Abstract
Transplantation of hematopoietic stem/progenitor cells (HSPC) modified with a lentiviral vector bearing a potent nontoxic short hairpin RNA (sh1005) directed to the HIV coreceptor CCR5 is capable of continuously producing CCR5 downregulated CD4+ T lymphocytes. Here, we characterized HIV-1 resistance of the sh1005-modified CD4+ T lymphocytes in vivo in humanized bone marrow/liver/thymus (hu BLT) mice. The sh1005-modified CD4+ T lymphocytes were positively selected in CCR5-tropic HIV-1–challenged mice. The sh1005-modified memory CD4+ T lymphocytes (the primary target of CCR5-tropic HIV-1) expressing sh1005 were maintained in lymphoid tissues in CCR5-tropic HIV-1–challenged mice. Frequencies of HIV-1 p24 expressing cells were significantly reduced in the sh1005-modified splenocytes by ex vivo cell stimulation confirming that CCR5 downregulated sh1005 modified cells are protected from viral infection. These results demonstrate that stable CCR5 downregulation through genetic modification of human HSPC by lentivirally delivered sh1005 is highly effective in providing HIV-1 resistance. Our results provide in vivo evidence in a relevant small animal model that sh1005 is a potent early-step anti-HIV reagent that has potential as a novel anti-HIV-1 HSPC gene therapeutic reagent for human applications.
Keywords: CCR5, shRNA, HIV-1 Gene Therapy, Hematopoietic stem cells, Humanized mouse
Introduction
Hematopoietic stem/progenitor cell (HSPC) based gene therapy strategies aim to confer long term HIV-1 resistance through genetic modification of HSPC capable of continuously producing HIV-1–resistant progeny.1,2,3,4,5,6,7 In 2009, the first clinical case of an HIV-1 cure was reported.8 This was achieved by bone marrow stem cell transplants with a Δ32/Δ32 homozygous gene mutation in the HIV-1 coreceptor (c-c motif) chemokine receptor 5 (CCR5) in one HIV-1+ leukemic patient. Subsequent studies revealed only trace, possibly false positive, amounts of HIV-1 RNA and DNA in the patient over more than 5 years, suggesting the patient might be “functionally cured.”9 Although the first clinical cure was achieved using bone marrow from a naturally CCR5 homozygous (Δ32/Δ32) donor,8 the frequency of human leukocyte antigen (HLA) matched CCR5 homozygous (Δ32/Δ32) bone marrow donors is extremely rare.10,11,12,13 Thus, strategies to genetically modify patient HSPC by gene-based CCR5 inhibitors such as si/shRNA, ribozymes, and intrabodies have been developed.14,15,16,17 More recently, CCR5 gene targeting strategies include zinc finger nucleases, transcription activator like nucleases (TALENs) and clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 nucleases.18,19,20 We and others have investigated inhibiting the expression of CCR5 by shRNA-mediated RNA interference.16,21,22,23,24,25,26,27 In order to efficiently downregulate CCR5, we extensively screened a random CCR5-directed shRNA library using an enzymatic production of RNAi library (EPRIL) method and identified a potent short hairpin RNA (shRNA).16,28 Using transduction with lentiviral vectors, this shRNA (sh1005) was found to be nontoxic and highly efficient for stable downregulation of CCR5 expression in human primary T lymphocytes in vitro and in rhesus T lymphocytes in vivo following modified HSPC transplantation.16 In vivo transplantation of sh1005-transduced human HSPC in the humanized bone marrow/liver/thymus (hu BLT) mouse model demonstrated that gene-modified human HSPC could differentiate into multilineage hematopoietic cells including T lymphocytes, memory T cells, B lymphocytes, and monocyte/macrophage populations in lymphoid tissues, including gut-associated lymphoid tissue.24 Great diversity in human T-cell receptor (TCR) vβ rearrangements in gene-modified human thymocytes in the transplanted human thymus tissue can also be achieved in this mouse model. Also, CCR5 expression was stably downregulated in gene-modified HSPC-derived CD4+ T lymphocytes and monocytes/macrophages in lymphoid tissues in the transplanted hu BLT mice.24 Our previous study provided an extensive characterization of stable CCR5 downregulated sh1005-modified cells in lymphoid tissues in the hu BLT mice,24 but demonstrated only ex vivo HIV-1 inhibition using splenocytes isolated from the hu BLT mice.
In this study, we characterized selective protection of sh1005-mediated CCR5 downregulated CD4+ T lymphocytes in vivo in HIV-1–challenged hu BLT mice. Our results showed that CD4/CD8 ratios in sh1005-gene modified populations were stably maintained in peripheral blood and lymphoid tissues in CCR5 (R5) tropic HIV-1–challenged BLT mice. sh1005-modified memory CD4+ T cells were also well maintained in lymphoid tissues, suggesting that sh1005-mediated CCR5 downregulation can protect memory CD4+ T cells, the primary target of R5-tropic HIV-1. The frequencies of HIV-1 infected cells were significantly reduced in the sh1005-modified splenocytes, measured by viral reactivation by ex vivo cell stimulation. These results demonstrated that sh1005 is a potent early-step anti-HIV reagent that provides HIV-1 resistance to CD4+ T lymphocytes by stable CCR5 downregulation and can be effective in HSPC gene therapy strategies for HIV-1 disease.
Results
Hematopoietic reconstitution of sh1005-transduced HSPC in hu BLT mice
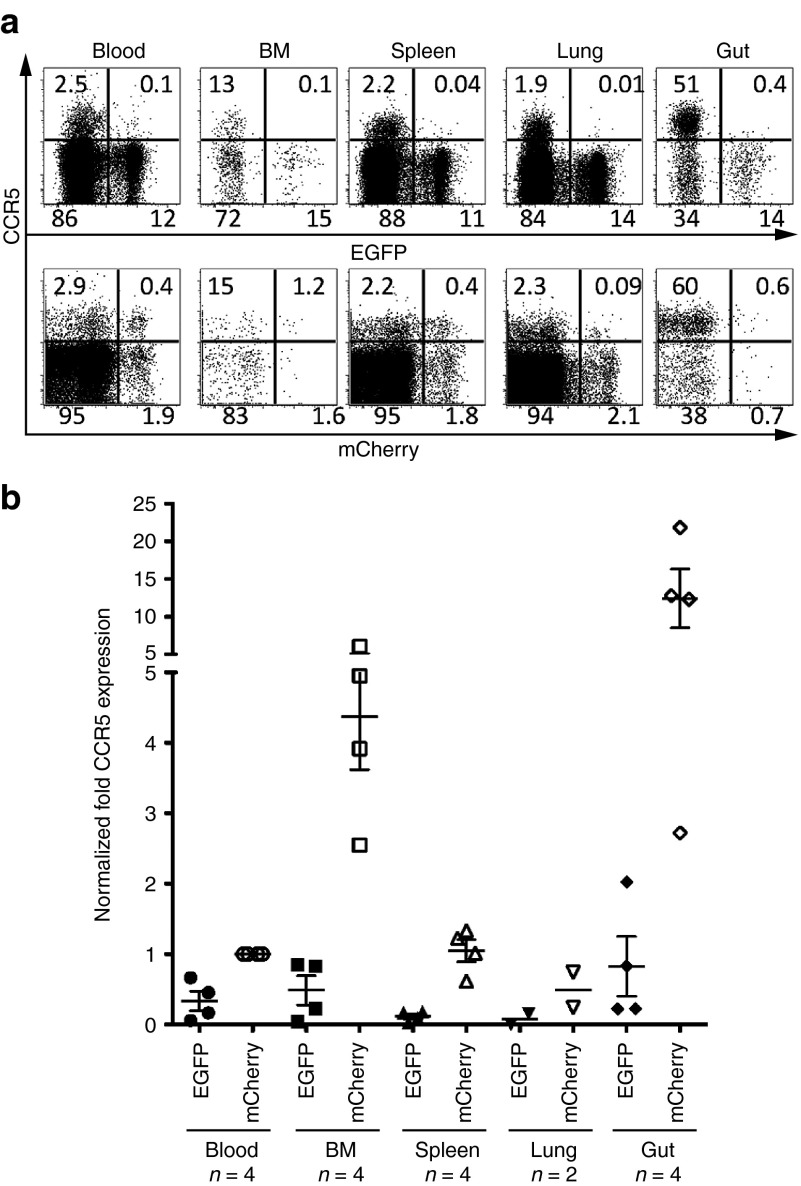
In order to effectively evaluate in vivo HIV-1 resistance mediated by sh1005 in the hu BLT mice, an sh1005-expressing lentiviral vector was marked with an EGFP green fluorescent marker while a non-shRNA control lentiviral vector was marked using an mCherry red fluorescent marker (Supplementary Figure S1). Both vectors were pseudotyped with Vesicular Stomatitis Virus glycoprotein (VSV–G). Human fetal liver-derived CD34+ HSPCs were separately transduced with either the sh1005 vector or the control vector overnight without cytokine stimulation. An equal mix of the sh1005- and the control vector-transduced human CD34+ HSPCs were suspended in Matrigel, and then transplanted along with human thymus pieces under the kidney capsule of NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice, which had been myeloablated by either busulfan or total body irradiation (TBI).24 To improve engraftment, these mice were also subsequently injected intravenously with similar mixtures of CD34+ cells transduced with sh1005 and control vector. Since therapeutic and control vector transduced cells can be monitored with different fluorescent markers within the same animal, this experimental design allowed us to effectively evaluate the anti-HIV gene (sh1005) expressing cells (EGFP+) and control vector-expressing cells (mCherry+) by multicolor flow cytometric analysis, as previously described.24 A small aliquot of vector transduced CD34+ HSPCs was cultured with cytokines (stem cell factor, IL-3 and IL-6) for 5 days in order to assess the efficiency of vector transduction, which yielded frequencies of 63.4 ± 24% (n = 5), and 52.3 ± 25% (n = 5) for sh1005- and control vector-transduced HSPC, respectively (Supplementary Figure S2). Prior to in vivo HIV-1 challenge, the efficiency of human hematopoietic cell reconstitutions were evaluated by detecting human CD45+ lymphoid population in peripheral blood in the transplanted mice at 8 weeks after transplantation in each experiment. No significant difference of human reconstitution between the three independent experiments was observed (Supplementary Figure S3). The average of therapeutic and control vector expression levels in human CD45+ populations were similar (mean value: % EGFP 20.7 ± 11%, % mCherry 26.5 ± 17%, P value > 0.05). CCR5 expression was downregulated in EGFP+ marked CD4+ T cells in tissues at levels comparable to our previous published results (Figure 1).24
Figure 1.
CCR5 downregulation in EGFP+ CD4+ T cells in the reconstituted hu BLT mice. (a) Representative data for CCR5 expression in CD4+ T cells. Upper panel shows EGFP+ sh1005 vector marked CD4+ T cells and lower panel shows mCherry+ control vector marked CD4+ T cells. Numbers in each quadrant indicate the percentage. (b) The level of CCR5 expression was compared in EGFP+ and mCherry+ human CD4+ T cells in lymphoid tissues from multiple transplanted hu BLT mice. We normalized CCR5 expression level using the mean CCR5 expression in mCherry+ cells from peripheral blood (Blood) as 1. Samples were obtained between 21 and 26 weeks after human Thy/Liv implantation. Bar represents mean value; n indicates number of mice.
Maintenance of CD4/CD8 ratio in sh1005-modified CD4+ T lymphocytes in vivo in R5-tropic HIV-1–challenged BLT hu mice
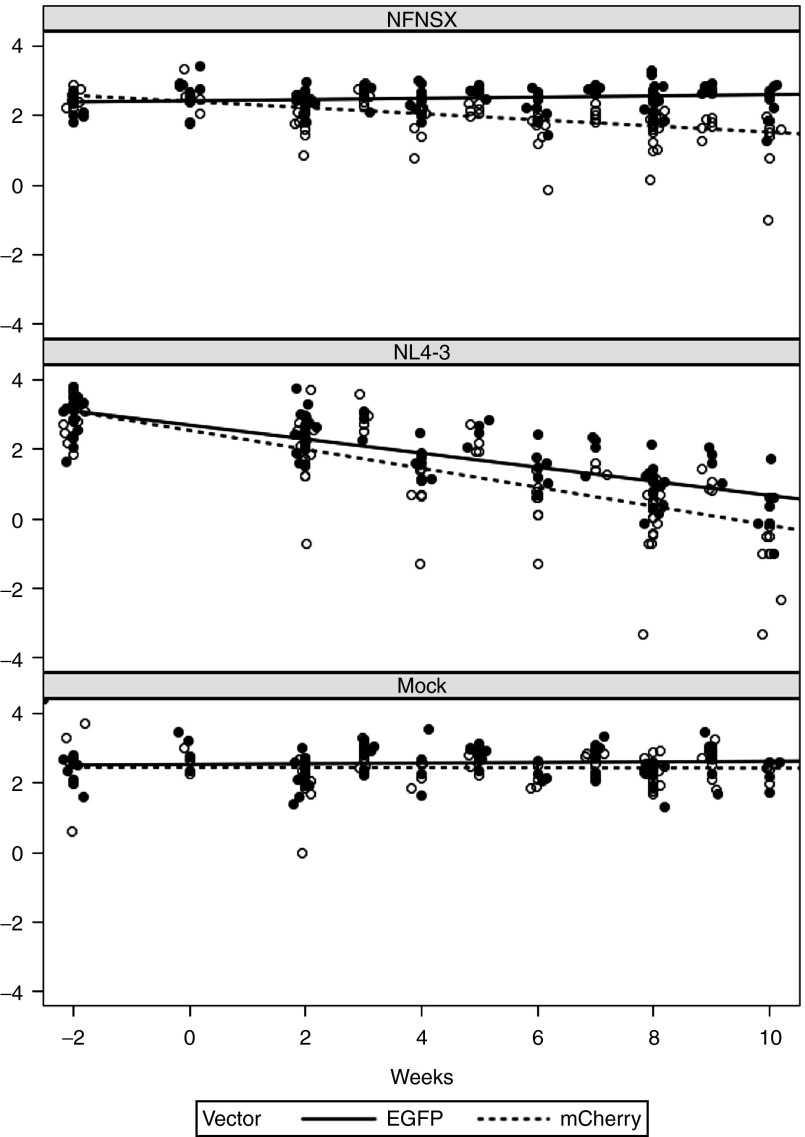
To investigate HIV-1 resistance in vivo, reconstituted BLT mice were challenged with R5-tropic HIV-1NFNSX through the retro-orbital intravenous route (dose = 200 ng of p24) in three independent cohort experiments. R5-tropic HIV-1NFNSX is a recombinant HIV-1 that has the envelope of R5 tropic HIV-1JRFL in HIV-1NL4-3 backbone.29 As controls, separate sets of mice were either mock-challenged or challenged with CXCR4 (X4) tropic HIV-1NL4-3.30 The CD4/CD8 ratio of sh1005- (EGFP+) was maintained relative to the CD4/CD8 ratio of no-shRNA control- (mCherry+) modified T lymphocytes in peripheral blood in R5-tropic HIV-1NFNSX–challenged mice in each of the three cohort experiments (Figure 2). In order to assess the statistical significance, the data were analyzed by including all data from the three cohort experiments in a linear mixed effects model with the mouse as a random effect and challenge, vector and time (in weeks) as fixed effects (Figure 3; see Statistical methods). The CD4/CD8 ratio in EGFP+ CD3+ T lymphocyte population remained stable while that of the mCherry+ CD3+ T lymphocyte population declined following R5-tropic HIV-1 challenge (Figure 3, top panel; EGFP+ slope = −0.01, mCherry+ slope = −0.09, Bonferroni-corrected difference in slopes P value = 0.0006). In contrast, both the EGFP+ and mCherry+ CD4+ T lymphocyte populations gradually decreased in X4-tropic HIV-1–challenged mice, (Figure 3, middle panel; EGFP+ slope = −0.21 (P < 0.0001), mCherry+ slope = −0.27 (P < 0.0001), Difference in slopes P value > 0.05). EGFP and mCherry expressing CD4+ T lymphocytes remained relatively stable in mock-infected mice (Figure 3, bottom panel; EGFP+ slope = −0.01 (P = 0.4), mCherry+ slope = −0.02 (P = 0.2), Difference in slopes P value > 0.05). These results demonstrate that sh1005-mediated CCR5 downregulation can maintain CD4/CD8 ratios by conferring resistance to R5-tropic HIV-1–induced CD4+ T lymphocyte depletion in peripheral blood in the hu BLT mice, providing a selective advantage.
Figure 2.
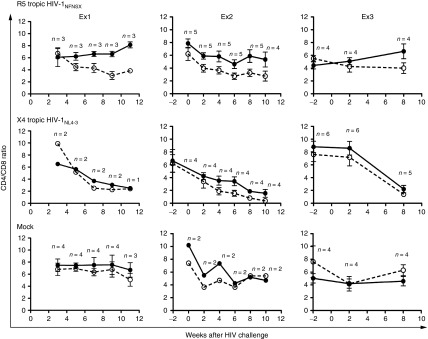
Selective advantage of sh1005-modified CD4+ T cells in peripheral blood following R5 tropic HIV-1 challenge. Hu BLT mice were challenged with R5-tropic HIV-1NFNSX (top panel), X4-tropic HIV-1NL4-3 (middle panel) or mock (bottom panel) in 3 independent cohort of experiments (Ex1, EX2 and EX3). Relative change in CD4/CD8 ratio kinetics in EGFP+ (solid circle and solid line) and in mCherry+ (open circle and dashed line) human CD3+ T lymphocytes in peripheral blood were compared between −2 and 11 weeks after HIV-1 challenge. Error bar shows standard deviation. n, number of mice.
Figure 3.
Model slopes of CD4/CD8 ratio in peripheral blood. The relative changes in CD4/CD8 ratio of sh1005- (EGFP+) and no-shRNA control- (mCherry+) modified CD4+ T lymphocytes in peripheral blood were compared using a linear mixed effects model by including all data from the three cohort experiments (Ex1, EX2 and EX3). CD4/CD8 ratio in shRNA-transduced cells (EGFP+, dotted line) and control cells (mCherry, solid line) overlayed on raw values (EGFP+, open circle; mCherry+, solid circle) were shown.
Maintenance of CD4/CD8 ratio in tissues in the R5-tropic HIV-1–challenged hu BLT mice
To assess the HIV-1 resistance of sh1005-modified CD4+ T lymphocytes in lymphoid tissues, hu BLT mice were euthanized at 8–12 weeks after HIV-1 challenge. CD4 and CD8 cell surface expression on total human CD3+ T lymphocyte populations was assessed in lymphoid tissues (Figure 4a) and CD4/CD8 ratios of EGFP+ and mCherry+ CD3+ T lymphocyte populations were compared (Figure 4b). Overall, the mean of CD4/CD8 ratio was higher in EGFP+ populations than in mCherry+ populations in all tissue analyzed. In particular, the difference between EGFP+ and mCherry+ populations was significantly larger in intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) in both small and large intestine in R5-tropic HIV-1–challenged mice (small intestine IEL and LPL P value < 0.001; large intestine IEL P value = 0.0005, LPL P value < 0.0001) after correcting for multiple comparisons (Figure 4b; top panel). In contrast, the CD4/CD8 ratios were not significantly different between EGFP+ and mCherry+ populations in tissues isolated from X4-tropic HIV-1– and mock-challenged mice except for bone marrow (P = 0.0003) and small intestine IEL (P = 0.0008) in X4-tropic HIV-1 (Figure 4b; middle and bottom panels). These results suggest that sh1005-mediated CCR5 downregulation could provide HIV-1 resistance in CD4+ T lymphocytes against R5-tropic HIV-1–induced cell depletion and is therefore capable of maintaining CD4/CD8 ratios in lymphoid tissues in HIV-1–infected hu BLT mice.
Figure 4.
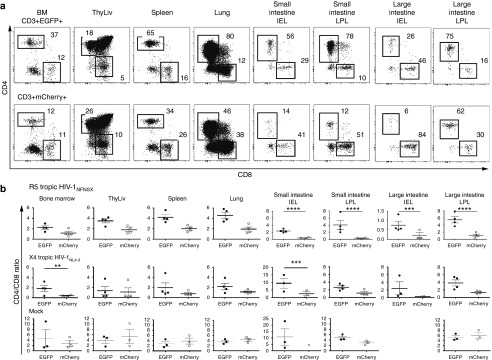
Maintenance of CD4/CD8 ratio in sh1005-modified CD4+ T cells in lymphoid tissue. (a) Representative data for percentage of CD4 and CD8 expression in EGFP+ (top panels) and mCherry+ (bottom panels) CD3+ T lymphocytes in bone marrow (BM), spleen, lung, small and large intestine (SI and LI) and intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) in a R5 tropic HIV-1NFNSX–challenged hu BLT mice. CD4+ and CD8+ populations were gated and percentages are shown. (b) The data of CD4/CD8 ratio in EGFP+ CD3+ T lymphocytes (solid circle) and in mCherry+ lymphocytes (open circle) from all analyzed mice are shown. Samples were obtained between 8 and 12 weeks after HIV-1 challenge. A bar represents the mean value. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001.
Protection of memory CD4+ T lymphocytes by sh1005 in lymphoid tissues
CCR5 expression is differentially regulated within T-cell subsets.31 CCR5 is mainly expressed in memory T cells in vivo.32,33 The level of CCR5 expression can be upregulated during T-cell activation ex vivo.34 CCR5 is expressed at low or undetectable levels in naïve T cells. Therefore, memory CD4+ T lymphocytes are highly susceptible and are the primary target of R5 tropic HIV-1.35 To assess HIV-1 resistance of sh1005-modified memory CD4+ T lymphocytes, cells isolated from lymphoid tissues were stained with monoclonal antibodies specific for CD27 and CD45RA. The percentages of EGFP+ and mCherry+ populations were compared in central (CD27+/CD45RA-) and effector (CD27-/CD45RA-) memory T lymphocyte populations (Figure 5). EGFP+ central and effector memory CD4+ T lymphocytes were maintained in BM, spleen, lung, and small and large intestine in R5-tropic HIV-1–challenged mice (Figure 5a,b; top panel). In contrast, mCherry+ central and effector memory CD4+ T lymphocytes were depleted (Figure 5a; bottom panel). The remaining unprotected mCherry+ control populations were mainly naïve CD4+ T cells (CD27+/CD45RA+) that do not express CCR5 and are not susceptible to R5-tropic HIV-1 infection (Figure 5a; bottom panel). Percentages of total memory CD4+ T lymphocytes were significantly higher in the EGFP+ populations than in the mCherry+ populations in all analyzed tissues (mean differences between EGFP+ and mCherry+: bone marrow 45%, spleen 35%, lung 42%, SI LPL 63%, LI LPL 65%; P < 0.0001 for all tissues) in R5-tropic HIV-1–challenged mice, but not in X4-tropic HIV-1–challenged mice (Figure 5b). One exception was that % of total memory CD4+ T lymphocytes was higher in the EGFP+ populations than in the mCherry+ populations in bone marrow in mock infected group. However, it was only in bone marrow but not in other tissues.
Figure 5.
Protection of CCR5 downregulated CD4+ memory T lymphocytes from R5-tropic HIV-1. (a) Percentage of central memory (CD45RA-/CD27+) in EGFP+ (Top panel) or mCherry+ (Bottom panel) CD4+ T lymphocytes in bone marrow (BM), spleen, lung, small and large intestine (SI and LI) lamina propria lymphocytes (LPL) and intraepithelial lymphocytes (IEL) from a R5 tropic HIV-1 infected hu BLT mouse. TCM indicates central memory T cells; TEM, effector memory T cells; and TTD, terminally differentiated cells. (b) Comparison of the level of % memory CD4+ T lymphocytes in EGFP+ (solid circle) and mCherry+ (open circle) from all analyzed mice (HIV-1NFNSX infected mice; n = 4, HIV-1NL4-3 infected mice; n = 4, mock infected mice; n = 3). A bar represents the mean value. ***P ≤ 0.001. ****P ≤ 0.0001.
The CD4/CD8 ratios were higher in EGFP+ populations than in mCherry+ populations in the total memory T-cell (CD27+/- and CD45RA-) population in bone marrow (BM; P = 0.0008), spleen (P = 0.001), lung (P < 0.0001), lamina propria lymphocytes (LPL) in both small and large intestine in R5-tropic HIV-1–challenged mice (P < 0.0001 for both) (Figure 6a,b). In contrast, the CD4/CD8 ratios were not significantly different between EGFP+ and mCherry+ populations in tissues except large intestine LPL (P = 0.001) isolated from X4-tropic HIV-1– or mock-challenged mice (Figure 6b; middle and bottom panels). The CD4/CD8 ratios were not statistically different in EGFP+ populations and in mCherry+ populations in naïve CD4+ T-cell population (Supplementary Figure S4). We also confirmed CCR5 expression was downregulated in memory CD4+ T cells in mock-infected mice (Supplementary Figure S5; top panel). The observed CCR5 decrease in R5-tropic HIV-1–challenged mice was primarily due to killing of CCR5+ cells by HIV-1 (Supplementary Figure S5; bottom). These results suggest that sh1005-mediated CCR5 downregulation effectively protects highly R5 tropic HIV-1–susceptible, CCR5-expressing memory CD4+ T lymphocytes in lymphoid tissues from HIV-1–induced cell depletion.
Figure 6.
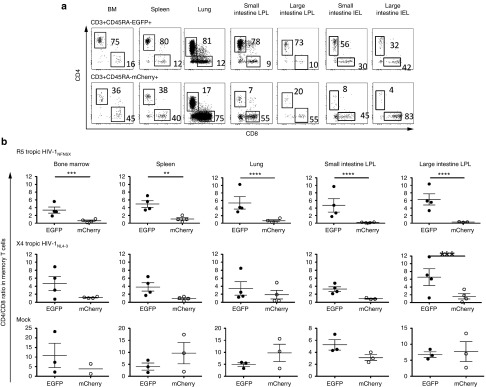
Maintenance of CD4/CD8 ratio in sh1005 modified memory T cells in lymphoid tissues. (a) Representative data showing percentage of CD4 and CD8 expression in EGFP+ (top panels) and mCherry+ (bottom panels) memory T lymphocytes in bone marrow (BM), spleen, lung, small and large intestine (SI and LI) lamina propria lymphocytes (LPL) and intraepithelial lymphocytes (IEL) in a R5 tropic HIV-1NFNSX–challenged hu BLT mice. CD4+ and CD8+ populations were gated and percentages are shown. (b) CD4/CD8 ratios in EGFP+ memory T lymphocytes (solid circle) and in mCherry+ lymphocytes (open circle) from all analyzed mice are shown. A bar represents the mean value. **P ≤ 0.01. ***P ≤ 0.001. ****P ≤ 0.0001.
Prevention of HIV-1 infection sh1005 modified CD4+ T splenocytes
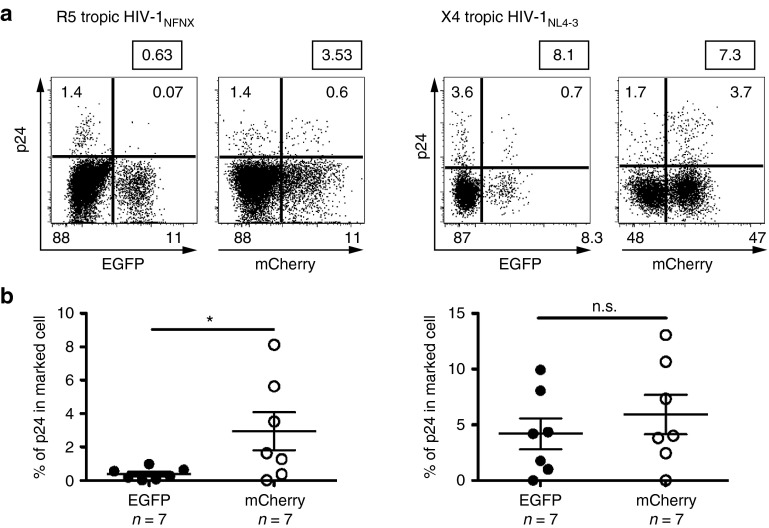
HIV establishes latent infection mainly in resting memory CD4+ T lymphocytes.36,37,38 These latently infected cells cannot be effectively eliminated by current antiretroviral drug therapy (ART).37,39 We and others recently established the hu BLT mouse model to characterize latently infected CD4+ T lymphocytes in spleens of HIV-infected hu BLT mice.40,41 In our previous study, over 2% of total human lymphocytes recovered from spleens in HIV-1–infected hu BLT mice harbored HIV-1 that could be induced by ex vivo cell stimulation. The ex vivo reactivated virus can be readily quantified in the lymphocytes by flow cytometric analysis for intracellular p24 expression.40 Since CCR5 downregulation by sh1005 is capable of inhibiting R5-tropic HIV-1 infection at viral entry, we examined the effectiveness of sh1005 in preventing HIV-1 infection in sh1005 modified CD4+ T cells using the ex vivo assay. Splenocytes from HIV-1–challenged hu BLT mouse were isolated 7 weeks after HIV-1 challenge. We found significant reduction of p24-expressing cells in EGFP+ sh1005 modified CD4+ T lymphocyte populations relative to mCherry+ populations after 5 days of ex vivo PHA/IL2 stimulation (Figure 7a,b). These results suggest that sh1005-mediated CCR5 downregulation can protect splenocytes from R5-tropic HIV-1.
Figure 7.
Protection of sh1005-modified splenocytes. Comparison of p24 reactivation in sh1005 (EGFP+) and no shRNA (mCherry+) population ex vivo. Splenocytes were isolated from R5-tropic HIV-1NFNSX–challenged hu BLT mice (n = 7) or X4-tropic HIV-1NL4-3–challenged hu BLT mice (n = 7). Splenocytes were isolated, stimulated with PHA/IL-2 for 5 days to reactivate HIV-1 and stained with anti-HIV-1 p24 gag monoclonal antibodies. (a) Representative p24 intracellular staining data in CD45+CD3+CD8- cell population were shown. (b) Results of p24 reactivation in EGFP+ population (solid circle) and in mCherry+ population (open circle) from all analyzed mice are shown. The percentages of p24 expression within EGFP+ or mCherry+ populations were normalized based on % each marker (EGFP or mCherry) expression for the comparison. *P ≤ 0.05. n.s., not significant.
No detectable emergence of X4-tropic HIV-1
To investigate a switch in viral coreceptor usage to CXCR4 under the pressure of CCR5 downregulation, splenocytes from R5 tropic HIV-1–challenged mice isolated at 8–12 weeks after in vivo HIV-1 challenge were cocultured with PHA/IL2 activated PBMC ex vivo to amplify HIV-1. HIV-1–containing coculture supernatants were used to infect Ghost/CXCR4 indicator cell line to test CXCR4 usage. The % EGFP expression levels were similar in the Ghost/CXCR4 indicator cells among coculture supernatants from R5 tropic HIV-1–infected mice, mock and R5 tropic HIV-1NFNSX at 5 days after infection (Supplementary Table S1). Of note, we recognized a limitation of the short duration of the experiment. Therefore, it is unknown if the lack of coreceptor usage conversion may or may not occur in longer observation period. These results demonstrate that viral evolution to X4-tropic HIV-1 did not occur in our experimental setting.
Discussion
In this study, we characterized in vivo HIV-1 resistance in CCR5 downregulated CD4+ T lymphocytes provided by sh1005 in R5-tropic HIV-1–challenged hu BLT mice. We examined the effectiveness of sh1005 in peripheral blood and lymphoid tissues by comparing sh1005-modified EGFP-expressing and control vector-modified mCherry-expressing CD4+ T lymphocytes by multicolor flow cytometric analysis. Our results demonstrate a selective advantage of sh1005-modified CCR5 downregulated CD4+ T lymphocytes in the R5-tropic HIV-1–challenged mice. sh1005 expression was effective in maintaining memory CD4+ T lymphocytes in lymphoid tissues. sh1005-modified splenocytes from R5-tropic HIV-1–challenged hu BLT mice were resistant to HIV-1 infection during ex vivo HIV-1 reactivation and spread. Taken together, our results suggest that stable CCR5 downregulation by sh1005 through lentiviral vector transduction of CD34+ HSPC is an effective strategy to reconstitute and continuously provide gene modified CD4+ T lymphocytes resistant to HIV-1 infection in the hu BLT mice.
One of the major achievements in the history of HIV-1 therapeutic research is the development of antiretroviral drug therapy (ART) that has significantly reduced morbidity and mortality of HIV-1–infected individuals.42 Unfortunately, ART cannot eliminate the infection due to the presence of ART refractory latently infected memory T-cell populations.43,44
Genetic modification of HSPC to inhibit the HIV-1 coreceptor (c-c motif) chemokine receptor 5 (CCR5) expression is capable of blocking an early stage of infection and may be able to stably protect HSPC and their progeny from HIV-1 infection. An advantage of CCR5 directed gene therapy approaches that inhibit the virus at entry is that the gene-modified cells should also be refractory to HIV-1. In this study, we have built on this prior work by showing that ex vivo stimulation of isolated sh1005-expressing cells generates significantly lower levels of productively infected cells than with cells expressing a control vector within the same mice. These data indicate that sh1005 modified cells are protected from establishment of HIV infection in vivo and can reduce HIV-1 spread from cell activation-induced HIV-1 in ex vivo culture.
Strategies to genetically modify CCR5 gene and its expression have been developed using various technologies, si/shRNA, ribozymes, intrabodies.14,15,16,17 More recently CCR5 gene targeting strategies, including zinc finger, TAREN and CRISPR/CAS9 nucleases have been studied.18,19,20 These genome-editing technologies have an advantage over si/shRNA, ribozymes, intrabodies by inactivating CCR5 gene by transient expression. However, these technologies may have off target gene editing activities.19,45 The advantage of shRNAs over other anti-HIV reagents is that shRNAs are relatively small and feasible to coexpress from a lentiviral vector. CCR5 inhibition is an effective strategy to protect cells from R5-tropic HIV-1 infection; however, it is not effective against X4-tropic HIV-1 infection. A single reagent directed against CCR5 may not be effective in preventing the emergence of resistant X4-tropic HIV-1 strains by escape mutations. This limitation could be circumvented by using relatively simple-to-design combinations of anti-HIV-1 genes as previously described.7,22,46,47 We have recently demonstrated that sh1005 can be coexpressed along with a shRNA targeting the HIV-1 LTR.48 Thus, sh1005 can be combined with other gene-based HIV therapeutics for future multipronged anti-HIV-1 HSPC gene therapy strategies.
Anti-HIV-1 gene therapy vectors require careful in vivo investigation in animal model settings. The goal of anti-HIV HSPC therapy is to produce life-long HIV resistance by genetic engineering of HSPC and their progenies, ultimately controlling HIV-1 infection using a single treatment. Such anti-HIV-1 HSPC gene therapy strategies require suitable animal model systems to test the long-term safety and efficiency of gene-modified human HSPC transplant procedures. Among available humanized mouse models, the hu BLT supports robust human multilineage hematopoietic cell reconstitution due to its use of NSG mice and cotransplantation of human thymus-like organoid (thy/liv) and CD34+ HSPC.49 The human hematopoietic cells reconstituted in the hu BLT mouse model are multilineage differentiated, including naïve and memory T lymphocytes developed from thymocytes generated in the human thymus implant. Our results provide evidence that sh1005 can stably produce HIV-1 resistant cells in vivo in a relevant small animal model. Thus, these findings demonstrate that sh1005 has the potential to be a novel anti-HIV-1 HSPC gene therapeutic reagent for human application. Recently, sh1005 has been entered in a phase 1/2 clinical trial along with a HIV fusion inhibitor, C46 peptide (ClinicalTrials.gov Identifier:NCT01734850). This clinical trial may reveal feasibility and safety for HIV-1 gene therapy treatment using sh1005 gene-modified hematopoietic progenitor/stem cells and CD4+ T lymphocytes.
Materials and Methods
Lentiviral vector construction and production. The construction of a lentiviral vector for an sh1005 expression (FG12 H1shRNACCR5) and a control lentiviral vector for mCherry expression (FG11FmCherry) was previously described16,23,24. VSV-G pseudotyped lentiviral vectors were prepared by calcium phosphate plasmid DNA transfection in 293T cells as previously described.23 The concentrated vector stocks were titered on 293T cells based on % EGFP or mCherry expression.
Lentiviral vector transduction. Fetal liver derived CD34+ cells (0.5 × 106) were seeded into 20 μmol/l RetroNectin (Clontech Laboratories, Mountain View, CA) coated plates with 2% bovine serum albumin in Yssel's medium (GEMINI Bio Products, West Sacrament, CA). After 1-hour incubation, cells were infected with either the FG12 H1shRNACCR5 or FG11FmCherry lentiviral vector at MOI of 2 (Ex 1), 3 (Ex 2), and 4 (Ex 3) for overnight without cytokine stimulation. Vector transduced CD34+ cells were mixed for transplantation into mice.
Generation of hu BLT mice. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice were maintained at UCLA facilities in accordance with protocol approved by the UCLA Animal Research Committee. Hu BLT mice were generated as previously described24 with modifications for the myeloablative procedure and for the timing of transplants. Briefly, NSG mice (6–8 weeks old) were myeloablated either by total body irradiation (300 cGy by 60Co-irradiator) for Ex1 or intraperitoneal busulfan injections for Ex2 and Ex3 (two injections, 25 mg/kg each at 2 days and 1 day before transplant and 35 mg/kg 1 day before transplant). sh1005 vector (EGFP+) transduced and the control (mCherry+) vector transduced CD34+ cells (0.5 × 106) were mixed, solidified with matrigel (BD Bioscience, San Jose, CA). CD34- cells were also mixed in the matrigel as feeder cells. The matrigel solidified cell mix was implanted with a piece of thymus under the kidney capsule. On the same day, mice were injected with the vector transduced human CD34+ cells (1 × 106) using a 27-gauge needle through the retro-orbital vein.
HIV challenge in the hu BLT mice. The HIV-1 stocks were prepared by a calcium phosphate plasmid DNA transfection method as previously described.24 After confirming peripheral blood reconstitution with EGFP or mCherry expressing cells, HIV-1NFNSX or HIV-1NL4-3 (200 ng /p24) were injected through the retro-orbital vein using a 27-gauge needle at 9–12 weeks after HSPC transplant.
Cell isolation from peripheral blood and mouse tissues and flow cytometry. Isolation of cells from peripheral blood (PBMC), bone marrow (BM), thy/liv implant (thy/liv), lung, spleen (SL), and the Gut were described previously.24 Peripheral blood- and tissue- derived mononuclear cells were stained with monoclonal antibodies to human CD45-biotin (HI30: eBioscinece, San Diego, CA) or eFluor 450 (HI30, eBioscinece), CD3-PerCP (SK7: BD Pharmingen, Franklin, NJ) or APC-H7(SK7: BD Pharmingen), CD4-PE (RPA-T4: eBioscience) or APC(OKT4: eBiosciences), and CD8-APC (RPA-T8: BD Pharmingen) or PerCP Cy5.5 (SK1: BioLegend), CD45RA-PECy7 (HI100: BioLegend) or PECy5, CD27-APCCy7 (O323: BioLegend) and CCR5-PECy7 (2D7: BD Pharmingen) or PECy5. Red blood cells were lysed with red cell lysis buffer after being stained with streptavidine-AlexaFluoro350 (Invitrogen, Grand Island, NY). Stained cells were fixed with 1% formaldehyde in PBS and examined with LSRII or Fortessa (BD Biosciences) flow cytometers. The data were analyzed by FlowJo (TreeStar, Ashland, OR) software.
Ex vivo splenocyte stimulation for the activation of intracellular HIV-1 p24 expression. Splenocytes were ex vivo isolated and stimulated with PHA (Sigma) and human IL-2 for 5 days. They were stained for human CD45, CD3 and CD8 cell surface markers, fixed, and permeabilized according to manufacturer's instructions using the Fix and Perm buffer (BD Biosciences). Fixed cells were stained for intracellular HIV-1 p24 antigen using KC57-RD-1 (Beckman Coulter, Indianapolis, IN) antibody and analyzed by Fortessa flow cytometer. Data were analyzed with FlowJo software.
Statistical methods. The relative changes in CD4/CD8 ratio of sh1005- (EGFP+) and no-shRNA control- (mCherry+) modified CD4+ T lymphocytes in peripheral blood were compared using a linear mixed effects model with a random mouse effect and challenge, experiment (mock, NL4-3, NFNSX) and time (in weeks) fixed effects, with heterogeneous variances by sampling cohort. CD4/CD8 ratio was log-transformed prior to modeling. Time was modeled as a continuous variable. All two-way and three-way interaction effects between challenge, vector and time were included in the model. Estimates of the slopes and difference in slopes between EGFP+ and mCherry+ were produced by modeled contrasts. To compare the CD4/CD8 ratios, percentages in memory T lymphocyte populations and CD4/CD8 ratio in total memory T cell in lymphoid tissues of EGFP+ and mCherry+, a linear mixed effects model with a random mouse effect and challenge (mCherry+ or EGFP+), experiment (mock, NL4-3, NFNSX), and tissue as fixed effects was used. All two-way and three-way interaction effects between challenge, vector and tissue were included in the model. Estimates of the difference between EGFP+ and mCherry+ were produced from the modeled contrasts. Bonferroni correction was used to account for the multiple comparison tests of all the tissue challenge interactions depending on the number of tissues in each experiment. P values < 0.05 were considered statistically significant. SAS version 9.4 was used for all statistical analyses.
Coreceptor usage. Isolated mouse splenocytes were cultured with human PBMC in PHA and IL-2 medium. After 3 days, supernatants were collected and cultured with Ghost/CXCR4 for 5 days. The percentage of EGFP was measured by flow cytometry.
SUPPLEMENTARY MATERIAL Figure S1. Construction of a lentiviral vector for delivering shRNA against human CCR5. Figure S2. The efficiency of vector transduction in CD34+ cells. Figure S3. Human CD45+ lymphoid cell reconstitution in vivo. Figure S4. CD4/CD8 ratio in sh1005 modified naïve T cells in lymphoid tissues. Figure S5. CCR5 expression in EGFP+ and mCherry+ memory CD4T+ cells in tissues. Table S1. No coreceptor changing.
Acknowledgments
We thank Lauren Pokomo, Josh Boyer, Anna Sahakyan, Emily Lowe, Nuttee Suree, Patrick Kim, Masakazu Kamata, Fadi Kandarian, Erica Eggers, Xiaomeng Wu, Min Liang, Yiming Xie, and Munetoshi Narukawa for their technical support and helpful discussions. We thank Janet Chung for critical reading of our manuscript. This research was supported by the NHLBI 1R01HL086409, NIAID 1R01AI100652-01A1, California Institute for Regenerative Medicine (CIRM grant DR1-01431 to I.S.Y.C.), NIAID AI70010 (J.A.Z.), NIAID U19AI096113, (project 3.4, to J.A.Z.), the UCLA AIDS Institute and the UCLA Center for AIDS Research NIH/NIAID AI028697, NIH/NCATS/UC:LA CTSI grant UL1TR000124UCLA (Clinical and Translational Science Institute). We thank the UCLA CFAR Gene and Cellular Therapy Core Facility for providing fetal tissues and CD34+ HSPC, the CFAR Humanized Mouse Core Facility for the animal housing, CFAR Virology Core Lab at UCLA(5P30 AI028697) for HIV p24 assay and UCLA Clinical and Translational Science Institute for statistics analysis. The authors declare no conflict of interest.
Supplementary Material
References
- Kiem HP, Jerome KR, Deeks SG, McCune JM. Hematopoietic-stem-cell-based gene therapy for HIV disease. Cell Stem Cell. 2012;10:137–147. doi: 10.1016/j.stem.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen SG, Shimizu S, An DS. Stem cell-based anti-HIV gene therapy. Virology. 2011;411:260–272. doi: 10.1016/j.virol.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoxie JA, June CH.2012Novel cell and gene therapies for HIV Cold Spring Harb Perspect Med 2(10)doi:10.1101/cshperspect.a007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988;335:395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Bauer G. Fighting HIV with stem cell therapy: one step closer to human trials. Expert Rev Anti Infect Ther. 2012;10:1071–1073. doi: 10.1586/eri.12.105. [DOI] [PubMed] [Google Scholar]
- Rossi JJ, June CH, Kohn DB. Genetic therapies against HIV. Nat Biotechnol. 2007;25:1444–1454. doi: 10.1038/nbt1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Zaia JA, Rossi JJ. Creating genetic resistance to HIV. Curr Opin Immunol. 2012;24:625–632. doi: 10.1016/j.coi.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hütter G, Nowak D, Mossner M, Ganepola S, Müssig A, Allers K, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- Allers K, Hütter G, Hofmann J, Loddenkemper C, Rieger K, Thiel E, et al. Evidence for the cure of HIV infection by CCR5?32/?32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, et al. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM, et al. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- Bai J, Gorantla S, Banda N, Cagnon L, Rossi J, Akkina R. Characterization of anti-CCR5 ribozyme-transduced CD34+ hematopoietic progenitor cells in vitro and in a SCID-hu mouse model in vivo. Mol Ther. 2000;1:244–254. doi: 10.1006/mthe.2000.0038. [DOI] [PubMed] [Google Scholar]
- Feng Y, Leavitt M, Tritz R, Duarte E, Kang D, Mamounas M, et al. Inhibition of CCR5-dependent HIV-1 infection by hairpin ribozyme gene therapy against CC-chemokine receptor 5. Virology. 2000;276:271–278. doi: 10.1006/viro.2000.0536. [DOI] [PubMed] [Google Scholar]
- An DS, Donahue RE, Kamata M, Poon B, Metzger M, Mao SH, et al. Stable reduction of CCR5 by RNAi through hematopoietic stem cell transplant in non-human primates. Proc Natl Acad Sci USA. 2007;104:13110–13115. doi: 10.1073/pnas.0705474104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger P, Andris-Widhopf J, Bühler B, Torbett BE, Barbas CF., 3rd Functional deletion of the CCR5 receptor by intracellular immunization produces cells that are refractory to CCR5-dependent HIV-1 infection and cell fusion. Proc Natl Acad Sci USA. 2000;97:805–810. doi: 10.1073/pnas.97.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, et al. A TALE nuclease architecture for efficient genome editing. Nat Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting ß-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41:9584–9592. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt N, Wang J, Kim K, Friedman G, Wang X, Taupin V, et al. Human hematopoietic stem/progenitor cells modified by zinc-finger nucleases targeted to CCR5 control HIV-1 in vivo. Nat Biotechnol. 2010;28:839–847. doi: 10.1038/nbt.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Walker J, Nolta JA, Bauer G. Specific transduction of HIV-susceptible cells for CCR5 knockdown and resistance to HIV infection: a novel method for targeted gene therapy and intracellular immunization. J Acquir Immune Defic Syndr. 2009;52:152–161. doi: 10.1097/QAI.0b013e3181b010a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Chen RX, McGee J, Nacey C, Pollard RB, Abedi M, et al. Generation of an HIV-1-resistant immune system with CD34(+) hematopoietic stem cells transduced with a triple-combination anti-HIV lentiviral vector. J Virol. 2012;86:5719–5729. doi: 10.1128/JVI.06300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Kamata M, Kittipongdaja P, Chen KN, Kim S, Pang S, et al. Characterization of a potent non-cytotoxic shRNA directed to the HIV-1 co-receptor CCR5. Genet Vaccines Ther. 2009;7:8. doi: 10.1186/1479-0556-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Hong P, Arumugam B, Pokomo L, Boyer J, Koizumi N, et al. A highly efficient short hairpin RNA potently down-regulates CCR5 expression in systemic lymphoid organs in the hu-BLT mouse model. Blood. 2010;115:1534–1544. doi: 10.1182/blood-2009-04-215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Kamata M, Chen KN, Pariente N, An DS, Chen IS. Inhibition of HIV-1 infection by a unique short hairpin RNA to chemokine receptor 5 delivered into macrophages through hematopoietic progenitor cell transduction. J Gene Med. 2010;12:255–265. doi: 10.1002/jgm.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamhane M, Akkina R. Stable gene transfer of CCR5 and CXCR4 siRNAs by sleeping beauty transposon system to confer HIV-1 resistance. AIDS Res Ther. 2008;5:16. doi: 10.1186/1742-6405-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin XF, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183–188. doi: 10.1073/pnas.232688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirane D, Sugao K, Namiki S, Tanabe M, Iino M, Hirose K. Enzymatic production of RNAi libraries from cDNAs. Nat Genet. 2004;36:190–196. doi: 10.1038/ng1290. [DOI] [PubMed] [Google Scholar]
- O'Brien WA, Koyanagi Y, Namazie A, Zhao JQ, Diagne A, Idler K, et al. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, et al. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald-Richter K, Grill SM, Leelawong M, Tseng M, Kalams SA, Hulgan T, et al. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Jiao YM, Wang R, Ji YX, Zhang HW, Zhang YH, et al. High CCR5 density on central memory CD4+ T cells in acute HIV-1 infection is mostly associated with rapid disease progression. PLoS ONE. 2012;7:e49526. doi: 10.1371/journal.pone.0049526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert LM, McColl SR. Up-regulation of CCR5 and CCR6 on distinct subpopulations of antigen-activated CD4+ T lymphocytes. J Immunol. 2002;168:65–72. doi: 10.4049/jimmunol.168.1.65. [DOI] [PubMed] [Google Scholar]
- Riley JL, Levine BL, Craighead N, Francomano T, Kim D, Carroll RG, et al. Naïve and memory CD4 T cells differ in their susceptibilities to human immunodeficiency virus type 1 infection following CD28 costimulation: implicatip6s for transmission and pathogenesis. J Virol. 1998;72:8273–8280. doi: 10.1128/jvi.72.10.8273-8280.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden MD, Kovochich M, Suree N, Shimizu S, Mehta R, Cortado R, et al. HIV latency in the humanized BLT mouse. J Virol. 2012;86:339–347. doi: 10.1128/JVI.06366-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton PW, Olesen R, Choudhary SK, Archin NM, Wahl A, Swanson MD, et al. Generation of HIV latency in humanized BLT mice. J Virol. 2012;86:630–634. doi: 10.1128/JVI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DD. Time to hit HIV, early and hard. N Engl J Med. 1995;333:450–451. doi: 10.1056/NEJM199508173330710. [DOI] [PubMed] [Google Scholar]
- Siliciano RF, Greene WC. HIV latency. Cold Spring Harb Perspect Med. 2011;1:a007096. doi: 10.1101/cshperspect.a007096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coiras M, López-Huertas MR, Pérez-Olmeda M, Alcamí J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. doi: 10.1038/nrmicro2223. [DOI] [PubMed] [Google Scholar]
- Gabriel R, Lombardo A, Arens A, Miller JC, Genovese P, Kaeppel C, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nat Biotechnol. 2011;29:816–823. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- Li MJ, Bauer G, Michienzi A, Yee JK, Lee NS, Kim J, et al. Inhibition of HIV-1 infection by lentiviral vectors expressing Pol III-promoted anti-HIV RNAs. Mol Ther. 2003;8:196–206. doi: 10.1016/s1525-0016(03)00165-5. [DOI] [PubMed] [Google Scholar]
- ter Brake O, ‘t Hooft K, Liu YP, Centlivre M, von Eije KJ, Berkhout B. Lentiviral vector design for multiple shRNA expression and durable HIV-1 inhibition. Mol Ther. 2008;16:557–564. doi: 10.1038/sj.mt.6300382. [DOI] [PubMed] [Google Scholar]
- Ringpis GE, Shimizu S, Arokium H, Camba-Colón J, Carroll MV, Cortado R, et al. Engineering HIV-1-resistant T-cells from short-hairpin RNA-expressing hematopoietic stem/progenitor cells in humanized BLT mice. PLoS ONE. 2012;7:e53492. doi: 10.1371/journal.pone.0053492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus MW, Estes JD, Padgett-Thomas A, Gatlin J, Denton PW, Othieno FA, et al. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.