Abstract
We recently reported a new method to purify the induced pluripotent stem (iPS)-derived osteoprogenitors (iPSop). In this paper, we optimized the procedure and characterized cells at each process step. We observed that 10 days of treatment with FGF-2, IGF-1 and TGF-β (FIT) resulted in early-phase osteoblasts and 14 days of treatment resulted in late-phase osteoblasts. We found that treatment with 1,25(OH)2 vitamin D3 increased expression of osteocalcin and decreased expression of tissue-non-specific alkaline phosphatase and runt-related transcription factor 2 (RUNX2) in iPSop-day14 cells (cells treated with FIT for 14 days). Therefore, iPSop-day14 cells were promoted to mature osteoblasts by 1,25(OH)2 vitamin D3 treatment. In addition, we found that 1,25(OH)2 vitamin D3 treatment for 14 days enhanced not only mineralization but also expression of osteocyte markers, including dentin matrix protein-1 and fibroblast growth factor-23, in iPSop cells. Therefore, 1,25(OH)2 vitamin D3 is a potent promoter of osteoblast–osteocyte transition. The results of this study suggest that it is possible to evaluate both early- and late-phase osteoblasts and to apply cells to drug screening for anabolic drugs that stimulate bone formation.
Keywords: induced pluripotent stem cells, vitamin D, osteoblasts, osteocytes, drug assessment
2. Introduction
Induced pluripotent stem cells (iPSCs) can generate a variety of patient-specific cells and are used to explore disease mechanisms and novel therapeutic molecular targets in drug development [1–4]. Recent studies have reported on a number of different types of cells generated from iPSCs [5–7]. We have previously developed an effective procedure to generate not only osteoblastic cells but also osteocyte-like cells [8,9]. Osteocytes, once considered as silent cells trapped in mineralized bone, are now identified as key regulators of bone remodelling [10,11]. Osteocytes are postmitotic, terminally differentiated osteoblasts and many reside in mineralized matrices. The most abundant cell in bone and central to bone remodelling, osteocytes secrete many soluble factors that not only target cells on the bone surface but also target other organs. Osteocytes most abundantly express receptor activator of nuclear factor kappa-B ligand, which functions as a key factor in osteoclast differentiation and activation. Therefore, any drugs targeting osteocyte function and signalling pathways will have a major impact on the bone remodelling process. Currently, much research into drug development, particularly for osteoporosis, is focused on targeting osteocytes. For this reason, the generation of osteocytes has become particularly important.
Osteoporosis is a very common health problem among elderly people and is thought to affect more than 200 million people worldwide [12]. With ageing populations in many countries, the incidence of osteoporosis will increase further. Therefore, it is important to develop novel drugs for the treatment of osteoporosis [13–15]. Osteoporosis is caused by an uncoupling of bone resorption from bone formation and is associated with reductions in bone strength determined by bone mineral density and bone quality. Reductions in bone strength occur because of an imbalance between the activity of osteoclasts and osteoblasts. In addition, bone formation is impaired in elderly people [16–18], and loss of bone mineral density is caused by a decrease in calcium absorption with ageing [19]. Consequently, the microarchitecture of trabecular bone deteriorates [20].
Osteoporosis drugs are categorized into two classes: antiresorptive drugs that inhibit bone resorption and anabolic drugs that stimulate bone formation. The bisphosphonates (BPs) are antiresorptive drugs and are most commonly prescribed for the treatment of osteoporosis [15,16,21,22]. However, BPs may cause bisphosphonate-related osteonecrosis of the jaw, which is a rare but well recognized serious side effect of long-term bisphosphonate use [23–27]. Therefore, there is a need for anabolic drugs that could effectively modulate osteoblast or osteocyte activity to regenerate bone. iPSC technology can be applied to drug assessment and to patient-specific approaches to examine individual differences in pharmacokinetic and pharmacodynamic features.
All therapeutic management strategies for the prevention and treatment of osteoporosis include recommendations for calcium and vitamin D supplementation [28]. The active form of vitamin D, 1,25(OH)2 vitamin D3, contributes to a wide range of biological activities by binding to the nuclear vitamin D receptor. It is well established that the appropriate use of vitamin D can prevent the progression of osteoporosis. Many studies have shown that vitamin D has both anabolic and catabolic roles in bone homeostasis. However, the effects of vitamin D on bone tissue and bone cells have not yet been entirely evaluated. It is important to assess the precise effects of vitamin D on the osteolineage cells, osteoblasts and osteocytes. Here, we show that human osteolineage cells derived from human iPSCs (hiPSCs) can provide an ideal model to analyse the effects of 1,25(OH)2 vitamin D3 [9,29–33]. Our results suggest that these osteolineage cells could be used for high-throughput screening technologies to develop new drugs to treat osteoporosis. In a recent advance in drug discovery for osteoporosis using treatment targeting sclerostin, it is indicated that the osteocyte must be one of the major targets [29].
In this study, we treated tissue-non-specific alkaline phosphatase (TNAP) positive osteolineage cells derived from hiPSCs, referred to as iPS-derived osteoprogenitors (iPSop), with 1,25(OH)2 vitamin D3. We compared iPSop cells with TNAP positive osteolineage cells derived from human mesenchymal stem cells (hMSCs), referred to as MSC-derived osteoprogenitors (MSCop). We found that osteocalcin (OCN), a late-phase marker for osteoblasts, was increased, and several osteocyte markers were detectable in iPSop cells but not in MSCop cells, after 1,25(OH)2 vitamin D3 treatment. Therefore, we suggest that iPSop cells can respond to osteogenic agents and may be a useful tool for drug assessment. Furthermore, this model could be used to develop new agents to promote osteogenic progression.
3. Material and methods
3.1. Cell culture and reagents
Human iPSCs (line 201B7; Riken Cell Bank, Tsukuba, Japan) were maintained with SNL76/7 feeder cells, clonally derived from a mouse fibroblast STO cell line transformed with neomycin resistance genes and murine LIF genes, in human ES medium (Dulbecco's modified Eagle's medium, nutrient mixture F-12 (DMEM/F-12, Invitrogen, Carlsbad, CA) with 20% knockout serum replacement (Invitrogen) supplemented with 1× non-essential amino acids solution (Chemicon, Temecula, CA), 2 mM l-glutamine (Chemicon), 1 mM 2-mercaptoethanol (Wako Pure Chemical Industries Ltd., Osaka, Japan), 1% penicillin/streptomycin (Invitrogen) and 5 ng ml−1 human fibroblast growth factor (FGF)-2 (ReproCELL Incorporated, Yokohama, Japan)). hMSCs were purchased Lonza (Basel, Switzerland), cultured in BulletKit MSC growth medium (Lonza) and used at passage 3 to 5. The 1,25(OH)2 vitamin D3 was purchased from Kyowa Hakko Kirin Co., Ltd. (Tokyo, Japan). Insulin-like growth factor (IGF)-1 and transforming growth factor (TGF)-β were purchased from Wako Pure Chemical Industries Ltd.
3.2. Embryoid body formation and in vitro osteogenic differentiation
The hiPSC colonies were dissociated with a cell scraper and transferred to low attachment Petri dishes to generate embryoid bodies (EBs). EBs were maintained in suspension in human ES medium without FGF-2 for 6 days. On day 6, the EBs were treated with 2 μM thiazovivin (Wako Pure Chemical Industries Ltd.) in human ES medium without FGF-2 for 1 h at 37°C and collected and dissociated in 0.5 mg ml−1 collagenase type IV (Wako Pure Chemical Industries Ltd.) for 20 min at 37°C, followed by treatment with 0.05% trypsin–EDTA (Invitrogen) for 5 min at 37°C. Cell suspensions were rinsed with α-MEM (Invitrogen, Carlsbad, CA) with 10% FBS, and cells were seeded onto cell culture dishes and cultured in osteoblast medium (OBM; α-MEM with 10% FBS, 50 μg ml−1 l-ascorbic acid (Wako Pure Chemical Industries Ltd.), 10 mM β-glycerophosphate (Wako Pure Chemical Industries Ltd.) and 10 nM dexamethasone (Wako Pure Chemical Industries Ltd.)). FGF-2, IGF-1 and TGF-β (FIT) were added on the following day (day 0) at a final concentration of 25 ng ml−1 FGF-2, 100 ng ml−1 IGF-1 and 1 ng ml−1 TGF-β. After culture in OBM with FIT for 0 (iPSop-day0), 4 (iPSop-day4), 10 (iPSop-day10) or 14 days (iPSop-day14), the cells were analysed and isolated by flow cytometry. In addition, the hMSCs were cultured in OBM with FIT and then isolated by flow cytometry on day 4 (MSCop-day4) or day 14 (MSCop-day14).
3.3. Flow cytometrical analysis and cell sorting
After washing with phosphate-buffered saline (PBS), the cells were treated with 0.05% trypsin–EDTA for 10 min at 37°C. The trypsinized cells were stained with phycoerythrin-conjugated anti-human alkaline phosphatase (ALP) antibody (R&D Systems, Minneapolis, MN) at a concentration of 10 μl/2 × 105 cells for 45 min on ice. After staining, cells were washed three times with PBS, suspended in PBS containing 0.5% FBS, passed through a 40-μm mesh filter and maintained at 4°C until FACS sorting. We defined TNAP positive cells from hiPSCs and hMSCs as iPSop and MSCop, respectively. Dead cells were excluded by propidium iodide staining (2 μg ml−1) and forward scatter. Flow cytometrical analysis and cell sorting were performed on a FACS Aria (Becton-Dickinson, San Jose, CA).
3.4. Induction of osteoblast differentiation in osteoprogenitor-derived hiPSCs and hMSCs with 1,25(OH)2 vitamin D3
After culture in OBM with FIT for 14 days (iPSop-day14), the cells were isolated by flow cytometry, and 1,25(OH)2 vitamin D3 at a concentration of 10 or 50 nM was added on the following day (day 0). The medium was refreshed every 3 days, and the cells were analysed on day 6 and day 12. In addition, after culture in OBM with FIT for 4 days (iPSop-day4, MSCop-day4) or 14 days (iPSop-day14, MSCop-day14), iPSop and MSCop cells were isolated by flow cytometry and were treated with 50 nM 1,25(OH)2 vitamin D3. The medium was refreshed every 3 days, and the cells were analysed on day 6 and day 12.
3.5. mRNA expression
Total RNA was extracted using QIAzol reagent (Qiagen Inc., Valencia, CA) according to the manufacturer's instructions. cDNA was synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real-time RT-PCR (qRT-PCR) was performed using Premix Ex Taq reagent (Takara Bio Inc., Shiga, Japan) according to the manufacturer's instructions. Target genes included type I collagen (COL1A1), TNAP, runt-related transcription factor 2 (RUNX2), OCN and osterix (OSX). 18S rRNA was used as an internal control. All primers and probes are presented in table 1. Relative expression of genes of interest was estimated using the ΔΔCt method.
Table 1.
Primers used for qRT-PCR.
| gene symbol | GenBank accession no. | forward primer sequence | reverse primer sequence |
|---|---|---|---|
| COL1A1 | NM_000088.3 | 5′-gggattccctggacctaaag-3′ | 5′-ggaaacctcgctctcca-3′ |
| TNAP | NM_000478.3 | 5′-caaccctggggaggagac-3′ | 5′-gcattggtgttgtacgtcttg-3′ |
| RUNX2 | NM_001024630.2 | 5′-gtgcctaggcgcatttca-3′ | 5′-gctcttcttactgagagtggaagg-3′ |
| OCN | NM_007541.2 | 5′-agactccggcgctacctt-3′ | 5′-ctcgtcacaagcagggttaag-3′ |
| OSX | NM_152860.1 | 5′-catctgcctggctccttg-3′ | 5′-caggggactggagccata-3′ |
| 18SrRNA | M11188.1 | 5′-cggacaggattgacagattg-3′ | 5′-cgctccaccaactaagaacg-3′ |
RT-PCR was performed to examine some of the osteocyte-specific markers using ExTaq DNA polymerase (Takara Biotechnology, Shiga, Japan). Target genes included dentin matrix protein-1 (DMP-1), fibroblast growth factor-23 (FGF-23), matrix extracellular phosphoglycoprotein (MEPE) and podoplanin (PDPN). β-Actin was used as an internal control. Amplified PCR products were electrophoresed on 2% agarose gels. PCR primers used are listed in table 2.
Table 2.
Primers used for RT-PCR.
| gene symbol | GenBank accession no. | forward primer sequence | reverse primer sequence |
|---|---|---|---|
| DMP-1 | NM_001079911 | 5′-caggagcacaggaaaaggag-3′ | 5′-ctggtggtatcttgggcact-3′ |
| FGF-23 | NM_020638.2 | 5′-tatttcgacccggagaactg-3′ | 5′-ggtatgggggtgttgaagtg-3′ |
| PHEX | NM_000444.5 | 5′-aagaggaccctgggagaaaa-3′ | 5′-gggactgtgagcaccaattt-3′ |
| MEPE | NM_001184694 | 5′-ccctttctgaagccagtgag-3′ | 5′-ttttcttcccccaggagttt-3′ |
| PDPN | NM_001006624 | 5′-ccagcgaagaccgctataag-3′ | 5′-acgatgattgcaccaatgaa-3′ |
| β-actin | NM_001101.3 | 5′-gggaaatcgtgcgtgacatta-3′ | 5′-ggcagtgatctccttctgcat-3′ |
3.6. Mineralization
The iPSop-day14 cells were treated with 1,25(OH)2 vitamin D3 for 2 weeks. The medium was removed, and the cells were rinsed in PBS and fixed in 4% paraformaldehyde for 5 min at room temperature. For mineralization, plates were treated with alizarin red solution for 5 min at room temperature. After 5 min, plates were rinsed in PBS and visually examined.
3.7. Statistical analysis
All data are expressed as the mean ± standard deviation (s.d.). When ANOVA indicated differences among the groups, multiple comparisons between each experimental group were performed using the Bonferroni test. Statistical significance was defined as p < 0.05.
4. Results
4.1. Induction of iPSop cells
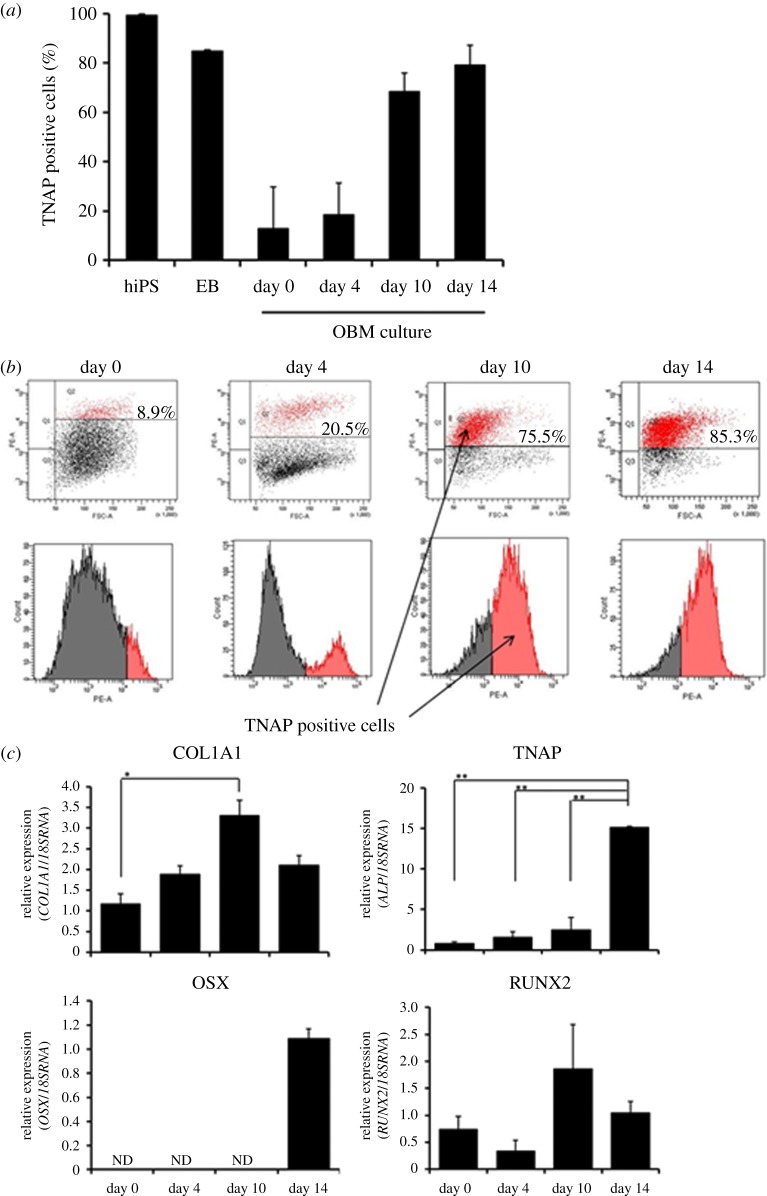
As expected, the hiPSCs expressed a large amount of TNAP. We found that cells separated from EBs rapidly lost TNAP expression, but FIT treatment greatly increased TNAP expression (figure 1a,b). We isolated TNAP positive cells by FACS sorting and defined them as iPSop cells. Furthermore, we estimated osteoblast-specific markers in iPSop cells at each differentiation stage during culture in OBM. COL1A1 and TNAP expression increased over time. In iPSop-day10 cells, the expression of TNAP and RUNX2 was two times that in iPSop-day0 cells, but OSX expression was not detected. In iPSop-day14 cells, TNAP expression was further increased, RUNX2 expression was decreased and OSX expression sharply increased. Therefore, culture in OBM led to the differentiation of iPSop cells into osteoblasts over time (figure 1c). These results demonstrated that we could obtain osteoblasts at various stages of differentiation from iPSCs.
Figure 1.
The expression of TNAP during hiPSC-to-osteogenic cell differentiation and the expression of osteoblast markers. (a) The frequency of TNAP positive cells in hiPSCs, EBs and single cells derived from EBs cultured in OBM with FGF-2/IGF-1/TGF-β (FIT). (b) Flow cytometrical analysis was performed on hiPSCs, EBs and single cells cultured in OBM with FIT for 0, 4, 10 and 14 days. (c) qRT-PCR analysis of COL1A1, TNAP, OSX and RUNX2 was performed on iPSop-day0, 4, 10 and 14 cells. mRNA levels were normalized to those of 18S rRNA. Experiments were performed in triplicate. Values represent the mean ± s.d., n = 3. Bonferroni correction for multiple comparisons was applied. *p < 0.05, **p < 0.01.
4.2. 1,25(OH)2 vitamin D3 promotes osteogenic differentiation of iPSop cells
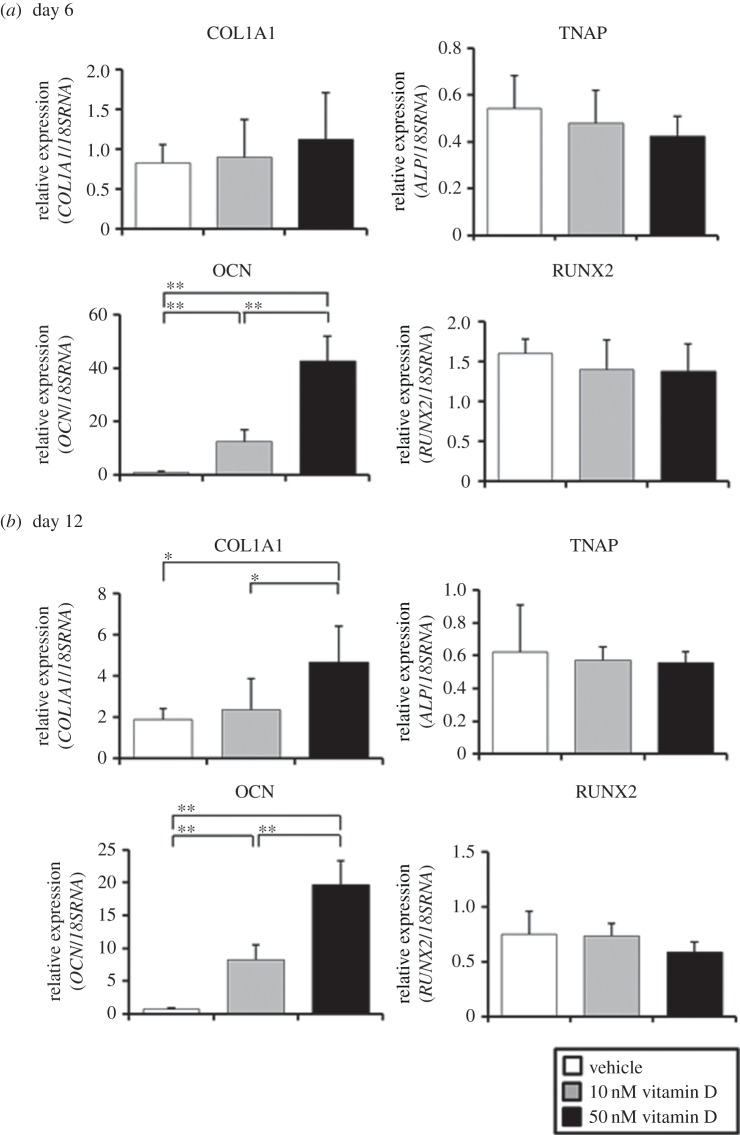
The treatment of iPSop-day14 cells with 1,25(OH)2 vitamin D3 for 6 days increased the expression of COL1A1 and OCN and decreased the expression of TNAP and RUNX2 (figure 2a). The increases or decreases in osteoblast markers occurred in a dose-dependent manner, and the longer the treatment, the more significant the change in osteoblast marker expression (figure 2b).
Figure 2.
Expression of osteoblast markers after 1,25(OH)2 vitamin D3 treatment. (a) After isolation by flow cytometry, iPSop-day14 cells were treated with vehicle (control), 10 nM or 50 nM 1,25(OH)2 vitamin D3 for 6 days or (b) 12 days. Expression of COL1A1, TNAP, OCN and RUNX2 was analysed with qRT-PCR, and mRNA levels were normalized to those of 18S rRNA. Experiments were performed in triplicate. Values represent the mean ± s.d., n = 4. Bonferroni correction for multiple comparisons was applied. *p < 0.05, **p < 0.01.
4.3. iPSop cells are more reactive to 1,25(OH)2 vitamin D3 than MSCop cells
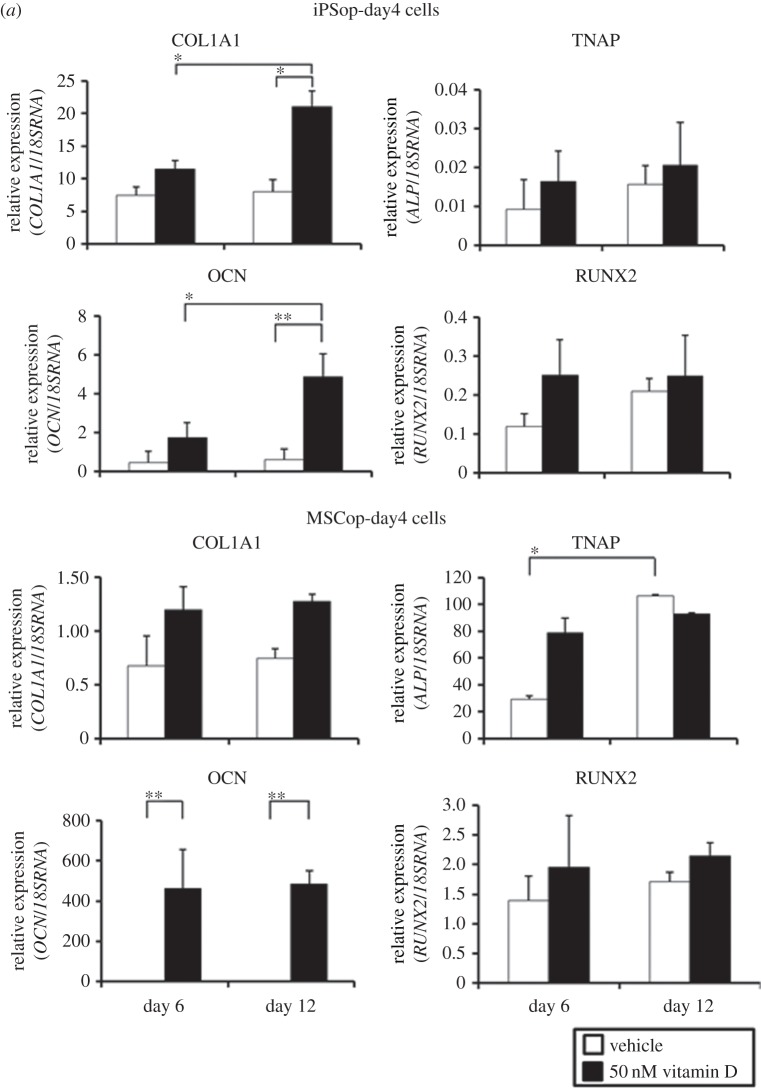
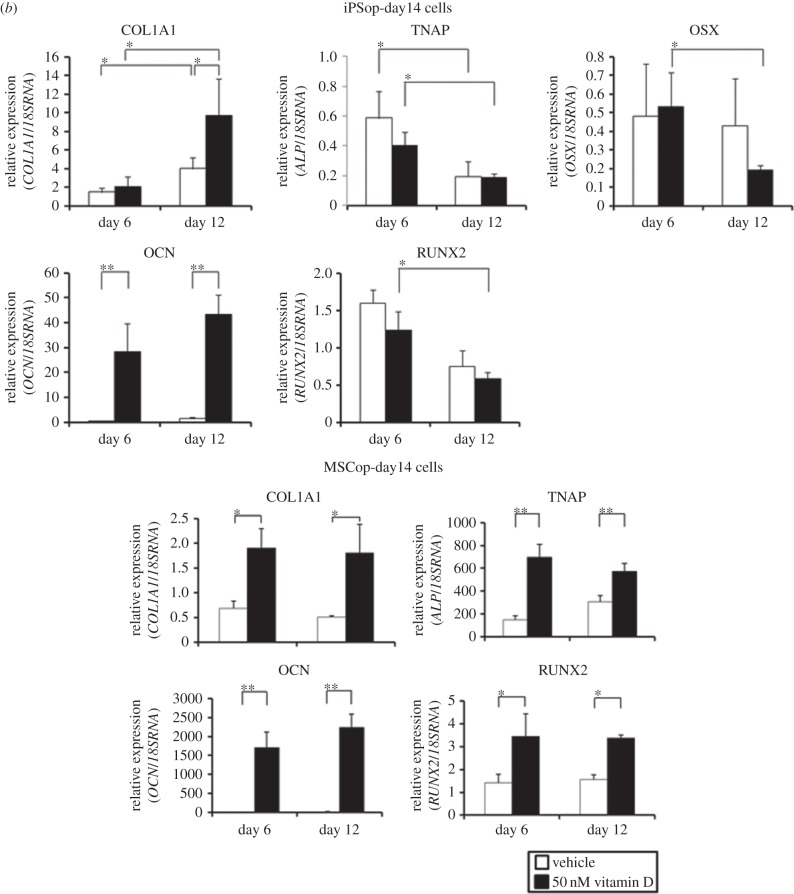
Furthermore, we investigated the difference in response to 1,25(OH)2 vitamin D3 between iPSop cells and MSCop cells. We treated iPSop-day4 and MSCop-day4 cells, which were isolated by flow cytometry after culture in OBM with FIT for 4 days, with 1,25(OH)2 vitamin D3. Every osteoblast marker in MSCop-day4 cells increased but there was little difference between 6 and 12 days of 1,25(OH)2 vitamin D3 treatment (figure 3a). In iPSop-day4 cells, again every osteoblast marker increased with 1,25(OH)2 vitamin D3 treatment and marker expression continued to increase over time (figure 3a). In iPSop-day14 cells, expression of COL1A1 and OCN increased and expression of TNAP, RUNX2 and OSX decreased over time with 1,25(OH)2 vitamin D3 treatment (figure 3b). However, TNAP and RUNX2 expression in MSCop-day14 cells did not decrease with 1,25(OH)2 vitamin D3 treatment (figure 3b). These results suggest that iPSop cells were more reactive to 1,25(OH)2 vitamin D3 treatment than MSCop cells, and iPSop-day14 cells were advancing to a late phase of osteoblast differentiation.
Figure 3.
Expression of osteoblast markers in iPSop and MSCop cells at different differentiation stages after 1,25(OH)2 vitamin D3 treatment. iPSop and MSCop cells express various osteoblast markers. Comparison of expression of osteoblast marker genes between cells treated with vehicle and 1,25(OH)2 vitamin D3. (a) In iPSop- and MSCop-day4 cells. (b) In iPSop- and MSCop-day14 cells. These cells were treated with vehicle (white bar) or 50 nM 1,25(OH)2 vitamin D3 (black bar) for 6 or 12 days. Expressions of COL1A1, TNAP, OCN, RUNX2 and OSX were analysed with qRT-PCR, and mRNA levels were normalized to those of 18S rRNA. Experiments were performed in triplicate. Values represent the mean ± s.d., n = 3. Bonferroni correction for multiple comparisons was applied. *p < 0.05, **p < 0.01.
4.4. iPSop cells expressed various osteocyte marker genes with 1,25(OH)2 vitamin D3 treatment
Osteocyte-specific markers, such as DMP-1, FGF-23 and MEPE, were detected in iPSop-day14 cells treated with 1,25(OH)2 vitamin D3 for 14 days (figure 4a). In addition, 1,25(OH)2 vitamin D3 promoted mineralization in iPSop-day14 cells, when compared with vehicle alone (figure 4b). Therefore, these cells had proceeded to an early phase of osteocyte differentiation.
Figure 4.
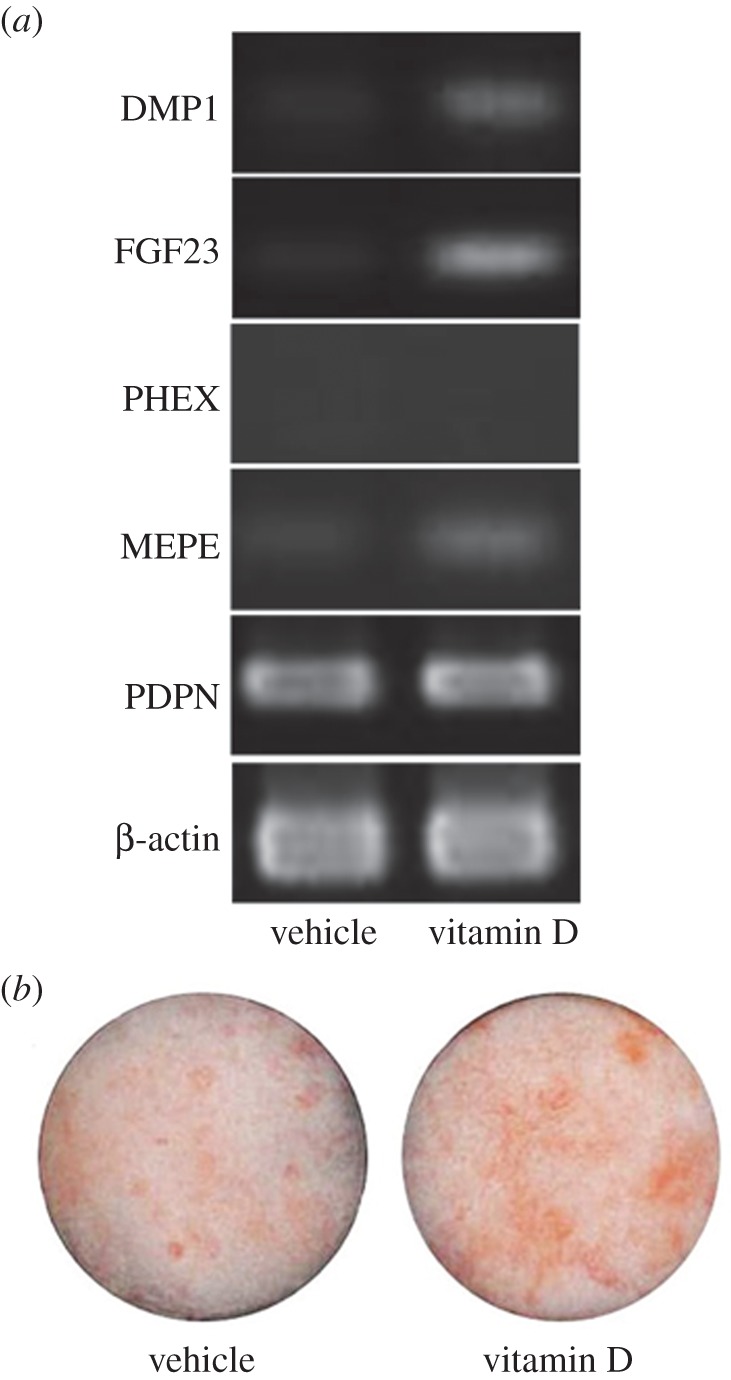
Expression of osteocyte-specific marker genes and the mineralization by alizarin red staining. (a) Expression of osteocyte-specific markers in iPSop-day14 cells treated with vehicle (vitamin D−) and 50 nM 1,25(OH)2 vitamin D3 (vitamin D+) for 14 days. The expression of dentin matrix protein-1 (DMP-1), fibroblast growth factor-23 (FGF-23), matrix extracellular phosphoglycoprotein (MEPE) and podoplanin (PDPN) were analysed by RT-PCR. β-Actin was used as an internal control. RT-PCR was performed using ExTaq DNA polymerase. Amplified PCR products were electrophoresed on 2% agarose gels. PCR primers are presented in table 2. (b) Mineralization in iPSop-day14 cells treated with vehicle (vitamin D−) and 50 nM 1,25(OH)2 vitamin D3 (vitamin D+) for 14 days with alizarin red staining.
5. Discussion
Osteoporosis is a very common disease normally associated with ageing and caused by bone resorption overtaking bone formation. Dietary or supplemental calcium and activated vitamin D are required to reduce the risk of osteoporosis [15]. In clinical use, the intestinal calcium absorption effect of the prodrug alfacalcidol (1-hydroxyvitamin D3) becomes active at a daily dosage of 1.0 μg and then suppresses parathyroid hormone and inhibits bone resorption in adults. However, vitamin D has a narrow therapeutic window and bone resorption can occur at higher doses. Therefore, vitamin D may have both anabolic and catabolic effects on osteolineage cells. Although the mechanism underlying this biphasic effect is clinically important, to date, it is not well understood. The study of osteoremodelling by 1,25(OH)2 vitamin D3 has been limited by difficulty in obtaining osteocytes, which have a central role in that process.
We recently reported a new method to purify osteoprogenitors from hiPSCs. One of the unique advantages of our method is the ability to generate osteocyte-like cells. In this study, we used our system to evaluate the effects of 1,25(OH)2 vitamin D3 on osteoblast differentiation. First, we identified the optimal timing to obtain iPSop cells by sorting TNAP positive cells at different time points. Osteoblast marker gene expression differed over time. Cells liberated from hiPSC-derived EBs after 10 days of culture in OBM with FIT were sorted as TNAP positive cells and these cells showed increased expression of RUNX2. Four days later, after 14 days of culture in OBM with FIT, OSX expression was significantly increased (figure 1c). Therefore, it appears that cells liberated from hiPSC-derived EBs after 14 days of culture in OBM with FIT are differentiated into late-phase osteoblasts [34]. Next, we investigated the effects of 1,25(OH)2 vitamin D3 on these iPSop cells. We found that iPSop cells responded to 1,25(OH)2 vitamin D3 and the expression of osteoblast markers in these cells was affected in a dose-dependent manner. The effects of 1,25(OH)2 vitamin D3 on osteoblast marker gene expression suggested that iPSop-day4 cells were early-phase osteoblasts and iPSop-day14 cells were late-phase osteoblasts. Treatment with 1,25(OH)2 vitamin D3 increased all osteoblast markers in iPSop-day4 cells and MSCop-day4 cells. However, 1,25(OH)2 vitamin D3 treatment decreased the expression of some osteoblast markers (TNAP, RUNX2 and OSX) and increased OCN expression in iPSop-day14 cells but not in MSCop-day14 cells. Moreover, we observed an increase of mineralization in iPSop-day14 cells after 14 days of 1,25(OH)2 vitamin D3 treatment. We previously showed that it took 40 days for iPSop cells to express osteocyte markers [9]. The current observations clearly showed that 1,25(OH)2 vitamin D3 could be a potent accelerator of osteodifferentiation, particularly from late- to early-phase osteocytes. However, we did not detect an increase in sclerostin (an osteocyte-derived inhibitor of cultured osteoblasts) expression in this study. We suggest that high dose 1,25(OH)2 vitamin D3 treatment could induce sclerostin expression in newly formed osteocytes or existing osteocytes, which, in turn, could cause inhibition of Wnt signalling and accelerate the resorption of bone tissue.
Another important point is that this method could be used for research into new drugs for osteoporosis. Human-cell-based in vitro assay systems are a basic requisite for drug discovery and iPSCs reprogrammed from somatic cells can provide an opportunity to establish these systems [3,4]. Recent research has revealed that osteocytes are part of the command centre for osteoremodelling, and one reason is that sclerostin is exclusively secreted from osteocytes [35]. A neutralizing antibody to sclerostin has been shown to be effective in initial preclinical and early clinical trials. Postmenopausal women treated with a neutralizing antibody to sclerostin showed an increase in markers of bone formation [29]. Therefore, agents regulating osteocyte functions such as sclerostin secretion could be effective in the treatment of osteoporosis. However, screening methods using animal models may not reflect the human situation and some compounds found to be successful in cellular or animal models have failed to show effectiveness in clinical trials [35,36]. It has been postulated that drug screening using iPSCs could identify compounds that increase differentiation and promote maturation in target cells [36]. To date, there are few examples of the use of high-throughput screening methods to assess differentiation. We believe that the iPSop cells generated using our method could be a valuable cell source for high-throughput screening to research new drugs for a range of diseases, including osteoporosis.
6. Conclusion
Treatment with 1,25(OH)2 vitamin D3 could promote osteogenic differentiation in iPSop cells and could accelerate the expression of osteocyte marker genes. This is the first report showing that 1,25(OH)2 vitamin D3 can promote osteocyte generation. Because iPSCs expand into a large number of cells, this method could be used for high-throughput screening of new drugs to treat osteoporosis. We hope that our method will provide us a new platform for research into the treatment of osteoporosis and other bone diseases.
Acknowledgements
The human iPS cell line 201B7 (HPS0063) was provided by Riken, BRC through the National Bio-Resource Project of the MEXT, Japan.
Author contributions
H.K. carried out the molecular laboratory work, participated in data analysis, participated in the design of the study, carried out the statistical analyses and drafted the manuscript. H.O.-S., S.O., A.S. and T.S. participated in data analysis and participated in the design of the study. T.A. conceived the study, designed the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Funding statement
This work was supported by JSPS KAKENHI (C) grant no. 25462900 and by JSPS KAKENHI for young scientist (B) grant nos. 25861761, 26861686 and 26861560.
Competing interests
We have no competing interests.
References
- 1.Kondo T, et al. 2013. Modeling Alzheimer's disease with iPSCs reveals stress phenotypes associated with intracellular Aβ and differential drug responsiveness. Cell Stem Cell 12, 487–496 (doi:10.1016/j.stem.2013.01.009) [DOI] [PubMed] [Google Scholar]
- 2.Saito A, Ochiai H, Okada S, Miyata N, Azuma T. 2013. Suppression of Lefty expression in induced pluripotent cancer cells. FASEB J. 27, 2165–2174 (doi:10.1096/fj.12-221432) [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Yamanaka S. 2011. The use of induced pluripotent stem cells in drug development. Clin. Pharmacol. Ther. 89, 655–661 (doi:10.1038/clpt.2011.38) [DOI] [PubMed] [Google Scholar]
- 4.Yahata N, et al. 2011. Anti-Aβ drug screening platform using human iPS cell-derived neurons for the treatment of Alzheimer's disease. PLoS ONE 6, e25788 (doi:10.1371/journal.pone.0025788) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilousova G, et al. 2012. Osteoblasts derived from induced pluripotent stem cells form calcified structures in scaffolds both in vitro and in vivo. Stem Cells 29, 206–216 (doi:10.1002/stem.566) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashiro K, Inamura M, Kawabata K, Sakurai F, Yamanishi K, Hayakawa T, Mizuguchi H. 2009. Efficient adipocyte and osteoblast differentiation from mouse induced pluripotent stem cells by adenoviral transduction. Stem Cells 27, 1802–1811 (doi:10.1002/stem.108) [DOI] [PubMed] [Google Scholar]
- 7.Li F, Bronson S, Niyibizi C. 2010. Derivation of murine induced pluripotent stem cells (iPS) and assessment of their differentiation toward osteogenic lineage. J. Cell. Biochem. 109, 643–652 (doi:10.1002/jcb.22440) [DOI] [PubMed] [Google Scholar]
- 8.Ochiai H, Okada S, Saito A, Hoshi K, Yamashita H, Takato T, Azuma T. 2012. Inhibition of insulin-like growth factor-1 (IGF-1) expression by prolonged transforming growth factor-β1 (TGF-β1) administration suppresses osteoblast differentiation. J. Biol. Chem. 287, 22 654–22 661 (doi:10.1074/jbc.M111.279091) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ochiai-Shino H, Kato H, Sawada T, Onodera S, Saito A, Takato T, Shibahara T, Muramatsu T, Azuma T. 2014. A novel strategy for enrichment and isolation of osteoprogenitor cells from induced pluripotent stem cells based on surface marker combination. PLoS ONE 9, e99534 (doi:10.1371/journal.pone.0099534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pajevic PD. 2013. Recent progress in osteocyte research. Endocrinol. Metab. 28, 255–261 (doi:10.3803/EnM.2013.28.4.255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonewald LF. 2011. The amazing osteocyte. J. Bone Miner. Res. 26, 229–238 (doi:10.1002/jbmr.320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper C, Campion G, Melton LJ., 3rd 1992. Hip fractures in the elderly: a world-wide projection. Osteoporos. Int. 2, 285–289 (doi:10.1007/BF01623184) [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura N, et al. 2009. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J. Bone Miner. Metab. 27, 620–628 (doi:10.1007/s00774-009-0080-8) [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura N, Muraki S, Oka H, Kawaguchi H, Nakamura K, Akune T. 2010. Cohort profile: research on osteoarthritis/osteoporosis against disability study. Int. J. Epidemiol. 39, 988–995 (doi:10.1093/ije/dyp276) [DOI] [PubMed] [Google Scholar]
- 15.Sambrook P, Cooper C. 2006. Osteoporosis. Lancet 367, 2010–2018 (doi:10.1016/S0140-6736(06)68891-0) [DOI] [PubMed] [Google Scholar]
- 16.Rachner TD, Khosla S, Hofbauer LC. 2011. Osteoporosis: now and the future. Lancet 377, 1276–1287 (doi:10.1016/S0140-6736(10)62349-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimura N, Hashimoto T, Sakata K, Morioka S, Kasamatsu T, Cooper C. 1999. Biochemical markers of bone turnover and bone loss at the lumbar spine and femoral neck: the Taiji study. Calcif. Tissue Int. 65, 198–202 (doi:10.1007/s002239900682) [DOI] [PubMed] [Google Scholar]
- 18.Iki M, Akiba T, Matsumoto T, Nishino H, Kagamimori S, Kagawa Y, Yoneshima H. 2004. Reference database of biochemical markers of bone turnover for the Japanese female population. Japanese population-based osteoporosis (JPOS) study. Osteoporos. Int. 15, 981–991 (doi:10.1007/s00198-004-1634-1) [DOI] [PubMed] [Google Scholar]
- 19.Nordin BEC, Need AG, Morris HA, O'Loughlin PD, Horowitz M. 2004. Effect of age on calcium absorption in postmenopausal women. Am. J. Clin. Nutr. 80, 998–1002. [DOI] [PubMed] [Google Scholar]
- 20.Seeman E, Delmas PD. 2006. Bone quality: the material and structural basis of bone strength and fragility. N. Engl. J. Med. 354, 2250–2261 (doi:10.1056/NEJMra053077) [DOI] [PubMed] [Google Scholar]
- 21.Black D, Cummings S, Karpf D. 1996. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348, 1535–1541 (doi:10.1016/S0140-6736(96)07088-2) [DOI] [PubMed] [Google Scholar]
- 22.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR. 2000. Fracture risk reduction with alendronate in women with osteoporosis: the fracture intervention trial. FIT research group. J. Clin. Endocrinol. Metab. 85, 4118–4124 (doi:10.1210/jcem.85.11.6953) [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Gupta H, Mandhyan D, Srivastava S. 2013. Bisphophonates related osteonecrosis of the jaw. Natl J. Maxillofac. Surg. 4, 151–158 (doi:10.4103/0975-5950.127643) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma D, Ivanovski S, Slevin M, Hamlet S, Pop TS, Brinzaniuc K, Petcu EB, Miroiu RI. 2013. Bisphosphonate-related osteonecrosis of jaw (BRONJ): diagnostic criteria and possible pathogenic mechanisms of an unexpected anti-angiogenic side effect. Vasc. Cell 5, 1 (doi:10.1186/2045-824X-5-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fleisch H. 1998. Bisphosphonates: mechanisms of action. Endocr. Rev. 19, 80–100 (doi:10.1210/edrv.19.1.0325) [DOI] [PubMed] [Google Scholar]
- 26.Marx RE. 2003. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J. Oral Maxillofac. Surg. 61, 1115–1117 (doi:10.1016/S0278-2391(03)00720-1) [DOI] [PubMed] [Google Scholar]
- 27.Khosla S, et al. 2007. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 22, 1479–1491 (doi:10.1359/jbmr.0707onj) [DOI] [PubMed] [Google Scholar]
- 28.Das S, Crockett JC. 2013. Osteoporosis: a current view of pharmacological prevention and treatment. Drug Des. Devel. Ther. 7, 435–448 (doi:10.2147/DDDT.S31504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evenepoel P, D'Haese P, Brandenburg V. 2014. Romosozumab in postmenopausal women with osteopenia. N. Engl. J. Med. 370, 1664–1665 (doi:10.1056/NEJMc1402396) [DOI] [PubMed] [Google Scholar]
- 30.van Driel M, Pols HA, van Leeuwen JP. 2004. Osteoblast differentiation and control by vitamin D and vitamin D metabolites. Curr. Pharm. Des. 10, 2535–2555 (doi:10.2174/1381612043383818) [DOI] [PubMed] [Google Scholar]
- 31.Geng S, Zhou S, Bi Z, Glowacki J. 2013. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metabolism 62, 768–777 (doi:10.1016/j.metabol.2013.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou S, LeBoff MS, Glowacki J. 2010. Vitamin D metabolism and action in human bone marrow stromal cells. Endocrinology 151, 14–22 (doi:10.1210/en.2009-0969) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woeckel VJ, Bruedigam C, Koedam M, Chiba H, van der Eerden BCJ, van Leeuwen JPTM. 2013. 1α,25-dihydroxyvitamin D3 and rosiglitazone synergistically enhance osteoblast-mediated mineralization. Gene. 512, 438–443 (doi:10.1016/j.gene.2012.07.051) [DOI] [PubMed] [Google Scholar]
- 34.Van Dinther M, et al. 2013. Anti-sclerostin antibody inhibits internalization of sclerostin and sclerostin-mediated antagonism of Wnt/LRP6 signaling. PLoS ONE 8, e62295 (doi:10.1371/journal.pone.0062295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue H, Nagata N, Kurokawa H, Yamanaka S. 2014. iPS cells: a game changer for future medicine. EMBO J. 33, 409–417 (doi:10.1002/embj.201387098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ebert AD, Svendsen CN. 2010. Human stem cells and drug screening: opportunities and challenges. Nat. Rev. Drug Discov. 9, 367–372 (doi:10.1038/nrd3000) [DOI] [PubMed] [Google Scholar]