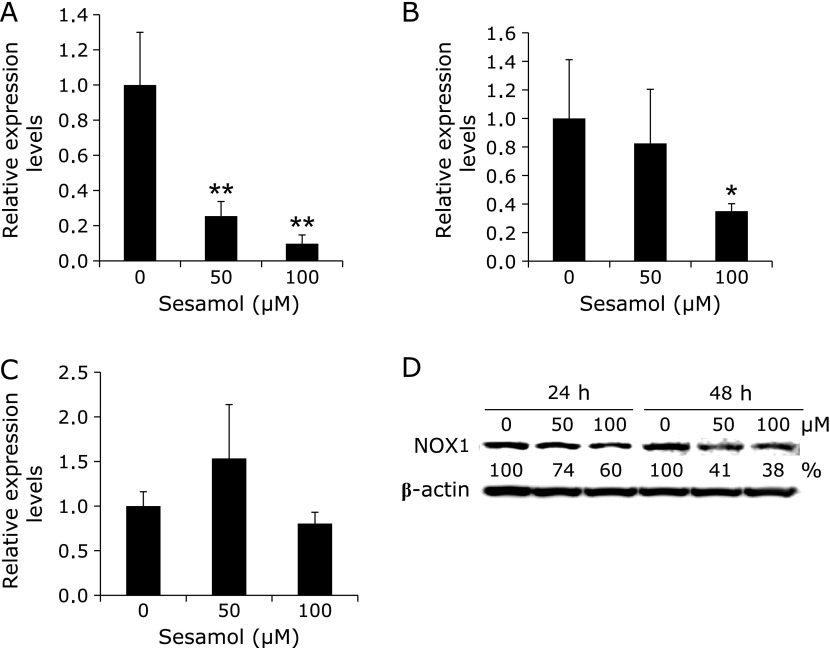
To evaluate the effect of sesamol suppressing COX-2 transcriptional activity on expression of NADPH oxidase, several mRNA of NADPH oxidase components were investigated in human colon cancer cells with or without sesamol (Fig. 2). Treatment of DLD-1/COX-2-B2-βGal-BSD cells with 50 or 100 µM sesamol for 48 h revealed that sesamol significantly suppressed NOX1 and NOX2 mRNA levels in a dose-dependent manner (Fig. 2A and B). As in the case of p22phox, mRNA levels were not clearly changed by sesamol treatment (Fig. 2C). The expression levels of NOX1 protein were also confirmed by western blotting, and the levels were reduced in a dose- and time-dependent manner (Fig. 2D).
Fig. 2.
Expression levels of components of NADPH oxidase in human colon cancer cells with or without sesamol treatment. DLD-1/COX-2-B2-βGal-BSD cells were seeded in 24-well multi-well plates at a density of 2.0 × 105 cell/well and cultured in medium containing 50 and 100 µM sesamol for 48 h. After 48 h treatment, quantitative real-time PCR analysis was performed to determine NOX1 (A), NOX2 (B), p22phox(C) mRNA levels. Data are normalized with GAPDH. The data are representative of more than three independent experiments. Data are mean ± SD, n = 3. *p<0.01, **p<0.001 vs 0 µM. After indicated dose and time treatment of sesamol, Western blot analysis was performed to determine NOX1 (D) protein levels. β-actin was proved as a loading control. Densities of the bands were calculated and are shown under the band as % of control. NOX; nicotinamide adenine dinucleotide phosphate oxidase.
Knockdown of NOX1 by siRNA transfection suppressed COX-2 transcriptional activity in human colon cancer cells
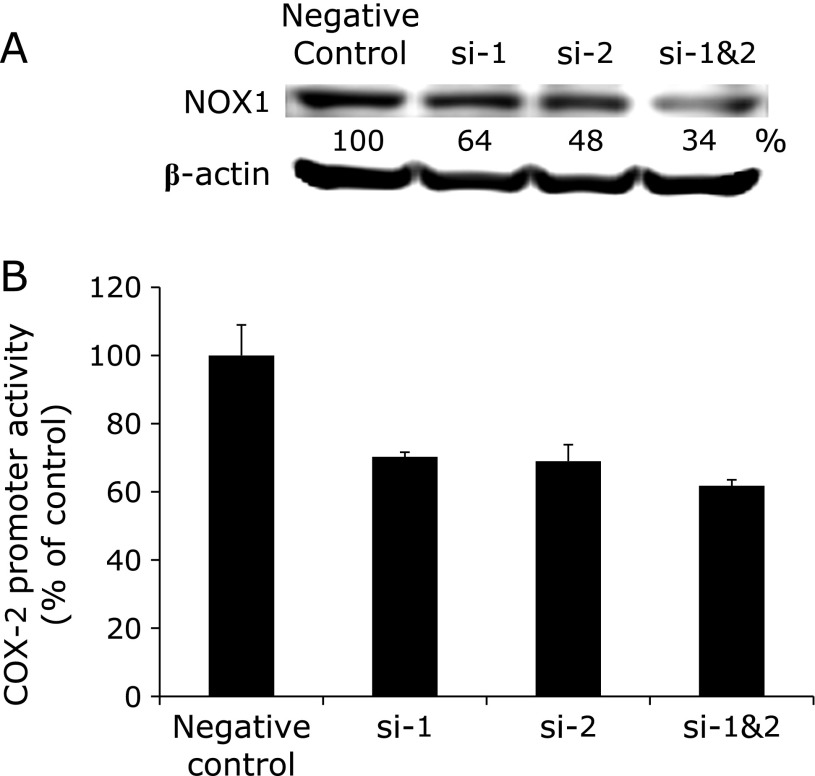
As NOX1 mRNA was strongly suppressed by sesamol, we next focused on NOX1 and created NOX1-specific siRNAs to test for the ability to suppress COX-2 transcriptional activity. Western blot analysis showed that transfection of NOX1 siRNAs (si-1, si-2 and si-1&2) decreased the protein levels of NOX1 up to 34% of the control (Fig. 3A). At the same timing of protein depression, we evaluated COX-2 transcriptional activity in DLD-1/COX-2-B2-βGal-BSD cells. NOX1 knockdown with si-1, si-2 or si-1&2 resulted in decreased COX-2 transcriptional activities to 60% of the control (Fig. 3B).
Fig. 3.
Knockdown of NOX1 by siRNA transfection inhibits NADPH oxidase activities in human colon cancer cells. DLD-1/COX-2-B2-βGal-BSD cells were transfected with negative control or NOX1 siRNAs (si-1, si-2 and si-1&2), and cultured for 6 days. (A) NOX1 protein levels were analyzed by western blot. β-actin was proved as a loading control. Densities of the bands were calculated and are shown under the band as % of control. (B) COX-2 transcriptional activity was evaluated by β-galactosidase activity and was normalized with viable cell numbers assessed by MTT assay.
Decrease of NADPH oxidase mRNA levels in intestinal polyp parts of Min mice by sesamol administration
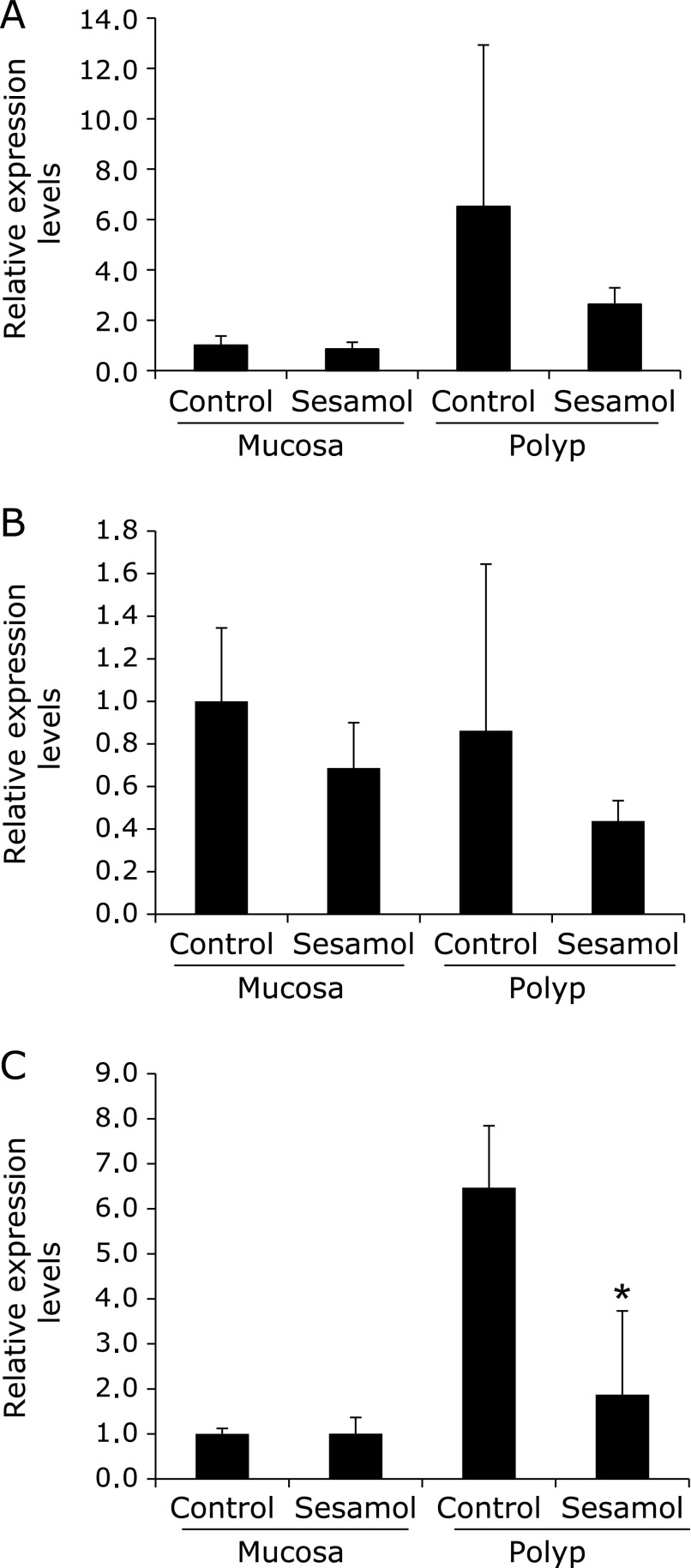
The components of NADPH oxidase mRNA expression in the mice intestinal tissue obtained from the previous experiment were investigated (Fig. 4).(6) Real-time PCR revealed that treatment with 500 ppm sesamol for 8 weeks significantly suppressed p22phox mRNA levels in the intestinal polyp parts (Fig. 3C). NOX1 mRNA levels tended to be reduced in intestinal polyp parts by sesamol (Fig. 3A). The components of NADPH oxidase mRNA expression levels in non-polyp parts of the small intestine were not suppressed by sesamol treatment.
Fig. 4.
Expression levels of component of NADPH oxidase in intestinal non-polyp mucosa parts and/or polyp parts of Min mice. Quantitative real-time PCR analysis was performed to determine NOX1 (A), NOX2 (B), p22phox (C) mRNA expression levels in the polyps or non-polyp mucosa parts of Min mice, given diets containing sesamol at doses of 500 ppm for 8 weeks. Data are normalized with GAPDH expression level. Data are mean ± SD, n = 6. *p<0.01 vs 0 ppm.
Discussion
Recently, we reported that sesamol inhibits COX-2 transcriptional activity in human colon cancer cells. In the present study, we found that COX-2 transcriptional activity in human colon cancer cells was suppressed by NADPH oxidase inhibitors, and sesamol decreased NADPH oxidase mRNA levels in human colon cancer cells. Moreover, knockdown of NOX1, a component of NADPH oxidase, successfully suppressed COX-2 transcriptional activity.
As far as we know, this is the first report demonstrating the suppression of COX-2 transcriptional activity in human colon cancer cells by NADPH oxidase inhibitors, apocynin, benzaldehyde and vanillin. In recent years, it has been suggested that lipopolysaccharides stimulate NADPH oxidase activation, and resultant reactive oxygen species (ROS) generation were playing a role on COX-2 expression.(15) In this literature, NADPH oxidase inhibitor, apocynin reduced COX-2 synthesis. Moreover, it has been reported that NOX1-derived ROS activates crucial components for mitogen signaling, including p38 mitogen-activated protein kinase (MAPK), Akt/PI3K, and tyrosine phosphatase.(16) We found the involvement of NADPH oxidase function on COX-2 transcription levels without any ROS-inducing stimulation. In our experiments, it is not clear that NADPH oxidase inhibitors directly blocked the COX-2 inducing pathways or indirect pathways through ROS reduction.
In the next experiments, we demonstrated a decrease of NADPH oxidase expression levels in human colon cancer cells by sesamol treatments. This is the first report that presents significant suppression of NOX1 and NOX2 mRNA expression levels by sesamol. We have not examined whether long-term sesamol treatment inhibits cell growth or not. Our previous study demonstrated that sesamol inhibits intestinal polyp formation in Apc-mutant Min mice, in which β-catenin signaling is activated. Recent studies have shown that Wnt-induced activation of β-catenin signaling and cell growth was inhibited by NOX1 ablation.(17) It is expected that blocking a complex of NADPH oxidase components may decrease the activity of NADPH oxidase.(18) Thus, down-regulation of NOX1 by sesamol could be an additional approach against β-catenin signaling-dependent cancers.
In our experiments, NOX1 mRNA was strongly suppressed by sesamol. Thus, we next focused on the role of NOX1. NOX1-specific siRNAs successfully suppressed COX-2 transcriptional activity. This data demonstrated that NOX1 plays an important role in the regulation of COX-2 transcription. However, inhibition of COX-2 transcriptional activity was not strong as expected by NOX1 protein expression level knockdown by siRNA of NOX1. Thus, other factors, such as NOX2 and MAPK pathways,(19–21) could be involved in the regulation of COX-2 expression.
In conclusion, we investigated the relationship between sesamol and NADPH oxidase in this study, and suggest that NOX1 is one of the molecules that is responsible for the inhibition of COX-2 transcriptional activity by sesamol.
Acknowledgments
This work was supported by Grants-in-Aid for the Third-Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour, and Welfare of Japan, and also from Grants-in-Aid for Project Future, Relay for Life (Japan Cancer Society), and also supported by the National Cancer Center Research Core Facility.
Abbreviations
- COX
cyclooxygenase
- FBS
fetal bovine serum
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- NOX1
nicotinamide adenine dinucleotide phosphate oxidase 1
- ONPG
o-nitrophenyl-β-d-galactopyranoside
- PCR
polymerase chain reaction
- PGs
prostaglandins
- ROS
reactive oxygen species
- siRNA
short interfering RNA
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Mutoh M, Takahashi M, Wakabayashi K. Roles of prostanoids in colon carcinogenesis and their potential targeting for cancer chemoprevention. Curr Pharm Des. 2006;12:2375–2382. doi: 10.2174/138161206777698972. [DOI] [PubMed] [Google Scholar]
- 2.Rigas B, Goldman IS, Levine L. Altered eicosanoid levels in human colon cancer. J Lab Clin Med. 1993;122:518–523. [PubMed] [Google Scholar]
- 3.Hagiwara A, Kokubo Y, Takesada Y, et al. Inhibitory effects of phenolic compounds on development of naturally occurring preneoplastic hepatocytic foci in long-term feeding studies using male F344 rats. Teratog Carcinog Mutagen. 1996;16:317–325. doi: 10.1002/(SICI)1520-6866(1996)16:6<317::AID-TCM4>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Hirose N, Doi F, Ueki T, et al. Suppressive effect of sesamin against 7,12-dimethylbenz[a]-anthracene induced rat mammary carcinogenesis. Anticancer Res. 1992;12:1259–1265. [PubMed] [Google Scholar]
- 5.Salerno JW, Smith DE. The use of sesame oil and other vegetable oils in the inhibition of human colon cancer growth in vitro. Anticancer Res. 1991;11:209–215. [PubMed] [Google Scholar]
- 6.Shimizu S, Fujii G, Takahashi M, et al. Sesamol suppresses cyclooxygenase-2 transcriptional activity in colon cancer cells and modifies intestinal polyp development in ApcMin/+ mice. J Clin Biochem Nutr. 2014;54:95–101. doi: 10.3164/jcbn.13-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 8.Lawrece JC, Jr, Larner J. Activation of glycoge synthase in rat adipocytes by insulin and glucose involves increased glucose transport and phosphorylation. J Biol Chem. 1978;253:2104–2113. [PubMed] [Google Scholar]
- 9.Mukherjee SP, Lane RH, Lynn WS. Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol. 1978;27:2589–2594. doi: 10.1016/0006-2952(78)90332-5. [DOI] [PubMed] [Google Scholar]
- 10.Laurent E, McCoy JW, 3rd, Macina RA, et al. Nox1 is over-expressed in human colon cancers and crrelates with activating mutations in K-Ras. Int J Cancer. 2008;123:100–107. doi: 10.1002/ijc.23423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbiser JL, Yeung R, Weiss SW, et al. The generation and characterization of a cell line derived from a sporadic renal angiomyolipoma: use of telomerase to obtain stable popuration of cells from benign neoplasms. Am J Pathol. 2001;159:483–491. doi: 10.1016/S0002-9440(10)61720-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suh YA, Arnold RS, Lassegue B, et al. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 13.Jacklin A, Ratledge C, Welham K, Bilko D, Newton CJ. The sesame seed oil constituent, sesamol, induces growth arrest and apoptosis of cancer and cardiovascular cells. Ann N Y Acad Sci. 2003;10:374–380. doi: 10.1196/annals.1299.068. [DOI] [PubMed] [Google Scholar]
- 14.Mutoh M, Takahashi M, Fukuda K, et al. Suppression of cyclooxygenase-2 promoter-dependent transcriptional activity in colon cancer cells by chemopreventive agents with a resorcin-type structure. Carcinogenesis. 2000;21:959–963. doi: 10.1093/carcin/21.5.959. [DOI] [PubMed] [Google Scholar]
- 15.Lin WN, Lin CC, Cheng HY, et al. Regulation of cyclooxygenase-2 and cytosolic phospholipase A2 gene expression by lipopolysaccharide through the RNA-binding protein HuR: involvement of NADPH oxidase, reactive oxygen species and mitogen-activated protein kinases. Br J Pharmacol. 2011;163:1691–1706. doi: 10.1111/j.1476-5381.2011.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarty MF. Minimizing the cancer-promotional activity of cox-2 as a central strategy in cancer prevention. Med Hypotheses. 2012;78:45–57. doi: 10.1016/j.mehy.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 17.Kajla S, Mondol AS, Nagasawa A, et al. A crucial role for Nox 1 in redox-dependent regulation of Wnt-β-catenin signaling. FASEB J. 2012;26:2049–2059. doi: 10.1096/fj.11-196360. [DOI] [PubMed] [Google Scholar]
- 18.Sancho P, Martín-Sanz P, Fabregat I. Reciprocal regulation of NADPH oxidases and the cyclooxygenase-2 pathway. Free Radic Biol Med. 2011;51:1789–1798. doi: 10.1016/j.freeradbiomed.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Shao J, Evers BM, Sheng H. Prostaglandin E2 synergistically enhances receptor tyrosine kinase-dependent signaling system in colon cancer cells. J Biol Chem. 2004;279:14287–14293. doi: 10.1074/jbc.M313276200. [DOI] [PubMed] [Google Scholar]
- 20.Lin CC, Lee IT, Wu WL, Lin WN, Yang CM. Adenosine triphosphate regulates NADPH oxidase activity leading to hydrogen peroxide production and COX-2/PGE2 expression in A549 cells. Am J Physiol Lung Cell Mol Physiol. 2012;303:L401–L412. doi: 10.1152/ajplung.00090.2012. [DOI] [PubMed] [Google Scholar]
- 21.Kamizato M, Nishida K, Masuda K, et al. Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol. 2009;44:1172–1184. doi: 10.1007/s00535-009-0119-6. [DOI] [PubMed] [Google Scholar]