Abstract
Introduction
Allogeneic hematopoietic stem cell transplantation is a potentially curative treatment modality for hematological malignancies. We evaluated the outcome of patients suffering from hematological malignancies, including acute leukemias, chronic myeloid leukemia and myelodysplastic syndrome after allogeneic transplantation.
Methods
All patients having hematological malignancies with HLA identical sibling donors who underwent allogeneic transplantation were included. Pre-transplant workup consisted of complete blood counts, evaluation of liver, kidneys, lungs, infectious profile, chest X-ray, paranasal sinus roentgenograms and dental review. Donors were given G-CSF at a dose of 5-10 μg/kg/twice daily for five days prior to harvest. The conditioning regimens included cyclophosphamide, busulfan and total body irradiation.
Results
A total of 41 allogeneic transplants were performed for hematological malignancies from April 2004 to December 2012. There were 31 males and 10 females. Median age ± SD was 28 ± 11.7 years (range 8 – 54 years). A mean of 7.7×108±1.5 mononuclear cells/kg were infused (range:6.2-9.2×108/kg). The median time to white cell recovery was 19±4 days (range:15-23 days). Transplant related mortality was 19.5%. The median overall survival was 53.6 months. Overall survival at a median follow up of 37 months was 67%.
Conclusion
Allogeneic stem cell transplantation is an effective treatment option in patients with hematological malignancies. Our outcomes are comparable with results from neighboring countries as well as the western world.
Keywords: Allogeneic transplantation, Hematopoietic stem cell transplantation, Hematological malignancies, Treatment Outcome
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective treatment modality constituting the management of a variety of benign and malignant hematological disorders, especially hematological malignancies like leukemias, lymphomas and myelodysplastic syndromes.1, 2 However, hematopoietic stem cell transplantation (HSCT) is an extremely high risk procedure and its benefits are often offset by the severe post-transplant complications that follow. Some of these complications include, but are not limited to, profound compromise of the immune system, sepsis and a variety of potentially fatal infections, failure of multiple organ systems, bleeding and graft-versus-host disease (GvHD). These complications can take place acutely in the post-transplant period and/or chronically after discharge from the hospital.2-4
Some studies previously conducted in Pakistan reported transplant related mortality (TRM) of 18% to 22.7% in patients with hematological malignancies undergoing allo-HSCT, whereas the overall long-term survival of these patients ranges from 40% to 77%.5-7 A review of the first decade of HSCT procedures performed in Pakistan stated an overall TRM of 18% in patients undergoing allo-HSCT for hematological malignancies, whereas the overall long-term survival in these patients was up to 54%.8
These findings are comparable with outcomes in neighboring countries from our region. For example, a study in China reported TRM of 15.66% with an overall survival (OS) of 73.49%.9 The overall mortality in patients undergoing allo-HSCT for hematological malignancies in Iran was 28.5%, with an OS of 71%.10 Data from India also exhibits similar results, with mortality of approximately 52% and OS of 48.2%.11
More or less similar outcomes have been reported from other countries, particularly the Western world. A multicenter study done by the EBMT showed a TRM of 14.7% with OS of 58%.12
We present the outcome of patients undergoing allo-HSCT for hematological malignancies at our center.
MATERIALS AND METHODS
Patients with malignant hematological disorders having an HLA matched sibling donor were evaluated. Hematological malignancies included were acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), acute biphenotypic leukemia (ABPL), chronic myeloid leukemia (CML) and myelodysplastic syndrome (MDS). These patients were stratified into standard and high risk groups, based on age, sex, disease stage, time interval between diagnosis and transplant, response to therapy and donor’s gender according to the European Group for Blood and Bone Marrow Transplantation risk stratification.13 All patients with AML, ALL and ABPL were in either first or second complete remission. All patients with CML were in chronic phase of the disease. Out of the patients with MDS, one patient had hypoplastic MDS, 2 patients had MDS with refractory cytopenia with multi-lineage dysplasia (RCMD) and 4 had MDS with refractory anemia with excess blasts-1 (RAEB-1).
Pre-transplant workup and characteristics
Pre-transplant work up for all patients and donors consisted of standard blood work, including complete blood counts (CBC), liver function tests, blood grouping and coagulation profile. Serological tests for hepatitis B surface antigen, hepatitis C antibody, human immunodeficiency virus antibody, Cytomegalovirus IgG and IgM antibodies, mantoux test and chest X-ray were also performed for patients and donors. Additionally, echocardiography, pulmonary function tests and dental evaluation were performed in patients as part of pre-transplant work up.
All patient-donor pairs were positive for Cytomegalovirus IgG. All patients received complete HLA-matched allografts from siblings except three AML patients, who each had one mismatched HLA allele in the allograft from sibling donors.
Transplant environment
All patients undergoing allo-HSCT were kept in protective isolation equipped with High-efficiency particulate air (HEPA) filters, positive pressure and laminar air flow ventilation. Food for the patients was cooked and packaged using terminal pressure and neutropenic precautions. Double lumen Hickman or peripherally inserted catheters were used and dressed once every week by trained nursing staff till the day of discharge. Patients from outside the city were advised to stay near the hospital for the first 100 days after discharge for ease of access to health care and surveillance.
Antimicrobial prophylaxis
Standard prophylaxis with ciprofloxacin (500mg twice daily or 20-30mg/kg/two divided doses), fluconazole (200mg once daily or 6mg/kg/day) and valacyclovir (500mg twice daily or 10mg/kg twice daily) were started for antibacterial, antifungal and antiviral prophylaxis, respectively, in all patients on day -5.
Stem cell source
In all patients filgrastim (recombinant G-CSF) mobilized peripheral blood progenitor cells were used as the only source of stem cells. All donors received filgrastim at a dose of 5μg/kg twice daily for five days prior to stem cell harvest.
Conditioning regimens
Patients with, Philadelphia negative ALL, ABPL, CML and MDS received busulfan (4mg/kg/day for 4 days from day -7 to day -4; total dose: 16mg/kg) and cyclophosphamide (60mg/kg/day for 2 days from day -3 to day -2; total dose: 120mg/kg). Patients with Philadelphia positive ALL, AML and one antigen mismatch donors received cyclophosphamide (60mg/kg/day for 2 days from day -6 to day -5; total dose: 120mg/kg) and total body irradiation (1.5cGY twice daily for 4 days from day -3 to day 0).
Graft versus host disease prophylaxis
Patients were started on intravenous cyclosporine on day – 1. Drug levels were maintained at 200-250ng/dl and 150-200ng/dl for adult and pediatric patients, respectively. Doses were adjusted accordingly. Methotrexate administered at a dose of 15mg/m2 on day +1 was followed by a dose of 10mg/m2 on days +3 and +6. Irradiated and leukocyte reduced blood products were used for supportive treatment during hospital admission and post-transplant period. Grading was done according to the Glucksberg classification.
Statistical analysis
All the data was entered SPSS version 19 (SPSS Inc., Chicago, IL, USA) for computing means, standard deviations and range of all descriptive variables.
RESULTS
A total of n=114 allogeneic transplants were performed at the Aga Khan University Hospital from April 2004 to December 2012. Out of these, n=41 were done for hematological malignancies and were included in this study. There were 31 males and 10 females. The remaining n=73 transplants were for other indications and were not included in the study.
The indications for allo-HSCT were AML (n=14), ALL (n=8), ABPL (n=1), CML (n=11) and MDS (n=7). Thirty eight patients were of adult age group (>15 years of age) while the remaining 3 were of pediatric age group (<15 years of age). Median age ± SD was 28 ± 11.7 years (range 8 – 54 years).
A mean of 7.7 × 108 ± 1.5 mononuclear cells/kg were infused (range: 4.2 – 10×108/kg). The median time of WBC recovery was 19 ± 4 days (range: 15 – 23 days).
Eight patients expired by day 100, making the transplant related mortality (TRM) 19.5%. Of the remaining n=33 patients who survived beyond day 100, n=8 had relapse of primary disease, out of which n=7 patients died of relapse. Incidence of GvHD was 34.1%. It was not the cause of death in any of the patients.
The OS at a median follow up of 37 months was 67%. The median duration of OS was 53.6 months (Range: 39.2 – 68.0 months). Details of outcomes according to diagnosis are displayed in Table 1. Mean survival at day 100 according to disease is given in Table 2. The survival curves of the entire patient population and each individual diagnosis are given in Figures 1 and 2, respectively.
Table 1.
Outcome of allogeneic hematopoietic stem cell transplants
| Diagnosis | No. of transplants | GvHD | Causes of TRM | RR/ RRM |
|---|---|---|---|---|
| All patients | 41 | 14 | 8 | 8/7 |
| Skin: 6, Gut: 6, Liver: 2 | Sepsis: 3, CRT:1, Graft rejection: 1, CMV: 1, CMV+RF: 1, IC bleed+RF: 1 | |||
| AML | 14 | 5 | 4 | 4/4 |
| Skin: 2, Gut: 2, Liver: 1 | Sepsis: 1, Graft rejection: 1, CMV+RF: 1, IC bleed+RF: 1 | |||
| ALL | 8 | 4 | 2/2 | |
| Skin: 1, Gut: 3 | ||||
| ABPL | 1 | 1 | 0 | |
| Skin: 1 | ||||
| CML | 11 | 2 | 3 | 1/1 |
| Skin: 1, Liver: 1 | Sepsis:1, CMV:1, CRT: 1 | |||
| MDS | 7 | 2 | 1 | 1/0 |
| Skin: 1, Gut: 1 | Sepsis: 1 |
AML: acute myeloid leukemia, ALL: acute lymphoblastic leukemia, ABPL: acute biphenotypic leukemia, CML: chronic myeloid leukemia, MDS: myelodysplastic syndrome, GvHD: graft-versus-host disease, TRM: transplant related mortality, RR: relapse rate, RRM: relapse related mortality, CRT: conditioning related toxicity, CMV: cytomegalovirus infection, RF: renal failure, IC bleed: intracranial bleed
Table 2.
Mean overall survival at day 100
| Diagnosis | Estimate ± Std. Error | 95% Confidence Interval | |
|---|---|---|---|
| Lower Bound | Upper Bound | ||
| All patients | 27.7 ± 4.6 | 18.7 | 36.9 |
| AML | 14.5 ± 4.6 | 5.6 | 23.4 |
| ALL | 26.2 ± 6.6 | 13.3 | 39.1 |
| CML | 33.2 ± 13.2 | 7.3 | 59.1 |
| MDS | 42.6 ± 10.2 | 22.5 | 62.7 |
AML: acute myeloid leukemia, ALL: acute lymphoblastic leukemia, CML: chronic myeloid leukemia, MDS: myelodysplastic syndrome
Figure 1.
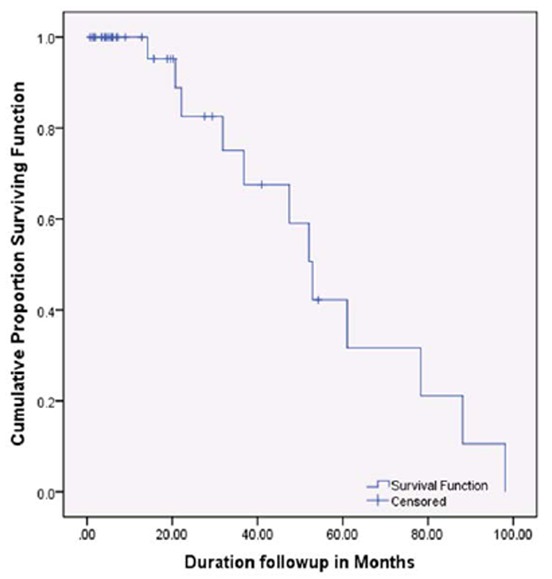
Median overall survival of all patients transplanted
Figure 2.
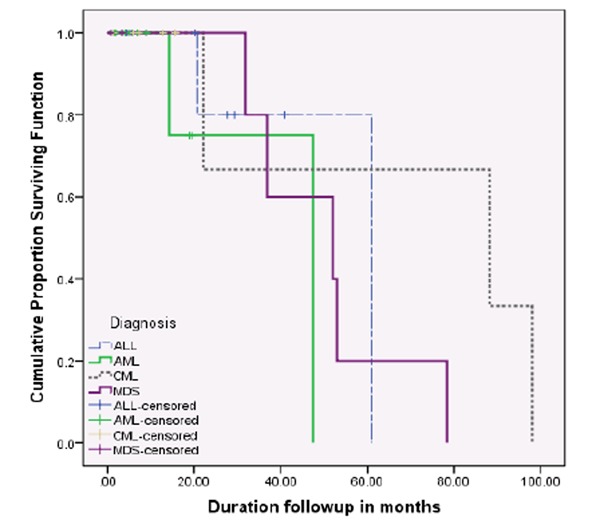
Median overall survival according to diagnosis
DISCUSSION
Combination chemotherapy with antineoplastic agents is the mainstay of treatment for hematological malignancies, inducing remission in a majority of patients.14 However, relapse of underlying malignancy is a common occurrence, resulting in failure of treatment after chemotherapy. Neutropenia complicated by sepsis and other infections, bleeding, anemia and tumor lysis related metabolic derangements are other causes accounting for morbidity and mortality in such patients.6, 15 Allo-HSCT has proven to be a superior treatment modality in terms of OS and disease-free survival in randomized control clinical trials16, 17 when compared with combination chemotherapy alone.
Allo-HSCT was commenced at our center in April 2004. The stem cell transplant unit comprises of highly qualified medical specialists and specially trained nursing staff, HEPA air filters with positive pressure ventilation, infection control protocols and extensive laboratory and blood bank services. Treatment is offered for both benign as well as malignant hematological conditions.18
Several complications ensue after allo-HSCT, leading to substantial morbidity and mortality in these patients. Following bone marrow ablation to engraftment and hematopoietic recovery, profound compromise of the immune system leaves patients vulnerable to fatal infections. After hematopoietic recovery, donor T-lymphocytes in the graft recognize the host tissues as foreign and initiate an immune mediated inflammatory response, giving rise to GvHD, which itself is a feared and potentially fatal complication following allo-HSCT if not treated properly. Despite its dangerous and potentially lethal clinical course, milder forms of GvHD are often welcomed. A certain aspect of GvHD, known as graft-versus-leukemia effect, attacks and destroys any remaining cancer cells in the body and accounts for the highly curative potential of allo-HSCT.3 Nevertheless, GvHD remains a noteworthy cause of morbidity and mortality following transplantation. The opposite is also an important, albeit infrequent, cause in treatment failure in which the host body rejects the stem cell graft, leading to graft failure.2-4 Graft failure was the cause of TRM in one of our patients, and has been reported as a cause of mortality at a few other centers.9, 11, 19
In our study, sepsis and CMV infection were the major causes of TRM within the first 100 days. Infectious complications have already been previously reported to be the major causes of death in patients undergoing allo-HSCT in Pakistan,5-7 as well as other countries in the region, like China9 and Iran.10 Some western countries have also reported infections and sepsis as the chief reasons for mortality,12, 20, 21 but in some instances, GvHD has proven to contribute equally or more to TRM than infections or other post-transplant complications.5-7, 11, 19, 22 Although graft failure was the cause of TRM in one of our patients, GvHD was not the cause of TRM in any patients in our study.
In our series, the overall TRM was 19.5%. Studies conducted at other centers offering stem cell transplantation in Pakistan have shown TRM of 22.7%,5 32.6%7 and 14.3%.6 After reviewing the outcomes of the first 10 years of allo-HSCT done for hematological malignancies in Pakistan, Shamsi et al8 found the overall TRM to be 18%. Chen et al 9 from China reported TRM of 15.66%, while Nagafuji et al23 stated TRM of 8.5% in Japanese patients. A multicenter study conducted by EBMT12, including patients from several countries like Italy, Turkey, Finland, etc., described TRM of 14.7%, while Ringdén et al,24 in another multicenter study, in patients with AML and ALL, from several countries found TRM to be as high as 29%. These figures from national and international centers, as well as several others, are comparable to the TRM at our center. For example, TRM of 24.2% was observed in large cohort of patients published by the IBMTR and EBMT.25
Relapse/progression of primary malignancy after allo-HSCT is not an infrequent occurrence and is a noteworthy cause of treatment failure, morbidity and mortality. Previous studies from Pakistan have reported relapse rates of 4.5%5, 14%7 and 14.3%.6 In these studies, relapse was mostly observed in patients with acute leukemias, and these patients also had a higher rate of relapse related mortality in comparison to CML and MDS. Same was the case in our study, where n=8 patients (19.5%) had relapse of primary disease, out of which n=7 died of the relapse. Six of these patients had acute leukemias. Similar rates of relapse have been reported from India,11 Iran10 and China9 i.e. 16.7%, 11.4% and 21.76%, respectively. These figures are comparable to rates described from other countries like Japan23 and Brazil22, i.e. 24.4% and 16.7%, respectively. However, lower relapse rates have been observed in several multicenter studies from western countries, ranging from 8.2% to 14.1%.12, 20, 25 Ringdén et al24 have described a relapse rate as high as 40% in one multicenter study, although this study comprised of patients with AML and ALL only, accounting for the high incidence of relapse due to the aggressive nature and clinical course of these malignancies. In most of the aforementioned studies, most patients died of relapse of primary disease, majority of which were patients with AML or ALL.9, 10, 12, 20, 22, 24
International CIBMTR data26 has shown CML to be associated with the best prognosis following allogeneic stem cell transplantation, with an OS of up to 62.9%. Data from Pakistan is very encouraging, showing similar trends in OS and low relapse rate among CML patients after transplantation. Hashmi et al5 and Ullah et al7 showed an OS of 77.2% and 69.7% in CML, respectively. There was only 1 case of relapse of CML after transplant in each series, making relapse incidence of 4.5% and 3%, respectively. In our series the median duration of OS was 88 months in patients with CML. Only 1 out of 11 patients (9%) relapsed and eventually succumbed to the disease. Similarly, low relapse rates and increased OS have been observed in India, where OS was 50% with a relapse rate of 6.7% i.e. n=2 patients.11 Iranian patients with CML showed an OS of 75% with no cases of relapse,10 whereas Chinese patients had an OS of 83.87% at 5 years with only n=1 of these dying of the relapse.9 Similar results have been reported from the western world. Oehler et al27 from United States of America (USA) reported an OS of 81% with a relapse rate of 3.1%(n=1). An EBMT trial12 showed OS of 80% and 71% at 1 year and 3 years post-transplant, respectively. Couban et al20 reported an OS of 79% at the end of 3 years in patients from Canada and New Zealand. A study from Germany has reported an OS of 94.6% with relapse incidence of 3%(n=1).28 Imamura et al29 reported an OS of 53.3% from Japan. Relapse rate in CML patients in another paper published by the IBMTR and EBMT was 4.2%.25
International CIBMTR data26 has shown lower survival rates in patients with AML (42.7%) and ALL (41.4%) after allo-HSCT. Data from Pakistan shows similar trends in survival among patients with either AML or ALL post-transplant. Ullah K et al7 showed an OS of 37.5% in patients with acute leukemias. In this series survival was 100% in n=3 AML patients. However, relapse rate and relapse related mortality was observed in 100% of the n=5 patients with ALL. In our series the median duration of OS for patients with AML and ALL was 47 and 61 months, respectively. Four out of n=14 patients with AML and two out of n=8 patients with ALL had relapse of underlying malignancy (relapse rates 28.6% and 25%, respectively). All n=6 of these patients eventually died of relapse. In India, patients with acute leukemias had an OS of 51.1%, however, in this study; ALL was associated with comparatively better outcomes than AML, with a higher OS (70% vs 45.7%). The relapse rate, however, was lower among AML patients in comparison to patients with ALL (22.9% vs 40%).11 In Iran, patients with acute leukemias showed an OS of 71.2% (AML 79.4% and ALL 55.6%). Lower relapse rates were observed in AML as compared to ALL (20.6% vs 33.3%).10 Mortality was 100% among relapsed patients. Imamura et al from Japan29 also reported better outcomes in patients with AML when compared with ALL (OS of 44.2% and 33.7%). Similar results have been demonstrated in the western world. An EMBT trial12 showed OS of 65.7% and 54.1% with relapse rates of 35.3% and 47% at 1 year and 3 years post-transplant, respectively. In this trial, AML was associated with better outcomes in terms of OS and relapse rate in comparison to ALL. A multinational study supported by EBMT24 reported an OS of 50% in patients with acute leukemia with a relapse rate of 40%. Patients with AML had better outcomes when compared to those with ALL in terms of OS (53% vs 47%) and relapse rate (34.9% vs 53.6%). Relapse rate in another paper published by IBMTR and EBMT was 12.5% in patients with acute leukemias.25
International CIBMTR data26 has shown the lowest survival rates in MDS when compared with other myeloid malignancies, with the OS after allogeneic stem cell transplant being 40%. Data from Pakistan shows similar trends in survival among patients with either AML or ALL after transplantation. Out of the two patients with MDS transplanted in the study published by Ullah K et al,7 one patient died of veno-occlusive disease of the liver, whereas the other patient survived without relapse of primary disease. In our series the median duration of OS was 52 months in patients with MDS. Only one patient died during the pre-engraftment phase due to E. coli sepsis. In India, two out of the eight patients with MDS that were transplanted survived with an OS of 25%. However, the follow up duration for these patients was very short.11 Iravani et al from Iran10 showed results similar to Ullah K et al from Pakistan. One out of the two patients with MDS expired due to GvHD. The other patient was surviving without relapse of primary disease. Imamura et al29 reported an OS of 37.3% in MDS patients from Japan. Similar results have been demonstrated in the western world. An EMBT trial12 showed OS of 50% and 38% at 1 year and 3 years post-transplant, respectively. Couban et al20 reported a comparatively higher OS of 61% at the end of 3 years in MDS patients from Canada and New Zealand.
In our study, the OS at a median follow up of 37 months was 67%, which is comparable to survival trends reported previously from other centers in Pakistan. For example, Ullah K et al7 reported an OS of 65.1% at the end of 5 years of follow up. Similarly, Hashmi et al5 and Shamsi et al6 described OS of 77.2% at a median follow up of 212 days and 66.7% at a median follow up of 678 days, respectively. A review of the data from the first decade from centers across Pakistan showed an OS of 54%.8 These findings are comparable to those in neighboring countries. While OS in our patients was relatively higher than in India (OS of 48.2%),11 higher survival rates have been reported in Iranian patients (OS of 71% at a median follow up of 17months)10 and Chinese patients (OS of 73.49% at the end of 5 years of follow up).9 The Japanese cohorts have shown an OS of 50.2% at the end of 3 years.23 Patients from Brazil showed an OS of 47% at 1000 days follow up.22 Finally, CIBMTR26 has shown a total of OS of 46.5% in patients undergoing allo-HSCT for hematological malignancies, a survival rate lower than what has been reported in this study.
CONCLUSION
Allo-HSCT is a very effective treatment option for patients with hematological malignancies who have HLA matched donors. The median time to engraftment was 19 days. The TRM was 19.5%, with infectious complications being the leading cause of death in our patients. Although the incidence of GvHD is similar to figures reported in other studies, none of our patients died of GvHD. OS at a median follow up of 37 months was 67%. These figures contribute to our national data and are comparable to previous studies reporting similar outcomes in Pakistan and international literature.
Footnotes
CONFLICT OF INTEREST
The authors declare they have no conflicts of interest.
References
- 1.Ali N, Adil SN, Shaikh MU, Masood N. Frequency and Outcome of Graft versus Host Disease after Stem Cell Transplantation: A Six-Year Experience from a Tertiary Care Center in Pakistan. ISRN hematology. 2013 doi: 10.1155/2013/232519. http://www.ncbi.nlm.nih.gov/pubmed/23936661. [DOI] [PMC free article] [PubMed]
- 2.Huynh TN, Weigt SS, Belperio JA, Territo M, Keane MP. Outcome and prognostic indicators of patients with hematopoietic stem cell transplants admitted to the intensive care unit. Journal of transplantation. 2009;2009 doi: 10.1155/2009/917294. http://www.ncbi.nlm.nih.gov/pubmed/20130763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Copelan EA. Hematopoietic stem-cell transplantation. New England Journal of Medicine. 2006;354(17):1813–26. doi: 10.1056/NEJMra052638. http://www.ncbi.nlm.nih.gov/pubmed/16641398. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan KM, Storb R. Allogeneic marrow transplantation. Cancer investigation. 1984;2(1):27–38. doi: 10.3109/07357908409020284. http://www.ncbi.nlm.nih.gov/pubmed/6367899. [DOI] [PubMed] [Google Scholar]
- 5.Hashmi K, Khan B, Ahmed P, Hussain I, Raza S, Iqbal H, et al. Allogeneic Stem Cell Transplantation in Chronic Myeloid Leukaemia- A 2 ½ Year Experience. Journal of the Pakistan Medical Association. 2005;55(11):478–82. http://www.ncbi.nlm.nih.gov/pubmed/16304866. [PubMed] [Google Scholar]
- 6.Shamsi TS, Irfan M, Ansari SH, Farzana T, Khalid MZ, Panjwani VK, et al. Allogeneic peripheral blood stem cell transplantation in patients with haematological malignancies. Journal of the College of Physicians and Surgeons--Pakistan: JCPSP. 2004;14(9):522–6. http://www.ncbi.nlm.nih.gov/pubmed/15353134. [PubMed] [Google Scholar]
- 7.Ullah K, Ahmed P, Raza S, Satti T, Nisa Q, Mirza S, et al. Allogeneic stem cell transplantation in hematological disorders: single center experience from Pakistan. Transplantation proceedings. 2007 doi: 10.1016/j.transproceed.2007.08.099. http://www.ncbi.nlm.nih.gov/pubmed/18089384. [DOI] [PubMed]
- 8.Shamsi TS, Hashmi K, Adil S, Ahmad P, Irfan M, Raza S, et al. The stem cell transplant program in Pakistan - the first decade. Bone marrow transplantation. 2008;42:S114–S7. doi: 10.1038/bmt.2008.137. http://www.ncbi.nlm.nih.gov/pubmed/18724282. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Xu Y, Zhu Y, Fu G, Liu Y, Peng J, et al. Clinical analysis of 104 patients with hematological malignancy after allogeneic hemotopoietic stem cell transplantation. Zhong nan da xue xue bao Yi xue ban= Journal of Central South University Medical sciences. 36(9):859–64. doi: 10.3969/j.issn.1672-7347.2011.09.008. http://www.ncbi.nlm.nih.gov/pubmed/21946205. [DOI] [PubMed] [Google Scholar]
- 10.Iravani M, Evazi MR, Mousavi SA, Shamshiri AR, Tavakoli M, Ashouri A, et al. Fludarabine and busulfan as a myeloablative conditioning regimen for allogeneic stem cell transplantation in high-and standard-risk leukemic patients. Bone marrow transplantation. 2007;40(2):105–10. doi: 10.1038/sj.bmt.1705685. http://www.ncbi.nlm.nih.gov/pubmed/17468775. [DOI] [PubMed] [Google Scholar]
- 11.Chandy M, Srivastava A, Dennison D, Mathews V, George B. Allogeneic bone marrow transplantation in the developing world: experience from a center in India. Bone marrow transplantation. 2001;27(8):785–90. doi: 10.1038/sj.bmt.1702869. http://www.ncbi.nlm.nih.gov/pubmed/11477434. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz N, Beksac M, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, et al. Filgrastim-mobilized peripheral blood progenitor cells versus bone marrow transplantation for treating leukemia: 3-year results from the EBMT randomized trial. haematologica. 2005;90(5):643–8. http://www.haematologica-thj.org/content/90/5/643.full.pdf. [PubMed] [Google Scholar]
- 13.Gratwohl A. The EBMT risk score. Bone marrow transplantation. 47(6):749–56. doi: 10.1038/bmt.2011.110. http://www.ncbi.nlm.nih.gov/pubmed/21643021. [DOI] [PubMed] [Google Scholar]
- 14.Bishop JF. The treatment of adult acute myeloid leukemia. Semin Oncol. 1997 Feb;24(1):57–69. http://www.ncbi.nlm.nih.gov/pubmed/9045305. [PubMed] [Google Scholar]
- 15.Bensinger WI, Weaver CH, Appelbaum FR, Rowley S, Demirer T, Sanders J, et al. Transplantation of allogeneic peripheral blood stem cells mobilized by recombinant human granulocyte colony-stimulating factor. Blood. 1995;85(6):1655–8. http://www.ncbi.nlm.nih.gov/pubmed/7534140. [PubMed] [Google Scholar]
- 16.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. New England Journal of Medicine. 2001;344(3):175–81. doi: 10.1056/NEJM200101183440303. http://www.ncbi.nlm.nih.gov/pubmed/11172139. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz N, Bacigalupo A, Hasenclever D, Nagler A, Gluckman E, Clark P, et al. Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European Group for Blood and Marrow Transplantation. Bone marrow transplantation. 1998;21(10) doi: 10.1038/sj.bmt.1701234. http://www.ncbi.nlm.nih.gov/pubmed/9632272. [DOI] [PubMed] [Google Scholar]
- 18.Bone Marrow Transplant Clinic - The Aga Khan University Hospital. Available at: http://hospitals.aku.edu/karachi/hospitaldepartments/medicine/Pages/specialservicesbonemarrow.aspx.
- 19.Wu HX, Qian SX, Hong M, Zhang YP, Lu H, Zhang R, et al. Allogeneic peripheral blood stem cell transplantation for 75 cases of hematologic malignancies. Zhongguo shi yan xue ye xue za zhi/Zhongguo bing li sheng li xue hui= Journal of experimental hematology/Chinese Association of Pathophysiology. 2008;16(6):1330–3. http://www.ncbi.nlm.nih.gov/pubmed/19099638. [PubMed] [Google Scholar]
- 20.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100(5):1525–31. doi: 10.1182/blood-2002-01-0048. http://www.ncbi.nlm.nih.gov/pubmed/12176866. [DOI] [PubMed] [Google Scholar]
- 21.Gratwohl A, Brand R, Frassoni F, Rocha V, Niederwieser D, Reusser P, et al. Cause of death after allogeneic haematopoietic stem cell transplantation (HSCT) in early leukaemias: an EBMT analysis of lethal infectious complications and changes over calendar time. Bone marrow transplantation. 2005;36(9):757–69. doi: 10.1038/sj.bmt.1705140. http://www.ncbi.nlm.nih.gov/pubmed/16151426. [DOI] [PubMed] [Google Scholar]
- 22.Vigorito AC, Azevedo WM, Marques JFC, Azevedo AM, Eid KAB, Aranha FJP, et al. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone marrow transplantation. 1998;22(12) doi: 10.1038/sj.bmt.1701510. http://www.ncbi.nlm.nih.gov/pubmed/9894716. [DOI] [PubMed] [Google Scholar]
- 23.Nagafuji K, Matsuo K, Teshima T, Mori S-i, Sakamaki H, Hidaka M, et al. Peripheral blood stem cell versus bone marrow transplantation from HLA-identical sibling donors in patients with leukemia: a propensity score-based comparison from the Japan Society for Hematopoietic Stem Cell Transplantation registry. International journal of hematology. 91(5):855–64. doi: 10.1007/s12185-010-0581-1. http://www.ncbi.nlm.nih.gov/pubmed/20464644. [DOI] [PubMed] [Google Scholar]
- 24.Ringden O, Labopin M, Bacigalupo A, Arcese W, Schaefer UW, Willemze R et al. Transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical siblings in adult patients with acute myeloid leukemia and acute lymphoblastic leukemia. J Clin Oncol. 2002 Dec 15;20(24):4655–64. doi: 10.1200/JCO.2002.12.049. http://www.ncbi.nlm.nih.gov/pubmed/12488410. [DOI] [PubMed] [Google Scholar]
- 25.Champlin RE, Schmitz N, Horowitz MM, Chapuis B, Chopra R, Cornelissen JJ, et al. Blood stem cells compared with bone marrow as a source of hematopoietic cells for allogeneic transplantation. Blood. 2000;95(12):3702–9. http://www.ncbi.nlm.nih.gov/pubmed/10845900. [PubMed] [Google Scholar]
- 26.Disease-specific HCT indications and outcome - CIBMTR. Available at: https://bethematchclinical.org/Transplant-Indications-and-Outcomes/Disease-Specific-Indications-and-Outcomes/
- 27.Oehler VG, Radich JP, Storer B, Blume KG, Chauncey T, Clift R, et al. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biology of Blood and Marrow Transplantation. 2005;11(2):85–92. doi: 10.1016/j.bbmt.2004.09.010. http://www.ncbi.nlm.nih.gov/pubmed/15682068. [DOI] [PubMed] [Google Scholar]
- 28.Elmaagacli AH, Basoglu S, Peceny R, Trenschel R, Ottinger H, Lollert A, et al. Improved disease-free survival after transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical unrelated donors in patients with first chronic phase chronic myeloid leukemia. Blood. 2002;99(4):1130–5. http://www.ncbi.nlm.nih.gov/pubmed/11830457. [PubMed] [Google Scholar]
- 29.Imamura M, Asano S, Harada M, Ikeda Y, Kato K, Kato S, et al. Current status of hematopoietic cell transplantation for adult patients with hematologic diseases and solid tumors in Japan. Int J Hematol. 2006 Feb;83(2):164–78. doi: 10.1532/IJH97.05134. http://www.ncbi.nlm.nih.gov/pubmed/16513537. [DOI] [PubMed] [Google Scholar]


