Abstract
Objective
The aim of this study was to investigate voluntary wheel running behavior in the unilateral 6-hydroxydopamine (6-OHDA) rat model.
Methods
Male Sprague-Dawley rats were assigned to 2 groups : 6-OHDA group (n=17) and control group (n=8). The unilateral 6-OHDA rat model was induced by injection of 6-OHDA into unilateral medial forebrain bundle using a stereotaxic instrument. Voluntary wheel running activity was assessed per day in successfully lesioned rats (n=10) and control rats. Each behavioral test lasted an hour. The following parameters were investigated during behavioral tests : the number of running bouts, the distance moved in the wheel, average peak speed in running bouts and average duration from the running start to the peak speed.
Results
The number of running bouts and the distance moved in the wheel were significantly decreased in successfully lesioned rats compared with control rats. In addition, average peak speed in running bouts was decreased, and average duration from the running start to the peak speed was increased in lesioned animals, which might indicate motor deficits in these rats. These behavioral changes were still observed 42 days after lesion.
Conclusion
Voluntary wheel running behavior is impaired in the unilateral 6-OHDA rat model and may represent a useful tool to quantify motor deficits in this model.
Keywords: Parkinson's disease, Voluntary wheel running behavior, 6-hydroxydopamine, Medial forebrain bundle, Rat
INTRODUCTION
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease21). It is characterized pathologically by the selective and progressive loss of dopaminergic neurons in the substantia nigra pars compacta (SNc) and the consequent deficiency of dopamine in the striatum16). The dopaminergic deficit causes most of the motor manifestations associated with the disease, including resting tremor, rigidity, bradykinesia, gait disturbance and postural instability11).
The unilateral 6-hydroxydopamine (6-OHDA) rat model has been widely used in PD research for decades. The model reproduces the major symptoms of PD, including akinesia, posture abnormality, tremor and dyskinesia5). Many of motor deficits in this model can be measured using various behavioral tests, such as paw retraction test, apomorphine-induced rotations, locomotor activity, treadmill locomotion task and forelimb use asymmetry test6,9).
In rodents, voluntary wheel running task was used to monitor both the absolute levels of motor activity and the circadian rhythmicity of this activity in many studies13,15,26,27). Numerous factors can influence voluntary wheel running behavior in animals20,28). Recently, Leng et al.18) used voluntary wheel running task to evaluate motor dysfunctions in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) mouse model of PD. By blocking the de novo DA biosynthesis, they found a reduction of voluntary wheel running activity in MPTP-treated mice, which suggested that voluntary wheel running task might provide a useful tool to assess motor deficits in rodent model of PD.
In the present study, we investigated whether voluntary wheel running behavior is impaired in the unilateral 6-OHDA rat model induced by injection of 6-OHDA into unilateral medial forebrain bundle (MFB) using a stereotaxic instrument.
MATERIALS AND METHODS
Animals
Twenty-five adult male Sprague-Dawley rats (250-300 g) obtained from the Laboratory Animal Center of the Academy of Military Medical Sciences were used in this experiment. Animals were housed individually in cages under a room temperature of 22±2℃ with a 12 h light-dark cycle (lights on at 7 a.m.) and were handled on a daily basis to familiarize them with handling. Food and water were provided ad libitum.
Surgery
Rats were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.; Sigma-Aldrich Corp., St. Louis, MO, USA). Penicillin G Sodium (16000 U per rat, i.m.) was given prior to the surgery. A stainless steel infusion guide cannula (23 gauge) was stereotaxically implanted with the tip placed 2 mm above the right MFB using the following coordinates : 2.0 mm posterior, 2.0 mm lateral and 6.1 mm ventral to the bregma23), to allow injection of 6-OHDA later in the experiment. The guide cannula was fixed to the skull with three stainless steel screws and dental cement. Each cannula was kept patent with a sterile obturator until the time of drug administration. Rats were allowed to recover from surgery for at least 7 days before being subjected to the behavioral experiment.
Experimental procedures
In behavioral experiment, rats were placed in a running wheel (diameter of drum : 330 mm, width of drum : 80 mm, diameter of grid rods : 6 mm, distance between rods : 20 mm), the edge of which was symmetrically mounted with four small iron cubes. When these iron cubes passed a sensor, they could be detected by it. The signals produced by the sensor were recorded and stored by the data acquisition software Magnet (Biographics Inc., Winston-Salem, NC, USA). Behavioral testing, which was performed under dim light, was videotaped with an infrared camera for off-line analysis of wheel running behavior. Prior to testing, animals were habituated to the running wheel 1 hour per day for 5 days, in order to obtain a stable wheel running behavior. Then rats were tested for voluntary wheel running behavior per day for 3 days. Each behavioral test lasted 1 hour.
After these tests, each rat was lightly anesthetized with sodium pentobarbital (20 mg/kg, i.p.; Sigma-Aldrich Corp., St. Louis, MO, USA) and received an injection of desipramine hydrochloride (15 mg/kg, i.p.; Sigma-Aldrich Corp., St. Louis, MO, USA), a norepinephrine uptake blocker. Thirty minutes later, 10 µg 6-OHDA (free base in 4 µL 0.2% ascorbic acid saline solution; Sigma-Aldrich Corp., St. Louis, MO, USA) was injected into the right MFB of rats in 6-OHDA group (n=17) over an 8-min period. The injection needle (30 gauges) was left in the cannula for at least 5 minutes after the completion of the injection to prevent leaking and allow diffusion of the drug into the MFB. In control group (n=8), the corresponding volume of 0.2% ascorbic acid saline solution was injected.
Unilateral dopamine (DA) depletion was confirmed with a traditional rotation test performed 14 days after 6-OHDA injections. Each rat was placed in a spherical-shaped behavioral chamber that enabled it to turn freely. The number of contralateral rotation was counted 10 min after administration of apomorphine (0.25 mg/kg, s.c.; Sigma-Aldrich Corp., St. Louis, MO, USA) for 30 min. Animals scoring over 7 rpm (displaying at least 7 full-body contralateral rotations per minute) are considered to be successfully lesioned4).
After rotation test, voluntary wheel running activity was assessed per day for 10 days in successfully lesioned rats and control rats. Each test lasted 1 hour. To investigate the long-term effect of MFB lesion on voluntary wheel running behavior, rats were tested on days 39-42 after 6-OHDA lesion.
Immunohistochemistry
Following the behavioral tests, immunohistochemistry was performed as described previously22). Briefly, midbrain samples were sectioned coronally with a cryostat (CM 1900, Leica, Nussloch, Germany) at a thickness of 30 µm. Sections were collected in sequence and were immunostained with mouse monoclonal anti-tyrosine hydroxylase (TH) antibody (TH, 1 : 1000; Sigma-Aldrich Corp., St. Louis, MO, USA). After TH staining, the number of TH-immunopositive neurons in the SNc was counted bilaterally on four adjacent sections between 4.92 mm and 5.04 mm posterior from the bregma23). For each animal, neuronal survival in the SNc was expressed as the percentage of TH-immunopositive neurons on the lesioned side, with respect to the intact side; this approach was chosen to avoid methodological biases due to interindividual differences and is widely used to assess the extent of 6-OHDA-induced lesion in the SNc12).
Statistical analysis
The data of behavioral experiment were analyzed offline using Microsoft Excel and NeuroExplorer (Nex Technologies, Littleton, MA, USA). The following parameters were investigated : the number of running bouts, the distance moved in the wheel, average peak speed in running bouts and average duration from the running start to the peak speed. Data obtained from both successfully lesioned rats and control rats were used in statistical analysis. All values are expressed as mean±standard error of the mean. The wheel running parameters were compared using repeated measures analysis of variance with testing time as Within-Subjects Factor and treatment as Between-Subjects Factor. The number of TH-positive cells in both groups was compared using independent-samples t-test. Differences were considered significant at p<0.05. These statistics were performed using the SPSS 13.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Apomorphine-induced rotation test
Ten out of the 17 rats were successfully lesioned after the injection of 6-OHDA into MFB. The success rate was about 58.8%. These rats became bradykinetic and adopted a stooped posture. In control rats, no spontaneous behavioral change was observed. Administration of 0.25 mg/kg apomorphine hydrochloride (s.c.) to control rats 2 weeks after surgery had no obvious effect on motor behavior. However, in successfully lesioned rats, apomorphine hydrochloride produced a reversal of motor dysfunction with increased motor activity beginning about 5 min after treatment. Furthermore, apomorphine induced rapid contralateral rotations in these rats.
Voluntary wheel running test
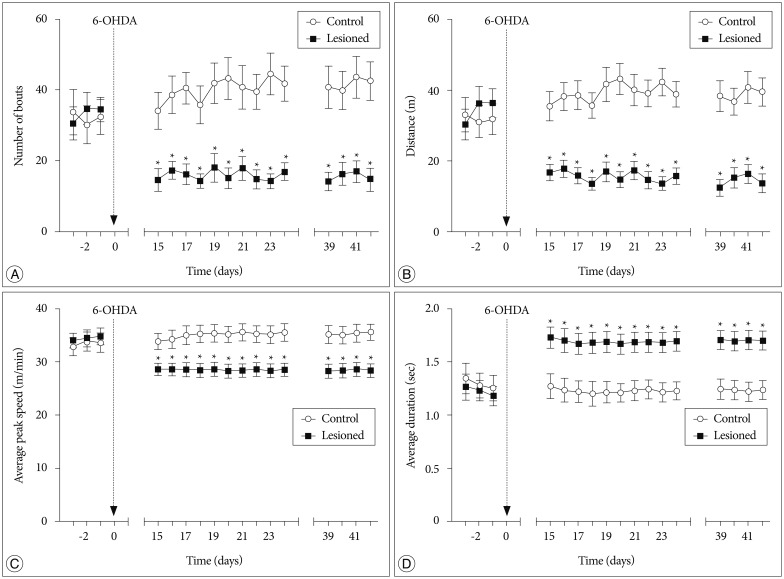
In voluntary wheel running behavioral test, a significant interaction of treatment×testing time was found in two parameters : the number of running bouts [F (16, 256)=4.612, p=0.002] (Fig. 1A) and the distance moved in the wheel [F (16, 256)=6.700, p=0.000] (Fig. 1B). Fifteen to twenty-four days after the treatment, the two parameters were significantly decreased in successfully lesioned rats compared with control rats (p<0.05). These results reflected that successfully lesioned rats showed a reduced voluntary wheel running activity. Furthermore, the reduction could be observed on days 39-42 after the treatment.
Fig. 1. Voluntary wheel running performance during 1 h testing session. The number of bouts (A), the distance moved in the wheel (B), and average peak speed (C) were significantly decreased in successfully lesioned rats (n=10). D : Average duration from the running start to the peak speed was significantly increased in successfully lesioned rats (n=10). All values are expressed as mean±standard error of the mean. *p<0.05, compared to control rats (n=8). 6-OHDA : 6-hydroxydopamine.
A significant interaction of treatment×testing time was also found in the other two parameters : average peak speed in running bouts [F (16, 256)=8.477, p=0.006] (Fig. 1C) and average duration from the running start to the peak speed [F (16, 256)=14.472, p=0.000] (Fig. 1D). Fifteen to twenty-four days after the treatment, average peak speed was decreased in successfully lesioned rats (p<0.05), and average duration from the running start to the peak speed was also significantly increased in these rats (p<0.05). In addition, these changes could be detected on days 39-42 after the treatment. These findings might indicate motor deficits in successfully lesioned rats.
In tests before the treatment, there were no significant differences in these four parameters between the two groups (p>0.05) (Fig. 1).
Immunohistochemistry
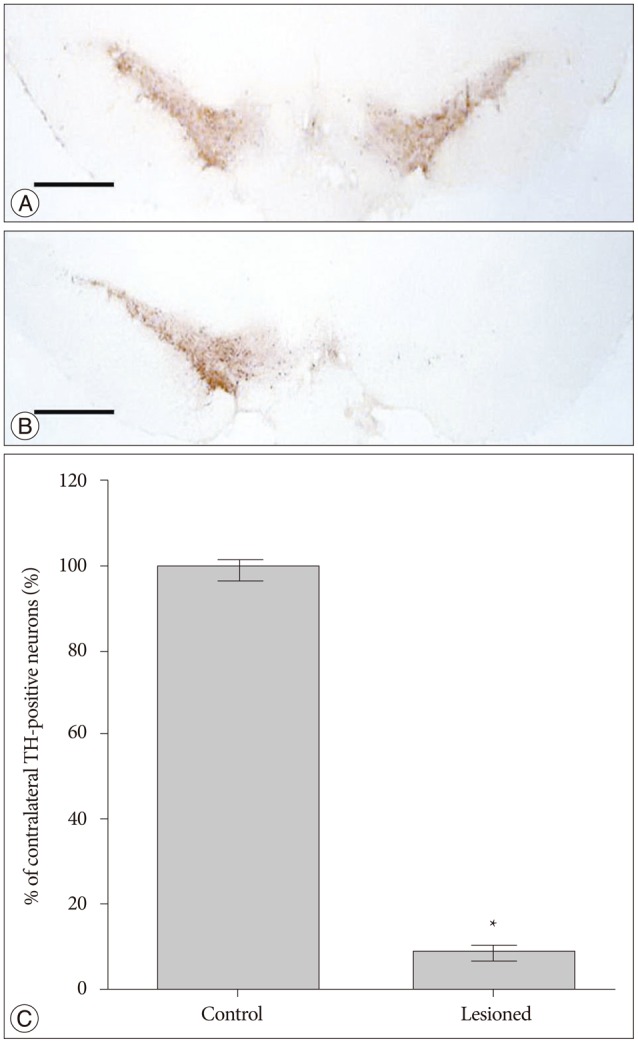
After application of the anti-TH antibody in control rats, our data show a large number of TH-positive cells in both sides of SNc (Fig. 2A). In contrast, a significant loss of TH-positive cells was found in the SNc ipsilateral to the lesion side in successfully lesioned rats (Fig. 2B). TH-positive cell counts in the left and right SNc are shown in Fig. 2C. The number of TH-positive cells in the right SNc was decreased by -91% compared to the left SNc.
Fig. 2. Tyrosine hydroxylase (TH) immunostaining of the substantia nigra pars compacta (SNc) in control rats and successfully lesioned rats. A : There were a large number of TH-positive cells in both sides of the SNc in control rats. B : A significant loss of TH-positive cells was found in the SNc ipsilateral to the lesion side in successfully lesioned rats. C : TH-positive cell counts in SNc. All values are expressed as mean±standard error of the mean. *p<0.001, compared to control rats; Scale bar=1 mm.
DISCUSSION
In the present study, we found that voluntary wheel running behavior was impaired in the unilateral 6-OHDA rat model of Parkinson's disease. Successfully lesioned rats showed a decreased voluntary wheel running activity. Furthermore, motor deficits of these rats could be detected during the task. These behavioral changes remained in these rats 42 days after lesion.
Up to date, the animal model of PD that has contributed the most in preclinical PD research is the unilateral 6-OHDA lesion of the MFB in rats9). Injection of 6-OHDA into unilateral MFB can cause a total destruction of A9 and A10 cell groups, resulting in near total depletion of DA in the ipsilateral caudate-putamen complex (CPu) and parkinsonian-like motor impairments including akinesia and sensorimotor deficits5,29). To confirm whether the rats are successfully lesioned, typical apomorphine-induced rotation test is usually performed2). Only rats displaying rapid contralateral rotations after administration of apomorphine (typically over 7 rpm) are considered to be successfully lesioned4).
In this study, ten successfully lesioned rats were obtained from seventeen rats. The success rate was about 58.8%. This result is consistent with usually reported success rates of 50-70%8). In these rats, unilateral DA depletion was further confirmed by using TH immunostaining of SNc, and we found an extensive loss (-91%) of dopaminergic neurons in the SNc on the lesion side, which results in postsynaptic supersensitivity in ipsilateral CPu and an imbalance in DA activity between the both sides of the CPu. When challenged with drugs acting on the DA system the rats will rotate away from the side of greater activity30). So, apomorphine which is a dopamine agonist induces a rotation contralateral to the lesion side due to the postsynaptic supersensitivity in ipsilateral CPu.
In addition to drug-induced rotation test, various behavioral tests have been designed to evaluate different behavioral deficits in the unilateral 6-OHDA rat model including the paw retraction test for akinesia, the adjusting steps task for rigidity, the staircase test for fine motor control and Morris water escape test for cognitive and sensorimotor impairments9). However, to the best of our knowledge, voluntary wheel running behavior has never been investigated in the unilateral 6-OHDA rat model. Voluntary wheel running is perhaps the most widely reported behavior performed by captive animals28). Several species, including rodents, are highly motivated to perform wheel running. Voluntary wheel running, which is usually considered as a spontaneous activity, is a highly plastic behavior and can be varied or regulated by internal and external causal factors28). Here, we found that unilateral 6-OHDA lesion of the MFB reduced voluntary wheel running activity in rats, which was measured as the number of running bouts and the distance moved in the wheel.
The mechanisms underlying this phenomenon are still unknown. Considering that voluntary wheel running is believed to be possibly rewarding or motivation-driven1,25,28) and that dopaminergic system plays an essential role in reward, motivation and motor function3,14,32), it is reasonable to assume that the decrease in voluntary wheel running activity may represent diminished capacity to initiate movement, decreased motivation to run or both. Similar to our findings, previous studies using open-field test have reported decreased locomotor activity in unilateral 6-OHDA rats with severe DA depletion10,24).
In addition, decreased average peak speed and prolonged average duration from the running start to the peak speed were observed in successfully lesioned rats. These results might indicate motor deficits in these rats. The motor deficits might include akinesia in the affected limbs and impaired motor coordination between affected limbs and unaffected limbs. Leng et al.18) recently reported that subchronically MPTP-treated mice did not show any persistent effects in a running wheel test, despite depletion of almost 90% of striatal dopamine. Compensatory mechanisms, like the development of supersensitivity17,31) or the increased de novo synthesis of D18), have been thought to mask behavioral deficits in the subchronic MPTP mouse model. It remains difficult to reliably detect behavioral motor equivalent for PD in this model8,19). Conversely, the unilateral 6-OHDA rat model is a relatively stable model and shows persistent motor deficits in several behavioral tests9). In our study, motor deficits could be detected in unilateral 6-OHDA rats with severe DA depletion (about 91% of dopaminergic neurons were lost in lesioned SNc).
Voluntary wheel running test might represent a sensitive tool to characterize and quantify motor impairments in the unilateral 6-OHDA rat model of PD. The task has the advantage that the influence of the investigator is largely excluded since the data collection is fully automated. Labour costs for voluntary wheel running task are likewise minimized because of its high level of automation. This task might be used in experiments that run through a long period of time, because behavioral changes during the task could be observed in lesioned rats 42 days after lesion in our study. Voluntary wheel running test can also be used together with forced wheel running test to investigate the different neural mechanisms underlying central motor control between internally triggered, spontaneous movement and externally triggered, forced movement due to the same experimental environment and similar running behaviors7).
CONCLUSION
Our results demonstrate that voluntary wheel running behavior is impaired in the unilateral 6-OHDA rat model. Voluntary wheel running test may represent a useful tool to quantify motor deficits in this model.
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 30872662) and Key Laboratory of Mental Health, Chinese Academy of Sciences.
References
- 1.Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats : a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Bové J, Perier C. Neurotoxin-based models of Parkinson's disease. Neuroscience. 2012;211:51–76. doi: 10.1016/j.neuroscience.2011.10.057. [DOI] [PubMed] [Google Scholar]
- 3.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control : rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carman LS, Gage FH, Shults CW. Partial lesion of the substantia nigra : relation between extent of lesion and rotational behavior. Brain Res. 1991;553:275–283. doi: 10.1016/0006-8993(91)90835-j. [DOI] [PubMed] [Google Scholar]
- 5.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits : how relevant is the rat? Nat Rev Neurosci. 2002;3:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 6.Chang JY, Shi LH, Luo F, Woodward DJ. High frequency stimulation of the subthalamic nucleus improves treadmill locomotion in unilateral 6-hydroxydopamine lesioned rats. Brain Res. 2003;983:174–184. doi: 10.1016/s0006-8993(03)03053-1. [DOI] [PubMed] [Google Scholar]
- 7.Chang JY, Shi LH, Luo F, Zhang WM, Woodward DJ. Studies of the neural mechanisms of deep brain stimulation in rodent models of Parkinson's disease. Neurosci Biobehav Rev. 2008;32:352–366. doi: 10.1016/j.neubiorev.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8.da Conceição FS, Ngo-Abdalla S, Houzel JC, Rehen SK. Murine model for Parkinson's disease : from 6-OH dopamine lesion to behavioral test. J Vis Exp. 2010;35:1376. doi: 10.3791/1376. http://dx.doi.org/10.3791/1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats : an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175:303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- 10.Fornaguera J, Schwarting RK. Early behavioral changes after nigro-striatal system damage can serve as predictors of striatal dopamine depletion. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1353–1368. doi: 10.1016/s0278-5846(99)00071-8. [DOI] [PubMed] [Google Scholar]
- 11.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inden M, Taira T, Kitamura Y, Yanagida T, Tsuchiya D, Takata K, et al. PARK7 DJ-1 protects against degeneration of nigral dopaminergic neurons in Parkinson's disease rat model. Neurobiol Dis. 2006;24:144–158. doi: 10.1016/j.nbd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Ito C, Onodera K, Sakurai E, Sato M, Watanabe T. Effect of cocaine on the histaminergic neuron system in the rat brain. J Neurochem. 1997;69:875–878. doi: 10.1046/j.1471-4159.1997.69020875.x. [DOI] [PubMed] [Google Scholar]
- 14.Iversen SD, Iversen LL. Dopamine : 50 years in perspective. Trends Neurosci. 2007;30:188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara T, Kubota M, Miyauchi T, Noda Y, Mouri A, Nabeshima T, et al. Mice with neuron-specific accumulation of mitochondrial DNA mutations show mood disorder-like phenotypes. Mol Psychiatry. 523;11:577–593. doi: 10.1038/sj.mp.4001824. [DOI] [PubMed] [Google Scholar]
- 16.Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136(Pt 8):2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau YS, Fung YK. Pharmacological effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on striatal dopamine receptor system. Brain Res. 1986;369:311–315. doi: 10.1016/0006-8993(86)90541-x. [DOI] [PubMed] [Google Scholar]
- 18.Leng A, Mura A, Hengerer B, Feldon J, Ferger B. Effects of blocking the dopamine biosynthesis and of neurotoxic dopamine depletion with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on voluntary wheel running in mice. Behav Brain Res. 2004;154:375–383. doi: 10.1016/j.bbr.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Liebetanz D, Baier PC, Paulus W, Meuer K, Bähr M, Weishaupt JH. A highly sensitive automated complex running wheel test to detect latent motor deficits in the mouse MPTP model of Parkinson's disease. Exp Neurol. 2007;205:207–213. doi: 10.1016/j.expneurol.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 20.Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci. 2014;281:20140210. doi: 10.1098/rspb.2014.0210. http://dx.doi.org/10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakata Y, Yasuda T, Mochizuki H. Recent progress in gene therapy for Parkinson's disease. Curr Mol Med. 2012;12:1311–1318. doi: 10.2174/156652412803833580. [DOI] [PubMed] [Google Scholar]
- 22.Niu C, Mei J, Pan Q, Fu X. Nigral degeneration with inclusion body formation and behavioral changes in rats after proteasomal inhibition. Stereotact Funct Neurosurg. 2009;87:69–81. doi: 10.1159/000202972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. ed 6. New York: Academic Press; 2007. [Google Scholar]
- 24.Reglodi D, Lubics A, Tamás A, Szalontay L, Lengvári I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 25.Rhodes JS, Gammie SC, Garland T., Jr Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integr Comp Biol. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- 26.Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010;44:229–237. doi: 10.1016/j.alcohol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Rosenwasser AM, Fixaris MC. Chronobiology of alcohol : studies in C57BL/6J and DBA/2J inbred mice. Physiol Behav. 2013;110-111:140–147. doi: 10.1016/j.physbeh.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherwin CM. Voluntary wheel running : a review and novel interpretation. Anim Behav. 1998;56:11–27. doi: 10.1006/anbe.1998.0836. [DOI] [PubMed] [Google Scholar]
- 29.Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson's disease. Neurotox Res. 2007;11:151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- 30.Ungerstedt U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta Physiol Scand Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 31.Weihmuller FB, Bruno JP, Neff NH, Hadjiconstantinou M. Dopamine receptor plasticity following MPTP-induced nigrostriatal lesions in the mouse. Eur J Pharmacol. 1990;180:369–372. doi: 10.1016/0014-2999(90)90324-y. [DOI] [PubMed] [Google Scholar]
- 32.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]