Abstract
A 45-year-old female patient with a diagnosis of ulcerative colitis complicated with composite lymphoma in the spleen and para-aortic lymph node presented with a one-month history of malaise, weakness and fatigue. Only mesalamine kept ulcerative colitis under control. In physical examination, splenomegaly was revealed and pancytopenia was obtained from laboratory data. Computed tomography scan revealed para-aortic mediastinal lymphadenopathy with splenomegaly. Splenectomy and excisional biopsy of abdominal lymph node were performed and disease was diagnosed as composite lymphoma, consisting of diffuse large B-cell lymphoma and nodular sclerosing Hodgkin lymphoma.
Keywords: Composite lymphoma, Nodular sclerosing Hodgkin lymphoma, Diffuse large B-Cell lymphoma, Inflammatory bowel disease, Ulcerative colitis
INTRODUCTION
Physicians have shown great concern on the relationship between lymphoma and inflammatory bowel disease (IBD) over the years. In 1928, Bargen described initial report of lymphosarcoma in a patient with ulcerative colitis.1 Since then, numerous case reports and meta-analyses have been presented, but composite lymphoma in IBD patients had not previously been reported. This is the first case report of ulcerative colitis complicated with composite lymphoma.
Composite lymphoma is a rare malignancy2 and defined as the presence of two or more distinct cytological and histological variant of lymphoma in the same tissue.3 This combination consists of Hodgkin lymphoma (HL) with non-Hodgkin lymphoma (NHL) or B-cell NHL with T -cell NHL, or two distinct subtype of B-cell or T-cell NHL.3-7 In this study, we present a case of a composite diffuse large B-cell lymphoma and nodular sclerosing HL in para-aortic lymph node and spleen in a 45-year- old female patient diagnosed with ulcerative colitis.
Case presentation
The patient was a 45-year-old female with a 2-year history of left-sided ulcerative colitis. Only mesalamine was responsible for keeping the disease under control and no other immunosuppressive agents such as azathioprine and anti TNF were administered. The patient was referred to hematologic ward with a one-month history of malaise, weakness and fatigue. Upon physical examination, massive splenomegaly without hepatomegaly or lymphadenopathy was revealed. The patient also appeared pale in physical examination. Hematologic findings consisted of pancytopenia, elevated erythrocyte sedimentation Rate (ESR) and lactate dehydrogenase (LDH). Results of renal and liver function tests, electrolytes, urine analysis and HIV test were within the normal range. Mesalamine was stopped and low-dose prednisolone was administered. Contrast-enhanced computed tomography scan of the chest and abdominopelvic regions showed lymphadenopathy of middle and posterior mediastinum, para-aortic lymphadenopathy and splenomegaly with heterogeneous density (Fig.1).
Figure 1.
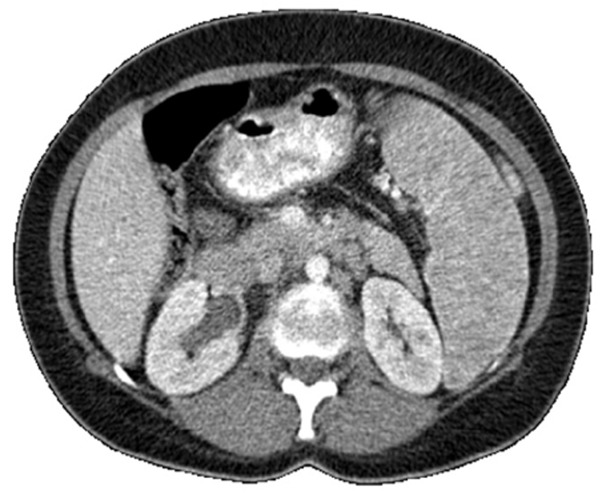
Splenomegaly with heterogeneous density in abdominal CT scan
To achieve a definite diagnosis, splenectomy, excisional biopsy of para-aortic lymph node, aspiration and biopsy of marrow were performed. Histomorphological evaluation of the spleen and para aortic lymph node (Fig: 2) show focal involvement by classical Hodgkin’s Lymphoma and nodular sclerosis variant, wherein typical nodules are seen in which the lacunar cells and classic Red-Sternberg (R-S) cells are surrounded by small mature lymphocytes with a few eosinophils and plasma cells. However, in other areas, diffuse sheets of centroblastic and (less) immunoblastic cells are also evident, which is in keeping with diffuse large B-cell lymphoma (DLBCL).
Figure 2.
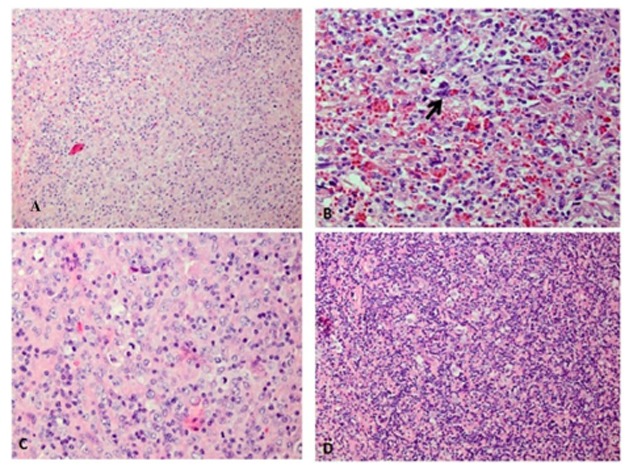
A/ Spleen with focal involvement by DLBCL (H&E X200), B/ Spleen with focal involvement by HL (H&E X400), C/ Hilar Lymph node involved by DLBCL (H&E X400). D/ Hilar lymph node involved by HL (H&E X200)
On immunohistochemical (IHC) staining (Fig. 3), the lacunar cells and R-S cells in the HL component show negative Leukocyte common antigen (LCA), CD20 and positive Ki-A10 (one of the HL associated markers) although CD30 and CD15 are negative. Accordingly, there is strong positivity of LCA and CD20 in the DLBCL component.
Figure 3.
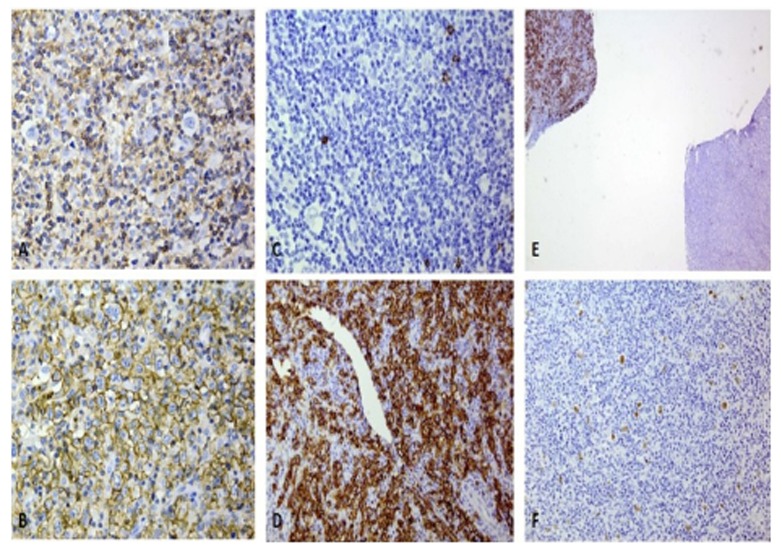
Immunohistochemical staining of the case showing: A/ LCA in HL component, the lacunar cells are negative (X400), B/ LCA in the DLBCL components, the sheets of centroblasts are strongly positive,(X400), C/ CD20 in HL component, the lacunar cells are negative (X400), D/ CD20 in DLBCL component, the sheets of centroblasts are strongly positive (X400), E/ CD20 staining in both components, the DLBCL (upper left) is diffusely positive, whereas HL component (lower right) shows some scattered dots (X10), F/ Ki-A10 in HL component, highlighting the lacunar and R-S cells (X200)
Histopathological and IHC study of the bone marrow trephine biopsy (Fig. 4) show interstitial infiltration of the CD20 positive centroblasts.
Figure 4.
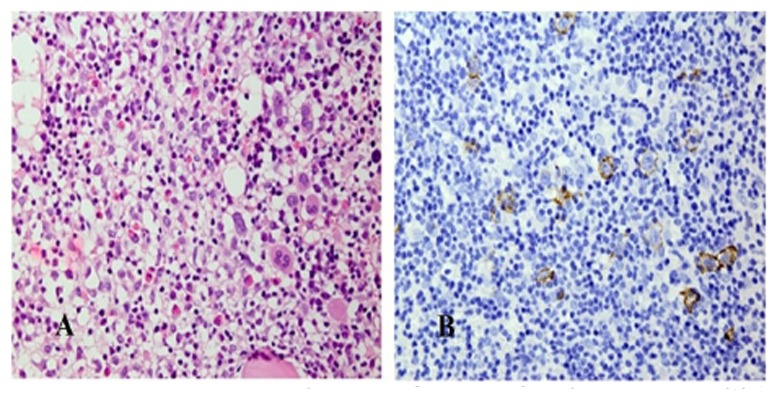
Bone marrow biopsy of the case showing: A/Interstitial infiltration of the large centroblastic cells (H&E X400), B/ The mentioned large tumor cells are positive for CD20 (X400)
Treatment of composite lymphoma depends on histological grading of components and is towards the high grade histology. The patient was treated with R-CHOP- based chemotherapy (Rituximab, Cyclophosphamide, Adriamycin, Vincristine and Prednisolone). After eight courses of chemotherapy with R-CHOP and one course of high-dose methotrexate, complete remission was achieved. No evidence of disease was seen within six-months of follow-up.
DISCUSSION
Inflammatory bowel disease is a chronic disorder of gastrointestinal tract including two main diseases: ulcerative colitis and crohn’s disease. The etiology of these diseases is unknown, but evidence suggests that genetically susceptible patients’ immune dysfunction triggered by environmental factors is responsible for disease.8 The risk of lymphoma in IBD patients has been a great concern in the medicine for many years. Environmental, genetic, infectious and iatrogenic factors in the patients with inflammatory bowel disease can predispose them to increase the risk of lymphoma. Overall risk of lymphoma in IBD patients is not higher than general population,9-11 but the basic question is whether treatment with immunomedulatory agents can lead to an increased risk of lymphoma. The use of azathioprine /6-mercaptopurine increases the incidence of lymphoma in patients with inflammatory bowel disease9, 10, 12 Although usefulness of azathioprine therapy outweigh the risk of lymphoma has been suggested in decision analysis model.13 Infection with Epstein–Barr Virus is another risk factor. EBV in the patients with IBD who received azathioprine/6-mercaptopurine associated with increased risk of lymphoma.14 Methotrexate is another immunomedulatory drug which is used as alternative treatment in moderate to severe crohn’s disease. Unlike azathioprine, there is no repeated and reliable data for the risk of lymphoma in IBD patients treated with methotrexate. In the CESAME study conducted by Beaugerie et al., (2009), methotrexate had not increased the risk of lymphoma.15
Some studies like those conducted on rheumatoid arthritis provide some information on the effect of methotrexate. In these studies, there was no increase in the risk of lymphoma among patients treated with methotrexate.16, 17 At that time, anti-TNF agents such as infliximab were approved for the treatment of crohn’s disease. There is a great concern over safety of drugs; moreover, the assessment of specific risk of these agents on lymphoma development is difficult because most patients had a previous exposure to methotrexate and azathioprine. A comprehensive meta-analysis that was conducted by Siegel et al., (2009) showed that the rate of NHL in patients with Crohn’s disease who treated with anti-TNF agents in combination with immunomedulators was not increased.18
Non-Hodgkin’s lymphoma, particularly diffuse large B-cell lymphoma, appears to be more common than other types of lymphoma in the patients with inflammatory bowel disease.11
The disease of our patient was controlled only with mesalamine in this study. She had no exposure to azathioprine, methotrexate or anti TNF agents. Despite the large number of IBD patients with lymphoma, no report has been released on composite lymphoma in IBD patients. It is the first case report. Clinical manifestation of composite lymphoma is similar to other types of lymphoma. The incidence rate of composite lymphoma, known as rare malignancy, is estimated to be 1 to 4.7% of all other types of lymphoma. Most composite lymphomas involve follicular lymphomas and diffuse large B-cell lymphomas.3 In contrast, the combination of non-Hodgkin’s lymphoma and Hodgkin’s disease rarely occurs. Nodular lymphocyte-predominant Hodgkin’s lymphoma and diffuse B-cell lymphoma are more frequently found among these combinations.3
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
References
- 1.Bargen JA. Chronic ulcerative colitis associated with malignant disease. Dis Colon Rectum. 1994;37:727–30. doi: 10.1007/BF02054420. [DOI] [PubMed] [Google Scholar]
- 2.Thirumala S, Esposito M, Fuchs A. An unusual variant of composite lymphoma: a short case report and review of the literature. Arch Pathol Lab Med. 2000;124:1376–8. doi: 10.5858/2000-124-1376-AUVOCL. [DOI] [PubMed] [Google Scholar]
- 3.Kim H. Composite lymphoma and related disorders. Am J Clin Pathol. 1993;99:445–51. doi: 10.1093/ajcp/99.4.445. [DOI] [PubMed] [Google Scholar]
- 4.Kim H, Hendrickson R, Dorfman RF. Composite lymphoma. Cancer. 1977;40:959–76. doi: 10.1002/1097-0142(197709)40:3<959::aid-cncr2820400302>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 5.Dargent JL, Lespagnard L, Meiers I, Bradstreet C, Heimann P, De Wolf- Peeters C. Composite follicular lymphoma and nodular lymphocyte predominance Hodgkin’s disease. Virchows Arch. 2005;447:778–80. doi: 10.1007/s00428-005-0008-1. [DOI] [PubMed] [Google Scholar]
- 6.Caleo A, Sánchez A, Rodríguez S, Dotor AM, Beltrán L, de Larrinoa AF, et al. Composite Hodgkin lymphoma and mantle cell lymphoma: two clonally unrelated tumors. Am J SurgPathol. 2003;27:1577–80. doi: 10.1097/00000478-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Kaleem Z, McGuire MH, Caracioni AC, Leonard RL, Pathan MH, Lessmann EA, et al. Composite B-cell and T-cell non-Hodgkin lymphoma of the tibia. Am J ClinPathol. 2005;123:215–21. [PubMed] [Google Scholar]
- 8.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–66. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 9.Vos AC. Risk of malignant lymphoma in patients with inflammatory bowel diseases: a Dutch nationwide study. Inflamm Bowel Dis. 2011;17:1837–45. doi: 10.1002/ibd.21582. [DOI] [PubMed] [Google Scholar]
- 10.Ashworth LA, Billett A, Mitchell P, Nuti F, Siegel C, Bousvaros A. Lymphoma risk in children and young adults with inflammatoryboweldisease: analysis of a large single-center cohort. Inflamm Bowel Dis. 2012;18:838–43. doi: 10.1002/ibd.21844. [DOI] [PubMed] [Google Scholar]
- 11.Chiorean MV, Pokhrel B, Adabala J, Helper DJ, Johnson CS, Juliar B. Incidence and risk factors for lymphoma in a single-center inflammatory bowel disease population. Dig Dis Sci. 2011;56:1489–95. doi: 10.1007/s10620-010-1430-z. [DOI] [PubMed] [Google Scholar]
- 12.Beaugerie L, Brousse N, Bouvier AM, Colombel JF, Lemann M, Cosnes J, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet. 2009;374:1617–25. doi: 10.1016/S0140-6736(09)61302-7. [DOI] [PubMed] [Google Scholar]
- 13.Lewis JD, Schwartz JS, Lichtenstein GR. Azathioprine for maintenance of remission in Crohn’s disease: benefits outweigh the risk of lymphoma. Gastroenterology. 2000;118:1018–24. doi: 10.1016/s0016-5085(00)70353-2. [DOI] [PubMed] [Google Scholar]
- 14.Dayharsh GA, Loftus EV, Jr, Sandborn WJ, Tremaine WJ, Zinsmeister AR, Witzig TE, et al. Epstein-Barr virus-positive lymphoma in patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine. Gastroenterology. 2002;122:72–77. doi: 10.1053/gast.2002.30328. [DOI] [PubMed] [Google Scholar]
- 15.Beaugerie L, Seksik P, Carrat F. Thiopurine therapy is associated with a threefold decrease in the incidence of advanced neoplaisa in IBD patients with longstanding extensive colitis: the CESAME prospective data. Journal of Crohn’s and Colitis. 2009 [Google Scholar]
- 16.Mariette X, Cazals-Hatem D, Warszawki J, Liote F, Balandraud N, Sibilia J. Lymphomas in rheumatoid arthritis patients treated with methotrexate: a 3-year prospective study in France. Blood. 2002;99:3909–15. doi: 10.1182/blood.v99.11.3909. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Michaud K. The effect of methotrexate and anti-tumor necrosis factor therapy on the risk of lymphoma in rheumatoid arthritis in 19,562 patients during 89,710 person-years of observation. Arthritis Rheum. 2007;56:1433–9. doi: 10.1002/art.22579. [DOI] [PubMed] [Google Scholar]
- 18.Siegel CA, Marden SM, Persing SM, Larson RJ, Sands BE. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulatortherapy for the treatment of Crohn’s disease: a meta-analysis. ClinGastroenterolHepatol. 2009;7:874–81. doi: 10.1016/j.cgh.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


