Abstract
Periconceptional supplementation with folic acid reduces the occurrence of neural tube defects (NTDs). The association between maternal abnormalities in homocysteine metabolism (e.g., hyperhomocysteinaemia, folate deficiency and low vitamin B12) and the risk of NTDs-affected pregnancies has been widely evaluated in recent years, although the results are conflicting. To investigate this inconsistency, we performed a meta-analysis of 32 studies, involving 1,890 NTD-affected mothers and 3,995 control mothers, to develop an understanding of the relationship between maternal biomarkers related to one-carbon metabolism and NTD. A random-effects model was used to calculate the ratio of means (RoM) between the cases and controls, along with the 95% confidence intervals (CIs). A significant increase in homocysteine levels was observed in NTD-affected mothers compared with controls (RoM: 1.16, 95% CI: 1.09–1.23, P = 1.8 × 10−6). The pooled analysis also revealed that NTD-affected mothers had significantly lower levels of folate (RoM: 0.93, 95% CI: 0.88–0.97, P = 0.002), vitamin B12 (RoM: 0.91, 95% CI: 0.87–0.95, P = 3.6 × 10−5) and red blood cell folate (RoM: 0.92, 95% CI: 0.86–0.98, P = 0.01). Therefore, altered plasma levels of biomarkers related to one-carbon metabolism are associated with NTD-affected pregnancies.
Neural tube defects (NTDs) are thought to be one of the most common congenital defects1. However, NTDs are caused by partial or complete failure of neural tube closure that causes a wide range of malformations, including anencephaly, encephalocoele and spina bifida, which further complicates the identification of the etiological factor(s)2. Although the underlying causes for the development and progression of NTDs have not been fully elucidated, accumulated evidence suggests that genetic variants interact with environmental factors, thereby modifying the phenotype2,3,4,5.
Numerous studies have demonstrated that periconceptional supplementation with folic acid reduces the occurrence and recurrence risk of NTDs by 50–75%6, although the underlying mechanisms have not been fully elucidated. However, one report has suggested that impaired homocysteine metabolism may be involved in the protective mechanism of folate7. Several epidemiologic studies have also reported that women with NTD-affected pregnancies have mildly elevated homocysteine levels8,9. Interestingly, homocysteine is known to be a teratogen, and can cause NTD in a chick embryo model10. Similarly, vitamin B12 is an active cofactor for methionine synthase, and is involved in homocysteine remethylation from the methyltetrahydrofolate donor11. Independent of folate, vitamin B12 deficiency has also been associated with the functional state of folate deficiency, hyperhomocysteinaemia and an increased risk of NTDs12,13,14.
Over the past decades, a considerable amount of research has investigated the relationship between disturbed maternal metabolism of folate and homocysteine and NTDs, hoping to identify biomarkers that could facilitate non-invasive prenatal diagnosis and prevent congenital defects. However, the maternal metabolic profile for the relevant components of the metabolic pathway are not currently known, due to the complexity of the metabolism and the lack of effective methods. Furthermore, existing studies have reported inconsistent results regarding the relationship between a disturbed maternal metabolic profile and risk of NTDs. This inconsistency may be due to insufficient power of their limited sample size, false-positive results, phenotypic heterogeneity and/or publication biases. The interpretation of these studies has also been complicated by their use of different ethnic populations, or bias in the study design. Therefore, we performed a meta-analysis of the published studies to clarify this inconsistency, and to develop an understanding of the relationship between maternal biomarkers of one-carbon metabolism and NTDs.
Results
Characteristics of the included studies
The search generated 1,348 publications that were available on electronic bibliographic databases (Supplementary Figure S1). Of these publications, 32 relevant studies were identified, involving 1,890 NTD-affected mothers and 3,995 control mothers; 28 assessed more than one biomarker8,9,13,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43. The studies were conducted in a wide range of ethnicities, including Caucasian (42.3% of NTD-affected mothers), Asian (43.0%) and other ethnicities (14.7%). The 32 studies contained 35 datasets, including 19 retrospective comparisons and 16 prospective comparisons. Detailed characteristics of each study are in Table 1.
Table 1. Characteristics of studies included in meta-analysis.
| First author | Year | Ethnic population | Biomarkers | Study design | No. of cases/controls | NTDs-affected mothers | Control mothers | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | Gestational age (week) | Age | Gestational age (week) | Matching | ||||||
| Steegers-Theunissen | 1995 | Dutch | tHcy, VB12, folate, RBC folate | Prospective | 27/31 | 30.5 | 22.3 | 37.8 | 15.9 | Location |
| Ubbink | 1999 | African | tHcy, VB12, folate, RBC folate | Retrospective | 54/54 | 26.5 | nr | 28.3 | nr | Location, socioeconomic |
| Groenen | 2004 | Dutch | tHcy, VB12, folate, RBC folate | Retrospective | 45/83 | 32.3 | nr | 33.3 | nr | Location |
| Zhang | 2008 | Chinese | tHcy, VB12, folate | Prospective | 46/44 | nr | nr | nr | nr | Location |
| Mills | 1995 | Irish | tHcy, VB12, folate, RBC folate | Prospective | 76/315 | nr | nr | nr | nr | Location |
| Lucock | 1998 | British | tHcy, VB12, folate, RBC folate | Retrospective | 21/28 | nr | nr | nr | nr | Location, age, social class |
| Martín | 2004 | Spanish | tHcy | Retrospective | 44/181 | 36.0 | nr | 39.0 | nr | Location |
| Martínez de Villarreal | 2001 | Mexican | folate, RBC folate | Retrospective | 36/31 | 33.6 | nr | 26.9 | nr | Ages, neighbourhoods |
| Wang | 2008 | Chinese | tHcy, folate | Prospective | 46/50 | nr | nr | nr | nr | Location |
| Christensen | 1999 | Canadian | tHcy, folate, RBC folate | Retrospective | 61/90 | nr | nr | nr | nr | Location |
| Shaw | 2009 | American | tHcy, VB12, folate | Prospective | 80/409 | 25–29 | 15–18 | 30–34 | >20 | Location, ethnicity |
| Arbour | 2002 | Norwegian | tHcy, RBC folate | Retrospective | 73/101 | nr | nr | nr | nr | Location, age, ethnicity |
| Félix | 2004 | Brazilian | tHcy, VB12, folate | Retrospective | 41/44 | 25.8 | nr | 25.6 | nr | Location |
| Zhao | 2006 | American | tHcy, VB12, folate | Retrospective | 43/160 | nr | nr | nr | nr | Location, ethnicity |
| Ceyhan | 2010 | Turkish | tHcy, VB12, folate | Prospective | 31/32 | 26.6 | nr | 28.3 | nr | Location, gestation |
| Guo | 2013 | Chinese | tHcy, folate | Prospective | 64/64 | nr | nr | nr | nr | Location, age, gestation |
| Ratan | 2008 | Indian | tHcy, VB12, folate, RBC folate | Retrospective | 35/24 | nr | nr | nr | nr | Location, socio-economic and nutritional status |
| Lacasaña | 2012 | Mexican | tHcy, VB12, folate, RBC folate | Prospective | 99/91 | nr | nr | nr | nr | Location, maternity clinic, date of birth |
| Mobasheri | 2010 | Iran | tHcy, VB12, folate | Prospective | 23/23 | 24.0 | nr | 26.5 | nr | Location |
| Gu | 2012 | Chinese | tHcy, VB12, folate | Prospective | 30/60 | 27.6 | 26.7 | 28.7 | 26.4 | Location |
| Huang | 2013 | Chinese | tHcy, folate | Prospective | 121/118 | nr | nr | nr | nr | Location |
| Zheng | 2007 | Chinese | tHcy | Retrospective | 63/66 | nr | nr | nr | nr | Location |
| Wu | 2011 | Chinese | tHcy | Prospective | 4/30 | nr | 24–32 | nr | 24–40 | Location |
| Steegers-Theunissen | 1994 | Dutch | tHcy, VB12, folate, RBC folate | Retrospective | 41/50 | 35.4 | nr | 33.3 | nr | Location |
| Molloy | 1985 | Irish | VB12, folate | Prospective | 32/384 | nr | nr | nr | nr | Location |
| Gaber | 2007 | Egyptian | tHcy, VB12, folate | Prospective & Retrospective | 36/35 | 27.0 | nr | nr | nr | Location |
| Mills | 1992 | Finnish | VB12, folate | Prospective | 89/178 | nr | nr | nr | nr | Location |
| Economides | 1992 | British | VB12, folate, RBC folate | Prospective | 8/24 | nr | 17.9 | nr | 18.9 | Location |
| Afman | 2003 | Dutch | tHcy, VB12, folate, RBC folate | Retrospective | 112/73 | 42.9 | nr | 35.6 | nr | Location |
| Boddie | 2000 | American | folate, RBC folate | Retrospective | 11/11 | 26.5 | nr | 27.2 | nr | Location |
| Godbole | 2011 | Indian | tHcy, VB12, folate | Retrospective | 309/689 | 24 | 23.0 | 25 | 37.3 | Location |
| Ray | 2007 | Canadian | folate | Prospective | 89/422 | 28.6 | nr | 29.8 | nr | Location |
tHcy: total homocysteine; VB12: vitamin B12; RBC: red blood cell; nr: not reported.
Association between maternal total homocysteine levels and neural tube defects
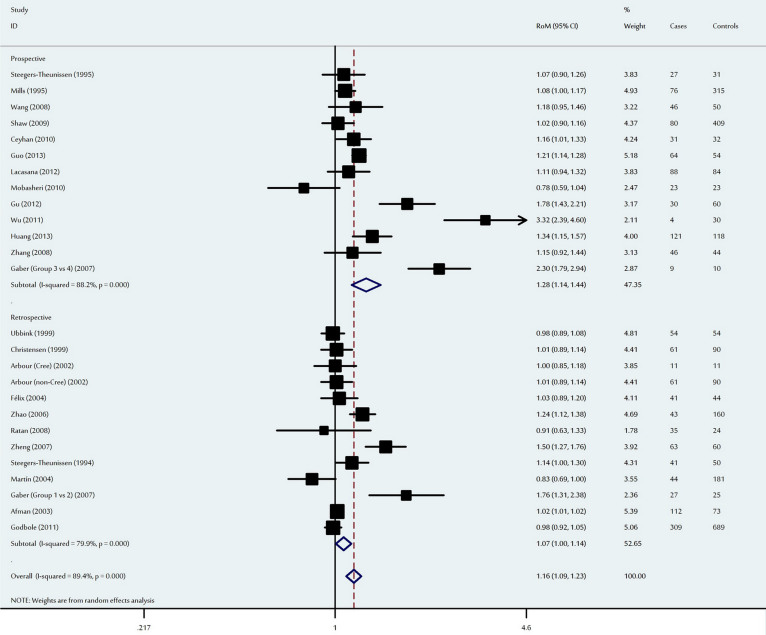
In the primary analysis of 26 studies, NTD-affected mothers had significantly higher mean total homocysteine levels compared to control mothers (RoM: 1.16, 95% CI: 1.09–1.23, P = 1.8 × 10−6). Ethnic subgroup analysis revealed that the pooled RoM for Asian mothers was 1.27 (95% CI: 1.11–1.46, P = 0.001), 1.05 for Caucasian mothers (95% CI: 1.00–1.11, P = 0.07) and 1.32 for the other ethnic populations (95% CI: 1.02–1.72, P = 0.04). When analysed according to study design, a significant increase in maternal homocysteine was observed among prospective studies (RoM: 1.28, 95% CI: 1.14–1.44, P = 3.2 × 10−5), with a marginally significant effect among retrospective studies (RoM = 1.07, 95% CI: 1.00–1.14, P = 0.04) (Figure 1). In addition, significant heterogeneity was observed among the 26 studies in both the overall and subgroup analysis (Table 2). To evaluate the possible sources of the heterogeneity, we conducted a meta-regression analysis of ethnicity, sample size, study design and age. Ethnicity (P < 10−4) and study design (P < 10−4) were significantly correlated with the magnitude of the effect, although age (P = 0.59), number of cases (P = 0.05) and number of controls (P = 0.44) were not.
Figure 1. Ratio of the mean (RoM) homocysteine levels in the NTD-affected mothers compared to the control mothers and the 95% confidence intervals, as stratified by the study design.
Table 2. Meta-analysis of maternal biomarkers related to the effect of one-carbon metabolism on risk of neural tube defects.
| Overall and subgroups analyses | tHcy | Folate | Vitamin B12 | RBC folate | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases/controls | RoM (95% CI) | P(Z) | P(Q) | I2 (%) | No. of cases/controls | RoM (95% CI) | P(Z) | P(Q) | I2 (%) | No. of cases/controls | RoM (95% CI) | P(Z) | P(Q) | I2 (%) | No. of cases/controls | RoM (95% CI) | P(Z) | P(Q) | I2 (%) | |
| All | 1,547/2,811 | 1.16 (1.09–1.23) | 1.8 × 10−6 | <10−5 | 89 | 1,694/3,690 | 0.93 (0.88–0.97) | 0.002 | <10−4 | 73 | 1,270/2,875 | 0.91 (0.87–0.95) | 3.6 × 10−5 | <10−3 | 57 | 628/827 | 0.92 (0.86–0.98) | 0.01 | <10−5 | 72 |
| Ethnicity | ||||||||||||||||||||
| Asian | 772/1,184 | 1.27 (1.11–1.46) | 0.001 | <10−5 | 90 | 699/1,091 | 0.88 (0.81–0.90) | 8.8 × 10−10 | 0.41 | 2 | 456/823 | 0.84 (0.70–1.02) | 0.07 | <10−4 | 84 | 35/24 | 0.74 (0.55–0.98) | 0.04 | NA | NA |
| Caucasian | 556/1,410 | 1.05 (1.00–1.11) | 0.07 | 0.003 | 64 | 666/2,147 | 0.93 (0.88–0.99) | 0.02 | 0.001 | 67 | 523/1,631 | 0.94 (0.92–0.95) | <10−10 | 0.60 | 0 | 405/627 | 0.90 (0.82–0.98) | 0.02 | <10−4 | 78 |
| Others | 219/217 | 1.32 (1.02–1.72) | 0.04 | <10−4 | 92 | 329/452 | 1.04 (0.84–1.28) | 0.74 | <10−5 | 87 | 291/421 | 0.83 (0.75–0.92) | 2.4 × 10−4 | 0.50 | 0 | 188/176 | 1.00 (0.93–1.08) | 0.99 | 0.47 | 0 |
| Study design | ||||||||||||||||||||
| Prospective | 645/1,300 | 1.28 (1.14–1.44) | 3.2 × 10−5 | <10−5 | 88 | 869/2,255 | 0.91 (0.86–0.96) | 0.001 | 0.05 | 40 | 546/1,580 | 0.90 (0.83–0.97) | 0.008 | 0.001 | 66 | 214/393 | 0.95 (0.80–1.14) | 0.58 | 0.03 | 67 |
| Retrospective | 902/1,511 | 1.07 (1.00–1.14) | 0.04 | <10−5 | 80 | 825/1,435 | 0.95 (0.86–1.04) | 0.24 | <10−5 | 84 | 724/1,295 | 0.91 (0.86–0.97) | 0.004 | 0.06 | 45 | 414/434 | 0.91 (0.84–0.98) | 0.02 | <10−5 | 74 |
P(Z): Z test used to determine the significance of the overall OR.
P(Q): Cochran's chi-square Q statistic test used to assess the heterogeneity in subgroups.
NA: not available; RBC: red blood cell; RoM: ratio of the mean; tHcy: total homocysteine.
We also conducted sensitivity analysis, and the results confirmed the significant difference between maternal homocysteine levels for NTD-affected cases and controls, with RoMs and 95% CIs ranging from 1.13 (95% CI: 1.07–1.20) to 1.17 (95% CI: 1.09–1.27) (Supplementary Figure S2). Funnel plots were used to identify small study effects, and the results did not indicate the presence of publication bias among the studies (Supplementary Figure S3). The results of Egger's test (P = 0.06) confirmed the absence of publication bias.
Association between maternal folate levels and neural tube defects
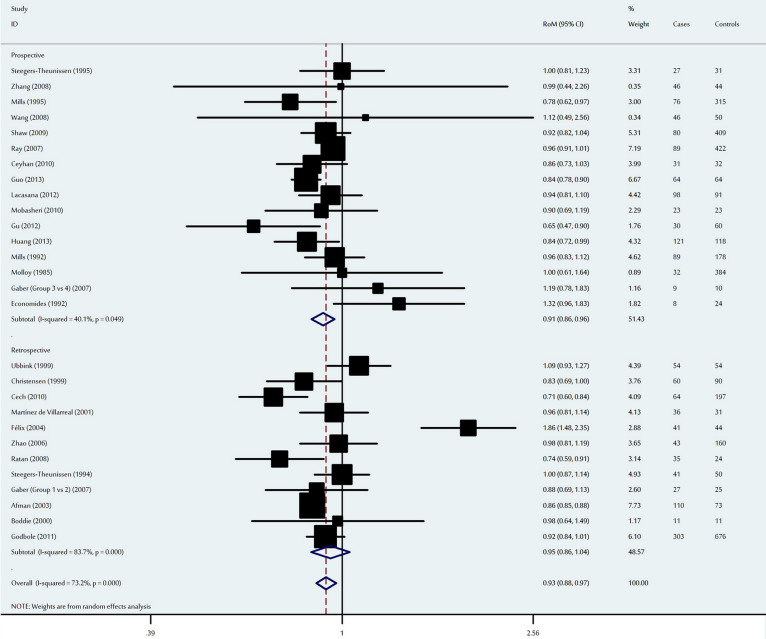
The meta-analysis detected a significant decrease in maternal folate levels among NTD-affected mothers, with an overall RoM of 0.93 (95% CI: 0.88–0.97, P = 0.002) (Figure 2) and statistically significant inter-study heterogeneity (P < 10−4). To determine the potential sources of this heterogeneity, we conducted subgroup analyses according to ethnicity and study design. When stratified by ethnicity, a significant decrease in maternal folate levels was observed among Asian and Caucasian mothers, with RoMs of 0.88 (95% CI: 0.81–0.90, P = 8.8 × 10−10) and 0.93 (95% CI: 0.88–0.99, P = 0.02) respectively. However, no significant difference was detected among the other ethnic populations (Table 2). When analysed according to study design, a significant decrease in folate levels was observed among prospective studies (RoM: 0.91, 95% CI: 0.86–0.96, P = 0.001), although no significant difference was detected among retrospective studies (Table 2). Ethnicity, age, number of cases, number of controls and study design were not significant sources of inter-study heterogeneity (P > 0.05 for all).
Figure 2. Ratio of the mean (RoM) folate levels in the NTD-affected mothers compared to the control mothers and the 95% confidence intervals, as stratified by the study design.
Sensitivity analysis indicated that no single study qualitatively influenced the pooled RoM, which suggests that the results of this meta-analysis were statistically robust (Supplementary Figure S4). The shape of the funnel plots were symmetrical (Supplementary Figure S5), and Egger's test did not reveal publication bias (P = 0.05).
Association between maternal vitamin B12 levels and neural tube defects
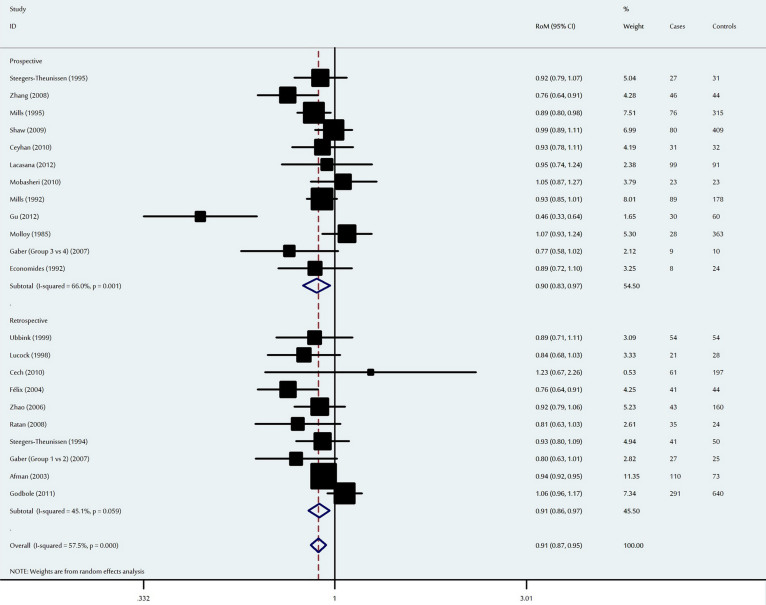
The meta-analysis detected a significant decrease in maternal vitamin B12 levels among NTD-affected mothers, with an overall RoM of 0.89 (95% CI: 0.84–0.94, P = 4.9 × 10−5) (Figure 3). When stratified by ethnicity, Asian mothers had a RoM of 0.79 (95% CI: 0.64–0.99, P = 0.04), Caucasian mothers had a RoM of 0.93 (95% CI: 0.90–0.98, P = 0.002), and mothers of other ethnicities had a RoM of 0.83 (95% CI: 0.75–0.92, P = 2.4 × 10−4). When analysed according to study design, the effects were significant for both prospective and retrospective studies, with RoMs of 0.90 (95% CI: 0.83–0.97, P = 0.008) and 0.86 (95% CI: 0.81–0.93, P = 4.5 × 10−5), respectively. In the meta-regression analysis, ethnicity (P = 0.07), age (P = 0.25), study design (P = 0.13), number of cases (P = 0.31) and number of controls (P = 0.13) explained the minimal level of heterogeneity.
Figure 3. Ratio of the mean (RoM) vitamin B12 levels in the NTD-affected mothers to compared to the control mothers and the 95% confidence intervals, as stratified by the study design.
One-way sensitivity analyses confirmed that no single study qualitatively influenced the pooled RoM (Supplementary Figure S6). The shape of the funnel plots were symmetrical (Supplementary Figure S7), and Egger's test confirmed the absence of significant publication bias (P = 0.16).
Association between maternal red blood cell folate levels and neural tube defects
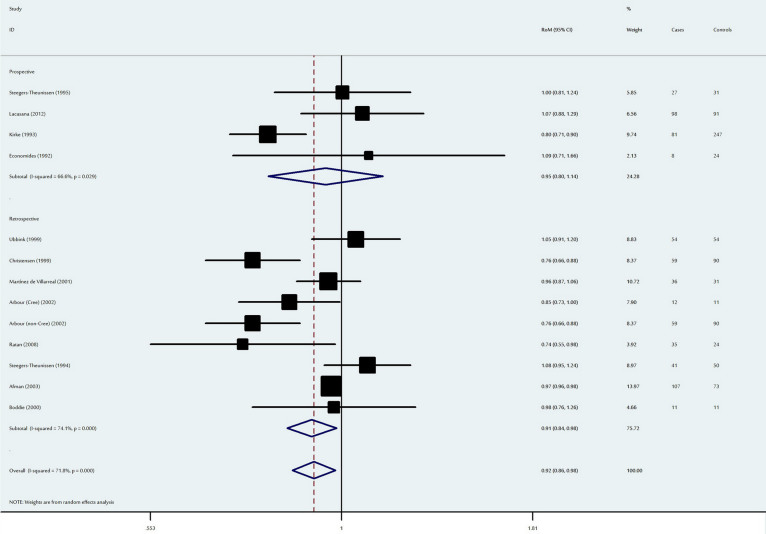
Using the random effect model, a marginally significant decrease in maternal RBC folate levels was observed among NTD-affected mothers (RoM: 0.92, 95% CI: 0.86–0.98, P = 0.01) (Figure 4). When stratified by ethnicity, maternal RBC folate levels were significantly lower among Asian (P = 0.04) and Caucasian (P = 0.02) NTD-affected mothers; no significant difference was detected for the other ethnic populations (Table 2). When analysed according to study design, prospective studies had a RoM of 0.95 (95% CI: 0.80–1.14, P = 0.58), while retrospective studies had a RoM of 0.91 (95% CI: 0.84–0.98, P = 0.02).
Figure 4. Ratio of the mean (RoM) red blood cell folate levels in the NTD-affected mothers compared to the control mothers and the 95% confidence intervals, as stratified by the study design.
Sensitivity analysis indicated that the results of this meta-analysis were stable, with RoMs and 95% CIs ranging from 0.903 (95% CI: 0.841–0.969, P = 0.005) to 0.934 (95% CI: 0.876–0.996, P = 0.038) (Supplementary Figure S8). No publication bias (Egger's test, P = 0.22) was observed for this overall meta-analysis (Supplementary Figure S9).
Discussion
This meta-analysis of 32 association studies, involving 1,890 NTD-affected mothers and 3,995 control mothers, provides the first comprehensive assessment of the relationship between disturbed maternal folate and homocysteine metabolism and the risk of NTDs. Our results indicate that NTD-affected mothers have higher levels of homocysteine, and lower levels of folate, vitamin B12 and RBC folate, compared to mothers with unaffected offspring.
To identify and evaluate potential sources of heterogeneity, we performed several subgroup analyses according to ethnicity and study design. As we detected significant heterogeneity between the studies included in our meta-analysis, the results must be interpreted with caution. However, the high degree of inter-study heterogeneity may be complicated by several factors. First, heterogeneity may exist in the NTD groups, as the phenotypic severity varies with the type and level of the lesion. Second, variations in vitamin supplement content and use by the studied populations may contribute to these different results. Furthermore, the nutritional habits and dietary folic acid intake may have varied across different study populations. Third, differences in sample collection protocols (occasionally several years postpartum) may also have affected the results. The optimal period for blood sampling is in the first several weeks of pregnancy, before or soon after the failed closure of the neural tube, as nutritional changes may occur after delivery. Finally, differences in the sensitivity and/or specificity of the analytical techniques, or sample degradation during storage, may also have affected the results.
Among the prospective studies, we observed a significant increase in homocysteine metabolism in NTD-affected women. If congenital susceptibility to NTDs is partially mediated by metabolic derangement, it is possible that exposure to high levels of homocysteine may affect the closure of the neural tube at its rostral pole (i.e., during the third or fourth week after conception). Although most blood samples in the prospective studies were collected during pregnancy, they were generally collected several weeks after closure of the neural tube. Therefore, if the resulting measurement error biased our results, it likely resulted in an underestimation of the measured effects.
Several studies have previously implied that excess homocysteine, regardless of the source of its elevation, might play an independent role in the development of NTDs9,29. Other studies have reported that homocysteine overload could affect the embryo's development by interfering with the embryonic cell cycle or inducing apoptosis, thereby leading to NTDs44. Recently, Rosenquist et al. have reported that homocysteine increased the number of NTDs when administered to chicken embryos in ovo45. However, another study reported that homocysteine failed to induce NTDs in a standard mouse model46. Therefore, it has been suggested that homocysteine may indirectly affect the development of NTDs47, possibly through a mechanism whereby homocysteinylation damages proteins and alters their function48.
When we stratified our analysis by study design, significantly lower levels of folate and vitamin B12 were observed in NTD-affected mothers (vs. controls) from the prospective studies. Maternal folic acid intake substantially reduces the probability of occurrence or recurrence of NTDs2,6, and our results support this benefit. In addition, folate responsiveness is important in the development of NTDs49, and we observed decreased levels of RBC folate in NTD-affected mothers. RBC folate reflects the level of intracellular folate and folate turnover during the previous 4 months, and is generally considered a more useful indicator of folate status than serum folate. Similar to our results, Daly et al. have suggested that decreased maternal RBC folate in early pregnancy is a marker of NTD risk50, in a concentration-dependent manner. Interestingly, vitamin B12 is an important coenzyme in the metabolic pathway of homocysteine, and affects homocysteine levels by maintaining the activity of folate. Therefore, either deficiency in folate or vitamin B12 can result in elevated plasma levels of homocysteine. As vitamin B12 is mainly absorbed from dietary intake, it is possible that mothers suffer from vitamin B12 deficiency due to changes in dietary patterns during pregnancy43, thus further increasing the risk of NTDs. As abnormality in homocysteine metabolism is present in NTD-affected women, our findings suggest that periconceptional supplements of vitamin B12 and folic acid may be more effective in NTD prevention.
As a retrospective study, the current meta-analysis is subject to the methodological deficiencies of the included studies, and several specific details merit consideration. First, heterogeneity is a potential factor must be considered when interpreting our results. Although we failed to identify the main sources of the heterogeneity in effect size, a meta-analysis of reported data has little capacity to do so. Ideally, we would prefer to pool individual-level data to allow for efficient assessment of the sources of heterogeneity, although this would be impractical for the present study. Second, populations from geographically distinct countries were pooled according to ethnicity in the subgroup meta-analyses, which may have generated a fluctuation effect. Third, our results were based on unadjusted estimates. If each individual's raw data were available, a more precise analysis could be conducted, which could be adjusted for other co-variants, such as age, obesity, folate supplementation and other lifestyle factors.
Despite these limitations, the findings of the present study indicate that altered plasma levels of biomarkers related to one-carbon metabolism are associated with NTD-affected pregnancies. To confirm these findings, future studies should involve a prospective design, strict selection of cases and larger studies of diverse ethnic populations.
Methods
Identification and screening of relevant studies
Given the limited evidence available regarding other metabolism biomarkers, the present meta-analysis was restricted to studies that evaluated maternal total homocysteine, folate, vitamin B12 and red blood cell (RBC) folate. We used computer-based searches to identify epidemiologic association studies published before September 2014, which investigating at least one of the four metabolism biomarkers in NTD-affected pregnancies. The databases we searched included PubMed, ISI Web of Science, EMBASE, EBSCO, the Cochrane Library databases, and the Chinese National Knowledge Infrastructure. No language restrictions were imposed on our search.
The search included keywords relating to NTDs (e.g., “neural tube defects”, “anencephaly”, “encephalocele” and “spina bifida”) in combination with words related to the maternal biomarkers (e.g., “homocysteine”, “hyperhomocysteinemia”, “folic acid”, “folate”, “vitamin B12” and “red blood cell folate”). The titles and abstracts from retrieved articles were screened to determine their relevance, and irrelevant studies were excluded without further evaluation. For the remaining articles, the full texts were evaluated to determine whether they contained data of interest. The reference lists from all relevant publications were also hand-searched for additional eligible reports.
For inclusion, studies were required to meet all of the following criteria: (1) investigated maternal one-carbon metabolism and the risk of NTDs (2) original human studies with independent data; (3) followed a case-control or cohort study design; (4) maternal biomarkers were measured separately for cases and controls using a reliable assay; (5) described the assessment methods, equipment and protocols, or provided reference to them; (6) the results were expressed as, or could be estimated into, mean and standard deviation (SD). Major exclusion criteria were: (1) case-only studies and (2) overlapping data. Case reports, editorials, and review articles were also excluded. If more than one paper was published studying the same sample series, only the study with the largest sample size and the most detailed information was included. Studies with different ethnic groups were considered as individual studies for our analyses.
NTD-affected pregnancies were defined as women whose children had NTDs, including live births, stillbirths, and prenatally diagnosed foetuses. Control subjects were defined as healthy women with unaffected pregnancies, or those who had a normal child-bearing history without any history of congenital malformation.
Data extraction
The two authors independently extracted the following information from each study according to a fixed protocol: first author, year of publication, ethnicity of the study population, clinical characteristics, matching criteria, study design, quantified method of biochemical analyses, diagnostic criteria for NTDs, number of cases and controls, collection strategy for maternal biological specimens and the mean and SD values for metabolites from the cases and controls. When only median and range were reported in the text, a conversion formula51 was used to calculate the mean and SD. Data reports from the two reviewers were than compared to identify any inconsistency, and differences were resolved by further discussion among all authors through consensus.
Statistical analysis
As metabolite concentrations were measured using different methods and reported in various units across different studies, we therefore used a ratio method to meta-analyse continuous values for each metabolite52,53,54. In brief, the metabolite concentrations difference in the means between NTD cases and controls for each study was expressed as the ratio of the mean (RoM). RoM was defined as the mean value of the NTD group divided by that of the control group. The variance of RoM was estimated using the delta method55, and the values were pooled using inverse-variance weighting. Heterogeneity across studies was assessed using Cochran's chi-square Q test and the I2 test56. Random effects models were used to calculate the overall results, as these assume a genuine diversity in the results of various studies, and typically provide wider confidence intervals (CIs) when significant inter-study heterogeneity exists57. The 95% CIs were constructed using Woolf's method, and the significance of the overall RoM was determined using the Z-test.
Sources of heterogeneity were investigated by stratified meta-analyses based on ethnicity and study design (prospective vs. retrospective study). A prospective study was defined as a study where the maternal blood sample was collected before delivery, and a retrospective study was defined as a study where the maternal blood sample was collected postpartum. Ethnic groups were defined as Asian (i.e., Chinese, Japanese, and Indian), Caucasian (i.e., subjects of European ancestry) or other ethnic populations. In addition, ethnicity, sample size and study design were analysed as covariates in a meta-regression. To assess the stability of the results, sensitivity analysis was performed by removing each individual study in turn from the total, and re-analysing the remaining studies. Funnel plots and Egger's linear regression test were used to evaluate potential publication bias58. The type I error rate was set at 0.05 for two-sided analysis. All the analyses were performed using Stata 10.0 software (STATA Corp., College Station, TX, USA).
Author Contributions
Conceived and designed the study: K.F.T.; Performed the experiments: K.F.T., Y.L.L. and H.Y.W.; Contributed material/analysis tools: K.F.T. and Y.L.L.; Statistical analyses and paper writing, revising: K.F.T. and H.Y.W.
Supplementary Material
Supplementary information
Acknowledgments
This work was supported by the grants from the 973 Program (2013CB945403), the National Science Fund for Distinguished Young Scholars (81025003), the Doctoral Fund in Ministry of Education of China (20110071110026), and the Commission for Science and Technology of Shanghai Municipality (13JC1407600) to Dr. Wang; China Postdoctoral Science Foundation funded project (2013M530179) to Dr. Tang. We thank Chittaranjan Yajnik, Giriraj Chandak, Charu Joglekar and Koumudi Godbole for providing data from their studies for this meta-analysis.
References
- Verma I. C. High frequency of neural tube defects in North India. Lancet 1, 879–880 (1978). [DOI] [PubMed] [Google Scholar]
- Blom H. J., Shaw G. M., den Heijer M. & Finnell R. H. Neural tube defects and folate: case far from closed. Nat Rev Neurosci 7, 724–731 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y. P. et al. VANGL2 mutations in human cranial neural-tube defects. N Engl J Med 362, 2232–2235 (2010). [DOI] [PubMed] [Google Scholar]
- Kibar Z. et al. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356, 1432–1437 (2007). [DOI] [PubMed] [Google Scholar]
- Motulsky A. Nutritional ecogenetics: homocysteine-related arteriosclerotic vascular disease, neural tube defects, and folic acid. Am J Hum Genet 58, 17–20 (1996). [PMC free article] [PubMed] [Google Scholar]
- De Wals P. et al. Reduction in neural-tube defects after folic acid fortification in Canada. N Engl J Med 357, 135–142 (2007). [DOI] [PubMed] [Google Scholar]
- Dunlevy L. P. et al. Abnormal folate metabolism in foetuses affected by neural tube defects. Brain 130, 1043–1049 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegers-Theunissen R. P. et al. Maternal hyperhomocysteinemia: a risk factor for neural-tube defects? Metabolism 43, 1475–1480 (1994). [DOI] [PubMed] [Google Scholar]
- Mills J. L. et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet 345, 149–151 (1995). [DOI] [PubMed] [Google Scholar]
- Afman L. A., Blom H. J., Van der Put N. M. & Van Straaten H. W. Homocysteine interference in neurulation: a chick embryo model. Birth Defects Res 67, 421–428 (2003). [DOI] [PubMed] [Google Scholar]
- Thompson M. D., Cole D. E. & Ray J. G. Vitamin B-12 and neural tube defects: the Canadian experience. Am J Clin Nutr 89, 697S–701S (2009). [DOI] [PubMed] [Google Scholar]
- Kirke P. N. et al. Maternal plasma folate and vitamin B 12 are independent risk factors for neural-tube defects. QJM 86, 703–708 (1993). [PubMed] [Google Scholar]
- Groenen P. M. et al. Marginal maternal vitamin B12 status increases the risk of offspring with spina bifida. Am J Obstet Gynecol 191, 11–17 (2004). [DOI] [PubMed] [Google Scholar]
- Ray J. G. & Blom H. J. Vitamin B12 insufficiency and the risk of fetal neural tube defects. QJM 96, 289–295 (2003). [DOI] [PubMed] [Google Scholar]
- Steegers-Theunissen R. P. et al. Neural tube defects and elevated homocysteine levels in amniotic fluid. Am J Obstet Gynecol 172, 1436–1441 (1995). [DOI] [PubMed] [Google Scholar]
- Ubbink J. B. et al. Folate status, homocysteine metabolism, and methylene tetrahydrofolate reductase genotype in rural South African blacks with a history of pregnancy complicated by neural tube defects. Metabolism 48, 269–274 (1999). [DOI] [PubMed] [Google Scholar]
- Lucock M. D., Daskalakis I., Lumb C. H., Schorah C. J. & Levene M. I. Impaired regeneration of monoglutamyl tetrahydrofolate leads to cellular folate depletion in mothers affected by a spina bifida pregnancy. Mol Genet Metab 65, 18–30 (1998). [DOI] [PubMed] [Google Scholar]
- Martín I. et al. Oxidative stress in mothers who have conceived fetus with neural tube defects: the role of aminothiols and selenium. Clin Nutr 23, 507–514 (2004). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Simultaneous quantification of 11 pivotal metabolites in neural tube defects by HPLC-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 863, 94–100 (2008). [DOI] [PubMed] [Google Scholar]
- Christensen B. et al. Genetic polymorphisms in methylenetetrahydrofolate reductase and methionine synthase, folate levels in red blood cells, and risk of neural tube defects. Am J Med Genet 84, 151–157 (1999). [DOI] [PubMed] [Google Scholar]
- Shaw G. M. et al. Choline and risk of neural tube defects in a folate-fortified population. Epidemiology 20, 714–719 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang H. Y. et al. Neural tube defects and disturbed maternal folate- and homocysteine-mediated one-carbon metabolism. Exp Neurol 212, 515–521 (2008). [DOI] [PubMed] [Google Scholar]
- Martínez de Villarreal L. E. et al. Folate levels and N(5),N(10)-methylenetetrahydrofolate reductase genotype (MTHFR) in mothers of offspring with neural tube defects: a case-control study. Arch Med Res 32, 277–282 (2001). [DOI] [PubMed] [Google Scholar]
- Molloy A. M., Kirke P., Hillary I., Weir D. G. & Scott J. M. Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch Dis Child 60, 660–665 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour L. et al. Spina bifida, folate metabolism, and dietary folate intake in a Northern Canadian aboriginal population. Int J Circumpolar Health 61, 341–351 (2002). [DOI] [PubMed] [Google Scholar]
- Félix T. M., Leistner S. & Giugliani R. Metabolic effects and the methylenetetrahydrofolate reductase (MTHFR) polymorphism associated with neural tube defects in southern Brazil. Birth Defects Res A Clin Mol Teratol 70, 459–463 (2004). [DOI] [PubMed] [Google Scholar]
- Zhao W. et al. Neural tube defects and maternal biomarkers of folate, homocysteine, and glutathione metabolism. Birth Defects Res A Clin Mol Teratol 76, 230–236 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan S. T. et al. Serum vitamin B12 and homocysteine levels in pregnant women with neural tube defect. Gynecol. Endocrinol 26, 578–581 (2010). [DOI] [PubMed] [Google Scholar]
- Economides D. L. et al. Folate and vitamin B12 concentrations in maternal and fetal blood, and amniotic fluid in second trimester pregnancies complicated by neural tube defects. Br J Obstet Gynaecol 99, 23–25 (1992). [DOI] [PubMed] [Google Scholar]
- Afman L. A., Trijbels F. J. & Blom H. J. The H475Y polymorphism in the glutamate carboxypeptidase II gene increases plasma folate without affecting the risk for neural tube defects in humans. J Nutr 133, 75–77 (2003). [DOI] [PubMed] [Google Scholar]
- Godbole K. et al. Maternal one-carbon metabolism, MTHFR and TCN2 genotypes and neural tube defects in India. Birth Defects Res A Clin Mol Teratol 91, 848–856 (2011). [DOI] [PubMed] [Google Scholar]
- Guo J. et al. The maternal folate hydrolase gene polymorphism is associated with neural tube defects in a high-risk Chinese population. Genes Nutr 8, 191–197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratan S. K. et al. Evaluation of the levels of folate, vitamin B12, homocysteine and fluoride in the parents and the affected neonates with neural tube defect and their matched controls. Pediatr Surg Int 24, 803–808 (2008). [DOI] [PubMed] [Google Scholar]
- Lacasaña M. et al. Effect on risk of anencephaly of gene-nutrient interactions between methylenetetrahydrofolate reductase C677T polymorphism and maternal folate, vitamin B12 and homocysteine profile. Public Health Nutr 15, 1419–1428 (2012). [DOI] [PubMed] [Google Scholar]
- Mobasheri E., Keshtkar A. & Golalipour M. J. Maternal folate and vitamin b(12) status and neural tube defects in northern iran: a case control study. Iran J Pediatr 20, 167–173 (2010). [PMC free article] [PubMed] [Google Scholar]
- Boddie A. M. et al. Folate absorption in women with a history of neural tube defect-affected pregnancy. Am J Clin Nutr 72, 154–158 (2000). [DOI] [PubMed] [Google Scholar]
- Gu Q., Li Y., Cui Z. L. & Luo X. P. Homocysteine, folate, vitamin B12 and B6 in mothers of children with neural tube defects in Xinjiang, China. Acta Paediatr 101, e486–e490 (2012). [DOI] [PubMed] [Google Scholar]
- Huang M., Liang X. P., Liang Q. L., Wang M. Y. & Luo A. G. A comparative study among folic acid and its related metabolites on risk assessment and prediction of neural tube defects. Chinese Journal of Analytical Chemistry 41, 15–19 (2013). [in Chinese] [Google Scholar]
- Zheng M. L., Wang G. H. & Zhang G. L. Relationship of plasma homocysteine (HCY) and the gene polymorphism of metabolic enzymes with neural tube defects. Chinese Journal of Healthy Birth & Child Care 15, 158–161 (2007). [in Chinese] [Google Scholar]
- Wu X., Liu G. S., Wan B. & Feng Q. Relationship between maternal plasma homocysteine level and fetal birth defects and its clinical significance. J Appl Clin Pediatr 26, 1104–1107 (2011). [in Chinese] [Google Scholar]
- Gaber K. R., Farag M. K., Soliman S. E., El-Bassyouni H. T. & El-Kamah G. Maternal vitamin B12 and the risk of fetal neural tube defects in Egyptian patients. Clin Lab 53, 69–75 (2007). [PubMed] [Google Scholar]
- Mills J. L. et al. Maternal vitamin levels during pregnancies producing infants with neural tube defects. J Pediatr 120, 863–871 (1992). [DOI] [PubMed] [Google Scholar]
- Ray J. G. et al. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology 18, 362–366 (2007). [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. Methionine metabolism in mammals. J Nutr Biochem 1, 228–237 (1990). [DOI] [PubMed] [Google Scholar]
- Rosenquist T. H., Ratashak S. A. & Selhub J. Homocysteine induces congenital heart and neural tube defects: effect of folic acid. Proc Natl Acad Sci USA 93, 15227–1532 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G. D. et al. Failure of homocysteine to induce neural tube defects in a mouse model. Birth Defects Res B Dev Reprod Toxicol 77, 89–94 (2006). [DOI] [PubMed] [Google Scholar]
- Taparia S., Gelineau-van Waes J., Rosenquist T. H. & Finnell R. H. Importance of folate-homocysteine homeostasis during early embryonic development. Clin Chem Lab Med 45, 1717–1727 (2007). [DOI] [PubMed] [Google Scholar]
- Zinellu A. et al. S-homocysteinylated LDL apolipoprotein B adversely affects human endothelial cells in vitro. Atherosclerosis 206, 40–46 (2009). [DOI] [PubMed] [Google Scholar]
- Beaudin A. E. et al. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am J Clin Nutr 93, 789–798 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly L. E., Kirke P. N., Molloy A., Weir D. G. & Scott J. M. Folate levels and neural tube defects: implications for prevention. JAMA 274, 1698–1702 (1995). [DOI] [PubMed] [Google Scholar]
- Hozo S. P., Djulbegovic B. & Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5, 13 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich J. O., Adhikari N. K. & Beyene J. The ratio of means method as an alternative to mean differences for analyzing continuous outcome variables in meta-analysis: a simulation study. BMC Med Res Methodol 8, 32 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud S. et al. High frequency oscillation in patients with acute lung injury and acute respiratory distress syndrome (ARDS): systematic review and meta-analysis. BMJ 340, c2327 (2010). [DOI] [PubMed] [Google Scholar]
- Friedrich J. O., Adhikari N., Herridge M. S. & Beyene J. Meta-analysis: low-dose dopamine increases urine output but does not prevent renal dysfunction or death. Ann Intern Med 142, 510–524 (2005). [DOI] [PubMed] [Google Scholar]
- Friedrich J. O., Adhikari N. K. & Beyene J. Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J Clin Epidemiol 64, 556–564 (2011). [DOI] [PubMed] [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey Smith G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information