Abstract
Safety and probiotic properties make lactic acid bacteria (LAB) attractive hosts for surface display of heterologous proteins. Protein display on nonrecombinant microorganisms is preferred for therapeutic and food applications due to regulatory requirements. We displayed two designed ankyrin repeat proteins (DARPins), each possessing affinity for the Fc region of human IgG, on the surface of Lactococcus lactis by fusing them to the Usp45 secretion signal and to the peptidoglycan-binding C terminus of AcmA, containing lysine motif (LysM) repeats. Growth medium containing a secreted fusion protein was used to test its heterologous binding to 10 strains of species of the genus Lactobacillus, using flow cytometry, whole-cell enzyme-linked immunosorbent assay (ELISA), and fluorescence microscopy. The fusion proteins bound to the surfaces of all lactobacilli; however, binding to the majority of bacteria was only 2- to 5-fold stronger than that of the control. Lactobacillus salivarius ATCC 11741 demonstrated exceptionally strong binding (32- to 55-fold higher than that of the control) and may therefore be an attractive host for nonrecombinant surface display. Genomic comparison of the species indicated the exopolysaccharides of Lb. salivarius as a possible reason for the difference. Additionally, a 15-fold concentration-dependent increase in nonrecombinant surface display on L. lactis was demonstrated by growing bacteria with sublethal concentrations of the antibiotics chloramphenicol and erythromycin. Nonrecombinant surface display on LAB, based on LysM repeats, was optimized by selecting Lactobacillus salivarius ATCC 11741 as the optimal host and by introducing antibiotics as additives for increasing surface display on L. lactis. Additionally, effective display of DARPins on the surfaces of nonrecombinant LAB has opened up several new therapeutic possibilities.
INTRODUCTION
The ability to display heterologous proteins on bacterial surfaces is becoming increasingly important in various fields of biotechnology (1–3). Bacteria displaying heterologous proteins can be used as bioadsorbents, biosensors, biocatalysts, and oral vaccines and in antibody production, screening of peptide libraries, and detection of mutations (1–3). Lactic acid bacteria (LAB) are particularly attractive hosts due to their long-term usage in food, their industrial applicability, and the general acceptance of their probiotic properties (4). Several approaches to the display of heterologous proteins on the surfaces of LAB have been established, including the use of LPXTG-type domains (5), lysine motif (LysM) domains (6), surface layer proteins (7), and lipoprotein anchors (8). Display of heterologous proteins on the surfaces of LAB has been exploited for the preparation of mucosal vaccines (9, 10), for the delivery of binding molecules to the gastrointestinal tract (11), for the assembly of macromolecular enzyme complexes (12), and for bacterial immobilization (13).
The nonrecombinant approach to surface display is preferred for therapeutic and food applications. This can be achieved by noncovalent binding of recombinant proteins to the surfaces of nonrecombinant bacteria. The feasibility of such an approach has already been demonstrated by using the C-terminal part of the lactococcal AcmA protein (cA) as the cell wall anchor in lactobacilli (14) and in nonviable Gram-positive enhancer matrix (GEM) particles (15). AcmA is an autolysin (N-acetylmuramidase) with a vital role in cell division in Lactococcus lactis. It comprises an enzymatic domain at the N terminus and a peptidoglycan-binding domain at the C terminus that comprises 3 LysM repeats (14, 16, 17). cA has been used as a fusion partner for noncovalent attachment of heterologous proteins to the surfaces of LAB, e.g., attachment of malaria antigen to Lactobacillus sakei and Lactobacillus casei (14), attachment of α-amylase to Lactobacillus plantarum and Lactobacillus casei (18), attachment of chicken anemia virus coat proteins to Lactobacillus acidophilus (19), and attachment of a tumor necrosis factor alpha (TNF-α)-specific Affibody to Lactococcus lactis (11).
Other LysM repeat-containing proteins have also been applied as anchors for surface display on LAB, with similar levels of effectiveness. These include the bacteriophage endolysin Lyb5 (20) and the muropeptidase MurO from Lactobacillus plantarum (21). Differences in the ability to bind LysM repeat-containing proteins have been observed between Lactobacillus species, which reflect the differences in lactobacillus surfaces (22). Lb. casei bound LysM-containing proteins at the cell poles, Lb. sakei bound them over the entire surface, and Lactobacillus helveticus did not bind them at all (14). This suggests that a more detailed and quantitative comparison of various Lactobacillus species may yield species or strains that would be particularly suitable for LysM repeat-based surface display.
Binding of LysM-containing proteins to the bacterial surface can be hindered by surface structures that spatially obstruct the accessibility of peptidoglycan, such as lipoteichoic acid (LTA) or surface layer proteins (14). Bacterial surface structures and surface proteome composition can be perturbed by changing the growth conditions or exposing bacteria to exogenous substances, which may consequently lead to differences in LysM-mediated binding. Antibiotics can cause significant alterations in gene transcription at subinhibitory concentrations and stress responses at sublethal concentrations (23). Erythromycin (24) and chloramphenicol (25) altered the transcription of more than 600 genes in Enterococcus faecalis. Treatment with bile, which also has antimicrobial activity, altered the transcription of more than 300 genes and caused changes in several cell envelope-related functions in Lactobacillus rhamnosus (26).
We previously demonstrated LysM-mediated surface display of Affibody molecules (11). Here we introduced designed ankyrin repeat proteins (DARPins) that bind the Fc domain of human IgGs (27) as model proteins for testing LysM-mediated surface binding. DARPins are small, nonantibody protein scaffolds that can be selected from combinatorial libraries to bind numerous proteins (28). The first successful display of DARPins on the surfaces of LAB broadened the spectrum of successfully displayed binding proteins and opened up several new therapeutic and other possibilities.
The goal of the present study was to improve heterologous LysM-mediated nonrecombinant surface display on LAB. In order to achieve this, 10 strains of lactobacillus species were screened to select an optimal Lactobacillus carrier for heterologous nonrecombinant surface display. Additionally, erythromycin and chloramphenicol, two antibiotics commonly used in LAB research, were tested for their impacts on LysM-mediated surface display.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
The bacterial strains used in this study are shown in Table 1. L. lactis NZ9000 was grown at 30°C in M-17 medium (Merck) supplemented with 0.5% glucose (GM-17) without aeration. To maintain selection pressure on transformation, 10 μg/ml of chloramphenicol was added. To evoke surface changes in untransformed strains, 1, 2, or 3 μg/ml of chloramphenicol and 0.05, 0.075, or 0.1 μg/ml of erythromycin were added. Lactobacillus strains were grown in De Man, Rogosa, and Sharpe (MRS) medium (Merck, Darmstadt, Germany) at 37°C without aeration. Escherichia coli strain DH5α was grown at 37°C with aeration in LB medium supplemented with 100 μg/ml ampicillin or 10 μg/ml kanamycin.
TABLE 1.
Strains, plasmids, and genes used in this study
| Strain, plasmid, or gene | Relevant feature(s) or sequence (5′–3′)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli DH5α | endA1 glnV44 thi-1 recA1 relA1 gyrA96 deoR F− ϕ80dlacZΔM15 Δ(lacZYA-argF)U169 hsdR17(rK− mK−) λ− | Invitrogen |
| L. lactis NZ9000 | MG1363 nisRK ΔpepN | NIZO |
| Lb. acidophilus ATCC 4356 | Wild type | ATCC |
| Lb. delbrueckii subsp. bulgaricus ATCC 1184 | Wild type | ATCC |
| Lb. casei ATCC 393 | Wild type | ATCC |
| Lb. gasseri ATCC 33323 | Wild type | ATCC |
| Lb. gasseri K7 | Wild type | 44 |
| Lb. paracasei ATCC 25302 | Wild type | ATCC |
| Lb. plantarum ATCC 8014 | Wild type | ATCC |
| Lb. reuteri ATCC 55730 | Wild type | ATCC |
| Lb. rhamnosus ATCC 53103 | Wild type | ATCC |
| Lb. salivarius ATCC 11741 | Wild type | ATCC |
| Plasmids | ||
| pNZ8148 | pSH71 derivative; nisAp Cmr; nisin-controlled expression | NIZO |
| pSDLBA3b | pNZ8148 containing gene fusion of Usp45 signal peptide, B domain, and cA | 11 |
| pMA-T-I07 | pMA-T containing DARPin I_07 gene; Ampr | Gene Art |
| pMK-RQ-I_19 | pMK-RQ containing DARPin I_19 gene; Kanr | Gene Art |
| pSD_I07 | pNZ8148 containing gene fusion of Usp45 signal peptide, DARPin I_07, and cA | This work |
| pSD_I19 | pNZ8148 containing gene fusion of Usp45 signal peptide, DARPin I_19, and cA | This work |
| Genes | ||
| DARPin I_07 | GGATCCGATCTTGGTAAAAAACTTCTTGAAGCAGCACGAGCTGGACAAGACGACGAAGTACGTATCCTTATGGCTAACGGTGCCGATGTTAACGCATCAGATAAATCTGGTTATACTCCATTACATTTGGCCGCACATATTGGTCATTTAGAAATTGTTGAGGTATTATTGAAGCATGGTGCTGATGTTAATGCACATGATTCATGGGGAGACACTCCTCTTCATTTAGCCGCCACTTTTGGTCACTTAGAAATCGTAGAAGTACTTTTAAAACATGGTGCTGACGTTAATGCACAAGACAAATTTGGAAAAACAGCATTTGACATTTCAATTGATAACGGTAATGAAGACTTGGCAGAGATCTTACAAGAATTC | This work |
| DARPin I_19 | GGATCCGATTTAGGAAAGAAGTTATTAGAAGCAGCACGAGCAGGACAAGACGATGAGGTAAGAATCTTAATGGCTAATGGTGCTGACGTAAACGCTGATGATAACAAAGGAGATACTCCACTTCATTTAGCTGCATCATTCGGTCACTTAGAAATTGTCGAAGTACTTCTTAAAAACGGAGCTGATGTAAACGCAGACGATTACTTTGGAGATACACCATTACACTTAGCAGCTTGGTCTGGTCATCTTGAAATTGTTGAAGTTTTATTAAAATATGGTGCAGATGTAAATGCACAAGATCAACGTGGATTTACACCTTTACATCTTGCTGCTATCGCTGGACACCTTGAAATTGTAGAAGTTTTACTTAAATATGGAGCTGACGTTAACGCACAAGATAAGTTTGGAAAAACTGCTTTCGATATTAGTATTGATAATGGTAACGAAGACCTTGCAGAAATCTTACAAGAATTC | This work |
The introduced restriction sites in gene sequences are underlined.
Molecular cloning.
DARPins I_07 and I_19 (27) were used as model binding proteins for surface display on L. lactis. Amino acid sequences of DARPins were back translated and codon optimized for expression in L. lactis, yielding the DARPin I_07 and DARPin I_19 genes (Table 1). Genes were purchased from GeneArt Gene Synthesis (Regensburg, Germany) and cloned into plasmid pSDLBA3b (Table 1) by use of BamHI and EcoRI restriction enzymes (New England BioLabs, Beverly, MA), yielding plasmids pSD_I07 and pSD_I19. Plasmid DNA was isolated with a NucleoSpin plasmid kit (Macherey and Nagel, Düren, Germany), with an additional lysozyme treatment step for L. lactis. Lactococci were transformed by electroporation using a Gene Pulser II apparatus (Bio-Rad, Hercules, CA) according to the instructions of MoBiTec GmbH (Goettingen, Germany). All plasmids were sequenced by GATC (Constance, Germany).
Expression of DARPin-cA fusion proteins in L. lactis.
Overnight cultures of L. lactis NZ9000 harboring plasmids pSD_I07 and pSD_I19 were diluted (1:100) in 10 ml (or 100 ml) of fresh GM-17 medium and grown to an A600 of 0.80. Fusion protein expression was induced with 25 ng/ml nisin (Fluka AG, Buchs, Switzerland). After 3 h of incubation, 1 ml of culture was stored at 4°C for flow cytometric analysis. The remaining cell culture was centrifuged at 5,000 × g for 10 min. The cell pellet was resuspended in 500 μl of 0.1 M phosphate-buffered saline (PBS; pH 7.4) and stored at −20°C for SDS-PAGE analysis or resuspended in PBS to an A600 of 1.0 and stored at 4°C for whole-cell enzyme-linked immunosorbent assay (ELISA). The supernatant was decanted, filtered with a 0.22-μm-pore-size filter (Minisart; Millipore, Billerica, MA), aliquoted, and stored at −20°C for testing of DARPin-cA fusion protein binding to nonrecombinant bacteria.
SDS-PAGE and Western blotting.
SDS-PAGE was performed with a mini-Protean II apparatus (Bio-Rad, Hercules, CA). Samples were thawed in an ice bath, briefly sonicated with a UPS200S sonicator (Hielscher, Teltow, Germany), mixed with 2× Laemmli sample buffer and dithiothreitol (DTT), and denatured by heating at 100°C before loading. The Page Ruler Plus (Fermentas, St. Leon-Rot, Germany) prestained standard was used for molecular weight comparison. Proteins were stained with Coomassie brilliant blue or transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore). Each membrane was blocked in 5% skim milk and incubated overnight at 4°C with fluorescein isothiocyanate (FITC)-conjugated human IgG (1:1,000 dilution; Jackson ImmunoResearch, West Grove, PA). After washing with 0.05% TBST (50 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.5), fluorescence was detected with a ChemiDoc MP imaging system (Bio-Rad), using blue excitation (488 nm).
Whole-cell ELISA.
Whole-cell ELISA was performed as described previously (29). For testing of DARPin surface display, 750 μl of L. lactis or 1 ml of Lactobacillus sp. cell suspension in PBS, at an A600 of 1.0, was centrifuged (5,000 × g, 5 min, 4°C) and washed twice with 500 μl PBS. Cells were then resuspended in 200 μl of FITC-conjugated human IgG (diluted 1:500 in PBS; Jackson ImmunoResearch, West Grove, PA) and incubated for 1 h at room temperature (RT) with gentle shaking. Cells were then washed twice with PBS and resuspended in 200 μl of peroxidase-conjugated mouse anti-human IgG (diluted 1:2,500 in PBS; Jackson ImmunoResearch, West Grove, PA). After 1 h of incubation at RT with gentle shaking, cells were washed first with PBS and then with substrate buffer (150 mM Na2HPO4, 50 mM citric acid, pH 6.0). Cells were then resuspended in 1 ml of substrate buffer, and 100-μl aliquots of appropriate dilutions in substrate buffer (1:5 and 1:25) were loaded on a microtiter plate. One hundred microliters of 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma-Aldrich, Steinheim, Germany) was added, and the reaction was stopped after 15 min by the addition of 50 μl of 2 M sulfuric acid. Absorbances were read at 450 nm using an Infinite M1000 reader (Tecan, Salzburg, Austria).
A similar protocol was used, with a few modifications, for quantifying LTA on the surfaces of Lactobacillus species. A monoclonal antibody to LTA (clone 55; Hycult Biotech, Uden, The Netherlands) (diluted 1:250 in PBS) was used as the primary antibody. Peroxidase-conjugated goat anti-mouse IgG (Merck Millipore, Billerica, MA) (diluted 1:500 in PBS) was used as the secondary antibody. Color was developed for 20 min before stopping the reaction.
Flow cytometry and fluorescence microscopy.
For flow cytometry, 10 μl of cell culture in stationary phase was added to 500 μl of Tris-buffered saline (TBS; 50 mM Tris-HCl, 150 mM NaCl, pH 7.5) and centrifuged for 5 min at 5,000 × g and 4°C. The pellet was resuspended in 500 μl of TBS, and 1 μl of FITC-conjugated human IgG antibody (Jackson ImmunoResearch, West Grove, PA) was added. After 2 h of incubation at RT with constant shaking at 100 rpm, cells were washed three times with 200 μl 0.1% TBST and finally resuspended in 500 μl TBS. Samples were analyzed with a flow cytometer (FACSCalibur; Becton Dickinson, Franklin Lakes, NJ), using excitation at 488 nm and emission at 530 nm, in the FL1 channel. The geometric mean fluorescence intensity (MFI) of at least 20,000 bacterial cells in the appropriate gate was measured. The averages of results from at least two independent experiments were considered.
The protocol for preparing specimens for fluorescence microscopy was similar, except that the starting volume of the cell cultures was 20 μl and the final resuspension volume was 300 μl. Stained cells were fixed to a microscope slide by use of a StatSpine Cytofuge 2 centrifuge (Iris Sample Processing) for 10 min at maximum speed. An LSM 710 confocal microscope (Carl Zeiss, Oberkochen, Germany) was used for observation of prepared specimens. Alexa Fluor 488 was excited with an argon laser (488 nm), and the emission was filtered with a narrow-band 505- to 530-nm filter. All images were taken with the same settings and were analyzed using Carl Zeiss ZEN Lite 2012 software.
Binding of DARPIN-cA fusion proteins to nonrecombinant bacteria.
Cell cultures of L. lactis NZ9000 and Lactobacillus spp. were grown to an optical density of 2.0 to 3.0 (late exponential phase). For flow cytometry and fluorescence microscopy, 20 μl of cell culture was added to 500 μl of TBS and centrifuged for 5 min at 5,000 × g and 4°C, followed by resuspension of the pellet in 500 μl of filtered DARPin I_07-cA medium. Suspensions were incubated for 2 h at RT in a tube rotator. After incubation, cells were washed once with 500 μl TBS and stained as described above for flow cytometry and fluorescence microscopy. Control samples were stained without prior binding of the DARPin I_07-cA fusion to the bacterial surface.
For whole-cell ELISA, 1 ml of Lactobacillus sp. cell suspension in PBS, at an A600 of 1.0, was added to 20 ml of filtered DARPin I_07-cA medium and incubated for 2 h at RT in a tube rocker. Control bacteria were incubated with filtered growth medium of empty plasmid (pNZ8148)-containing bacteria and treated in the same fashion. After incubation, cells were centrifuged for 10 min at 5,000 × g and 4°C, resuspended in 500 μl of PBS, washed, and stained as described above for whole-cell ELISA.
Genomic comparison of Lactobacillus species.
Integrated Microbial Genomes (IMG) software (https://img.jgi.doe.gov/cgi-bin/w/main.cgi) was used to compare the 8 of 10 Lactobacillus species used in the study whose genomes have been sequenced. Additionally, the well-studied probiotic Lactobacillus salivarius UCC 118 and Lb. plantarum ATCC 14917 (a close relative of Lb. plantarum ATCC 8014 [30]) were included in the comparison. Clusters of orthologous groups (COGs) from the cell wall/membrane/envelope biogenesis category were compared. COGs that were present in Lb. salivarius alone or absent only from Lb. salivarius were identified and studied further in the genome of Lb. salivarius ATCC 11741 to elucidate their possible functional roles. BLAST analysis of the Lb. salivarius ATCC 11741 genome was performed to determine the presence of LTA biosynthesis genes and LTA d-alanylation genes. Genes from Lb. acidophilus NCFM, for which the LTA biosynthesis pathway was annotated (31), were used as query sequences for BLAST searches.
Statistics.
Statistical analyses were performed with GraphPad Prism 5.0 software. Student's t test was used to compare the significance of the differences between samples.
RESULTS
Expression and surface display of DARPIN-cA fusion proteins on L. lactis NZ9000.
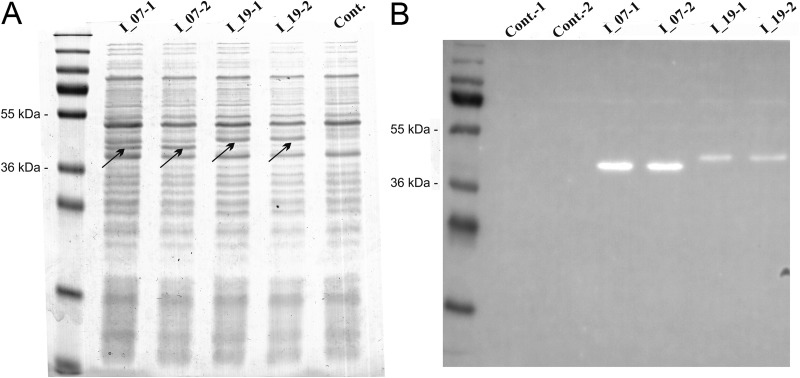
Genes for two DARPins (I_07 and I_19), both expressing affinity for the Fc region of human IgG, were codon optimized for expression in L. lactis and synthesized. Both genes were cloned, under the control of the NisA promoter, into our previously reported plasmid for surface display (11) that enables fusion to the Usp45 secretion signal and to the LysM repeat-containing C-terminal domain of the AcmA protein (cA). Expression of fusion proteins was induced by adding nisin to the growth medium and was confirmed in the cell lysates by SDS-PAGE and Coomassie brilliant blue staining (Fig. 1A). After Western blotting, functional DARPin-containing fusion proteins were detected indirectly via their ability to bind the Fc region of human IgG (Fig. 1B). Stronger binding was observed with DARPin I_07.
FIG 1.
SDS-PAGE (A) and Western blotting (B) of lysates of L. lactis cells expressing DARPins in fusion with the Usp45 secretion signal and the LysM-containing cA domain, stained with Coomassie brilliant blue. I_07, DARPin I_07-cA fusion; I_19, DARPin I_19-cA fusion; Cont., control containing empty plasmid pNZ8148. DARPin-cA fusion proteins are indicated with arrows.
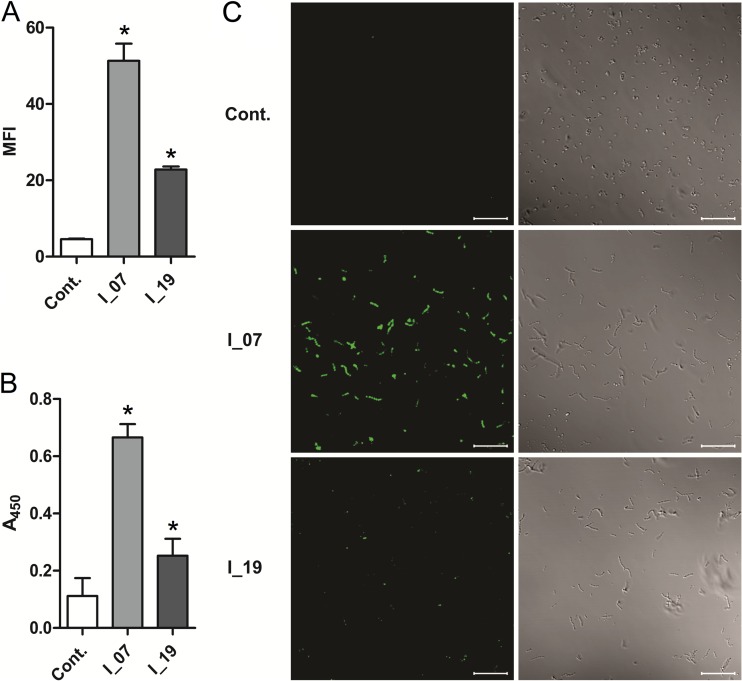
Surface display of both DARPins was also evaluated indirectly via their ability to bind the Fc region of human IgGs and was confirmed with flow cytometry (Fig. 2A), whole-cell ELISA (Fig. 2B), and fluorescence microscopy (Fig. 2C). The amount of IgG bound by bacteria with surface-displayed DARPin I_07 was at least 2-fold higher than that with DARPin I_19-displaying bacteria, as determined by both flow cytometry and whole-cell ELISA.
FIG 2.
Flow cytometry (A), whole-cell ELISA (B), and fluorescence microscopy (C) results for L. lactis cells expressing DARPins I_07 and I_19, detected with FITC-conjugated human IgG. Cont., control containing empty plasmid pNZ8148. MFI, mean fluorescence intensity; A450, absorbance at 450 nm. Error bars denote standard deviations. *, significant difference (t test; P < 0.05) in comparison to control. (C) (Left) Fluorescence images. (Right) Bright-field images. Bars = 20 μm.
Selection of the optimal Lactobacillus host for LysM-mediated nonrecombinant surface display of DARPin-cA fusion proteins.
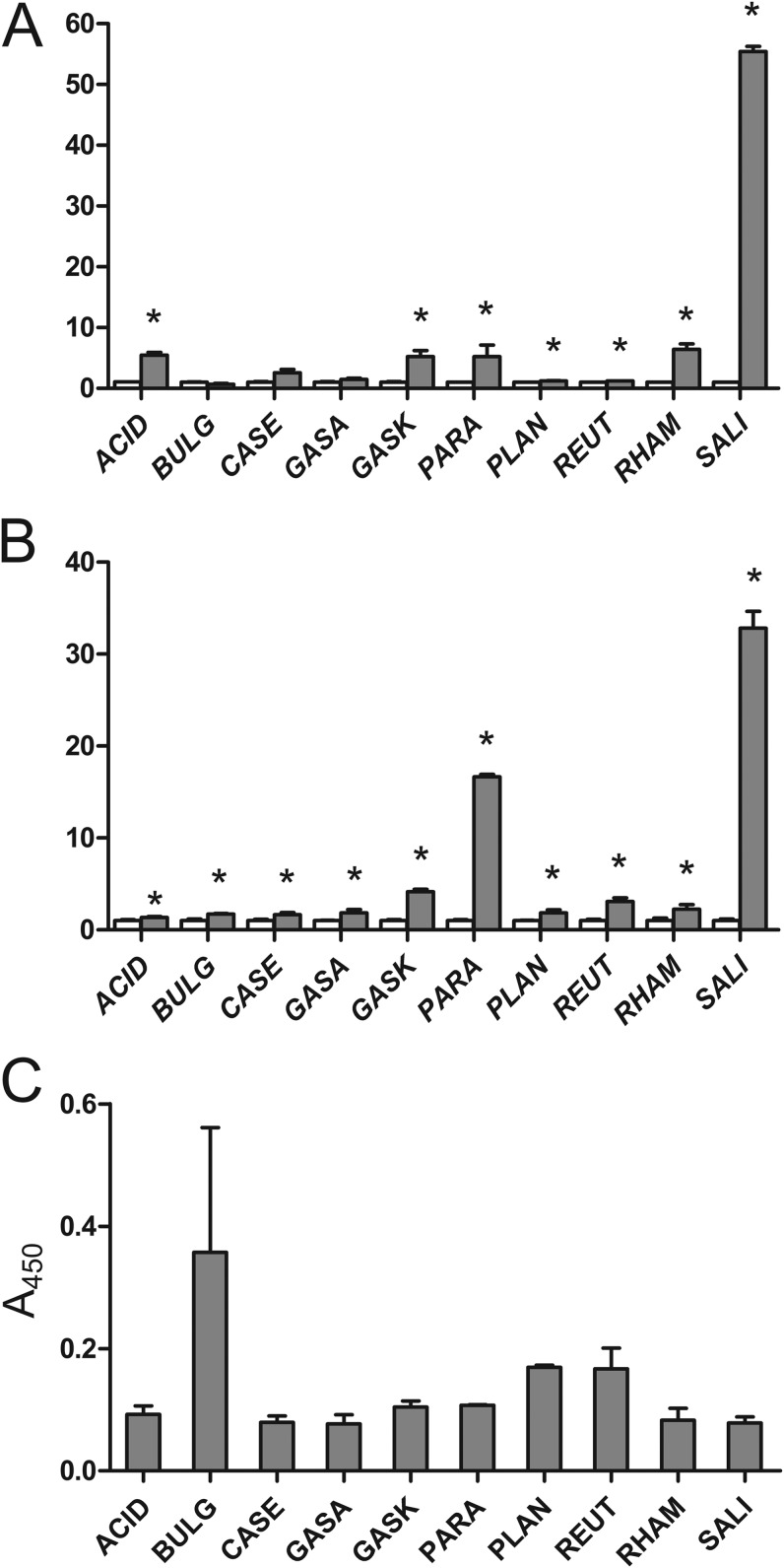
Sterile growth medium containing the DARPin I_07-cA fusion was incubated with 10 strains of the genus Lactobacillus (Table 1). Binding of the DARPin I_07-cA fusion to the bacterial surface was detected indirectly via the ability of DARPin I_07 to bind the Fc region of human IgG. DARPin-bound immunoglobulins were quantitated by flow cytometry and whole-cell ELISA. The mean fluorescence intensity (MFI) value, obtained by flow cytometry for each Lactobacillus species, was normalized to that of the control sample, in which bacteria were incubated with immunoglobulins without prior binding of the DARPin I_07-cA fusion to the bacterial surface (Fig. 3A). The A450 values obtained by whole-cell ELISA were normalized to that of the control sample, in which bacteria were incubated with growth medium of an empty plasmid-containing bacterial culture (Fig. 3B). Fluorescence microscopy (see Fig. S1 in the supplemental material) was used to observe the difference between DARPin I_07-cA-bound and control bacteria on the single-cell level.
FIG 3.
Flow cytometric (A) and whole-cell ELISA (B) analyses of Lactobacillus sp. cells after incubation with DARPin I_07-cA fusion-containing growth medium and detection with FITC-conjugated human IgG (gray bars). MFI and A450 values were normalized to those for the control samples (white bars). (C) Quantification of LTA on the surfaces of Lactobacillus spp. by whole-cell ELISA using an LTA-specific monoclonal antibody. ACID, Lb. acidophilus ATCC 4356; BULG, Lb. delbrueckii subsp. bulgaricus ATCC 1184; CASE, Lb. casei ATCC 393; GASA, Lb. gasseri ATCC 33323; GASK, Lb. gasseri K7; PARA, Lb. paracasei ATCC 25302; PLAN, Lb. plantarum ATCC 8014; REUT, Lb. reuteri ATCC 55730; RHAM, Lb. rhamnosus ATCC 53103; SALI, Lb. salivarius ATCC 11741. *, significant increase in IgG binding (t test; P < 0.05) in comparison to control.
Binding of the DARPin I_07-cA fusion to the surfaces of lactobacilli was confirmed for all bacterial species by whole-cell ELISA and for 7 of 10 species by flow cytometry. The majority of species exhibited weak to moderate binding (approximately 2- to 5-fold higher than that of the control), depending on the method of detection used. Stronger binding of Lactobacillus paracasei ATCC 25302 (>16-fold higher than that of the control) was determined with whole-cell ELISA but could not be confirmed with flow cytometry or fluorescence microscopy. The strongest binding was observed with Lb. salivarius ATCC 11741 and was confirmed with all three methods, i.e., flow cytometry (>55-fold higher MFI), whole-cell ELISA (>32-fold higher A450), and fluorescence microscopy (much higher fluorescence than that with any other Lactobacillus species).
To establish whether an increased content of LTA on the surfaces of lactobacilli correlates with less LysM-mediated surface binding, we quantified LTA on the surfaces of lactobacilli by using whole-cell ELISA with an LTA-specific monoclonal antibody (Fig. 3C). Though not statistically significant, the results suggest that larger amounts of LTA correlate with less LysM-mediated surface binding, as observed with Lactobacillus delbrueckii subsp. bulgaricus, Lb. plantarum, and Lactobacillus reuteri.
The exceptional surface properties of Lb. salivarius.
Genome sequences were compared to identify possible traits that separate Lb. salivarius from the rest of the Lactobacillus species tested. We narrowed the comparison to genes involved or predicted to be involved in synthesis of the cell wall, cell membrane, or exopolysaccharides (EPS). Four COGs were identified that were present in Lb. salivarius only, and 1 COG was present in all Lactobacillus species tested except Lb. salivarius (Table 2). The unique Lb. salivarius COGs contain 6 genes, among which 5 are included in the EPS biosynthesis cluster (Table 2), which suggests a role for EPS in LysM-mediated surface display.
TABLE 2.
IMG analysis of COGs unique to Lb. salivarius
| Locus tag | Gene product description | COG no. | COG description |
|---|---|---|---|
| HMPREF0545_0918 | UDP-glucose 6-dehydrogenase | COG1004 | Predicted UDP-glucose 6-dehydrogenase |
| HMPREF0545_0471a | UDP-glucose 6-dehydrogenase | COG1004 | Predicted UDP-glucose 6-dehydrogenase |
| HMPREF0545_0474a | Glycosyltransferase | COG3754 | Lipopolysaccharide biosynthesis protein |
| HMPREF0545_0475a | Glycosyltransferase | COG3754 | Lipopolysaccharide biosynthesis protein |
| HMPREF0545_0486a | Pyridoxal phosphate-dependent aminotransferase | COG0399 | Predicted pyridoxal phosphate-dependent enzyme apparently involved in regulation of cell wall biogenesis |
| HMPREF0545_0487a | Possible capsular polysaccharide biosynthesis protein | COG1086 | Predicted nucleoside-diphosphate sugar epimerases |
| NPb | NPb | COG1732 | Periplasmic glycine betaine/choline-binding (lipo)protein of an ABC-type transport system (osmoprotectant binding protein) |
Part of the EPS biosynthesis cluster.
NP, not present in Lactobacillus salivarius ATCC 11741.
To establish the possible influence of LTA (or its absence) on the surface properties of Lb. salivarius, the presence of LTA biosynthesis and LTA d-alanylation genes was confirmed with a high probability for the genome of Lb. salivarius by BLAST analysis (E values of <1.0 × 10−21), suggesting that Lb. salivarius is capable of producing LTA (Table 3).
TABLE 3.
BLAST analysis of Lb. salivarius ATCC 11741 genes
| Gene category and locus tag | Gene product description | Query sequencea | E value |
|---|---|---|---|
| Genes involved in LTA biosynthesis | |||
| HMPREF0545_1244 | Glycosyltransferase | LBA0444 | 3.0 × 10−144 |
| HMPREF0545_1243 | Glycosyltransferase | LBA0445 | 2.0 × 10−140 |
| HMPREF0545_1242 | Integral membrane protein | LBA0446 | 4.0 × 10−65 |
| HMPREF0545_1240 | Phosphatidylglycerol-membrane oligosaccharide glycerophosphotransferase | LBA0447 | 0 |
| Genes involved in LTA d-alanylation | |||
| HMPREF0545_0394 | d-Alanine-d-alanyl carrier protein ligase (DltA) | LBA1926 | 0 |
| HMPREF0545_0393 | DltB | LBA1925 | 1.0 × 10−166 |
| HMPREF0545_0392 | d-Alanine-poly(phosphoribitol) ligase subunit (DltC) | LBA1924 | 1.0 × 10−21 |
| HMPREF0545_0391 | DltD precursor | LBA1923 | 1.0 × 10−125 |
Query sequences are from Lactobacillus acidophilus NCFM.
Antibiotic-induced increase in LysM-mediated nonrecombinant surface display on L. lactis.
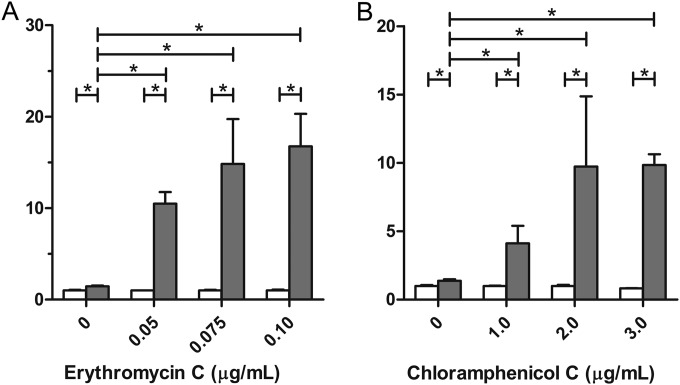
Nonrecombinant L. lactis cells were grown to late exponential phase in the presence of several concentrations of erythromycin or chloramphenicol, followed by incubation with the DARPin I_07-cA fusion. Surface binding was measured by flow cytometry. The presence of erythromycin or chloramphenicol in the growth medium caused significant, antibiotic concentration-dependent increases in LysM-mediated binding to the bacterial surface (Fig. 4). The largest increases in surface binding were observed with 0.1 μg/ml erythromycin (Fig. 4A) and 3 μg/ml chloramphenicol (Fig. 4B).
FIG 4.
Flow cytometric analysis of LysM-mediated nonrecombinant surface display on L. lactis after treatment of bacteria with different concentrations of the antibiotics erythromycin (A) and chloramphenicol (B). Binding of the DARPin I_07-cA fusion to the bacterial surface was detected with FITC-conjugated human IgG (gray bars). MFI values were normalized to those for the control samples (white bars), in which bacteria were incubated with immunoglobulins without prior binding of the DARPin I_07-cA fusion to the bacterial surface. Error bars denote standard deviations. *, significant difference (t test; P < 0.05) between the pair of samples indicated.
DISCUSSION
LysM-mediated binding to the surfaces of LAB was assessed by introducing two DARPins (I_07 and I_19) as model binding proteins, both expressing affinity for the Fc region of human immunoglobulins. DARPins that have already been developed against several other targets, such as lactococcal phage TP901-1 (32), HIV gp120 (33), and human IgE (34), are potentially applicable in LAB-mediated therapy. We genetically fused DARPins I_07 and I_19 to the C terminus of the AcmA protein and to the Usp45 secretion signal and expressed the resulting fusion proteins in L. lactis by using nisin-controlled expression (NICE). Both fusion proteins (I_07-cA and I_19-cA) were overexpressed in the cytoplasm (Fig. 1A) and were able to bind Fc of human IgG (Fig. 1B); however, stronger binding was observed with DARPin I_07. The secretion of both fusion proteins into the growth medium, subsequent surface binding, and human IgG Fc-binding ability were confirmed by flow cytometry, whole-cell ELISA, and fluorescence microscopy. I_07-cA bound more strongly to IgG Fc than did I_19-cA, as determined by all three methods (Fig. 2), and was therefore used for further testing of surface display. The stronger I_07-cA binding contrasts with the results of a previously reported study (27) in which I_19 was found to bind more strongly. The expression levels of the two DARPin fusion proteins were the same, and we attribute the stronger IgG binding with I_07-cA to the more complex folding of the larger DARPin, DARPin I_19 (containing 5 ankyrin repeats), than of DARPin I_07 (containing 4 ankyrin repeats). The display of human IgG-binding DARPins on the surface of L. lactis adds another class of LAB-displayed binders to the existing pool, which includes Affibody molecules, Nanobodies, and single-chain variable fragments (11, 35, 36).
A LysM repeat-containing fusion protein is secreted into the growth medium with the Usp45 secretion signal. Because not all of the fusion protein binds to the producer cells, the spent growth medium can be applied for heterologous binding of a LysM repeat-containing fusion protein to unmodified bacterial cells, as already demonstrated (14, 20). Heterologous binding can be considered to be nonrecombinant, which is a great advantage in view of the significant regulatory obstacles and low public acceptance of genetically modified organisms (GMOs) (37). Heterologous binding was very effective on naked peptidoglycan particles, while levels of binding to lactobacilli varied and were species dependent (14, 15). The differences between lactobacilli were quantified by flow cytometry and whole-cell ELISA, using the DARPin-cA fusion protein as a probe for surface binding mediated by the LysM repeats (Fig. 3). The results of flow cytometry were substantiated by those obtained using fluorescence microscopy (see Fig. S1 in the supplemental material). The DARPin-cA fusion protein bound to the surfaces of all lactobacillus species tested; however, for the majority of species, weak to moderate binding was observed. Stronger binding to the surface of Lb. paracasei ATCC 25302 was observed with whole-cell ELISA but not with flow cytometry or fluorescence microscopy, suggesting an intraspecies variability which might be growth phase dependent and would require further study. The strongest binding was observed with Lb. salivarius ATCC 11741 and was confirmed by all three methods. Lb. salivarius ATCC 11741 exhibited so far unique properties for LysM-mediated surface display, largely surpassing all other lactobacillus strains (>15-fold and 8.5-fold stronger binding than the average for other lactobacilli, as determined by flow cytometry and whole-cell ELISA, respectively). Lb. salivarius ATCC 11741 therefore has the potential to become a nonrecombinant surface display host, particularly as it has already been reported to be a probiotic with antipathogenic and TNF-α-stimulatory activities (38). However, its applicability will have to be verified in further studies. Our future work will therefore include testing of Lb. salivarius ATCC 11741 as a nonrecombinant vector for the intestinal delivery of cytokine- and toxin-binding proteins in mouse disease models.
The observed difference in surface display ability between Lb. salivarius ATCC 11741 and the other lactobacilli tested may be explained as a consequence of different surface structures or of their chemical composition. A higher content of cell wall LTA has already been shown to correlate with less LysM-mediated surface binding. We also observed that species containing more LTA on their surfaces (Lb. delbrueckii subsp. bulgaricus ATCC 1184, Lb. plantarum ATCC 8014, and Lb. reuteri ATCC 55730) exhibited a lower LysM-mediated display ability. However, this was not the case for all species and, in particular, could not explain the much larger LysM-mediated display ability observed with Lb. salivarius. We therefore performed a genomic comparison of Lb. salivarius and the rest of the species for which genomic information is available. There are no apparent differences in the presence of LTA biosynthetic genes between Lb. salivarius and the rest of the lactobacilli, although this comparison is hampered by differences in LTA biosynthesis and the lack of a complete understanding of LTA biosynthesis in lactobacilli. The presence of an LTA biosynthesis cluster homologous to that of Lb. acidophilus NCFM (31) and of an LTA d-alanylation cluster in Lb. salivarius has been confirmed. More general comparisons of genes involved in the synthesis of the cell wall, membrane, or envelope across the tested species revealed five Lb. salivarius-specific genes (present in unique COGs) that are located in one of the two Lb. salivarius EPS biosynthetic clusters (39). Lb. salivarius ATCC 11741 has already been described as a more intensive producer of EPS than other lactobacillus strains (40). The most prominent difference observed was the presence of the UDP-glucose-6-dehydrogenase (UGDH) gene (HMPREF0545_0471) in the Lb. salivarius ATCC 11741 genome. UGDH can convert UDP-glucose to UDP-glucoronate, which is rarely observed in lactobacillus EPS (41) but is a relatively common constituent of the capsule of pathogenic Gram-positive bacteria (42, 43). The interdependence of UDP-glucoronate monomers in the EPS structure and LysM-mediated binding remains to be confirmed, and more detailed characterization is therefore needed to confirm the possible involvement of UGDH or the EPS structure.
The level of LysM repeat-containing fusion protein on the surface was higher on a recombinant expression host than on nonrecombinant lactococci that bound the fusion protein from the spent growth medium of the recombinant producer. This can be attributed to partial removal of the LysM repeat-containing fusion protein from the growth medium by the producer cells, which, to a certain extent, limits the applicability of the procedure. We also observed that recombinant expression and display of the fusion protein were significantly increased if the producer cells contained a gene that conferred resistance to a second antibiotic (with the first being part of the NICE system) (data not shown). We therefore turned our attention to the influence of nonlethal concentrations of antibiotics on nonrecombinant lactococci. Surprisingly, a concentration-dependent, up-to-10-fold increase in LysM-mediated surface binding was observed when the bacterial culture was grown in the presence of chloramphenicol or erythromycin. This was unexpected, because both antibiotics are ribosome inhibitors and do not interfere with cell wall synthesis. However, stress (including antibiotic stress) can cause massive perturbations in patterns of bacterial gene expression, including that of genes in the cell surface homeostasis pathways (24, 25). In our experiments, treatment of cultures with antibiotics consistently resulted in greater LysM-mediated surface display, although the amount of the increase was less reproducible. Presumably there are factors that play an important role in antibiotic-induced surface changes that were not completely controlled in our experiments. Nevertheless, addition of antibiotics or, alternatively, more acceptable stress compounds may provide an important means of increasing LysM-mediated display on nonrecombinant bacteria.
The display of proteins on the surfaces of LAB usually requires their genetic modification, which limits or prevents its medical and food applications due to poor public acceptance and unresolved legal issues. Noncovalent binding of heterologous proteins from a recombinant host to the surfaces of nonrecombinant bacteria may constitute a regulatory bypass. In the present research, we improved nonrecombinant surface display in LAB. Ten species of lactobacilli were screened, and Lb. salivarius ATCC 11741 was shown to have the greatest LysM-mediated surface display ability, making it an attractive candidate for nonrecombinant LysM-mediated surface display. Additionally, sublethal concentrations of the antibiotics erythromycin and chloramphenicol have been shown to increase LysM-mediated surface display in L. lactis in a concentration-dependent manner. Addition of such stress factors may be used as a complementary strategy in improving LysM-mediated surface display in LAB. Finally, surface display of DARPins on various LAB species has been established, which offers an opportunity for new therapeutic applications.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Slovenian Research Agency grant P4-0127.
We are grateful to Roger Pain for a critical reading of the manuscript, to Irena Rogelj and Bojana Bogovič Matijašić for providing Lactobacillus species, and to Urša Pečar Fonović for help with fluorescence microscopy.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03694-14.
REFERENCES
- 1.Georgiou G, Stathopoulos C, Daugherty PS, Nayak AR, Iverson BL, Curtiss R III. 1997. Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat Biotechnol 15:29–34. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 2.Lee SY, Choi JH, Xu Z. 2003. Microbial cell-surface display. Trends Biotechnol 21:45–52. doi: 10.1016/S0167-7799(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 3.Stahl S, Uhlen M. 1997. Bacterial surface display: trends and progress. Trends Biotechnol 15:185–192. doi: 10.1016/S0167-7799(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 4.Berlec A, Ravnikar M, Strukelj B. 2012. Lactic acid bacteria as oral delivery systems for biomolecules. Pharmazie 67:891–898. doi: 10.1691/ph.2012.1705. [DOI] [PubMed] [Google Scholar]
- 5.Dieye Y, Usai S, Clier F, Gruss A, Piard JC. 2001. Design of a protein-targeting system for lactic acid bacteria. J Bacteriol 183:4157–4166. doi: 10.1128/JB.183.14.4157-4166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visweswaran GR, Leenhouts K, van Roosmalen M, Kok J, Buist G. 2014. Exploiting the peptidoglycan-binding motif, LysM, for medical and industrial applications. Appl Microbiol Biotechnol 98:4331–4345. doi: 10.1007/s00253-014-5633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu S, Kong J, Sun Z, Han L, Kong W, Yang P. 2011. Heterologous protein display on the cell surface of lactic acid bacteria mediated by the s-layer protein. Microb Cell Fact 10:86. doi: 10.1186/1475-2859-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zadravec P, Mavric A, Bogovic Matijasic B, Strukelj B, Berlec A. 2014. Engineering BmpA as a carrier for surface display of IgG-binding domain on Lactococcus lactis. Protein Eng Des Sel 27:21–27. doi: 10.1093/protein/gzt059. [DOI] [PubMed] [Google Scholar]
- 9.Bahey-El-Din M. 2012. Lactococcus lactis-based vaccines from laboratory bench to human use: an overview. Vaccine 30:685–690. doi: 10.1016/j.vaccine.2011.11.098. [DOI] [PubMed] [Google Scholar]
- 10.Berlec A, Malovrh T, Zadravec P, Steyer A, Ravnikar M, Sabotič J, Poljšak-Prijatelj M, Štrukelj B. 2013. Expression of a hepatitis A virus antigen in Lactococcus lactis and Escherichia coli and evaluation of its immunogenicity. Appl Microbiol Biotechnol 97:4333–4342. doi: 10.1007/s00253-013-4722-3. [DOI] [PubMed] [Google Scholar]
- 11.Ravnikar M, Strukelj B, Obermajer N, Lunder M, Berlec A. 2010. Engineered lactic acid bacterium Lactococcus lactis capable of binding antibodies and tumor necrosis factor alpha. Appl Environ Microbiol 76:6928–6932. doi: 10.1128/AEM.00190-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieczorek AS, Martin VJ. 2010. Engineering the cell surface display of cohesins for assembly of cellulosome-inspired enzyme complexes on Lactococcus lactis. Microb Cell Fact 9:69. doi: 10.1186/1475-2859-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Kyla-Nikkila K, Saris PE. 2011. Cell immobilization studies using a cellulose-binding domain fused to PrtP in Lactococcus lactis. Bioeng Bugs 2:160–162. doi: 10.4161/bbug.2.3.15348. [DOI] [PubMed] [Google Scholar]
- 14.Steen A, Buist G, Leenhouts KJ, El Khattabi M, Grijpstra F, Zomer AL, Venema G, Kuipers OP, Kok J. 2003. Cell wall attachment of a widely distributed peptidoglycan binding domain is hindered by cell wall constituents. J Biol Chem 278:23874–23881. doi: 10.1074/jbc.M211055200. [DOI] [PubMed] [Google Scholar]
- 15.Bosma T, Kanninga R, Neef J, Audouy SA, van Roosmalen ML, Steen A, Buist G, Kok J, Kuipers OP, Robillard G, Leenhouts K. 2006. Novel surface display system for proteins on non-genetically modified gram-positive bacteria. Appl Environ Microbiol 72:880–889. doi: 10.1128/AEM.72.1.880-889.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buist G, Kok J, Leenhouts KJ, Dabrowska M, Venema G, Haandrikman AJ. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J Bacteriol 177:1554–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steen A, Buist G, Horsburgh GJ, Venema G, Kuipers OP, Foster SJ, Kok J. 2005. AcmA of Lactococcus lactis is an N-acetylglucosaminidase with an optimal number of LysM domains for proper functioning. FEBS J 272:2854–2868. doi: 10.1111/j.1742-4658.2005.04706.x. [DOI] [PubMed] [Google Scholar]
- 18.Okano K, Zhang Q, Kimura S, Narita J, Tanaka T, Fukuda H, Kondo A. 2008. System using tandem repeats of the cA peptidoglycan-binding domain from Lactococcus lactis for display of both N- and C-terminal fusions on cell surfaces of lactic acid bacteria. Appl Environ Microbiol 74:1117–1123. doi: 10.1128/AEM.02012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moeini H, Rahim RA, Omar AR, Shafee N, Yusoff K. 2011. Lactobacillus acidophilus as a live vehicle for oral immunization against chicken anemia virus. Appl Microbiol Biotechnol 90:77–88. doi: 10.1007/s00253-010-3050-0. [DOI] [PubMed] [Google Scholar]
- 20.Hu S, Kong J, Kong W, Guo T, Ji M. 2010. Characterization of a novel LysM domain from Lactobacillus fermentum bacteriophage endolysin and its use as an anchor to display heterologous proteins on the surfaces of lactic acid bacteria. Appl Environ Microbiol 76:2410–2418. doi: 10.1128/AEM.01752-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu W, Huang M, Zhang Y, Yi X, Dong W, Gao X, Jia C. 2011. Novel surface display system for heterogonous proteins on Lactobacillus plantarum. Lett Appl Microbiol 53:641–648. doi: 10.1111/j.1472-765X.2011.03160.x. [DOI] [PubMed] [Google Scholar]
- 22.Kleerebezem M, Hols P, Bernard E, Rolain T, Zhou M, Siezen RJ, Bron PA. 2010. The extracellular biology of the lactobacilli. FEMS Microbiol Rev 34:199–230. doi: 10.1111/j.1574-6976.2009.00208.x. [DOI] [PubMed] [Google Scholar]
- 23.Davies J, Spiegelman GB, Yim G. 2006. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol 9:445–453. doi: 10.1016/j.mib.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Aakra A, Vebo H, Snipen L, Hirt H, Aastveit A, Kapur V, Dunny G, Murray BE, Nes IF. 2005. Transcriptional response of Enterococcus faecalis V583 to erythromycin. Antimicrob Agents Chemother 49:2246–2259. doi: 10.1128/AAC.49.6.2246-2259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aakra A, Vebo H, Indahl U, Snipen L, Gjerstad O, Lunde M, Nes IF. 2010. The response of Enterococcus faecalis V583 to chloramphenicol treatment. Int J Microbiol 2010:483048. doi: 10.1155/2010/483048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koskenniemi K, Laakso K, Koponen J, Kankainen M, Greco D, Auvinen P, Savijoki K, Nyman TA, Surakka A, Salusjarvi T, de Vos WM, Tynkkynen S, Kalkkinen N, Varmanen P. 2011. Proteomics and transcriptomics characterization of bile stress response in probiotic Lactobacillus rhamnosus GG. Mol Cell Proteomics 10:M110.002741. doi: 10.1074/mcp.M110.002741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steiner D, Forrer P, Pluckthun A. 2008. Efficient selection of DARPins with sub-nanomolar affinities using SRP phage display. J Mol Biol 382:1211–1227. doi: 10.1016/j.jmb.2008.07.085. [DOI] [PubMed] [Google Scholar]
- 28.Stumpp MT, Binz HK, Amstutz P. 2008. DARPins: a new generation of protein therapeutics. Drug Discov Today 13:695–701. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 29.Lindholm A, Smeds A, Palva A. 2004. Receptor binding domain of Escherichia coli F18 fimbrial adhesin FedF can be both efficiently secreted and surface displayed in a functional form in Lactococcus lactis. Appl Environ Microbiol 70:2061–2071. doi: 10.1128/AEM.70.4.2061-2071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molenaar D, Bringel F, Schuren FH, de Vos WM, Siezen RJ, Kleerebezem M. 2005. Exploring Lactobacillus plantarum genome diversity by using microarrays. J Bacteriol 187:6119–6127. doi: 10.1128/JB.187.17.6119-6127.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohamadzadeh M, Pfeiler EA, Brown JB, Zadeh M, Gramarossa M, Managlia E, Bere P, Sarraj B, Khan MW, Pakanati KC, Ansari MJ, O'Flaherty S, Barrett T, Klaenhammer TR. 2011. Regulation of induced colonic inflammation by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc Natl Acad Sci U S A 108(Suppl 1):S4623–S4630. doi: 10.1073/pnas.1005066107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veesler D, Dreier B, Blangy S, Lichiere J, Tremblay D, Moineau S, Spinelli S, Tegoni M, Pluckthun A, Campanacci V, Cambillau C. 2009. Crystal structure and function of a DARPin neutralizing inhibitor of lactococcal phage TP901-1: comparison of DARPin and camelid VHH binding mode. J Biol Chem 284:30718–30726. doi: 10.1074/jbc.M109.037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mann A, Friedrich N, Krarup A, Weber J, Stiegeler E, Dreier B, Pugach P, Robbiani M, Riedel T, Moehle K, Robinson JA, Rusert P, Pluckthun A, Trkola A. 2013. Conformation-dependent recognition of HIV gp120 by designed ankyrin repeat proteins provides access to novel HIV entry inhibitors. J Virol 87:5868–5881. doi: 10.1128/JVI.00152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann MJ, Eggel A, Amstutz P, Stadler BM, Vogel M. 2010. DARPins against a functional IgE epitope. Immunol Lett 133:78–84. doi: 10.1016/j.imlet.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Kruger C, Hu YZ, Pan Q, Marcotte H, Hultberg A, Delwar D, van Dalen PJ, Pouwels PH, Leer RJ, Kelly CG, van Dollenweerd C, Ma JK, Hammarstrom L. 2002. In situ delivery of passive immunity by lactobacilli producing single-chain antibodies. Nat Biotechnol 20:702–706. doi: 10.1038/nbt0702-702. [DOI] [PubMed] [Google Scholar]
- 36.Vandenbroucke K, de Haard H, Beirnaert E, Dreier T, Lauwereys M, Huyck L, Van Huysse J, Demetter P, Steidler L, Remaut E, Cuvelier C, Rottiers P. 2010. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol 3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 37.Foligne B, Daniel C, Pot B. 2013. Probiotics from research to market: the possibilities, risks and challenges. Curr Opin Microbiol 16:284–292. doi: 10.1016/j.mib.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Diaz MA, Bik EM, Carlin KP, Venn-Watson SK, Jensen ED, Jones SE, Gaston EP, Relman DA, Versalovic J. 2013. Identification of Lactobacillus strains with probiotic features from the bottlenose dolphin (Tursiops truncatus). J Appl Microbiol 115:1037–1051. doi: 10.1111/jam.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raftis EJ, Salvetti E, Torriani S, Felis GE, O'Toole PW. 2011. Genomic diversity of Lactobacillus salivarius. Appl Environ Microbiol 77:954–965. doi: 10.1128/AEM.01687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu CT, Hsu IT, Chou CC, Lo PR, Yu RC. 2009. Exopolysaccharide production of Lactobacillus salivarius BCRC 14759 and Bifidobacterium bifidum BCRC 14615. World J Microbiol Biotechnol 25:883–890. doi: 10.1007/s11274-009-9965-x. [DOI] [Google Scholar]
- 41.Harutoshi T. 2013. Exopolysaccharides of lactic acid bacteria for food and colon health applications, p 515–538. In Kongo JM. (ed), Lactic acid bacteria—R&D for food, health and livestock purposes. InTech, Rijeka, Croatia. [Google Scholar]
- 42.Cole JN, Aziz RK, Kuipers K, Timmer AM, Nizet V, van Sorge NM. 2012. A conserved UDP-glucose dehydrogenase encoded outside the hasABC operon contributes to capsule biogenesis in group A Streptococcus. J Bacteriol 194:6154–6161. doi: 10.1128/JB.01317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campbell RE, Sala RF, van de Rijn I, Tanner ME. 1997. Properties and kinetic analysis of UDP-glucose dehydrogenase from group A streptococci. Irreversible inhibition by UDP-chloroacetol. J Biol Chem 272:3416–3422. [DOI] [PubMed] [Google Scholar]
- 44.Bogovic-Matijasic B, Rogelj I. 2000. Lactobacillus K7—a new candidate for a probiotic strain. Food Technol Biotechnol 38:113–119. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.