Dear Editor,
It has been demonstrated that exposure to poultry in live poultry markets (LPMs) is strongly associated with human infections of avian influenza virus (AIV) subtype A(H7N9)1. In Hangzhou, the capital city of Zhejiang province, China, the first outbreak of human A(H7N9) infections emerged in April 2013 and was halted soon after the closure of LPMs in the main districts of the city2,3. These LPMs were reopened at the end of June 2013. The second outbreak of human A(H7N9) infections reemerged in January-February 2014 (Figure 1). In LPMs, diverse subtypes of AIVs are brought together by various types of poultry from many regions, which might facilitate the emergence and spread of new reassortants4,5. To deepen our understanding of the reemergence and evolution of the A(H7N9) virus, we conducted a survey on the dynamic diversities of AIV subtypes in LPMs before and during the second A(H7N9) outbreak in Hangzhou.
Figure 1.
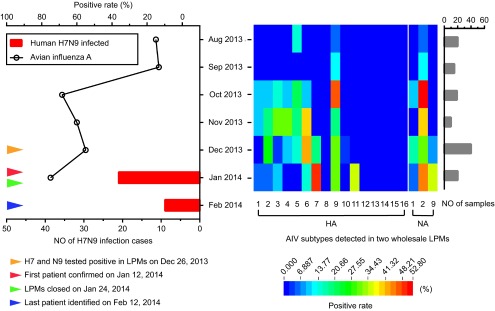
The number of human A(H7N9) infection cases in the second outbreak in Hangzhou and the positive rates of avian influenza viruses (AIVs) in environmental specimens from two live poultry markets (LPMs) before and during the second outbreak are shown by month in the left panel. The dates of H7 and N9 detection in LPMs, reporting the first and last patients with H7N9 infection, and LPM closure are noted separately using colored arrows in the panel. The monthly positive rates of AIV HA and NA subtypes in environment specimens from these two LPMs are presented in the middle panel, with different colors in the grids indicating the degree of positive rates. The numbers of environmental specimens tested by month are shown in the right panel.
A total of 124 environmental specimens, including poultry feces, poultry cage swabs and water used to remove feathers, were collected from two wholesale LPMs in Hangzhou during the period from August 2013 to January 2014. Sixteen hemagglutinin (HA) subtypes and three neuraminidase (NA) subtypes were tested using real-time reverse transcription polymerase chain reaction according to previously published methods6 and the protocols provided by the World Health Organization Collaborating Center for Reference and Research on Influenza at the Chinese National Influenza Center, Beijing, China. Five environmental specimens positive for H7 and N9 by reverse transcription polymerase chain reaction were utilized to determine H7 and N9 sequences using previously described methods7.
Up to ten AIV HA subtypes (H1, H2, H3, H4, H5, H6, H7, H9, H10 and H11) were detected at various rates in the experimental LPM samples. In August and September 2013, only two AIV HA subtypes (H5 and H9) were found; by contrast, at least seven AIV HA subtypes were detected in each month during the period from October 2013 to January 2014 (Figure 1). The H7 and N9 subtypes were absent in these two LPMs until December 2013; however, in 40 specimens collected on December 26, eight samples were positive for H7 and three for N9. Subsequently, the first local patient with A(H7N9) infection of the second outbreak, who developed illness on January 2, was laboratory-confirmed on January 13 (Figure 1). After 14 local patients had been reported, the LPMs in the main districts of Hangzhou were closed on January 24. The occurrence of human A(H7N9) infections lasted until February 11, 19 days after the LPM closure (Figure 1). A total of 30 laboratory-confirmed patients were recorded in the outbreak. Of 20 environmental specimens collected from the LPMs on January 23, ten were positive for H7 and seven for N9. In two specimens of poultry cage swabs positive for H7 and N9, partial sequences of H7 and N9 of A/Environment/Hangzhou/249/2014 and H7 of A/Environment/Hangzhou/231/2014 were obtained. These sequences were deposited in the Global Initiative on Sharing All Influenza Data Database under accession numbers EPI554540-EPI554542. Comparing the sequences of one human A(H7N9) strain in the first wave (A/Hangzhou/1/2013)2 and two human A(H7N9) strains in the second wave (A/Hangzhou/10-1/2014 and A/Hangzhou/17-1/2014)7, the H7 similarities of A/Environment/Hangzhou/249/2014 at the nucleic acid level are 99.53% (1258/1264), 99.92% (1263/1264) and 100.00% (1264/1264), respectively, and the N9 similarities of A/Environment/Hangzhou/249/2014 at the nucleic acid level are 98.92% (1005/1016), 99.51% (1011/1016) and 99.61% (1012/1016), respectively. In addition, the N9 similarities of A/Environment/Hangzhou/231/2014 with A/Hangzhou/1/2013, A/Hangzhou/10-1/2014 and A/Hangzhou/17-1/2014 at the nucleic acid level are 100.00% (385/385), 99.74% (384/385) and 99.74% (384/385), respectively. These high sequence similarities suggest a linkage between the A(H7N9) viruses detected in local LPMs and in human patients during the second wave in Hangzhou. Our results reveal a correlation between the emergence of A(H7N9) viruses in LPMs and the subsequent appearance of local patients with A(H7N9) infections, indicating an important role of AIV surveillance of LPMs in providing an early warning sign of human A(H7N9) infection outbreaks. It could be reasonably inferred that most of these 30 patients could have avoided their infections if the decision to close the LPMs was made immediately after the A(H7N9) viruses were detected in the LPM environment in December 2013 rather than on January 24.
Although the idea of substituting frozen poultry for live poultry in poultry trade at local markets in the main districts of Hangzhou is being promoted, a consensus of permanent LPM closure remains difficult to reach in most regions affected by A(H7N9) viruses in China. It is particularly important to conduct virus surveillance in the LPMs of these regions, in those that remain open this autumn and winter. In these regions, closing LPMs immediately after an early warning sign from AIV surveillance would minimize the occurrence of human A(H7N9) infections.
Interestingly, subtypes of H9 and N2 were continuously detected in the environment of the LPMs from August 2013 to January 2014 (Figure 1), suggesting that A(H9N2) viruses might prevail among poultry more often than other subtypes, even in summer. It is believed that A(H9N2) viruses provide internal gene segments for the novel reassortants of A(H7N9) viruses, and previous studies have shown that A(H9N2) viruses and A(H7N9) viruses coexist in poultry and continuously undergo reassortment5. These results support the proposal that A(H9N2) viruses circulating in poultry play an important role in the emergence and evolution of A(H7N9) viruses; however, the source of the H7 and N9 segments of the viruses in the second outbreak remains unknown. In addition to A(H7N9) and A(H5N1) viruses, AIV subtypes A(H1N1), A(H6N1), A(H10N8) and A(H5N6) have been recently found to be transmissible from poultry to humans in China8,9,10,11. The HA subtypes of H1, H5, H6 and H10 were also detected in the environment of these LPMs in Hangzhou. These HA subtypes can spread among poultry in LPMs and might also reassort with various NA subtypes in poultry. Thus, permanent closure of LPMs could not only effectively block the transmission of AIVs from poultry to humans but also reduce the chance for the emergence of new reassortants with enhanced transmissibility to humans.
Acknowledgments
This work was supported by the Hangzhou Key Medicine Discipline Fund for Public Health Laboratory sponsored by the Hangzhou government.
References
- Liu B, Havers F, Chen E, et al. Risk Factors for Influenza A(H7N9) Disease-China, 2013. Clin Infect Dis. 2014;59:787–794. doi: 10.1093/cid/ciu423. [DOI] [PubMed] [Google Scholar]
- Li J, Yu X, Pu X, et al. Environmental connections of novel avian-origin H7N9 influenza virus infection and virus adaptation to the human. Sci China Life Sci. 2013;56:485–492. doi: 10.1007/s11427-013-4491-3. [DOI] [PubMed] [Google Scholar]
- Ding H, Xie L, Sun Z, et al. Epidemiologic characterization of 30 confirmed cases of human infection with avian influenza A(H7N9) virus in Hangzhou, China. BMC Infect Dis. 2014;14:175. doi: 10.1186/1471-2334-14-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, He S, Walker D, et al. The influenza virus gene pool in a poultry market in South central China. Virology. 2003;305:267–275. doi: 10.1006/viro.2002.1762. [DOI] [PubMed] [Google Scholar]
- Yu X, Jin T, Cui Y, et al. Influenza H7N9 and H9N2 viruses: coexistence in poultry linked to human H7N9 infection and genome characteristics. J Virol. 2014;88:3423–3431. doi: 10.1128/JVI.02059-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde M, Aguero M, Buitrago D, et al. Rapid molecular haemagglutinin subtyping of avian influenza isolates by specific real-time RT-PCR tests. J Virol Methods. 2014;196:71–81. doi: 10.1016/j.jviromet.2013.10.031. [DOI] [PubMed] [Google Scholar]
- Li J, Kou Y, Yu X, et al. Human co-infection with avian and seasonal influenza viruses, China. Emerg Infect Dis. 2014;20:1953–1955. doi: 10.3201/eid2011.140897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qiao C, Tang X, Chen Y, Xin X, Chen H. Human infection from avian-like influenza A (H1N1) viruses in pigs, China. Emerg Infect Dis. 2012;18:1144–1146. doi: 10.3201/eid1807.120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I, Munoz O. Emergence of influenza viruses with zoonotic potential: open issues which need to be addressed. A review. Vet Microbiol. 2013;165:7–12. doi: 10.1016/j.vetmic.2013.01.044. [DOI] [PubMed] [Google Scholar]
- Wei SH, Yang JR, Wu HS, et al. Human infection with avian influenza A H6N1 virus: an epidemiological analysis. Lancet Respir Med. 2013;1:771–778. doi: 10.1016/S2213-2600(13)70221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Yuan H, Gao R, et al. Clinical and epidemiological characteristics of a fatal case of avian influenza A H10N8 virus infection: a descriptive study. Lancet. 2014;383:714–721. doi: 10.1016/S0140-6736(14)60111-2. [DOI] [PubMed] [Google Scholar]


