Abstract
This naturalistic telephone survey study compared perceptions of withdrawal severity in 67 daily cannabis users and 54 daily tobacco cigarette smokers who made quit attempts during the prior 30 days. A Withdrawal Symptom Checklist assessed the severity of abstinence symptoms and a Likert scale assessed perceived relations between abstinence symptoms and relapse. A composite Withdrawal Discomfort Score did not differ significantly between groups (M=13.0 for cannabis, vs. M=13.2 for tobacco). Individual symptom severity ratings were also of similar magnitude, except craving and sweating were slightly higher for tobacco. Both groups reported that withdrawal contributed substantially to relapse, and the strength of these ratings was similar across groups. The diverse convenience sample examined in this study adds external validity and generalizability to prior studies that included only users not planning to quit or excluded many common types of cannabis users. The comparable withdrawal experience from these heterogeneous cannabis and tobacco users supports previous findings from controlled laboratory studies, and indicates that real world, frequent cannabis users perceive that withdrawal symptoms negatively affect their desire and ability to quit.
Keywords: cannabis, marijuana, withdrawal, dependence, tobacco, smoking cessation, nicotine
1. Introduction
The addictive potential of cannabis remains controversial and is questioned by many in the clinical, scientific, and lay communities (Budney, 2006). During the past decade, controlled studies have documented a valid cannabis withdrawal syndrome following chronic exposure to cannabis and its primary psychoactive compound, delta-9-tetrahydracannabinol (THC) (reviewed in Budney, Hughes, Moore, & Vandrey, 2004; Hart, 2005). These studies have characterized a cannabis withdrawal syndrome with the most common symptoms being anger/aggression/irritability, anxiety, decreased appetite/weight loss, restlessness, and sleep difficulty/strange dreams. Depressed mood, stomach pain/nausea, shakiness, and sweating have also been observed, but appear less frequently. As with other substance withdrawal syndromes, most symptoms onset within 24 hours of abstinence, peak within 2-3 days, and last approximately 1-2 weeks.
Ambiguity regarding the clinical importance of this cannabis withdrawal syndrome is the primary reason that it is not recognized as a substance use disorder in the DSM-IV-TR (American Psychiatric Association, 2000). Cannabis withdrawal disorder is recognized in the ICD-10, but diagnostic criteria are lacking. Recent studies have provided growing support for the clinical importance of cannabis withdrawal. For example, the majority of adults and adolescents enrolled in outpatient treatment for cannabis dependence report having experienced withdrawal symptoms following a quit attempt, report difficulty achieving initial periods of abstinence, complain that withdrawal contributes to their inability to quit, and report using cannabis or taking other direct action to alleviate withdrawal symptoms (Budney, Novy, & Hughes, 1999; Budney, Radonovich, Higgins, & Wong, 1998; Coffey et al., 2002; Copeland, Swift, & Rees, 2001; Copersino et al., 2006; Crowley, MacDonald, Whitmore, & Mikulich, 1998; Stephens, Babor, Kadden, Miller, & the Marijuana Treatment Project Research Group, 2002; Vandrey, Budney, Moore, & Hughes, 2005).
In an attempt to gauge the clinical importance of cannabis withdrawal, in a previous archival study we compared a report of tobacco withdrawal symptoms (a syndrome with well-accepted clinical importance) with a report of cannabis withdrawal symptoms, and observed close similarities in the type, magnitude, and timecourse of symptoms (Vandrey et al., 2005). A more recent small (N=12) prospective laboratory study also observed comparable cannabis and tobacco withdrawal symptoms in a non-treatment seekers during 5-day periods of temporary abstinence (Vandrey, Budney, Hughes, & Liguori, 2008). This study had high internal validity, but included only select individuals who were not planning to quit using marijuana or tobacco (only quitting for the study), and many users were excluded in an effort to control for potential measurement confounds (active psychiatric disorders, use of psychoactive medications or psychoactive substances other than alcohol).
The present study used a telephone study to recruit a larger, more generalizable sample to compare the cannabis and tobacco withdrawal syndromes as experienced by a diverse set of adult cannabis and tobacco users who made a recent quit attempt in their home environments. The study focused on establishing external validity by including a wide range of heavy cannabis and tobacco that likely represent the general population of smokers who make quit attempts (e.g., those who use other substances and those who take psychiatric medications). Although this inclusive recruitment strategy raises issues with measurement error and attributions of causality, we believe that systematically obtaining perceptions of withdrawal from this previously excluded population is important for elucidating the clinical needs of the common heavy cannabis user. The survey assessed frequency and severity of withdrawal symptoms during the recent quit attempt, and added a novel assessment of beliefs about whether and how much these withdrawal symptoms contributed to relapse. The primary hypotheses were that perceived discomfort from withdrawal would not substantially differ between cannabis and tobacco quitters, and the groups would provide similar attributions about the relation between withdrawal and relapse.
2. Materials and Methods
2.1. Participants
Adults who reported attempting to quit daily use of either cannabis or tobacco during the past 30 days were recruited via newspaper advertisements in Albany, NY, Burlington, VT, Hartford, CT, Little Rock, AR, Providence RI, and Seattle, WA. The advertisements indicated that individuals 18 years and older who had recently tried to quit using marijuana or tobacco (separate ads) were needed for a 30-minute survey study; compensation of $25 was indicated. Inclusion criteria were 1) at least 18 years of age, 2) reported an attempt to quit use of either cannabis or tobacco cigarettes in the 30 days prior to telephone contact, 3) had used the substance daily for at least 6 months prior to the quit attempt (at least 25 days per month for cannabis because its illicit status results in occasional abstinence days), and 4) had achieved at least 2 consecutive days of complete abstinence following the quit attempt. The criterion of having achieved 2 consecutive days of abstinence ensured sufficient time for the onset of withdrawal symptoms (Budney et al., 2004; Hughes, Higgins, & Hatsukami, 1990). Participants who primarily used forms of tobacco other than cigarettes were excluded. Note that consistent with our goal to focus on external validity of the syndrome, participants were not excluded if they used another substance during the quit attempt nor if they quit another substance because this is represents a common scenario in this population. Survey data were collected on 67 adults who made a recent cannabis quit attempt and 54 who made a recent tobacco quit attempt.
2.2. Procedures
Participants called a toll free telephone number and were provided with a brief synopsis of the study. A short interview determined study eligibility. The Human Subjects Institutional Review Boards of the University of Vermont and the University of Arkansas for Medical Sciences approved a waiver of written informed consent, and oral informed consent was obtained by telephone. The survey was then completed in a single telephone interview.
Participants were recruited from the general population because participants in most prior studies were either marijuana users who were not interested in quitting, or were treatment seekers who likely representative the minority of users with more severe dependence and psychiatric problems (Stephens, Roffman, & Simpson, 1993; Tsogia, Copello, & Orford, 2001). All heavy marijuana users who reported a recent quit attempt regardless of psychiatric status or other substance use were included. Advertisements were used to recruit participants because given the prevalence of current marijuana dependence is < 1.5%, random-digit dialing recruitment was not feasible. We also did not exclude cigarette smokers who used nicotine replacement or other treatments because this group represents a significant minority (>25%) of those who stop smoking (Hughes, Marcy, & Naud, in press).
2.3. Measures
The survey included: socio-demographic information, detailed information about the most recent and prior cannabis or tobacco quit attempts, and minimal information on medical and mental health history, and past and current daily use of alcohol and illicit drugs (Table 1). Note that the outcome of the quit attempt was operationalized as “still abstinent”, “lapsed” (any use since the initiation of the quit attempt) or “relapsed” (return to daily use).
Table 1.
Sociodemographics, Substance Use History, Quit Attempt Characteristics
| Cannabis Group | Tobacco Group | Significance Level | |
|---|---|---|---|
| N | 67 | 54 | - |
| Age | 31.9 (11.2)* | 45.7 (15.3) | < .01 |
| Female | 41% | 60% | < .04 |
| Ethnicity | < .01*** | ||
| Caucasian | 66% | 89% | -- |
| African-American | 30% | 6% | -- |
| Global Symptom Index (from the BSI) | 28 (19, 53) | 21.5 (6, 34) | < .01 |
| Daily Use of Alcohol | |||
| Lifetime | 37% | 37% | -- |
| Current | 3% | 2% | -- |
| Years of cannabis / tobacco use | 12 (8, 27)** | 31.5 (15, 43) | < .01 |
| Frequency of Use | 4.5 (2.8) times / day | 16.8 (8.6) cigarettes / day | --- |
| # of past quit attempts | 3 (2, 6) | 5 (4, 10) | < .01 |
| Longest prior period of abstinence (days) | 60 (10, 248) | 75 (16, 365) | ns |
| % quitting another substance at same time | 24% | 13% | ns |
| # of days from quit date to interview | 18.4 ± 8.6 | 19.0 ± 8.4 | ns |
| # of days abstinent following quit attempt | 9.7 ± 8.4 | 8.0 ± 7.1 | ns |
| Reduced use before quitting (% yes) | 41% | 51% | ns |
| Lapse**** (% yes) | 60% | 83% | < .01 |
| Relapse**** | 30% | 36% | ns |
Mean (SD)
Median (quartiles) analyzed because of non-normal distribution
Reflects Chi-square test result comparing Caucasian vs. All Others including African-American
Lapse indicates any use of cannabis or tobacco since the initiation of the quit attempt. Relapse indicates return to daily use
The presence and magnitude of specific withdrawal symptoms were assessed using a version (17 items) of our Withdrawal Symptom Checklist, which includes common symptoms of both cannabis and tobacco withdrawal (Budney et al., 1999, 2001, 2003; Hughes 2007). Each symptom was rated on a 0-3 scale (0 = not at all, 1= mild, 2 = moderate, 3 = severe) based on their experience during their recent period of abstinence. The primary outcome measure, the Withdrawal Discomfort Score (WDS), was calculated by totaling scores from 9 items previously reported as common symptoms of withdrawal for both cannabis and tobacco: aggression, anger, appetite change (decreased for Cannabis, increased for Tobacco), depressed mood, irritability, anxiety/nervousness, restlessness, sleep difficulty, strange dreams (APA, 2000; Budney et al., 2004; Hughes, 2007; Vandrey et al., 2008). Prior studies have demonstrated that the individual Checklist items and the WDS scores change over time following abrupt cessation in a manner consistent with a true withdrawal syndrome (Budney et al, 2003, 2004; Hughes 2007), and the WDS has shown good internal consistency, α=.89 (Budney et al., 1999).
In addition to the severity rating, participants rated their beliefs about how much each symptom contributed to relapse during lifetime past quit attempts using a 10-point Likert scale where 1 indicated “very little” and 10 indicated “a lot”. The reference to lifetime attempts rather than the current quit attempt was used because some participants were still abstinent and others had relapsed at the time of the survey. A composite score comprised of the WDS items listed above was used to estimate the relation between overall withdrawal discomfort and relapse.
The Brief Symptom Index (BSI), a 53-item checklist that yields scores on nine subscales and a composite Global Severity Index, was used to assess current psychological functioning (Derogatis, 1993).
2.4. Statistical Analysis
All of the analyses were performed using SASv9 (SAS Institute Inc., Cary, NC). Between-group differences on socioeconomic, demographic, and substance use variables were examined using chi-square tests for categorical variables, Satterthwaite's t-tests for continuous variables with unequal variances across groups, and two-tailed t-tests for continuous variables with normal distributions and equal variances. Wilcoxon Rank Sums Tests were used to compare groups on continuous variables with skewed distributions.
The indicators of withdrawal severity, i.e., WDS mean score, mean individual symptom ratings, and mean ratings of the association between symptoms and relapse, were analyzed using linear regression models to test for group differences while controlling for variables that differed between groups at baseline: age, gender, race (Caucasian vs. other) and the Global Severity Index of the BSI. Because this study relied on a failure to find significant differences to argue for equivalence of the syndromes, it is of particular importance to note that the study had 80% power to detect a difference score of 1.4 on the WDS (primary severity measure), an 11.3% difference.
A supplementary analysis of point prevalence of experiencing each withdrawal symptom was performed by creating a dichotomy for each Withdrawal Checklist item, either not having the symptom (rating of 0) or having the symptom (rating of 1-3). A series of 2 × 2 chi square tests were performed to test group prevalence differences for each symptom.
3. Results
3.1. Participant characteristics and substance use
Participants in the Cannabis group (N=67) were younger, less likely to be female or Caucasian, and had more psychological symptoms than those in the Tobacco group (N=54) (Table 1). These variables were controlled for in subsequent analyses of withdrawal indicators. The Cannabis group reported fewer years of regular use and fewer quit attempts than did the Tobacco group. Also, individuals in the Cannabis group were less likely to report a lapse (use of any cannabis) following their recent quit attempt than those in the Tobacco group; however no group difference was observed in reported relapse rates (return to daily use). Overall participants averaged 8-9 days of abstinence and about two-thirds were still abstinent at the time of the interview.
3.2. Withdrawal Discomfort and Symptom Severity
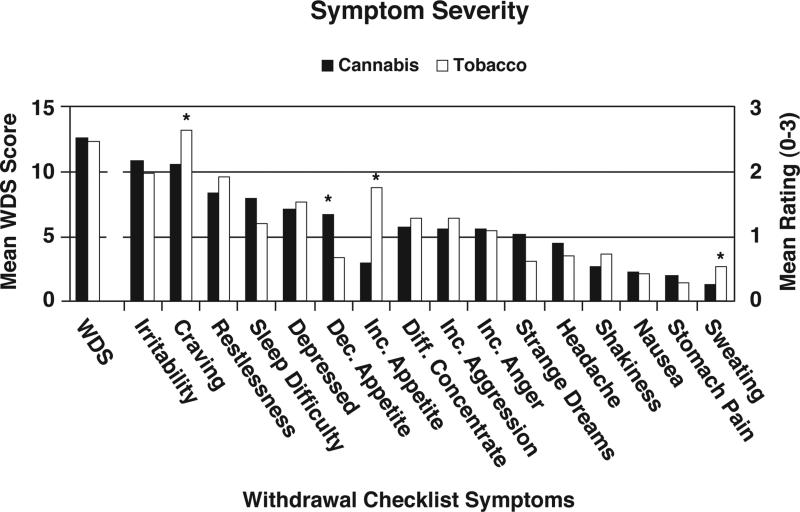
Figure 1 shows the mean WDS score and the mean individual Checklist item scores for both groups. The WDS means for cannabis and tobacco were similar [X =13.0 (SD = 6.4) vs. X = 13.2 (SD = 6.5). The regression analysis adjusting for age, gender, ethnicity, and psychological symptoms (GSI score) revealed no group effects on the WDS (p = .24).
Figure 1.
Group mean severity scores for the WDS (refer to Y-axis scale on left side of figure) and individual symptoms on the Withdrawal Symptom Checklist (refer to Y-axis scale on right side of figure). Asterisk indicates a significant difference between groups after controlling for age, gender, race, and Global Symptom Index score from the Brief Symptom Inventory in the linear regression models.
Regression analyses indicated that the groups differed significantly on only four individual symptoms. As expected differences were observed on appetite change; the Cannabis group reported greater decreased appetite and the Tobacco group reported greater increased appetite. Mean craving scores (2.1 vs. 2.6, p < .01) and mean sweating scores (0.3 vs. 0.5, p < .05) were also lower in the Cannabis group.
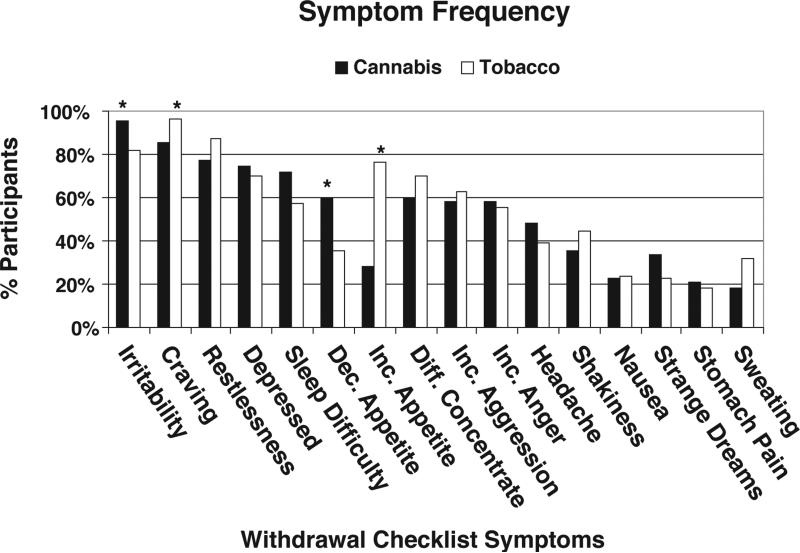
Figure 2 shows results from the supplementary prevalence analysis of the individual symptoms reported by each group. Prevalence rates were similar for most symptoms, and the mean number of symptoms reported was 9.4 (SD=4.0) for the Cannabis group and 9.7 (SD=4.0) for the Tobacco group. The only significant differences (p < .05) were greater prevalence of irritability in the Cannabis group (96% vs. 81%) and greater prevalence of craving in the Tobacco group (85% vs. 96%).
Figure 2.
Percentage of participants from each group that reported each withdrawal symptom, i.e., scoring greater or equal to 1 on the 0-3 point Withdrawal Checklist Scale. Asterisk indicates significant differences between groups on chi square analysis (p < .05).
3.3. Relation to relapse
The regression analyses comparing mean ratings of the strength of the relation between withdrawal symptoms (0-10 pt Likert scale) and relapse revealed no significant group effect for the WDS (Cannabis mean = 35.7, SD=23.4 vs. Tobacco mean = 35.7, SD=24.0). Only 3 of the 17 items showed a significant group effect. Craving (5.9, SD=4.0 vs. 7.9, SD=2.7), increased appetite (1.4, SD=2.4 vs. 4.6, SD=3.9), and sweating (0.5, SD=1.4) vs. 1.4, SD=2.6) were reported as less related to relapse by the Cannabis group. Greater than 50% of participants in each group reported that aggression, anger, anxiety, craving, depressed mood, difficulty concentrating, irritability, restlessness, and sleep difficulty had contributed to failed quit attempts. The only between-group differences in item prevalence ratings were fewer individuals in the Cannabis group reported that craving (79% vs. 96%), restlessness (70% vs. 87%) and increased appetite (33% vs. 72%) had contributed to relapse.
3.4. Potential Confounding Variables
A number of situational factors related to substance use and quit attempts that could impact withdrawal severity were evident in this convenience sample (Table 1). The duration of abstinence and rate of continued abstinence at the time of the interview varied across individuals but did not differ between the groups, and thus did not likely influence the comparative outcomes. Nonetheless, we compared those who had vs. had not relapsed at the time of the interview. Among all participants, those who relapsed had higher WDS mean scores than those who had not (15.7 vs. 11.9, p < .01), and within groups this trend held true (Cannabis: 16.3 vs. 11.6, p < .01; Tobacco: 15.2 vs. 12.1, p < .10)
Over half of the Cannabis group also used tobacco (61%) and approximately one third of the Tobacco group also used cannabis (31%). Both groups included individuals who reported that, in addition to quitting cannabis or tobacco, respectively, they were also trying to quit use of another substance simultaneously (Cannabis group 24%; Tobacco group 13%). In the Cannabis group, the other substances were tobacco (n=9), alcohol (n=2), opiates (n=2), benzodiazepines (n =2), and cocaine (n=1). In the Tobacco group, the other substances were marijuana (n=5), alcohol (n=1), and caffeine (n=1). Also, 44% (n=24) of those in the Tobacco group reported using some form of nicotine replacement therapy (NRT) during their quit attempt. Other treatment assistance modalities were also reported. In the Cannabis group, treatment modalities included: residential treatment (n=2), outpatient counseling or class (n = 14), and helpline (n=1). In the Tobacco group treatment modalities other than NRT included: acupuncture (n=4), outpatient counseling or class (n=6), helpline (n=8), and bupropion (n=2)
To explore how some of these factors may have influenced withdrawal severity, we compared mean WDS scores for (a) cannabis only users vs. cannabis and tobacco users; (b) tobacco users only vs. tobacco and cannabis users; (c) both groups: users who tried to quit only one drug (cannabis or tobacco) vs. those who tried to quit more than one drug; (d) Tobacco group: used NRT vs. no NRT. WDS scores for these each of these two group comparisons (t-tests or Rank Sum tests) did not significantly differ, except that those who used NRT reported more withdrawal (WDS mean=15.2, SD=5.9) than those who did not use NRT (WDS mean=11.6, SD=6.7) (p < 05).
Including NRT status in the regression model that controlled for other baseline variables also showed a significant NRT group effect (p < .05). Tukey's Studentized range tests were performed to compare WDS mean scores for cannabis withdrawal with tobacco withdrawal among NRT and no NRT users. The Cannabis group's WDS score (mean=13.0, SD=6.4) was not different from either the NRT group or the no NRT group (confidence intervals of the differences in means = -1.0, 5.3 and -1.5, 4.4, respectively).
4.0. Discussion
Withdrawal symptom reports following recent cannabis or tobacco quit attempts in this convenience sample of diverse users were consistent with that reported for each substance in prior controlled studies (Budney et al., 2004; Budney et al., 1999; Vandrey et al., 2008). Comparative findings suggest that participants’ perceptions of cannabis withdrawal were similar in scope and severity to those for tobacco withdrawal. This result serves as a naturalistic and conceptual replication of two prior studies indicating cannabis and tobacco withdrawal are of similar severity (Vandrey et al., 2008; Vandrey et al., 2005). The findings contribute to the growing literature on the characterization and clinical importance of the cannabis withdrawal syndrome in two important ways. First, quantitative ratings of cannabis withdrawal severity were obtained from a heterogeneous sample of quitters representative of many common types of users, many of which would have been excluded from prior studies of this phenomenon. Although this sampling method opened the study to multiple types of measurement bias, the findings regarding type and magnitude of withdrawal symptoms are consistent with that observed in prior studies and thus provide suggestive evidence of the robust nature of the cannabis withdrawal syndrome. Second, this is the first study to obtain quantitative ratings on the strength of the perceived relationship between specific withdrawal symptoms and relapse. Those trying to quit cannabis reported that withdrawal discomfort contributed substantially to relapse, and the strength of this relationship was of similar magnitude to that reported by the tobacco quitters. Again, inclusion of participants who were using or trying to quit multiple substances raises measurement issues, however, one could argue that it is remarkable that these participants consistently attribute relapse risk specifically to cannabis withdrawal symptoms.
That said, the convenience sample and measurement methods used in this survey study raise concerns about a number of potential confounds and limitations. First, the groups may not have been comparable in their respective substance use or dependence. Because no valid methods were available to equate severity of use or dependence across substances, the inclusion criteria of daily use (almost daily for cannabis) and the requirement of a quit attempt were used to recruit a convenience sample of those who probably have some dependence features and have recently tried to quit. Dependence criteria were not assessed because of time constraints.
The Cannabis and Tobacco groups each included individuals who tried to quit another substance at approximately the same time as cannabis or tobacco. Some cannabis users also continued to use tobacco and vice versa during the quit attempt. These scenarios likely represent the experience of the general population of adults who try to quit these substances, hence we did not exclude them in this study. Our reported attempts to evaluate whether these scenarios affected the cannabis vs. tobacco withdrawal comparisons suggest that these factors did not significantly alter the findings and the associated conclusion of comparable withdrawal syndromes. Nonetheless, our small laboratory study of concurrent tobacco and cannabis users suggested that other drug use or abstinence likely impacted withdrawal severity, but substantial individual differences were observed in the direction of such effects (Vandrey, et al., 2008). The impact of continuing to use other substances or quitting multiple substances simultaneously on withdrawal surely warrants further study.
Also warranting discussion was the inclusion of individuals who used NRT during their cessation attempts. The NRT users reported more, not less, withdrawal. This is likely due to “indication bias;” i.e., those who chose to use NRT have more severe tobacco dependence than those who tried to quit without NRT (Shiffman, Di Marino, & Sweeney, 2005). Importantly, the cannabis group did not report significantly less withdrawal than either the NRT users or nonusers, indicating cannabis withdrawal was perceived by participants to be similar in severity to tobacco withdrawal in both tobacco groups. Note that the analysis comparing withdrawal discomfort in the NRT user groups with the Cannabis group were performed with a relatively small sample size, and thus may not have provided adequate power to detect true differences.
A last methodological limitation of note is that the power calculations for the WDS measure reported in the Data Analysis section do not apply to the single symptom severity analyses or the analyses of the dichotomous prevalence measures. Thus, although we can state with some confidence that the Cannabis group's withdrawal discomfort level as measured by the WDS is either as large as or no more than 11% lower than the Tobacco group's discomfort level, we cannot assert this for the other measures.
Reports of craving reflect the one consistent withdrawal symptom rating difference between the Cannabis and Tobacco groups. The Cannabis group showed a lower prevalence of increased craving during abstinence (85% vs. 96%), reported less severe craving, and reported that craving was less of a contributor to relapse than the Tobacco group. Most conceptualizations of craving suggest withdrawal is only one, and perhaps not even the major, cause of craving (Sayette et al., 2000); thus, reports of lesser craving for the cannabis group may reflect non-withdrawal differences in cannabis and tobacco use. However, given that craving is one of the best predictors of relapse (Hughes 2007), further tests of differences in craving between cannabis and tobacco users is warranted.
Clinicians must be aware of both the type and severity the withdrawal symptoms that are commonly reported by cannabis users who are attempting to quit. Although such symptoms do not typically pose serious medical or psychiatric consequences, they are perceived by the user to be distressing and likely serve as a deterrent to successful quit attempts and precipitants of relapse. If such symptoms are indeed comparable in type and severity to those experienced by tobacco quitters, treatment providers can use this more familiar syndrome to educate their patients and to develop plans for coping with this aversive experience. Other health providers also need to consider such cannabis cessation symptoms when evaluating or treating heavy cannabis users that present for other medical, psychiatric, or substance abuse problems.
In summary, the findings from this survey study suggest similar withdrawal severity profiles for cannabis and tobacco use cessation that occurs in real world settings. These data support and converge with that from controlled laboratory studies to suggest the cannabis withdrawal syndrome experienced when frequent users of cannabis stop using cannabis has clinical importance. In addition, prior studies indicate that many cannabis users take direct action to relieve the distress experienced during abstinence, e.g., resume cannabis use or take other drugs (Coffey et al., 2002; Copersino et al., 2006), and the present study indicates that cannabis users clearly perceive that withdrawal symptoms negatively affect their desire and ability to quit.
Acknowledgements
This research was supported by research grants from the National Institute on Drug Abuse: DA12471, T32DA07242, K02-00109, K05-00450, and in part by the Arkansas Biosciences Institute, the major research component of the Tobacco Settlement Proceeds Act of 2000. Parts of this study were conducted as a segment of the doctoral dissertation of Ryan G. Vandrey at the University of Vermont. Preliminary findings form this study were presented at the annual conference of the College of Problems on Drug Dependence, 2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders (IV-TR ed.) American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Budney AJ. Are specific dependence criteria necessary for different substances: how can research on cannabis inform this issue? Addiction. 2006;101(Suppl. 1):125–133. doi: 10.1111/j.1360-0443.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey RG. The time course and significance of cannabis withdrawal. Journal of Abnormal Psychology. 2003;112:393–402. doi: 10.1037/0021-843x.112.3.393. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Novy P, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–1322. doi: 10.1046/j.1360-0443.1999.94913114.x. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Radonovich KJ, Higgins ST, Wong CJ. Adults seeking treatment for marijuana dependence: A comparison to cocaine-dependent treatment seekers. Experimental and Clinical Psychopharmacology. 1998;6(4):419–426. doi: 10.1037//1064-1297.6.4.419. [DOI] [PubMed] [Google Scholar]
- Coffey C, Carlin JB, Degenhardt L, Lynskey M, Sanci L, Patton GC. Cannabis dependence in young adults: An Australian population study. Addiction. 2002;97:187–194. doi: 10.1046/j.1360-0443.2002.00029.x. [DOI] [PubMed] [Google Scholar]
- Copeland J, Swift W, Rees V. Clinical profile of participants in a brief intervention program for cannabis use disorder. Journal of Substance Abuse Treatment. 2001;20:45–52. doi: 10.1016/s0740-5472(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, et al. Cannabis withdrawal among non-treatment-seeking adult cannabis users. Am J Addict. 2006;15(1):8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- Crowley TJ, MacDonald MJ, Whitmore EA, Mikulich SK. Cannabis dependence, withdrawal, and reinforcing effects among adolescents with conduct disorder symptoms and substance use disorders. Drug and Alcohol Dependence. 1998;50:27–37. doi: 10.1016/s0376-8716(98)00003-9. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory: Administration, scoring and procedures manual. National Computer Systems Inc.; Minneapolis, MN: 1993. [Google Scholar]
- Hart CL. Increasing treatment options for cannabis dependence: A review of potential pharmacotherapies. Drug & Alcohol Dependence. 2005;80(2):147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: Valid symptoms and time course. Nicotine and Tobacco Research. 2007;9(3):309–313. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Hatsukami D, editors. Effects of abstinence from tobacco: A critical review (Vol. 10) Plenum Press; New York: 1990. [Google Scholar]
- Hughes JR, Marcy TW, Naud S. Interest in treatments to stop smoking. Nicotine and Tobacco Research. doi: 10.1016/j.jsat.2008.04.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MS, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of craving. Addiction. 2000;95S2:S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Di Marino ME, Sweeney CT. Characteristics of selectors of nicotine replacement therapy. Tob Control. 2005;14(5):346–355. doi: 10.1136/tc.2004.009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RS, Babor TF, Kadden R, Miller M, The Marijuana Treatment Project Research Group The marijuana treatment project: Rationale, design, and participant characteristics. Addiction. 2002;97(S1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- Stephens RS, Roffman RA, Simpson EE. Adult marijuana users seeking treatment. J Consult Clin Psychol. 1993;61(6):1100–1104. doi: 10.1037//0022-006x.61.6.1100. [DOI] [PubMed] [Google Scholar]
- Tsogia D, Copello A, Orford J. Entering treatment for substance misuse: a review of the literature. Journal of Mental Health. 2001;10:481–499. [Google Scholar]
- Vandrey RG, Budney AJ, Hughes JR, Liguori A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug and Alcohol Dependence. 2008;92:48–54. doi: 10.1016/j.drugalcdep.2007.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey RG, Budney AJ, Moore BA, Hughes JR. A cross-study comparison of cannabis and tobacco withdrawal. The American Journal on Addictions. 2005;14:54–63. doi: 10.1080/10550490590899853. [DOI] [PubMed] [Google Scholar]