Abstract
The detection of Listeria monocytogenes from food is currently carried out using a double enrichment. For the ISO methodology, this double enrichment is performed using half-Fraser and Fraser broths, in which the overgrowth of L. innocua can occur in samples where both species are present. In this study, we analyzed the induction of phages and phage tails of Listeria spp. in these media and in two brain heart infusion (BHI) broths (BHIM [bioMérieux] and BHIK [Biokar]) to identify putative effectors. It appears that Na2HPO4 at concentrations ranging from 1 to 40 g/liter with an initial pH of 7.5 can induce phage or phage tail production of Listeria spp., especially with 10 g/liter of Na2HPO4 and a pH of 7.5, conditions present in half-Fraser and Fraser broths. Exposure to LiCl in BHIM (18 to 21 g/liter) can also induce phage and phage tail release, but in half-Fraser and Fraser broths, the concentration of LiCl is much lower (3 g/liter). Low phage titers were induced by acriflavine and/or nalidixic acid. We also show that the production of phages and phage tails can occur in half-Fraser and Fraser broths. This study points out that induction of phages and phage tails could be triggered by compounds present in enrichment media. This could lead to a false-negative result for the detection of L. monocytogenes in food products.
INTRODUCTION
The widespread prevalence of Listeria monocytogenes in food products, the severity of illness caused, and the number of reported outbreaks require fast and reliable detection of the pathogen (1). Moreover, the detection method has to be sensitive enough to detect L. monocytogenes at levels as low as 1 cell per gram of food material. This usually involves selective enrichment procedures due to both the low density of these microorganisms and the high levels of background microflora that are normally simultaneously present. For this detection, the ISO 11290-1 reference method is recommended; the first enrichment is performed in half-Fraser broth and the second in Fraser broth (2). Several studies showed that L. innocua and L. monocytogenes were found together in food, with L. innocua being more frequently isolated than L. monocytogenes (3, 4). This could result from the evolution of the ratio between these species during the enrichment process in an way that was advantageous for L. innocua. Competition between L. monocytogenes strains of different serotypes is also possible and has been demonstrated between serotypes 1 and 4 (5). These competitions could be due either to nutritional factors (components of the growth medium can differentially favor bacterial multiplication) or to the production of compounds inhibiting L. monocytogenes growth (3, 6–8). Kalmokoff et al. (9) showed that, among 300 Listeria strains tested, 71% produced phages or phage tails (68% of 50 L. innocua strains). Thus, during the enrichment steps necessary for the multiplication of the Listeria strains, the growth of nonpathogenic species could cause false-negative detection of low levels of L. monocytogenes (10). Until now, the influence of environmental factors on phage induction has been poorly described, with only a few publications concerning it. For example, NaCl induces phages in Escherichia coli (11, 12), lithium chloride (LiCl) in E. coli (12), phosphate in bacteria of marine origin (13–15), and pH in Nitrosospira multiformis (16). Antibiotics can also induce increases in phage titers, for example, ciprofloxacin in Pseudomonas aeruginosa (17); β-lactams, ciprofloxacin, and trimethoprim antibiotics in Staphylococcus aureus (18, 19); β-lactam and quinolone antibiotics in E. coli (20); norfloxacin in Shiga toxin-producing E. coli (11); and β-lactam antibiotics in Lactococcus lactis (21). Surprisingly, nothing has been described in the literature concerning Listeria spp.
During previous investigations, we observed that L. monocytogenes EGD-e phage tail induction occurred in brain heart infusion (BHI) broth from BioKar (France) (BHIK) but not in BHI broth from bioMérieux (France) (BHIM). It seemed interesting to analyze this phenomenon and to search for a putative inducing factor(s). Here, phage or phage tail induction levels were evaluated in the two BHI broths using 22 strains of Listeria spp. with different origins. Then, two treatments were assayed in order to perform fractionation of the BHIK medium, which should help in localizing the active compounds: ultrafiltration and adsorption on activated charcoal. Since phosphate presence was suspected to be involved in the phage or phage tail induction observed in BHIK, we evaluated the influence of both disodium hydrogen phosphate (Na2HPO4) addition in BHIM and the initial pH on phage tail induction in EGD-e cultures. Other putative inducers were also assayed under similar conditions: three selective agents, LiCl, acriflavine, and/or nalidixic acid. Finally, for comparison, the assessment of phages or phage tails was also performed with Listeria strains grown in half-Fraser broth and Fraser broth, the media recommended in the ISO reference method.
MATERIALS AND METHODS
Bacterial strains.
The Listeria strains used in this study are listed in Table 1. They were isolated in our laboratory (22) from cheeses, meat products, or human blood, except EGD-e, a reference strain that was provided by the Pasteur Institute Collection (Paris, France). These strains, except L. ivanovii RR3, produce either phages or phage tails. Genome analysis of L. monocytogenes EGD-e revealed one putative prophage (ΦEGD-e.2; lmo2271 to lmo2332) and one putative phage tail (ΦEGD-e.1; lmo0113 to lmo0129) (23). The laboratory strain L. ivanovii RR3 was used as an indicator strain for phage detection (22).
TABLE 1.
Bacterial strains used in this studya
| Listeria species | Strain code(s) | Phage or phage tails |
|---|---|---|
| L. innocua | 18.2, 22, 23, 25, 43, 45, F(d), NV2, P1, P6 | Phage |
| Pi2, Pi3, Pi53 | Phage tails | |
| L. seeligeri | RR4 | Phage |
| L. monocytogenes | 4F, 7F, 8F, 1S, H4, NV3, P8, Pi17, EGD-e | Phage tails |
| L. ivanovii | RR3 (indicator strain) | No production |
The Listeria strains and the indicator strain were used for phage or phage tail induction assays and for indirect bacteriophage assessment monitoring, respectively (22).
Bacterial growth conditions for phage or phage tail induction monitoring.
From frozen stocks (−80°C), all bacterial strains except L. ivanovii RR3 were individually grown for 48 h at 37°C on tryptic soy agar (TSA) plates (BioKar, France). One colony of each strain was transferred into 5 ml of BHI broth (BHIM; bioMérieux, France; 37 g/liter) (first culture). After incubation for 24 h at 30°C, 100 μl of the culture was transferred into 5 ml of BHIM and incubated for 24 h at 30°C (second culture). A similar procedure was applied using BHIK broth (BioKar, France; 37 g/liter).
Bacterial growth conditions for the screening of phage- or phage tail-inducing activities of different compounds (salts and selective agents).
From a second BHIM culture of EGD-e (described above), 20 μl was used to inoculate BHIM broth to which different reagents were added to assess their phage tail-inducing activities—Na2HPO4 (Prolabo, France) at 1, 2.5, 5, 10, 20, or 40 g/liter (6.75, 16.9, 33.8, 67.6, 135.2, and 270.3 mM, respectively) and LiCl (Sigma-Aldrich, France) at 3, 6, 9, 12, 15, 18, or 21 g/liter (70.8, 141.5, 212.3, 283, 354, 424.6, and 495.3 mM, respectively)—and the initial pHs (pHi values) of the media were initially adjusted to 5.5, 6.5, and 7.5 with HCl or NaOH solution. The incubations were conducted at 30°C for 24 h.
Similar conditions were applied using the strain L. innocua P1 to monitor the phage-inducing activities of acriflavine (Sigma-Aldrich, France; 3.125, 6.25, 12.5, or 25 μg/ml) and nalidixic acid (Sigma-Aldrich, France; 2.5, 5, 10, or 20 μg/ml) alone or in combination.
Bacterial growth conditions according to the ISO 11290-1 reference method for L. monocytogenes detection.
The induction of phages or phage tails was also evaluated in half-Fraser and Fraser broths (AES, France) according to the ISO 11290-1 reference method used for the detection of L. monocytogenes in food. Five lysogens (four strains of L. innocua, P1, 18.2, 43, and 45, and one of L. monocytogenes, EGD-e) were grown for 24 h at 30°C in 5 ml half-Fraser broth (AES, France). Then, 100 μl of these first enrichment cultures was transferred into 5 ml Fraser broth (AES, France) and incubated for 48 h at 37°C.
Bacterial growth conditions for the indicator strain, RR3 (used for all the bacteriophage assessments).
The indicator strain, L. ivanovii RR3, was grown for 48 h at 37°C on a tryptic soy agar plate (BioKar, France), and one colony was transferred into 5 ml of BHIM broth. After incubation for 24 h at 37°C, 100 μl of culture was transferred into 5 ml of BHIM broth and incubated for 24 h at 37°C. This strain was used for phage and phage tail assessment in the different assays.
Determination of bacterial concentrations.
Bacterial concentrations were determined by measuring the optical density at 600 nm (OD600) with a spectrophotometer (Biophotometer; Eppendorf). An OD600 of 1 corresponds to a bacterial concentration of 8.6 × 107 CFU/ml. Viable cells were also enumerated on tryptic soy agar plates. Thirty-microliter aliquots of culture were dispensed in 96-well microtiter plates (Nunc, Dutcher, France) containing 270 μl of 0.9% NaCl (Prolabo, France), and 10-fold serial dilutions of cultures were made up to a 1:106 dilution. Then, 20 μl of each dilution was spotted on plates. The plates were incubated for 24 h at 30°C. The colonies per spot were counted, and bacterial concentrations (CFU/ml) were determined.
Indirect phage or phage tail assessment (via the monitoring of bacterial lysis of L. ivanovii RR3 induced by a phage or phage tail suspension).
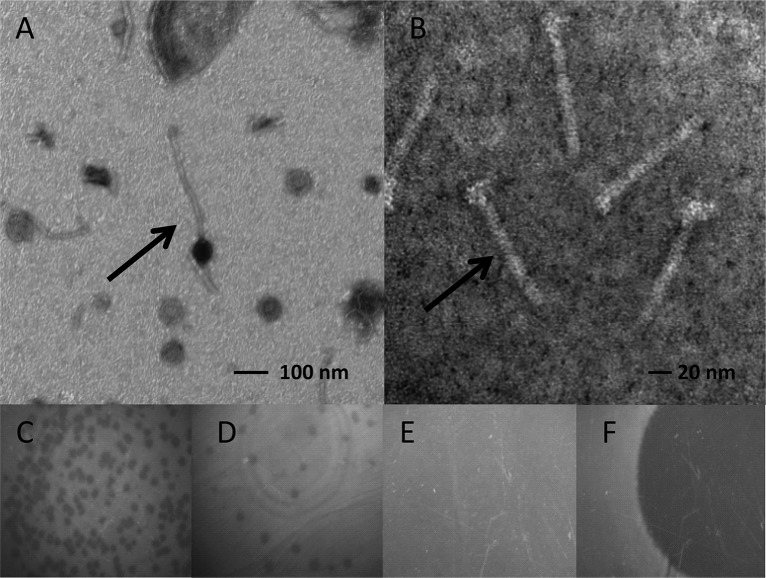
Phage or phage tail induction was investigated using the “spot on lawn technique” (24). For each culture of lysogens, a 1-ml aliquot was filtered through a sterile 0.45-μm-pore-size membrane filter (Nalgene, Dutcher, France). From 100-μl filtrates containing phages or phage tails, 2-fold serial dilutions were made up to a 1:256 dilution in 96-well microtiter plates (Nunc, Dutcher, France) containing 100 μl of 0.9% NaCl (Prolabo). The indicator strain L. ivanovii RR3 was spread onto tryptic soy agar plates (107 CFU per plate), and 20 μl of each phage or phage tail dilution was spotted on them. The plates were incubated for 24 h at 30°C. Lysis activity due to phages was associated with the presence of individual lysis plaques looking like holes in the bacterial lawn, which can be quantified (Fig. 1D), and the concentration of phages was expressed as PFU/ml of phage suspension (PFU). Lysis activity due to phage tails resulted in the presence of diffuse lysis zones, which cannot be enumerated. It was expressed as the highest dilution factor of the phage tail suspension for which the lysis zone could be detected (Fig. 1F).
FIG 1.
Phages and phage tails. Transmission electron micrographs of negatively stained bacteriophages from L. innocua F25 (phage; arrow) (A) and from L. monocytogenes EGD-e (phage tails; arrow) (B). The samples were stained using uranyl acetate as described in Materials and Methods. Bacterial lysis was observed at the deposited phage or phage tail spot (20 μl) on agar plates. (C to F) Induction by phage, >50 PFU (C) and 19 PFU (D), and by phage tails, diffuse lysis zone (F). (E) Control (no bacterial lysis).
BHIK fractionation and treatment with 0.2% activated charcoal.
The BHIK broth was ultrafiltered with a stirred ultrafiltration cell (Amicon, model 8050; Millipore, France) as follows: 25 ml of double-concentration BHIK (74 g/liter) was filtered at 4°C successively through a YM1 membrane (Amicon, NMWL 1000; Millipore, France) and a YC05 membrane (Amicon, NMWL 500; Millipore, France) using nitrogen gas pressure (44 lb/in2). Half of the resulting fraction was treated with activated charcoal (Sigma-Aldrich, France; 0.2% [wt/vol]) for 1 h at 37°C and filtered through a sterile 0.22-μm-pore-size membrane filter (Nalgene, Dutcher, France). The different fractions were stored at +4°C before being used to supplement BHIM broth (vol/vol) in order to assess their phage- or phage tail-inducing activities as described above.
Phosphate ion titration.
Phosphate concentrations in BHIK and BHIM were determined according to the method described by Murphy and Riley (25), and a calibration curve was obtained using an Na2HPO4 standard solution (Aldrich, France). The results are expressed as the phosphorus concentration (g/liter).
Transmission electron microscopy (TEM).
Negative staining was performed. A phage or phage tail suspension (5 μl) was dropped on collodion-coated and carbon-stabilized nickel microscope grids and left for 3 min to allow the phages or phage tails to bind. The grids were blotted with moist Whatman filter paper and stained with 10 μl of 1% (wt/vol) aqueous uranyl acetate for 10 s. The grids were dried and examined using a Hitachi H7500 transmission electron microscope (Hitachi Scientific Instruments Co., Tokyo, Japan) operating at 80 kV and equipped with an AMT camera driven by AMT software (AMT, Danvers, MA, USA).
RESULTS
Comparison of phage or phage tail induction levels for Listeria strains grown in BHIK or BHIM broth.
The 22 chosen Listeria strains produce either phages (10 strains) or phage tails (12 strains). The morphological difference between phages and phage tails is illustrated by transmission electron microscopy (Fig. 1A and B). For example, the phage of L. innocua 25, a member of the order Caudovirales, features a long, noncontractile tail characteristic of the family Siphoviridae, and the phage tail of L. monocytogenes EGD-e appears without a head. Both induce specific lysis on bacterial lawns. Lyses induced by phages are in the form of plaques (PFU), which can be confluent (Fig. 1C) or not (Fig. 1D). Lysis caused by phage tails appears as clear zones (Fig. 1F). The control plate is shown for comparison (Fig. 1E). The phage and phage tail induction levels, evaluated in the two BHI broths (BHIK and BHIM), are represented in Tables 2 and 3. In Table 2, it can be seen that, of the 10 strains of Listeria spp. producing phages (9 L. innocua and 1 L. seeligeri strains), all produced their phages in BHIK, whereas only 1 strain, L. innocua P6, produced them in both media. Phage titers in BHIK are highly variable: low for L. innocua 23 and 22 and L. seeligeri RR4 (between 5.33 × 102 and 6.50 × 102 PFU/ml); moderate for L. innocua 25, F(d), and 18.2 (between 1.57 × 103 and 2.57 × 103 PFU/ml); and high for L. innocua NV2, P1, and 43 (between 2.35 × 104 and 7.68 × 104 PFU/ml) and L. innocua P6 (5.46 105 PFU/ml). For the last strain, the phage titer is also high in BHIM (2.59 × 104 PFU/ml).
TABLE 2.
Comparison of phage induction of Listeria spp. in BHIK and BHIM
| Listeria strain | Phage titera (PFU/ml ± SD) |
|
|---|---|---|
| BHIK | BHIM | |
| L. innocua 23 | 5.33 × 102 A ± 1.00 × 102 | ND |
| L. innocua 22 | 5.83 × 102 A ± 58 | ND |
| L. seeligeri RR4 | 6.50 × 102 A ± 2.00 × 102 | ND |
| L. innocua 25 | 1.57 × 103 B ± 58 | ND |
| L. innocua F(d) | 2.30 × 103 B ± 8.23 × 102 | ND |
| L. innocua 18.2 | 2.57 × 103 B ± 3.06 × 102 | ND |
| L. innocua NV2 | 2.35 × 104 C ± 2.81 × 103 | ND |
| L. innocua P1 | 3.25 × 104 C ± 7.39 × 103 | ND |
| L. innocua 43 | 7.68 × 104 D ± 1.29 × 104 | ND |
| L. innocua P6 | 5.46 × 105 E ± 5.91 × 104 | 2.59 × 104 C ± 1.22 × 103 |
The Listeria strains were grown in BHIM or BHIK broth as two successive cultures (24 h at 30°C). The results are presented as means of three experiments. Values followed by different letters are significantly different. ND, not detected.
TABLE 3.
Comparison of phage tail inductions of Listeria spp. in BHIK and BHIM
| Listeria strain | Highest dilution factor of culture filtrate corresponding to the threshold of phage tail lysis activitya |
|
|---|---|---|
| BHIK | BHIM | |
| L. monocytogenes NV3 | 1 A | ND |
| L. monocytogenes 8F | 1 A | ND |
| L. monocytogenes 4F | 8 B | ND |
| L. monocytogenes 7F | 8 B | ND |
| L. monocytogenes H4 | 32 C | ND |
| L. monocytogenes 1S | 8 B | 1 A |
| L. monocytogenes P8 | 8 B | 2 A |
| L. monocytogenes Pi17 | 16 BC | 2 A |
| L. innocua Pi3 | 32 C | 2 A |
| L. innocua Pi53 | 32 C | 8 B |
| L. monocytogenes EGD-e | 64 CD | 2 A |
| L. innocua Pi2 | 128 D | 8 B |
The Listeria strains were grown in BHIK or BHIM broth as two successive cultures (24 h at 30°C). Two-fold serial dilutions of the Listeria culture filtrates were applied on L. ivanovii RR3 lawns. The level of phage tail induction is expressed as the highest dilution factor of filtrate that induced a detectable RR3 lysis zone. The results are representative of three experiments. Values followed by different letters are significantly different. ND, not detected.
Table 3 shows the highest dilution factors of Listeria culture filtrate containing phage tails for which an RR3 lysis zone was detectable. All 12 strains of Listeria spp. produced their phage tails in BHIK broth (9 strains of L. monocytogenes [NV3, 8F, 4F, 7F, H4, 1S, P8, Pi17, and EGD-e] and 3 strains of L. innocua [Pi3, Pi53, and Pi2]) with variable sensitivities; the phage tail induction was detected from the nondiluted filtrates to a 1:128 dilution. In BHIM, five L. monocytogenes strains (NV3, 8F, 4F, 7F, and H4) did not produce their phage tails even with the undiluted filtrates. In all cases, the induction of phage tails was more evident in BHIK than in BHIM. Thus, there are differences in BHI composition leading to a modulation of the phage or phage tail production. The compositions of both media, even if they are deemed to be quite similar, are complex, and BHIK comprises phage and phage tail inducers that BHIM does not have. In order to gain insight into the nature of the inducer(s), a fractionation and a treatment with activated charcoal were performed on the BHIK broth, and the phage- or phage tail-inducing activities of the fractions obtained were monitored.
Effect of BHIK fractionation on phage and phage tail induction.
A 500-Da BHIK fraction was obtained by ultrafiltration and treated with activated charcoal. BHIM broth was supplemented with charcoal-treated and untreated 500-Da BHIK fractions. A comparison of the phage and phage tail inductions is shown in Table 4, using two L. innocua strains (43 and NV2) and two L. monocytogenes strains (EGD-e and H4) for which phage and phage tail induction levels in BHIK broth were high. In BHIM broth, neither phages nor phage tails were detected. Supplementation of BHIM broth with the 500-Da BHIK fraction leads to production of both phages and phage tails, as is found in BHIK broth, totally or partially depending on the strains tested. The pretreatment of the 500-Da BHIK fraction with activated charcoal did not remove the active compound from BHIK broth.
TABLE 4.
Assessment of phage and phage tail induction by the 500-Da BHIK fraction treated or not with activated charcoala
| Condition assayed | Phage titer (PFU/ml ± SD) for L. innocua strain: |
Highest dilution factor of filtrate with phage tail lysis activity for L. monocytogenes strain: |
||
|---|---|---|---|---|
| 43 | NV2 | EGD-e | H4 | |
| BHIM | ND | ND | ND | ND |
| BHIK | 6.72 × 104 A ± 1.15 × 104 | 5.76 × 104 D ± 1.1 × 104 | 32 E | 8 F |
| BHIM supplemented by the 500-Da BHIK fraction | 1.07 × 104 B ± 1.22 × 103 | 4.75 × 104 D ± 1.03 × 104 | 16 E | 8 F |
| BHIM supplemented by the 500-Da BHIK fraction pretreated with activated charcoal | 1.71 × 104 C ± 4.03 × 103 | 4.96 × 104 D ± 6.40 × 103 | 16 E | 4 F |
Four Listeria strains were grown in BHIM, in BHIK, and in BHIM broth supplemented by the 500-Da BHIK fraction pretreated or not with 0.2% activated charcoal (1 h at 37°C). Two-fold serial dilutions of the Listeria strain culture filtrates were applied on L. ivanovii RR3 lawns. The phage titers and the highest dilution factor of filtrate with phage tail lysis activity were determined. For each strain, the results are presented as means of three experiments for Listeria phages or are representative of three experiments for phage tails. Values followed by different letters are significantly different (2 orders of dilution magnitude for dilution factors). ND, not detected.
Thus, it is possible to shift the inducible capacity of BHIK to BHIM by adding a 500-Da BHIK fraction. This fraction contains the major phage- or phage tail-inducing factor.
Na2HPO4 was presumed to be a candidate for the phage or phage tail inductions because it is present in the BHIK medium composition, as mentioned by the manufacturer. To evaluate this possibility, L. monocytogenes EGD-e was grown in BHIM supplemented with Na2HPO4 at different concentrations.
Influence of Na2HPO4 and pH on phage tail induction in EGD-e.
In order to evaluate the effect of Na2HPO4 on L. monocytogenes EGD-e phage tail induction, the salt was added at 6 different concentrations (ranging from 1 to 40 g/liter) to BHIM, and for each condition, the pH was adjusted to 5.5, 6.5, or 7.5 (Fig. 2). Without the addition of Na2HPO4 to BHIM broth, the induction of EGD-e phage tails at the three tested pHs (5.5, 6.5, and 7.5) did not occur. When Na2HPO4 was added to BHIM broth at increasing concentrations, the bacterial lysis associated with EGD-e phage tail induction was observed differentially depending on the pH: at pH 5, induction was detected only with 40 g/liter of Na2HPO4 (282 mM) from the 1:16 dilution; at pH 6.5, it was seen between 10 and 40 g/liter (70 mM and 282 mM) from the 1:32 and 1:64 dilutions; at pH 7.5, it was seen between 1 and 40 g/liter (7 mM and 282 mM) from the 1:8, 1:32, and 1:128 dilutions. Thus, at pH 7.5, phage tail induction occurs with a lower Na2HPO4 concentration (around 1 g/liter) and maximal intensities, since for the 1/128 dilution, lysis could be detected.
FIG 2.
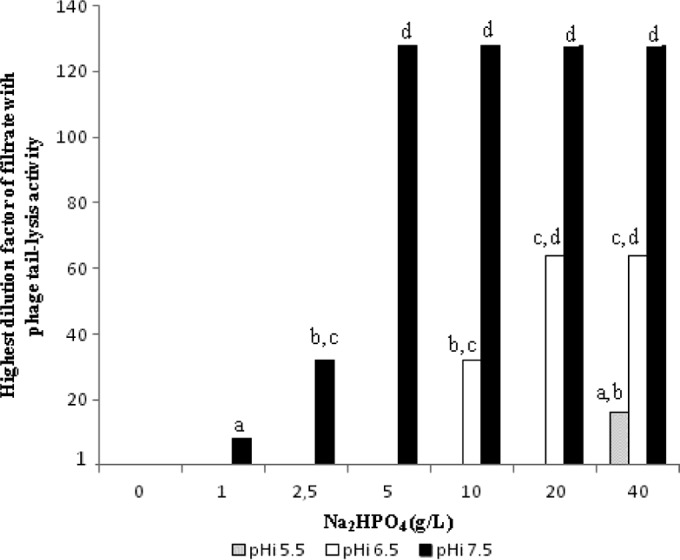
Effects of pH and Na2HPO4 on EGD-e phage tail induction in BHIM broth. Cultures were performed at 30°C for 24 h in BHIM broth supplemented with different Na2HPO4 concentrations and initial pH (pHi) values (5.5, 6.5, and 7.5). Twofold serial dilutions of the Listeria culture filtrates were applied on L. ivanovii RR3 lawns. The level of phage tail induction is expressed as the highest dilution factor of filtrate that induced a detectable RR3 lysis zone. The results are representative of three experiments. Different letters above the bars indicate values with significant differences (2 orders of dilution magnitude).
Phosphate ion concentrations in BHIK and BHIM broths were determined according to the method of Murphy and Riley. They are 822.2 ± 28 mg/liter and 189 ± 9.7 mg/liter for BHIK and BHIM broths, respectively, which correspond to 3.8 and 0.87 g/liter of Na2HPO4. This corroborates our guesswork based on Na2HPO4 involvement in the phage- and phage tail-inducing potential of BHIK broth and the 500-Da BHIK fraction. No decrease of cellular viability was observed in BHIM broth supplemented with Na2HPO4, whatever the concentrations used (data not shown). Thus, it appears that these differences in phage tail lysis activities are not due to a toxic effect of Na2HPO4. Na2HPO4 and the pH can contribute to induce phages of Listeria. Other compounds present in bacterial growth media, used as selective agents, could also be suspected to be involved with the production of phages.
Influence of selective agents on phage or phage tail induction.
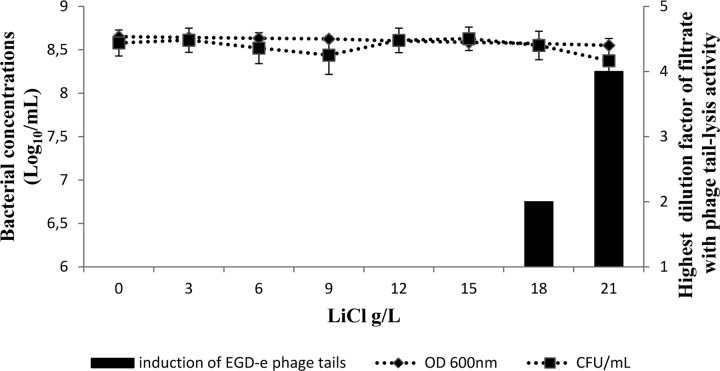
The main selective agents used for Listeria enrichments are LiCl, acriflavine, and nalidixic acid. They are present in half-Fraser and Fraser enrichment media. They were added to BHIM, and their effects on phage or phage tail induction were assayed with L. innocua P1 or L. monocytogenes EGD-e, respectively. The results of exposure of L. monocytogenes EGD-e to LiCl at concentrations ranging from 0 to 21 g/liter in BHIM are shown in Fig. 3. The EGD-e phage tail lysis activities were detected when the concentration reached 18 g/liter of LiCl (425 mM), and they increased between 18 and 21 g/liter (425 mM and 495 mM) with the highest dilution factor of filtrate (1:2 dilution and 1:4 dilution, respectively). No significant decrease in cellular viability was detected under these conditions (Fig. 3).
FIG 3.
Highest dilution factor of the EGD-e culture performed in the presence of LiCl with detectable induction of phage tails. The bars show induction of EGD-e phage tails at different LiCl concentrations in BHIM broth for 24 h at 30°C. Bacterial concentrations were determined both by measuring the OD600 and by counting CFU/ml. The results are expressed as the means ± standard deviations for triplicate experimental cultures for the bacterial concentrations, and the curve representing the threshold dilution with detectable lysis activity is representative of three experiments.
The effects of BHIM broth supplementation with nalidixic acid, acriflavine, or both on L. innocua P1 phage induction are shown in Table 5. With nalidixic acid, the phage release increased with the concentration (between 2.5 and 20 μg/ml) from 3.25 × 102 PFU/ml to 4.07 × 104 PFU/ml. With acriflavine, it was observed only for 25 μg/ml (2.83 × 103 PFU/ml). When both were added, the phage titers were moderate and maximal (7.13 × 103 PFU/ml) for 10/12.5 μg/ml nalidixic acid/acriflavine. For the 20/25-μg/ml nalidixic acid/acriflavine condition, the phage titer was lower than those obtained with each component alone. For the other combinations, the phage titers were slightly lower than or equal to that observed for nalidixic acid alone. Thus, the selective agents can induce phage production in BHIM broth. For comparison, an evaluation of phage or phage tail production was also performed in half-Fraser and Fraser broths.
TABLE 5.
L. innocua P1 phage lysis activities of nalidixic acid and/or acriflavine
| Selective agent concn (μg · ml−1) | Phage titera (PFU · ml−1 ± SD) |
|---|---|
| Nalidixic acid | |
| 0 | ND |
| 2.5 | 3.25 × 102 B ± 3.54 × 101 |
| 5 | 5.33 × 103 C ± 2.75 × 102 |
| 10 | 2.77 × 103 D ± 1.22 × 103 |
| 20 | 4.07 × 104 E ± 5.13 × 103 |
| Acriflavine | |
| 0 | ND |
| 3 0.125 | ND |
| 6.25 | ND |
| 12.5 | ND |
| 25 | 2.83 × 103 D ± 8.39 × 102 |
| Nalidixic acid/acriflavine | |
| 2.5/3.125 | 8.33 × 101 A ± 4.08 × 101 |
| 5/6.25 | 8.5 × 102 B ± 5.93 × 102 |
| 10/12.5 | 7.13 × 103 D ± 4.03 × 103 |
| 20/25 | 2.83 × 102 B ± 2.75 × 102 |
L. innocua P1 was grown (24 h at 30°C) in BHIM broth supplemented with the antibiotics. The results are presented as means of three experiments. Values followed by different letters are significantly different. ND, not detected.
Production of phages or phage tails in half-Fraser and Fraser broths.
Half-Fraser and Fraser broths, which are recommended media for Listeria detection in food products, contain disodium hydrogen phosphate (9.6 g/liter; initial pH 7.2 ± 0.2), LiCl (3 g/liter or 71 mM), and nalidixic acid and acriflavine (10/12.5 and 20/25 μg/ml for half-Fraser and Fraser broths, respectively). Phage or phage tail induction was analyzed for 4 L. innocua strains and L. monocytogenes EGD-e after the first enrichment in half-Fraser broth and after both enrichments (half-Fraser and Fraser broths). Table 6 shows that, in half-Fraser broth, the production of phages can be high (between 1.28 × 104 and 9.69 × 105 PFU/ml) for all the Listeria strains tested, whereas in Fraser broth, it occurs only for L. innocua 43 with an intensity lower than that observed with half-Fraser broth. For L. monocytogenes EGD-e, phage tail lysis activity was detected down to 1:64 dilution of half-Fraser culture and 1:4 dilution of Fraser culture. For all cases, it was higher in half-Fraser broth than in Fraser broth.
TABLE 6.
Comparison of Listeria phage or phage tail inductions after a first enrichment of Listeria sp. in half-Fraser broth and after both the first enrichment and culture in Fraser broth
| Listeria strain | Phage titer (PFU/ml ± SD) or highest dilution factor of filtrate with phage tail lysis activity aftera: |
|
|---|---|---|
| First enrichment in half-Fraser broth | Culture in Fraser broth | |
| L. innocua 45 | 1.28 × 104 A ± 4.00 × 103 | ND |
| L. innocua P1 | 7.17 × 105 B ± 1.55 × 105 | ND |
| L. innocua 18.2 | 8.23 × 105 B ± 8.52 × 104 | ND |
| L. innocua 43 | 9.69 × 105 B ± 8.52 × 104 | 1.49 × 104 A ± 4.89 × 103 |
| L. monocytogenes EGD-e | 64 C | 4 D |
The Listeria phage or phage tail induction was assayed after the first enrichment culture in half-Fraser broth for 24 h at 30°C and after both the first enrichment and culture in Fraser broth for 48 h at 37°C. Phage or phage tail lysis activity was determined on L. ivanovii RR3 lawns using 2-fold serial dilutions up to a 1:256 dilution. The results are presented as means of three experiments for phages or are representative of three experiments for phage tails (the highest dilution factor of filtrate with phage tail lysis activity). Values followed by different letters are significantly different (2 orders of dilution magnitude for the dilution factor). ND, not detected.
DISCUSSION
Detection of L. monocytogenes in food can be difficult, since the bacteria are normally found in very low numbers in the presence of a heterogeneous microflora. The most frequent Listeria spp. isolated from food are L. monocytogenes and L. innocua. Several studies have shown that L. innocua was found in food more frequently than L. monocytogenes (3, 4). This may not correspond to the reality. It has been proposed that the advantages of L. innocua over L. monocytogenes could be due to physiological characteristics, such as growth rates (6); to nutritional competition (26); or to the production of bacterial-growth-inhibiting factors (9), the majority of inhibitors are phages or phage tails. All Listeria-specific phages found to date are members of the Caudovirales, featuring the long, noncontractile tails of the family Siphoviridae or the complex contractile-tail machines of the family Myoviridae (27). Conditions for their induction have not been extensively explored.
We previously observed that, for L. monocytogenes EGD-e, phage tail induction occurred in BHIK broth (BioKar) but not in BHIM broth (bioMérieux). This prompted us to screen a set of strains and search for effectors of phage or phage tail induction in the composition of the bacterial growth media, both in key nutrients and in substances used in enrichment broths (half-Fraser and Fraser broths) to improve selective growth of targeted microorganisms.
The results described here show that the induction of phages occurred in BHIK broth for all the strains assayed with variable degrees of intensity, whereas such induction was detected for only one strain (L. innocua P6) in BHIM broth (Table 2). A similar trend was observed for the induction of phage tails (Table 3): in BHIK broth, phage tail induction was displayed for 12/12 Listeria strains; in BHIM broth, the ratio was 7/12. As was found with phages, induction of phage tails was variable; for example, in L. innocua Pi2, the phage tail lysis activity was detected down to the 1:128 dilution of the phage tail suspension of the bacterial culture. Even if the Listeria strains display differential sensitivities to these medium compositions, both productions are more evident in BHIK broth than in BHIM broth. BHIM broth supplementation with Na2HPO4 correlates with the increase in phage tail-induced bacterial lysis, and the effect was more pronounced at pH 7.5 than at 5.5 or 6.5 with a larger range of efficiencies (Fig. 2). This can explain the fact that phage and phage tail inductions do not occur in BHIM broth, since the phosphate concentration is too low (189 mg/liter, equivalent to 0.87 g/liter Na2HPO4), whereas they do occur in BHIK broth (822 mg/liter, equivalent to 3.8 g/liter Na2HPO4). Phosphate salt, present in BHIK broth and also in the 500-Da BHIK fraction, probably contributes to inducing phages and phage tails (Table 4). These results indicate that phosphate can be an effector of phage or phage tail induction. Prophage induction triggered by phosphate has been previously shown for bacterial communities in a marine environment (14, 15). The viral abundance could be increased by adding glucose to phosphate (14). Phosphate ions are generally present in enrichment broths for L. monocytogenes (half-Fraser and Fraser broths or UVM I and UVM II broths [Biokar], which contain disodium hydrogen phosphate [9.6 g/liter or 68 mM] and monopotassium hydrogen phosphate [1.35 g/liter] at pH 7.2 ± 0.2 [28]). They could contribute to inducing the production of phages or phage tails. These Listeria enrichment media also contain selective agents, such as LiCl, nalidixic acid, and acriflavine, which can be potential phage and phage tail inducers. The exposure to LiCl described here also led to phage tail induction, but at a final concentration of 18 g/liter to 21 g/liter (425 mM to 495 mM), which is higher than that present in half-Fraser and Fraser broths (3 g/liter, or 71 mM). The increase in phage tail titers was due to the induction of a small fraction of the bacterial population, since no significant decrease in the total bacterial population was observed. Shkilnyj and Koudelka (12) showed that Li+ (8.5 g/liter of LiCl, or 200 mM) increased the spontaneous induction frequency of prophage in E. coli by nearly 500-fold. For nalidixic acid and acriflavine, phage induction was higher when they were added alone to BHIM broth than when the association of the two compounds was tested. Moreover, in the latter case, inductions were slightly higher for 10/12.5 than 20/25 μg/ml nalidixic acid and acriflavine, which are the concentrations found in half-Fraser and Fraser broths, respectively. Even if the induction titers are not very high, the fact that the phage is replicative could lead to disturbed L. monocytogenes growth. In previous studies performed with quinolone antibiotics, nalidixic acid, ciprofloxacin, and norfloxacin were shown to significantly increase phage induction in E. coli, S. aureus, or P. aeruginosa (11, 17–20). Prophages may be induced under different stress conditions (antibiotics, H2O2, and lactococcin) through different mechanisms, one of which is the activation of the SOS response to DNA damage (19, 21, 29). However, other environmental conditions were shown to activate the lytic cycle of temperate phages without involvement of the SOS response (phosphate, LiCl, NaCl, and acyl-homoserine lactones) (12–15, 30). For example, it appears that lithium causes induction of λ bacteriophage by directly interfering with repressor-DNA interactions (12).
It was interesting to check phage and phage tail inductions in half-Fraser and Fraser broths, since they contain phosphate, LiCl, nalidixic acid, and acriflavine. To our knowledge, the levels of phages or phage tails in half-Fraser broth and Fraser broth have never been described before. The major phage and phage tail inducers in these media seem to be phosphate salts, nalidixic acid, and acriflavine. The fact that induction of Listeria phages or phage tails was found to be higher after the half-Fraser culture than after the two cultures (half-Fraser and Fraser broths) could be due to the temperature of 37°C for Fraser culture indicated in the ISO method. This is in accordance with previous data concerning the effect of the temperature of phage production (31, 32). The results presented here show that phages or phage tails of L. innocua could be responsible for the overgrowth of L. monocytogenes by L. innocua. Indeed, several authors showed that L. innocua was able to inhibit the growth of L. monocytogenes via the release of phages or phage tails (6, 33, 34). This phenomenon has been recently presented in a mathematical model (35). These criteria could be applied to the competition between serogroups/serotypes of L. monocytogenes. It was also mentioned (5) that competition could occur between L. monocytogenes strains involving monocins, which was a general term including different bacterial-lysis agents (phages, phage tails, and bacteriocins). L. monocytogenes serogroup 4 strains produce monocin type B, which is active only against L. monocytogenes serotypes 4a and 4c and L. ivanovii, whereas L. monocytogenes serogroup 1 strains produce monocin types C, D, and E, which are active against L. monocytogenes serotype 4b strains, in addition to serotypes 4a and 4c and L. ivanovii. This type of competition, along with other factors, such as nutrition (26), could explain the predominance of L. monocytogenes serogroup 1 found in food and in environmental sources and the involvement of phages or phage tails in overgrowth of L. monocytogenes by other Listeria species during enrichment steps of the detection assays.
This study shows that induction of phages or phage tails probably occurs and could be important in enrichment cultures for L. monocytogenes detection. The phage or phage tail production could explain the competition between Listeria species. The major inducers of phage and phage tail production seem to be disodium hydrogen phosphate and the pH, whereas selective agents trigger weak induction. It would be interesting to perform an evaluation of the roles of other buffers on phage or phage tail induction, for example, MOPS (morpholinepropanesulfonic acid) free acid and MOPS sodium salt. In this study, the buffer used in BHIM broth is not known. A buffer that does not trigger significant induction of phages or phage tails should be used to allow better enrichment of L. monocytogenes strains. This approach should also be considered for enrichments of other pathogens, such as Salmonella sp. or E. coli.
ACKNOWLEDGMENTS
We thank J. Lherminier (Centre de Microscopie INRA/UB) for technical assistance in electronic microscopy image capture of phages and A. Hartmann for interesting discussions.
REFERENCES
- 1.Ryser ET, Marth EH. 1999. Listeria, listeriosis and food safety, 2nd ed. Marcel Dekker, Inc., New York, NY. [Google Scholar]
- 2.Anonymous. 2004. Microbiology of food and animal feeding stuffs. Horizontal method for detection and enumeration of Listeria monocytogenes, part 1: detection method. Amendment 1: modification of the isolation media and the haemolysis test, and inclusion of precision data. EN ISO 11290-1:1996/A1:2004. European Committee for Standardization, Brussels, Belgium. [Google Scholar]
- 3.MacDonald F, Sutherland AD. 1994. Important differences between the generation times of Listeria monocytogenes and Listeria innocua in two Listeria enrichment broths. J Dairy Res 61:433–436. doi: 10.1017/S0022029900030879. [DOI] [PubMed] [Google Scholar]
- 4.Walsh D, Duffy G, Sheridan JJ, Blair IS, McDowell DA. 1998. Comparison of selective and non-selective media for the isolation of Listeria species from retail foods. J Food Saf 18:85–89. doi: 10.1111/j.1745-4565.1998.tb00205.x. [DOI] [Google Scholar]
- 5.Harvey J, Gilmour A. 2001. Characterization of recurrent and sporadic Listeria monocytogenes isolates from raw milk and nondairy foods by pulsed-field gel electrophoresis, monocin typing, plasmid profiling, and cadmium and antibiotic resistance determination. Appl Environ Microbiol 67:840–847. doi: 10.1128/AEM.67.2.840-847.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornu M, Kalmokoff M, Flandrois JP. 2002. Modeling the competitive growth of Listeria monocytogenes and Listeria innocua in enrichment broths. Int J Food Microbiol 73:261–274. doi: 10.1016/S0168-1605(01)00658-4. [DOI] [PubMed] [Google Scholar]
- 7.Oravcova K, Trncikova T, Kuchta T, Kaclikova E. 2008. Limitation in the detection of Listeria monocytogenes in food in the presence of competing Listeria innocua. J Appl Microbiol 104:429–437. doi: 10.1111/j.1365-2672.2007.03554.x. [DOI] [PubMed] [Google Scholar]
- 8.Carvalheira A, Eusébio C, Silva J, Gibbs P, Teixeira P. 2010. Influence of Listeria innocua on the growth of Listeria monocytogenes. Food Control 21:1492–1496. doi: 10.1016/j.foodcont.2010.04.021. [DOI] [Google Scholar]
- 9.Kalmokoff ML, Daley E, Austin JW, Farber JM. 1999. Bacteriocin-like inhibitory activities among various species of Listeria. Int J Food Microbiol 50:191–201. doi: 10.1016/S0168-1605(99)00097-5. [DOI] [Google Scholar]
- 10.Gnanou Besse N, Audinet N, Kérouanton A, Colin P, Kalmokoff M. 2005. Evolution of Listeria populations in food samples undergoing enrichment culturing. Int J Food Microbiol 104:123–134. doi: 10.1016/j.ijfoodmicro.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Los JM, Los M, Wegrzyn G, Wegrzyn A. 2009. Differential efficiency of induction of various lambdoid prophages responsible for production of Shiga toxins in response to different induction agents. Microb Pathog 47:289–298. doi: 10.1016/j.micpath.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Shkilnyj P, Koudelka GB. 2007. Effect of salt shock on stability of λimm434 lysogens. J Bacteriol 189:3115–3123. doi: 10.1128/JB.01857-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel L, Paul JH. 2005. Effect of nutrient addition and environmental factors on prophage induction in natural populations of marine Synechococcus species. Appl Environ Microbiol 71:842–850. doi: 10.1128/AEM.71.2.842-850.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandaa RA, Gomez-Consarnau L, Pinhassi J, Riemann L, Malits A, Weinbauer MG, Gasol JM, Thingstad TF. 2009. Viral control of bacterial biodiversity: evidence from a nutrient-enriched marine mesocosm experiment. Environ Microbiol 11:2585–2597. doi: 10.1111/j.1462-2920.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- 15.Williamson SJ, Houchin LA, McDaniel L, Paul JH. 2002. Seasonal variation in lysogeny as depicted by prophage induction in Tampa Bay, Florida. Appl Environ Microbiol 68:4307–4314. doi: 10.1128/AEM.68.9.4307-4314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi J, Kotay SM, Goel R. 2010. Various physico-chemical stress factors cause prophage induction in Nitrosospira multiformis 25196, an ammonia oxidizing bacteria. Water Res 44:4550–4558. doi: 10.1016/j.watres.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 17.Brazas MD, Hancock REW. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3222–3227. doi: 10.1128/AAC.49.8.3222-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sumby P, Waldor MK. 2003. Transcription of the toxin genes present within the staphylococcal phage ΦSa3ms is intimately linked with the phage's life cycle. J Bacteriol 185:6841–6851. doi: 10.1128/JB.185.23.6841-6851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goerke C, Köller J, Wolz C. 2006. Ciprofloxacin and trimethoprim cause phage induction and virulence modulation in Staphylococcus aureus. Antimicrob Agents Chemother 50:171–177. doi: 10.1128/AAC.50.1.171-177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comeau AM, Tétart F, Trojet SN, Prère MF, Krisch HM. 2007. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799. doi: 10.1371/journal.pone.0000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madera C, Garcia P, Rodriguez A, Suarez JE, Martinez B. 2009. Prophage induction in Lactococcus lactis by the bacteriocin Lactococcin 972. Int J Food Microbiol 129:99–102. doi: 10.1016/j.ijfoodmicro.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Lemaître JP, Delcourt A, Rousset A. 1997. Optimization of the detection of bacteriophages induced from Listeria spp. Lett Appl Microbiol 24:51–54. doi: 10.1046/j.1472-765X.1997.00346.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson KE, Fouts DE, Mongodin EF, Ravel J, Deboy RT, Kolonay JF, Rasko DA, Angiuoli SV, Gill SR, Paulsen IT, Peterson J, White O, Nelson WC, Nierman W, Beanan MJ, Brinkac LM, Daugherty SC, Dodson RJ, Durkin AS, Madupu R, Haft DH, Selengut J, Van Aken S, Khouri H, Fedorova N, Forberger H, Tran B, Kathariou S, Wonderling LD, Uhlich GA, Bayles DO, Luchansky JB, Fraser CM. 2004. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res 32:2386–2395. doi: 10.1093/nar/gkh562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagg JR, Dajani AS, Wannamaker LW. 1976. Bacteriocins of Gram-positive bacteria. Bacteriol Rev 40:722–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. doi: 10.1016/S0003-2670(00)88444-5. [DOI] [Google Scholar]
- 26.Gnanou-Besse N, Barre L, Buhariwalla C, Vignaud ML, Khamissi E, Decourseulles E, Nirsimloo M, Chelly M, Kalmokoff M. 2010. The overgrowth of Listeria monocytogenes by other Listeria spp. in food samples undergoing enrichment cultivation has a nutritional basis. Int J Food Microbiol 136:345–351. doi: 10.1016/j.ijfoodmicro.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 27.Klumpp J, Loessner MJ. 2013. Listeria phages: genomes, evolution, and application. Bacteriophage 3:e26861. doi: 10.4161/bact.26861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClain D, Lee WH. 1988. Development of USDA-FSIS method for isolation of Listeria monocytogenes from raw meat and poultry. J Assoc Off Anal Chem 71:660–664. [PubMed] [Google Scholar]
- 29.Selva L, Viana D, Regev-Yochay G, Trzcinski K, Corpa JM, Lasa I, Novick RP, Penadés JR. 2009. Killing niche competitors by remote-control bacteriophage induction. Proc Natl Acad Sci U S A, 106:1324–1338. doi: 10.1073/pnas.0812581106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh D, Roy K, Williamson KE, Srinivasiah S, Wommack KE, Radosevich M. 2009. Acyl-homoserine lactones can induce virus production in lysogenic bacteria: an alternative paradigm for prophage induction. Appl Environ Microbiol 75:7142–7152. doi: 10.1128/AEM.00950-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasinska S. 1964. Bacteriophages of lysogenic strains of Listeria monocytogenes. Acta Microbiol Polon 67:840–847. [PubMed] [Google Scholar]
- 32.Kim JW, Kathariou S. 2009. Temperature-dependent phage resistance of Listeria monocytogenes epidemic clone II. Appl Environ Microbiol 75:2433–2438. doi: 10.1128/AEM.02480-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoyama E, Maruyama S, Katsube Y. 1998. Production of bacteriocin-like-substance by Listeria innocua against Listeria monocytogenes. Int J Food Microbiol 40:133–137. doi: 10.1016/S0168-1605(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama E, Shibusawa Y, Maruyama S, Katsube Y, Mikami T. 2005. Influence of bacteriocin-like substance, generation times, and genetic profiles of Listeria innocua on the isolation of Listeria monocytogenes. Comp Immunol Microbiol Infect Dis 28:177–186. doi: 10.1016/j.cimid.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Fgaier H, Kalmokoff M, Ells T, Eberl HJ. 2014. An allelopathy based model for the Listeria overgrowth phenomenon. Math Biosci 247:13–26. doi: 10.1016/j.mbs.2013.10.008. [DOI] [PubMed] [Google Scholar]