Abstract
The evolutionarily conserved paired domain consists of the N-terminal PAI and the C-terminal RED domains, each containing a helix–turn–helix motif capable of binding DNA. Despite its conserved sequence, the physiological functions of the RED domain remain elusive. Here, we constructed a prd transgene expressing a truncated Paired (Prd) protein without the RED domain, and examined its rescue ability in prd mutants. We found that the RED domain is specifically required for the expression of Acp26Aa and sex peptide in male accessory glands, and the induction of female post-mating response. Our data thus identified an important physiological function for the evolutionarily conserved RED domain.
Keywords: accessory gland, post-mating response, Paired, RED domain, Drosophila
2. Introduction
The Drosophila gene paired (prd) belongs to the pair-rule gene family that exhibits a pair-rule expression pattern and participates in the determination of anterior–posterior axis during early embryogenesis [1–4]. Together with other pair-rule genes, prd regulates the expression of segment-polarity genes, including wingless (wg), gooseberry (gsb) and engrailed (en) [3,5–7]. In addition to the embryonic functions, prd is necessary for postembryonic viability, and male fertility through the regulation of accessory gland (AG) development [8–12]. The Drosophila male AG is a pair of dead-end tubes composed of a single-cell layer of two kinds of secretory cells: the ‘main cells’ spreading all over the lumen of the glands, and the ‘secondary cells’ restricted only to the distal region of each tube [13]. The AG is a secretory organ that is functionally analogous to the human prostate and seminal vesicle whose secretions (seminal fluid), together with sperm from the testes, play important roles in regulating female post-mating response (PMR) [11,13–17].
prd is the founding member of the Pax genes, which encode an evolutionarily conserved family of transcription factors with multiple DNA binding motifs and play key roles in animal development [18–21]. All members of the Pax family are defined by the presence of a highly conserved 128-amino-acid paired domain (PD) [22], which was first identified in Paired (Prd) [23]. The crystal structure demonstrates that PD is a bipartite module, which is further divided into the N-terminal PAI and the C-terminal RED subdomains (PAI + RED = PD) [24]. Each subdomain contains a helix–turn–helix (HTH) motif and has the ability to bind to DNA independently [24,25]. In addition to PD, some Pax proteins also contain a paired-type homeodomain (HD). According to the different combinations of these domains, the Pax proteins are classified into five subgroups [26]. Prd is homologous to the mammalian Pax3/Pax7 subgroup containing both a complete PD and an intact HD. It has been reported that both HD and PAI subdomain are simultaneously required within the same molecule to execute the early embryonic pair-rule function of Prd [8], whereas the RED subdomain appears dispensable for these functions either in vitro [27] or in vivo [8].
To investigate whether the RED subdomain is important for other Prd functions, we introduced a transgene, prd-PrdΔPBC, which encodes a truncated Prd protein with a deletion of the RED domain under the control of the complete cis-regulatory region, into a prd null mutant background. We found that the RED domain is dispensable for most Prd functions, including embryonic segmentation, postembryonic viability, AG development and male fertility, but is specifically required for the expression of Acp26Aa (also called Ovulin) and sex peptide (SP), two seminal fluid components essential for the induction of female PMR. Consequently, prd mutant males rescued by prd-PrdΔPBC failed to induce increased egg laying and decreased receptivity in wild-type females. Thus, we have characterized a specific function for the RED domain of Prd protein, which has shed light on the evolution of Pax genes.
3. Results and discussion
3.1. The RED domain is dispensable for the embryonic functions of Prd
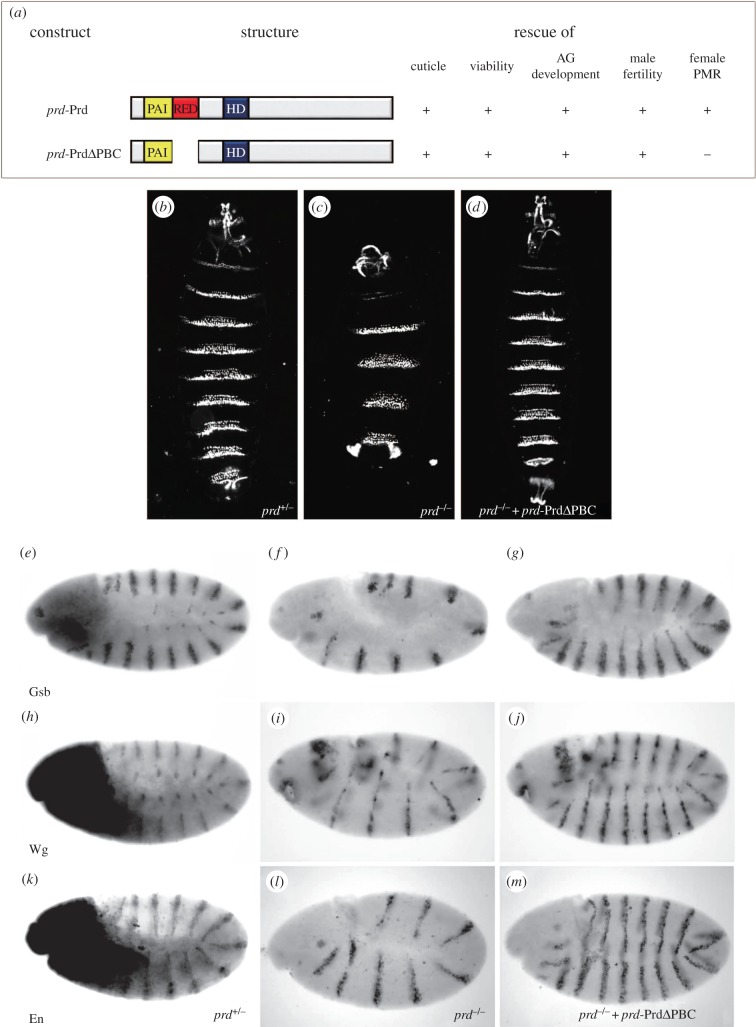
prd, initially identified as a pair-rule gene involved in embryonic segmentation, has been shown to perform multiple functions in development, including: (i) activation of segment-polarity genes and proper segmentation of the larval cuticle [5–7]; (ii) postembryonic viability [10]; (iii) AG development [10]; and (iv) male fertility [10,12]. A prd transgene consisting of the entire prd coding region as well as the full cis-regulatory elements, prd-Prd, was able to rescue all prd functions in prd mutants (figure 1a) [28]. To examine the specific contribution of RED domain to Prd functions in vivo, we introduced another transgene, prd-PrdΔPBC, into prd mutants and examined its ability to rescue the mutant phenotypes (figure 1a). prd-PrdΔPBC replaces the Prd coding region in prd-Prd by a truncated version with a deletion of the RED domain (amino acids 75–125, figure 1a). According to the crystal structure [24] and the in vitro experiments [27], this deletion of the RED domain should not affect the DNA-binding property of the PAI domain [24].
Figure 1.
The RED domain is dispensable for the embryonic functions of Prd. (a) Schematic of the coding structure of prd-Prd and prd-PrdΔPBC, and their ability to rescue Prd functions in Drosophila development. The rescue ability is scored by + if the transgene is sufficient for rescue or by − if no rescue is obtained. (b–d) The cuticle of a prd+/− (b), or a prd−/− (c) or a prd−/−; prd-PrdΔPBC/+ (d) embryo is shown under dark-field illumination with anterior up. prd-PrdΔPBC is able to rescue the cuticle phenotype in prd− (d). Expression patterns of Gsb (e–g), Wg (h–j) and En (k–m) in prd+/− (e,h,k), prd−/−embryo carrying no (f,i,l) or one copy of prd-PrdΔPBC (g,j,m) are shown during the extended germ band stage of embryogenesis. Embryos are oriented with their anterior to the left and dorsal side up. Expression of Gsb, Wg and En in prd mutants (f,i,l) is fully rescued by prd-PrdΔPBC (g,j,m).
The prd mutants used in this study are heterozygous for the deficiency Df(2L)Prl and the prd2.45 allele, which has a 1.1 kb insertion after amino acid 45 of the PD and acts as a null allele [29]. Compared with the heterozygous control (figure 1b), prd mutants lose half of the segmental equivalents and exhibit the classical pair-rule phenotype in larval cuticle (figure 1c) [1]. We found that prd-PrdΔPBC could fully rescue the cuticle phenotype of prd mutants (figure 1d), indicating that the RED domain is not essential for the cuticle function of Prd.
Prd is required for the activation of segment polarity genes in every other parasegment in early embryos. In prd mutant embryos, the expression patterns of Gsb, Wg and En are abolished with a double-segment periodicity (figure 1f,i,l) when compared with the control (figure 1e,h,k). Consistent with its ability to rescue the prd mutant cuticle phenotype, prd-PrdΔPBC is able to fully rescue the expression patterns of Gsb, Wg and En (figure 1g,j,m), demonstrating that the RED domain is dispensable for Prd to activate the transcription of segmental polarity genes in embryonic development.
We have shown in previous work that an evolutionary allele of prd, prd-Pax3, in which the Prd mouse homologue Pax3 is placed under the control of the entire prd cis-regulatory region, is able to rescue larval cuticles and target segment-polarity genes expression, but not the embryonic lethality, in prd− embryos [10]. Therefore, prd has a viability function independent of its cuticle functions to ensure the survival of embryos to adults [9,10]. Furthermore, prd− embryos rescued by two copies of prd-Gsb, another evolutionary allele of prd, are able to develop into adulthood, yet some of the adult flies display a distorted segment phenotype in the abdominal cuticle [10], suggesting a role of Prd in regulating adult segmentation. We found that prd-PrdΔPBC is able to rescue the lethality in prd mutant embryos to a similar extent as that of prd-Prd, and there is no significant difference when compared with that of heterozygous controls (figure 2a), demonstrating that the RED domain is unnecessary for the viability function of Prd. However, prd mutant flies rescued by prd-PrdΔPBC produce a distorted segmentation phenotype in adult cuticles (electronic supplementary material, figure S1b,e), which phenocopies that of prd mutants rescued by prd-Gsb [10], suggesting that the RED domain contributes to the adult cuticle function of Prd.
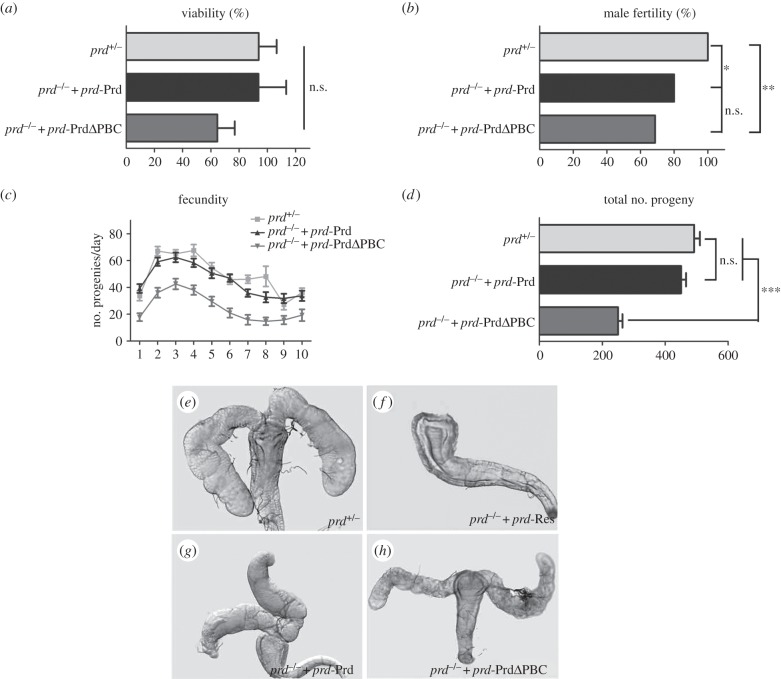
Figure 2.
The RED domain is dispensable for the viability and male fertility functions of Prd. (a) prd-PrdΔPBC is able to rescue prd mutants to adulthood at a similar rate as prd-Prd or the endogenous prd. Viability is scored by the actual number over the expected number of (i) prd+/−, (ii) prd−/−+prd-PrdΔPBC or (iii) prd−/− + prd-Prd from corresponding crosses. There is no significant difference in the viability among prd+/− (92/102 expected), prd−/−; prd-Prd/+ (72/64 expected) and prd−/−; prd-PrdΔPBC/+ (31/71 expected) flies. (b) prd-PrdΔPBC rescues the fertility of prd mutant males to a similar extent as prd-Prd, but slightly lower than that of endogenous prd. Number of flies examined (fertile/sterile): prd+/− (n = 24; 24/0), prd−/−; prd-Prd/+ (n = 44; 35/9) and prd−/−; prd-PrdΔPBC/+ (n = 22; 15/7). (c,d) prd mutant males rescued by prd-PrdΔPBC have a reduced fecundity when compared with those rescued by prd-Prd or the heterozygous controls. The number of progenies produced per vial per day (c) and the total number of progenies for 10 days (d) of prd+/− (n = 27), prd−/−; prd-Prd/+ (n = 24) and prd−/−; prd-PrdΔPBC/+ (n = 17) are shown as mean ± s.e.m. For statistical analysis: *p < 0.05; **p < 0.01; ***p < 0.001; n.s., not significant. (e–h) Light micrographs showing AG from a 3-day-old prd+/− (e), prd−/−; prd-Res/+ (f), prd−/−; prd-Prd/+ (g) or prd−/−; prd-PrdΔPBC/+ (h) male. The loss of AG phenotype in prd mutant males (f) was rescued by prd-Prd (g) or prd-PrdΔPBC (h).
3.2. The RED domain is dispensable for the male fertility function of Prd
Previous work reported that another transgene, prd-Res, in which the downstream sequence of prd-Prd is deleted, is sufficient to rescue prd mutants to adulthood, yet all males are sterile [8]. Additional studies confirmed that prd is required for the development of AGs, which secrete seminal fluid necessary for Drosophila male fertility [8,9,11,12]. The male fertility function of prd depends on a 0.8 kb Prd male fertility enhancer (PMFE) located in the prd downstream region [12], as prd mutant males rescued by prd-Res, which does not include PMFE, produces a loss of AG phenotype (figure 2f) [9,12]. We then examined the rescue ability of prd-PrdΔPBC in AG development and male fertility in prd mutants. We found that prd-PrdΔPBC could rescue AG development to a similar extent as that of prd-Prd (figure 2g,h). Furthermore, about 70% of prd mutant males rescued by prd-PrdΔPBC are fertile, which is not statistically different from the case with prd-Prd (figure 2b). Hence, we conclude that the RED domain is dispensable for the male fertility function of Prd.
However, prd− males rescued by prd-PrdΔPBC display a significantly reduced fecundity as they produce far less progen than the heterozygous controls or prd− males rescued by prd-Prd (figure 2c,d), suggesting the RED domain, though not necessary, still contributes to the male fertility. Prd is known to play a later function in AG maturation by regulating the expression of a variety of AG products, including SP and Acp26Aa, which stimulate female egg laying [12]. As shown below, these factors are significantly reduced in the AGs of prd mutant males rescued by prd-PrdΔPBC, which might account for the reduced number of eggs/progeny produced by their mates.
In addition, we also noted that prd− males rescued by prd-PrdΔPBC exhibited a lower mating success rate compared with those rescued by prd-Prd (electronic supplementary material, figure S2a), yet there is no significant difference in the climbing ability between these two groups of flies (electronic supplementary material, figure S2b), suggesting factors other than the locomotion skill are responsible for the different copulation outcome.
3.3. The RED domain is necessary for males to elicit post-mating response in mated females
Reproduction is one of the fundamental missions of life. Hence, many species have evolved an intricate variety of mechanisms to guarantee the success of procreation. In most insects, including Drosophila melanogaster, male-derived substances transferred during mating induce significant behavioural changes, so-called PMR, leading to an increased egg laying and decreased sexual receptivity in their mated partners [15,30–32].
Virgin females exhibit a high receptivity (figure 3a) and low rejection (figure 3b) towards males, and lay less than 10 eggs per day (figure 3c). The females show dramatically increased oviposition (up to about 50 eggs within 24 h) after mating to control males, and maintain this rate in the next few days with a gradual reduction (figure 3c). Meanwhile, mated females display a low receptivity (figure 3a) and high rejection (figure 3b) towards males after first copulation. However, females mated to prd mutant males rescued by prd-PrdΔPBC behave like virgins with low egg-laying (figure 3c), high receptivity (figure 3a) and low rejection (figure 3b). In contrast, prd mutant males rescued by prd-Prd are able to elicit PMR in their mates to a similar extent as control males (figure 3a–c). To rule out the possibility that the rescue failure of prd-PrdΔPBC is due to its lower expression level, we checked the transgene expression in male AGs by qRT-PCR, and found that prd-PrdΔPBC was expressed at a level comparable with that of prd-Prd or endogenous prd (figure 4g). Furthermore, adding another copy of prd-PrdΔPBC to prd mutant males does not improve PMR in their mates (electronic supplementary material, figure S3). Together, these data suggest that the RED domain is necessary for Prd to induce PMR in mated females.
Figure 3.
The RED domain is imperative for female post-mating response (PMR). (a,b) Females mated with prd−/−; prd-PrdΔPBC/+ males exhibit a virgin-like behaviour with a high receptivity (a) and low rejection (b) to second mating, whereas those that copulated with the heterozygous controls or prd−/−; prd-Prd/+ males exhibit an opposite behaviour with a low receptivity (a) and high rejection (b). (a) The percentage of virgin females (17/20) mated to naive w1118 males within 1 h or the percentage of non-virgin females previously mated to prd+/−(2/20), prd−/−; prd-PrdΔPBC/+ (19/20) or prd−/−; prd-Prd/+ (3/20) males successfully re-mating within 1 h. (b) The percentage of virgin females (2/20) not mated within 2 h or the percentage of non-virgin females previously mated to prd+/−(16/20), prd−/−; prd-PrdΔPBC/+ (1/20) or prd−/−; prd-Prd/+ (14/20) males not re-mated within 2 h. For statistical analysis: *p < 0.05; **p < 0.01; n.s., not significant. (c) Egg-laying of virgin females or females mated to (i) prd+/−, (ii) prd−/−; prd-Prd/+ or (iii) prd−/−; prd-PrdΔPBC/+ males (n = 20, respectively). Females mated with heterozygous controls or prd−/−; prd-Prd/+ show dramatic increase in egg-laying that lasts for a few days with a gradual reduction. However, females mated with prd−/−; prd-PrdΔPBC/+ males fail to trigger the increased oviposition and lay few eggs per day, as do virgin females. The statistical analysis of egg laying using one-way ANOVA followed by Bonferroni's multiple comparison test is shown on the right. Significant differences are indicated as *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 4.
The RED domain is required for the expression of Acp26Aa and SP in the AG. The expression of Acp26Aa (a–c) and SP (d–f) in AG from a 3-day-old prd+/− (a or d), prd−/−; prd-PrdΔPBC/+ (b or e) or prd−/−; prd-Prd/+ (c or f) male. The expression of Acp26Aa (b) or SP (e) in prd mutant males rescued by prd-PrdΔPBC was undetectable when compared with controls (a,d) or prd mutants rescued by prd-Prd (c,f). (g) qRT-PCR assay showing the transcription level of prd, acp26A and SP in AG of indicated genotypes (n = 10 per genotype). Significant differences are indicated as *p < 0.05; *** p < 0.001, n.s., not significant.
3.4. The RED domain is necessary for Prd to activate the expression of Acp26Aa and sex peptide in accessory glands
Female PMRs have been correlated with substances derived from the male AG [32,33], and among them Acp26Aa and SP have been extensively studied. Meanwhile, a dual role of prd in male AG development has been characterized [12]. Prd is required at an early developmental stage to promote cell proliferation and later for the expression of secretions, including Acp26Aa and SP, to ensure the maturation of the AG [12]. Acp26Aa stimulates the release of oocytes from the ovary [16], which is the first step of the egg-laying process essential for the initial increase of oviposition in females after mating [34]. Recent research indicated that Acp26Aa increases ovulation and egg laying through the OA neuronal signalling [35]. SP, a key component of AG secretions that is transferred into females with sperm, downregulates the excitability of SP sensory neurons (SPSNs) in the female reproductive tract and their input onto SAG neurons of the abdominal ganglion, which results in an increased oviposition and reduced sexual receptivity [36–38]. SP also binds to the sperm and induces a prolonged PMR in females by a gradual release from the sperm stored in mated females [39].
Owing to the virgin-like behaviour of females copulated to prd mutant males rescued by prd-PrdΔPBC (figure 3a–c and electronic supplementary material, figure S3), we examined the expression of Acp26Aa and SP in male AGs. In control AGs, Acp26Aa is expressed in the whole organ (figure 4a), whereas SP is detected only in the main cells by using a sp-LacZ reporter transgene (figure 4d) [12]. Although prd-PrdΔPBC is able to rescue AG development and male fertility in prd mutant males, it fails to rescue the expression pattern of Acp26Aa (figure 4b) and SP (figure 4e), when compared with prd-Prd (figure 4c,f). These results were confirmed by qRT-PCR assay (figure 4g). Thus, we conclude that the RED domain is indispensable for Prd to activate the expression of AG secretions, which are essential for PMR but not for male fertility.
Gsb, first identified as a member of segment-polarity genes [1] and activated by Prd during embryogenesis [3,40], is required for the establishment of positional information along the anterior–posterior axis in the epidermis. In addition, Gsb also plays an important role in the Drosophila central nervous system (CNS), including the specification of embryonic neuronal cell fate [41–45] and the maintenance of postembryonic synaptic homeostasis [46]. We previously found that Gsb is specifically expressed in the secondary cells located in the distal region of each AG tube (electronic supplementary material, figure S4a) [12], though the function of Gsb in the AG has remained unknown. We found that Gsb expression was rescued in prd− males by prd-Prd, but not prd-PrdΔPB (electronic supplementary material, figure S4b,c), which is confirmed by qRT-PCR assay (electronic supplementary material, figure S4d). Thus, the RED domain is also required for Prd to activate gsb expression in the AG.
It was proposed that the RED domain within the C-terminal of PD, though containing a functional DNA-binding motif, is dispensable for the physiological functions of Prd. In this study, we demonstrate that the RED domain is dispensable for most functions of Prd in development, yet it is specifically required for Prd to activate the expression of AG products Acp26Aa and SP, and thus to trigger PMR in mated females. However, the underlying mechanism by which the RED domain modulates Acp26Aa and SP expression, either through a direct transcriptional activation by binding to the cis-regulatory region or indirectly, requires further investigation.
4. Material and methods
4.1. Fly strains
All flies were raised on standard Drosophila media and maintained at 25°C. prd2.45, Df(2L)Prl, prd-Prd [9] and prd-Res [12] were described previously. Prd-PrdΔPBC was produced by inserting the coding sequence from hs-PrdΔPBC [47] into prd-0 [10]. The plasmid was injected with Δ2–3 helper plasmid into ry506 embryos and ry+ transformants were selected. All the strains are in the same ry506 background.
4.2. Immunostaining of embryos and cuticle preparation
Embryo collection, fixation and immunostaining were carried out as described [48]. Double-labelling of embryos for β-galactosidase and Gsb, Wg or En protein was performed as described [49]. Cuticles were prepared essentially as described [8].
4.3. Dissection, immunostaining and X-Gal staining of male accessory glands
AGs were dissected [11] and stained with anti-Acp26Aa [50] and anti-Gsb [45] as described. X-Gal staining was performed according to Bertram et al. [13].
4.4. Male fertility, fecundity, egg laying and receptivity assay
All flies were aged for 3 days after eclosion and then analysed. For male fertility assay, w1118 females, which were successfully mated by (i) prd2.45/+, (ii) Df(2L)Prl/prd2.45; prd-PrdΔPBC/+ or (iii) Df(2L)Prl/prd2.45; prd-Prd/+ males, were maintained separately on standard medium. The male was scored as fertile or sterile depending on the presence or absence of offspring, respectively. The data were calculated and presented as the ratio of fertile males. For male fecundity assay, each cross was of two virgin w1118 females placed by one (i) prd2.45/+, (ii) Df(2L)Prl/prd2.45; prd-PrdΔPBC/+ or (iii) Df(2L)Prl/prd2.45; prd-Prd/+ male and transferred into fresh vials with standard media every 24 h for 10 days. The number of progenies produced per vial was scored (figure 2c), and also the total number in 10 days was calculated (figure 2d). For egg laying, virgin or none virgin (successfully mated by the indicated males) w1118 females were transferred into fresh vials with standard media every 24 h and allowed to lay eggs for 5 days. The number of eggs laid by one individual female was scored every day. For receptivity assay, virgin or none virgin (successfully mated by the test males) w1118 females were housed individually for 24 h after first mating and then examined in a receptivity assay with naive w1118 males. The receptivity was classified as remating within 1 h, and rejection was categorized as no remating within 2 h, respectively. Final data were calculated as the ratio of each classification.
4.5. Rapid iterative negative geotaxis (climbing) assay
A modified version of Nichols's climbing assay was used [51] (electronic supplementary material, figure 2b). Briefly, 2-day-old flies were collected separately by gender within 24 h after eclosion. Ten to 15 flies per genotype were placed in a vertical vial (20 cm height, 2.5 cm diameter), and the vials were tapped at the bottom regularly. A picture was taken 5 s after each tapping and the average heights of flies climbing were calculated. Each analysis was repeated five times with 60 s resting intervals. The number of flies tested per genotype was n = 30 for females or n = 15 for males.
4.6. Statistical analysis
Statistical analysis of viability, male fecundity and egg laying assays was performed using one-way ANOVA followed by Bonferroni's multiple comparison test. Statistical analysis of male fertility, receptivity and rejection assays was performed using Fisher's exact test.
4.7. qRT-PCR
About 10 AGs were collected from 1-day-old males of indicated genotypes, and RT-PCR was performed as previously described [52]. Total RNA was extracted using the Ambion PureLink RNA mini kit according to the manufacturer's instructions. Primers used for qRT-PCR are as follows:
| actin88F | sense 5′- ATCGAGCACGGCATCATCAC-3′ |
| antisense 5′- CACGCGCAGCTCGTTGTA-3′ | |
| paired | sense 5′-CAGTCACGCCAACATTTCCG -3′ |
| antisense 5′-ACCCGGCATTATGTAGCTGG-3′ | |
| Acp26Aa | sense 5′-TCAAGGATCCAATCAAAGTGC-3′ |
| antisense 5′-ACGTTGGCTTCCTGAAACTG-3′ | |
| SP | sense 5′-GAATGGCCGTGGAATAGGAA-3′ |
| antisense 5′-GGCACCACTTATCACGAGGATT-3′ | |
| gsb | sense 5′-ATGACACCCTACTTTGGCGG-3′ |
| antisense 5′-TGCTGCCATCTCCACGATTT-3′ |
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to Dr Michael Weir for DNA plasmid and all members of the Xue laboratory for discussion and valuable comments. This work was initially started in the Institute for Molecular Biology, University of Zürich and supported by the Swiss National Science Foundation and the Kanton Zürich, later carried out in Tongji University and supported by the National Natural Science Foundation of China (30971681).
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Author contributions
L.L. and L.X. conceived, designed and conducted the experiments. L.L. P.L. and L.X. analysed the data and wrote the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Reference
- 1.Nusslein-Volhard C, Wieschaus E. 1980. Mutations affecting segment number and polarity in Drosophila. Nature 287, 795–801 (doi:10.1038/287795a0) [DOI] [PubMed] [Google Scholar]
- 2.Baumgartner S, Noll M. 1990. Network of interactions among pair-rule genes regulating paired expression during primordial segmentation of Drosophila. Mech. Dev. 33, 1–18 (doi:10.1016/0925-4773(90)90130-E) [DOI] [PubMed] [Google Scholar]
- 3.Ingham PW, Martinez Arias A. 1992. Boundaries and fields in early embryos. Cell 68, 221–235 (doi:10.1016/0092-8674(92)90467-Q) [DOI] [PubMed] [Google Scholar]
- 4.St Johnston D, Nusslein-Volhard C. 1992. The origin of pattern and polarity in the Drosophila embryo. Cell 68, 201–219 (doi:10.1016/0092-8674(92)90466-P) [DOI] [PubMed] [Google Scholar]
- 5.DiNardo S, O'Farrell PH. 1987. Establishment and refinement of segmental pattern in the Drosophila embryo: spatial control of engrailed expression by pair-rule genes. Genes Dev. 1, 1212–1225 (doi:10.1101/gad.1.10.1212) [DOI] [PubMed] [Google Scholar]
- 6.Ingham PW, Baker NE, Martinez-Arias A. 1988. Regulation of segment polarity genes in the Drosophila blastoderm by fushi tarazu and even skipped. Nature 331, 73–75 (doi:10.1038/331073a0) [DOI] [PubMed] [Google Scholar]
- 7.Bopp D, Jamet E, Baumgartner S, Burri M, Noll M. 1989. Isolation of two tissue-specific Drosophila paired box genes, Pox meso and Pox neuro. EMBO J. 8, 3447–3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bertuccioli C, Fasano L, Jun S, Wang S, Sheng G, Desplan C. 1996. In vivo requirement for the paired domain and homeodomain of the paired segmentation gene product. Development 122, 2673–2685. [DOI] [PubMed] [Google Scholar]
- 9.Xue L, Li X, Noll M. 2001. Multiple protein functions of Paired in Drosophila development and their conservation in the Gooseberry and Pax3 homologs. Development 128, 395–405. [DOI] [PubMed] [Google Scholar]
- 10.Xue L, Noll M. 1996. The functional conservation of proteins in evolutionary alleles and the dominant role of enhancers in evolution. EMBO J. 15, 3722–3731. [PMC free article] [PubMed] [Google Scholar]
- 11.Xue L, Noll M. 2000. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc. Natl Acad. Sci. USA 97, 3272–3275 (doi:10.1073/pnas.97.7.3272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue L, Noll M. 2002. Dual role of the Pax gene paired in accessory gland development of Drosophila. Development 129, 339–346. [DOI] [PubMed] [Google Scholar]
- 13.Bertram MJ, Akerkar GA, Ard RL, Gonzalez C, Wolfner MF. 1992. Cell type-specific gene expression in the Drosophila melanogaster male accessory gland. Mech. Dev. 38, 33–40 (doi:10.1016/0925-4773(92)90036-J) [DOI] [PubMed] [Google Scholar]
- 14.Chen PS. 1996. The accessory gland proteins in male Drosophila: structural, reproductive, and evolutionary aspects. Experientia 52, 503–510 (doi:10.1007/BF01969718) [DOI] [PubMed] [Google Scholar]
- 15.Wolfner MF. 1997. Tokens of love: functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 27, 179–192 (doi:10.1016/S0965-1748(96)00084-7) [DOI] [PubMed] [Google Scholar]
- 16.Heifetz Y, Lung O, Frongillo EA, Jr, Wolfner MF. 2000. The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr. Biol. 10, 99–102 (doi:10.1016/S0960-9822(00)00288-8) [DOI] [PubMed] [Google Scholar]
- 17.Ravi Ram K, Ramesh SR. 2003. Male accessory gland proteins in Drosophila: a multifaceted field [corrected]. Indian J. Exp. Biol. 41, 1372–1383. [PubMed] [Google Scholar]
- 18.Noll M. 1993. Evolution and role of Pax genes. Curr. Opin. Genet. Dev. 3, 595–605 (doi:10.1016/0959-437X(93)90095-7) [DOI] [PubMed] [Google Scholar]
- 19.Wang QY, Fang WH, Krupinski J, Kumar S, Slevin M, Kumar P. 2008. Pax genes in embryogenesis and oncogenesis. J. Cell. Mol. Med. 12, 2281–2294 (doi:10.1111/j.1582-4934.2008.00427.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson JA, Ziman M. 2011. Pax genes during neural development and their potential role in neuroregeneration. Prog. Neurobiol. 95, 334–351 (doi:10.1016/j.pneurobio.2011.08.012) [DOI] [PubMed] [Google Scholar]
- 21.Underhill DA. 2012. PAX proteins and fables of their reconstruction. Crit. Rev. Eukar. Gene 22, 161–177 (doi:10.1615/CritRevEukarGeneExpr.v22.i2.70) [DOI] [PubMed] [Google Scholar]
- 22.Treisman J, Harris E, Desplan C. 1991. The paired box encodes a second DNA-binding domain in the paired homeo domain protein. Genes Dev. 5, 594–604 (doi:10.1101/gad.5.4.594) [DOI] [PubMed] [Google Scholar]
- 23.Bopp D, Burri M, Baumgartner S, Frigerio G, Noll M. 1986. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell 47, 1033–1040 (doi:10.1016/0092-8674(86)90818-4) [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Rould MA, Jun S, Desplan C, Pabo CO. 1995. Crystal structure of a paired domain-DNA complex at 2.5 A resolution reveals structural basis for Pax developmental mutations. Cell 80, 639–650 (doi:10.1016/0092-8674(95)90518-9) [DOI] [PubMed] [Google Scholar]
- 25.Xu HE, Rould MA, Xu W, Epstein JA, Maas RL, Pabo CO. 1999. Crystal structure of the human Pax6 paired domain-DNA complex reveals specific roles for the linker region and carboxy-terminal subdomain in DNA binding. Genes Dev. 13, 1263–1275 (doi:10.1101/gad.13.10.1263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun H, Rodin A, Zhou Y, Dickinson DP, Harper DE, Hewett-Emmett D, Li W.H. 1997. Evolution of paired domains: isolation and sequencing of jellyfish and hydra Pax genes related to Pax-5 and Pax-6. Proc. Natl Acad. Sci. USA 94, 5156–5161 (doi:10.1073/pnas.94.10.5156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jun S, Desplan C. 1996. Cooperative interactions between paired domain and homeodomain. Development 122, 2639–2650. [DOI] [PubMed] [Google Scholar]
- 28.Gutjahr T, Vanario-Alonso CE, Pick L, Noll M. 1994. Multiple regulatory elements direct the complex expression pattern of the Drosophila segmentation gene paired. Mech. Dev. 48, 119–128 (doi:10.1016/0925-4773(94)90021-3) [DOI] [PubMed] [Google Scholar]
- 29.Frigerio G, Burri M, Bopp D, Baumgartner S, Noll M. 1986. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell 47, 735–746 (doi:10.1016/0092-8674(86)90516-7) [DOI] [PubMed] [Google Scholar]
- 30.Kubli E. 2003. Sex-peptides: seminal peptides of the Drosophila male. Cell. Mol. Life Sci. 60, 1689–1704 (doi:10.1007/s00018-003-3052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang CH, Rumpf S, Xiang Y, Gordon MD, Song W, Jan LY, Jan YN. 2009. Control of the postmating behavioral switch in Drosophila females by internal sensory neurons. Neuron 61, 519–526 (doi:10.1016/j.neuron.2008.12.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haussmann IU, Hemani Y, Wijesekera T, Dauwalder B, Soller M. 2013. Multiple pathways mediate the sex-peptide-regulated switch in female Drosophila reproductive behaviours. Proc. R. Soc. B 280, 20131938 (doi:10.1098/rspb.2013.1938) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DT, Hosken DJ, Ffrench-Constant RH, Wedell N. 2009. Variation in sex peptide expression in D. melanogaster. Genet. Res. 91, 237–242 (doi:10.1017/S0016672309000226) [DOI] [PubMed] [Google Scholar]
- 34.Herndon LA, Wolfner MF. 1995. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc. Natl Acad. Sci. USA 92, 10 114–10 118 (doi:10.1073/pnas.92.22.10114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubinstein CD, Wolfner MF. 2013. Drosophila seminal protein ovulin mediates ovulation through female octopamine neuronal signaling. Proc. Natl Acad. Sci. USA 110, 17 420–17 425 (doi:10.1073/pnas.1220018110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasemeyer M, Yapici N, Heberlein U, Dickson BJ. 2009. Sensory neurons in the Drosophila genital tract regulate female reproductive behavior. Neuron 61, 511–518 (doi:10.1016/j.neuron.2009.01.009) [DOI] [PubMed] [Google Scholar]
- 37.Rezaval C, Pavlou HJ, Dornan AJ, Chan YB, Kravitz EA, Goodwin SF. 2012. Neural circuitry underlying Drosophila female postmating behavioral responses. Curr. Biol. 22, 1155–1165 (doi:10.1016/j.cub.2012.04.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng K, Palfreyman MT, Hasemeyer M, Talsma A, Dickson BJ. 2014. Ascending SAG neurons control sexual receptivity of Drosophila females. Neuron 83, 135–148 (doi:10.1016/j.neuron.2014.05.017) [DOI] [PubMed] [Google Scholar]
- 39.Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. 2005. Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr. Biol. 15, 207–213 (doi:10.1016/j.cub.2005.01.034) [DOI] [PubMed] [Google Scholar]
- 40.Bouchard M, St-Amand J, Cote S. 2000. Combinatorial activity of pair-rule proteins on the Drosophila gooseberry early enhancer. Dev. Biol. 222, 135–146 (doi:10.1006/dbio.2000.9702) [DOI] [PubMed] [Google Scholar]
- 41.Bhat KM, van Beers EH, Bhat P. 2000. Sloppy paired acts as the downstream target of Wingless in the Drosophila CNS and interaction between sloppy paired and gooseberry inhibits sloppy paired during neurogenesis. Development 127, 655–665. [DOI] [PubMed] [Google Scholar]
- 42.Deshpande N, Dittrich R, Technau GM, Urban J. 2001. Successive specification of Drosophila neuroblasts NB 6–4 and NB 7–3 depends on interaction of the segment polarity genes wingless, gooseberry and naked cuticle. Development 128, 3253–3261. [DOI] [PubMed] [Google Scholar]
- 43.Duman-Scheel M, Li X, Orlov I, Noll M, Patel NH. 1997. Genetic separation of the neural and cuticular patterning functions of gooseberry. Development 124, 2855–2865. [DOI] [PubMed] [Google Scholar]
- 44.Skeath JB, Zhang Y, Holmgren R, Carroll SB, Doe CQ. 1995. Specification of neuroblast identity in the Drosophila embryonic central nervous system by gooseberry-distal. Nature 376, 427–430 (doi:10.1038/376427a0) [DOI] [PubMed] [Google Scholar]
- 45.Gutjahr T, Patel NH, Li X, Goodman CS, Noll M. 1993. Analysis of the gooseberry locus in Drosophila embryos: gooseberry determines the cuticular pattern and activates gooseberry neuro. Development 118, 21–31. [DOI] [PubMed] [Google Scholar]
- 46.Marie B, Pym E, Bergquist S, Davis GW. 2010. Synaptic homeostasis is consolidated by the cell fate gene gooseberry, a Drosophila pax3/7 homolog. J. Neurosci. 30, 8071–8082 (doi:10.1523/JNEUROSCI.5467-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai J, Lan Y, Appel LF, Weir M. 1994. Dissection of the Drosophila paired protein: functional requirements for conserved motifs. Mech. Dev. 47, 139–150 (doi:10.1016/0925-4773(94)90086-8) [DOI] [PubMed] [Google Scholar]
- 48.Gutjahr T, Frei E, Noll M. 1993. Complex regulation of early paired expression: initial activation by gap genes and pattern modulation by pair-rule genes. Development 117, 609–623. [DOI] [PubMed] [Google Scholar]
- 49.Lawrence PA, Johnston P, Macdonald P, Struhl G. 1987. Borders of parasegments in Drosophila embryos are delimited by the fushi tarazu and even-skipped genes. Nature 328, 440–442 (doi:10.1038/328440a0) [DOI] [PubMed] [Google Scholar]
- 50.Monsma SA, Harada HA, Wolfner MF. 1990. Synthesis of two Drosophila male accessory gland proteins and their fate after transfer to the female during mating. Dev. Biol. 142, 465–475 (doi:10.1016/0012-1606(90)90368-S) [DOI] [PubMed] [Google Scholar]
- 51.Nichols CD, Becnel J, Pandey UB. 2012. Methods to assay Drosophila behavior. J. Visual. Exp. 7, pii. 3795 (doi:10.3791/3795) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang MC, Bohmann D, Jasper H. 2003. JNK signaling confers tolerance to oxidative stress and extends lifespan in Drosophila. Dev. Cell 5, 811–816 (doi:10.1016/S1534-5807(03)00323-X) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.