Abstract
To study the similarity between a putative cloned mammalian release factor (RF) and tryptophanyl-tRNA synthetase (TRS), a recombinant rabbit RF fusion protein was expressed from prokaryotic expression vectors. Purified fractions of the fusion proteins were tested for TRS and RF activities. Addition of the fusion protein to a TRS assay increased the binding of tryptophan to tRNA(Trp). However, in an assay for RF activity, the addition of the fusion protein resulted in release of only 1-3% of formylmethionine from an fMet-tRNA-AUG-ribosome intermediate that had been provided with UAAA as message. To confirm this result, the coding region of the putative eukaryotic RF clone "eRF" was used for in vitro transcription and translation in a rabbit reticulocyte lysate system, resulting in the synthesis of a single 56-kDa protein. The influence of this 56-kDa protein on the termination of translation directed by tobacco mosaic virus was studied. Tobacco mosaic virus RNA produced a major 126-kDa protein and a minor 184-kDa readthrough protein in an in vitro translation system. The protein generated from the "eRF" coding region did not inhibit biosynthesis of the 184-kDa readthrough virus protein. Instead, it increased the yield of both viral proteins. This increase was presumably due to its TRS activity. Chromatography of proteins derived from human lymphoblasts separated RF from TRS activity. Thus, our results indicate that the previously cloned "eRF" clone encodes TRS and that rabbit reticulocyte RF activity lies in a different protein.
Full text
PDF

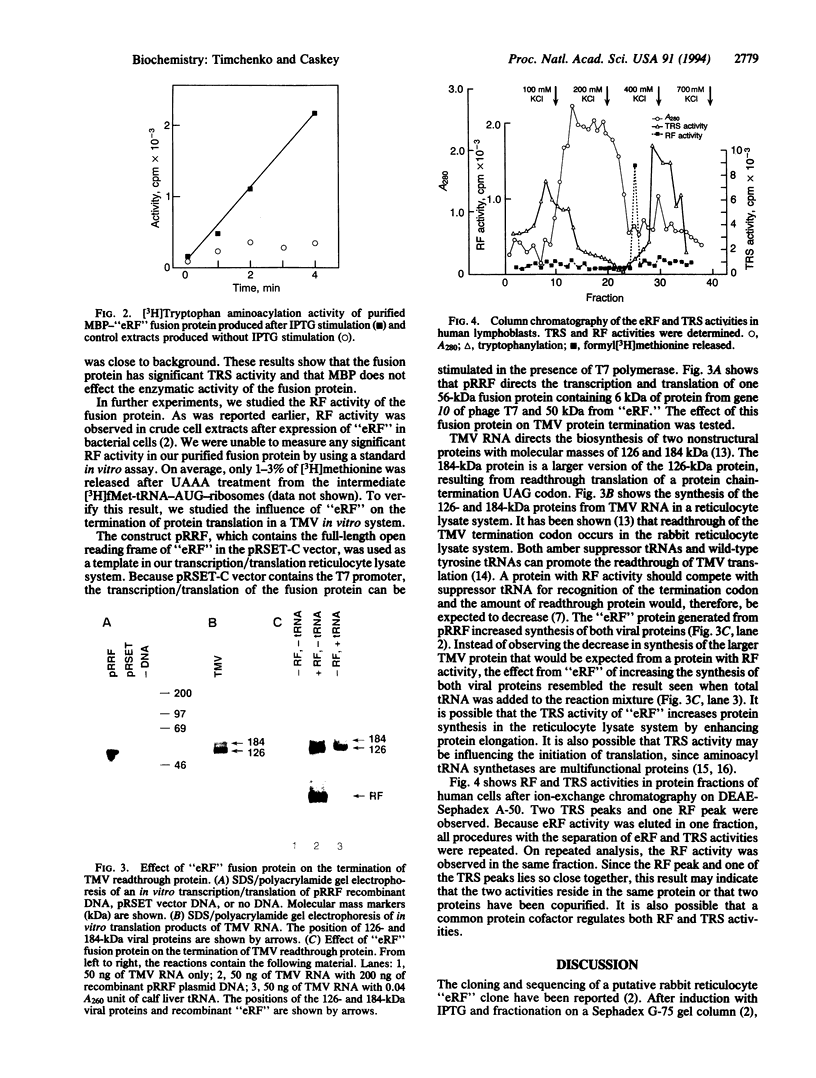

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. A protein required for splicing group I introns in Neurospora mitochondria is mitochondrial tyrosyl-tRNA synthetase or a derivative thereof. Cell. 1987 Jul 31;50(3):331–345. doi: 10.1016/0092-8674(87)90488-0. [DOI] [PubMed] [Google Scholar]
- Bange F. C., Flohr T., Buwitt U., Böttger E. C. An interferon-induced protein with release factor activity is a tryptophanyl-tRNA synthetase. FEBS Lett. 1992 Mar 30;300(2):162–166. doi: 10.1016/0014-5793(92)80187-l. [DOI] [PubMed] [Google Scholar]
- Buwitt U., Flohr T., Böttger E. C. Molecular cloning and characterization of an interferon induced human cDNA with sequence homology to a mammalian peptide chain release factor. EMBO J. 1992 Feb;11(2):489–496. doi: 10.1002/j.1460-2075.1992.tb05079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J. Does protein phosphorylation play a role in translational control by eukaryotic aminoacyl-tRNA synthetases? Trends Biochem Sci. 1990 May;15(5):172–175. doi: 10.1016/0968-0004(90)90153-3. [DOI] [PubMed] [Google Scholar]
- Craigen W. J., Lee C. C., Caskey C. T. Recent advances in peptide chain termination. Mol Microbiol. 1990 Jun;4(6):861–865. doi: 10.1111/j.1365-2958.1990.tb00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckner J., Rasmussen H. H., Justesen J. Human interferon gamma potently induces the synthesis of a 55-kDa protein (gamma 2) highly homologous to rabbit peptide chain release factor and bovine tryptophanyl-tRNA synthetase. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11520–11524. doi: 10.1073/pnas.88.24.11520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolova LYu, Fleckner J., Justesen J., Timms K. M., Tate W. P., Kisselev L. L., Haenni A. L. Are the tryptophanyl-tRNA synthetase and the peptide-chain-release factor from higher eukaryotes one and the same protein? Eur J Biochem. 1993 Mar 1;212(2):457–466. doi: 10.1111/j.1432-1033.1993.tb17682.x. [DOI] [PubMed] [Google Scholar]
- Frolova LYu, Sudomoina M. A., Grigorieva AYu, Zinovieva O. L., Kisselev L. L. Cloning and nucleotide sequence of the structural gene encoding for human tryptophanyl-tRNA synthetase. Gene. 1991 Dec 30;109(2):291–296. doi: 10.1016/0378-1119(91)90624-k. [DOI] [PubMed] [Google Scholar]
- Garret M., Pajot B., Trézéguet V., Labouesse J., Merle M., Gandar J. C., Benedetto J. P., Sallafranque M. L., Alterio J., Gueguen M. A mammalian tryptophanyl-tRNA synthetase shows little homology to prokaryotic synthetases but near identity with mammalian peptide chain release factor. Biochemistry. 1991 Aug 6;30(31):7809–7817. doi: 10.1021/bi00245a021. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Beaudet A. L., Caskey C. T. Peptide chain termination with mammalian release factor. Proc Natl Acad Sci U S A. 1970 Sep;67(1):99–106. doi: 10.1073/pnas.67.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann O. K., Ferenci T. Maltose-binding protein from Escherichia coli. Methods Enzymol. 1982;90(Pt E):459–463. doi: 10.1016/s0076-6879(82)90171-9. [DOI] [PubMed] [Google Scholar]
- Lee C. C., Craigen W. J., Muzny D. M., Harlow E., Caskey C. T. Cloning and expression of a mammalian peptide chain release factor with sequence similarity to tryptophanyl-tRNA synthetases. Proc Natl Acad Sci U S A. 1990 May;87(9):3508–3512. doi: 10.1073/pnas.87.9.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pel H. J., Maat C., Rep M., Grivell L. A. The yeast nuclear gene MRF1 encodes a mitochondrial peptide chain release factor and cures several mitochondrial RNA splicing defects. Nucleic Acids Res. 1992 Dec 11;20(23):6339–6346. doi: 10.1093/nar/20.23.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Rubin B. Y., Anderson S. L., Xing L., Powell R. J., Tate W. P. Interferon induces tryptophanyl-tRNA synthetase expression in human fibroblasts. J Biol Chem. 1991 Dec 25;266(36):24245–24248. [PubMed] [Google Scholar]