Abstract
Flavobacteria (members of the family Flavobacteriaceae) dominate the bacterial community in the Anopheles mosquito midgut. One such commensal, Elizabethkingia anophelis, is closely associated with Anopheles mosquitoes through transstadial persistence (i.e., from one life stage to the next); these and other properties favor its development for paratransgenic applications in control of malaria parasite transmission. However, the physiological requirements of E. anophelis have not been investigated, nor has its capacity to perpetuate despite digestion pressure in the gut been quantified. To this end, we first developed techniques for genetic manipulation of E. anophelis, including selectable markers, reporter systems (green fluorescent protein [GFP] and NanoLuc), and transposons that function in E. anophelis. A flavobacterial expression system based on the promoter PompA was integrated into the E. anophelis chromosome and showed strong promoter activity to drive GFP and NanoLuc reporter production. Introduced, GFP-tagged E. anophelis associated with mosquitoes at successive developmental stages and propagated in Anopheles gambiae and Anopheles stephensi but not in Aedes triseriatus mosquitoes. Feeding NanoLuc-tagged cells to A. gambiae and A. stephensi in the larval stage led to infection rates of 71% and 82%, respectively. In contrast, a very low infection rate (3%) was detected in Aedes triseriatus mosquitoes under the same conditions. Of the initial E. anophelis cells provided to larvae, 23%, 71%, and 85% were digested in A. stephensi, A. gambiae, and Aedes triseriatus, respectively, demonstrating that E. anophelis adapted to various mosquito midgut environments differently. Bacterial cell growth increased up to 3-fold when arginine was supplemented in the defined medium. Furthermore, the number of NanoLuc-tagged cells in A. stephensi significantly increased when arginine was added to a sugar diet, showing it to be an important amino acid for E. anophelis. Animal erythrocytes promoted E. anophelis growth in vivo and in vitro, indicating that this bacterium could obtain nutrients by participating in erythrocyte lysis in the mosquito midgut.
INTRODUCTION
Elizabethkingia species have attracted much interest because of their close biologic associations with Anopheles malaria vector and Aedes dengue fever vector mosquitoes (1–5). Elizabethkingia was detected in diverse sources of mosquitoes (field caught, seminatural reared, and insectary reared) sampled in different regions (Africa, Europe, and North America) (1, 3, 4). For instance, Elizabethkingia or Elizabethkingia-like bacteria were detected in 68% of field-caught mosquito populations collected in Cameroon (3). E. anophelis was isolated by Kämpfer et al. from the midgut of Anopheles gambiae G4 reared in an insectary as a predominant bacterial species (6). Wang et al. conducted a dynamic microbial community analysis of mosquitoes reared in seminatural microcosms (in Kenya) by using pyrosequencing methods and showed that Elizabethkingia spp. were more abundant in mosquitoes than in water of microcosms in which larvae were reared (1). Elizabethkingia spp. were frequently found to be associated with A. gambiae at various development stages (1). Similarly, Ngwa et al. found E. meningoseptica was the predominant bacterium in both larval and adult A. stephensi mosquitoes (7). Coon et al. also showed Elizabethkingia was common in all life stages in Aedes aegypti and A. gambiae (8). Based on the above observations, it is very likely that some Elizabethkingia species are symbionts for mosquitoes.
Paratransgenesis, a “Trojan horse” concept, entails transgenic symbionts or commensal microbes interfering with a pathogen's development inside insect vectors (3, 9, 10). It presents an alternative intervention strategy for vector-borne pathogen transmission (11). A candidate paratransgenesis agent should have the following characteristics: (i) it can be cultured in vitro and propagated; (ii) it can be genetically modified and introduced into female mosquitoes; (iii) it infects and stably persists in mosquitoes (12). Mosquito-associated commensal Elizabethkingia spp. may be excellent candidates for paratransgenesis tool development. For example, anti-Plasmodium activity was demonstrated in E. meningoseptica and E. anophelis in two recent studies. Ngwa et al. showed the ethyl acetate extracts of broth culture of E. meningoseptica in vitro had antiplasmodial activity (50% inhibitory concentration [IC50], 0.25 mg/ml) against the P. falciparum asexual blood stages, showed antigametocidal activity, and reduced 58% of P. falciparum density at the IC50 dose (7). Bahia et al. showed that E. anophelis had a prominent effect on Plasmodium parasite development when it was introduced at a low bacterial dose (103 cells/μl), reducing oocyst load (13). When Akhouayri et al. injected E. meningoseptica into Anopheles mosquitoes, the bacteria were highly virulent in adults, a process related to melanotic lesions in fat body tissues (5). Together, these studies demonstrate that Elizabethkingia species impact their host mosquitoes' physiology and interactions with malaria parasites. Recently, several genomes from E. meningoseptica and E. anophelis were sequenced and annotated (2, 14, 15). A number of genes, such as those related to sugar transportation/utilization, blood cell lysis, and the antioxidative system, were found and provided insights into possible flavobacterial symbiotic relationships with mosquito hosts (2). E. anophelis could therefore be a good model system to investigate how predominant bacteria interact with their hosts, vectored disease agents (parasites/viruses), and other associated microbes.
Despite their wide distribution in nature, potential importance in mosquito physiology, and paratransgenesis potential, the infection range, colonization mechanism(s) in mosquitoes, and nutrient requirements of E. anophelis remain poorly known. No genetic tools have been available for molecular manipulation of Elizabethkingia spp., impairing the study of symbiotic relationships with mosquitoes and interactions with other gut microbes and parasites. Genetic manipulation of flavobacterial members has been extremely difficult because the available genetic tools that are functional for proteobacteria do not function in flavobacteria, owing to the unique transcription initiation signals (promoter sequences) in the Bacteroidetes (16, 17). The objectives of the present study were as follows: (i) to develop molecular tools for tracking the fate of E. anophelis cells in mosquitoes; (ii) to investigate the mosquito host range of E. anophelis; (iii) to characterize nutrient requirements for E. anophelis growth in vivo and in vitro.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Strains and molecular reagents used in this study are listed in Table 1. Escherichia coli DH5α was used for cloning. E. coli S17(λ pir) was used for conjugation. E. coli EC100D pir+ was used for recovering transposon from E. anophelis. E. coli strains were routinely grown in Luria-Bertani (LB) broth (18). Elizabethkingia species were isolated from the mosquitoes Anopheles stephensi and A. gambiae sensu stricto kept in colonies at the insectary at Michigan State University. A primary isolate (from A. gambiae) used in experiments here was designated E. anophelis MSU001. M9 medium was slightly modified by adding yeast extract (0.5%, wt/vol) and peptone (1%, wt/vol). M9 medium, LB, or Casitone-yeast extract (CYE) medium was used for E. anophelis culture (18, 19). Liquid cultures were grown with shaking (ca. 200 rpm) at either 30°C (E. anophelis) or 37°C (E. coli). For solid LB medium, Bacto agar (Difco, Detroit, MI) was added to a final concentration of 20 g/liter with kanamycin (50 μg/ml) or ampicillin (100 μg/ml) added for plasmid selection in E. coli or erythromycin (Em) added (200 μg/ml) for transposon selection in E. anophelis. Various carbon sources and amino acids were added to modified M9 when growth tests were performed. Horse blood (Hemostat Lab, Dixon, CA) was supplemented in modified M9 medium to study its effect on bacterial growth.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics and/or plasmid constructiona | Reference or source |
|---|---|---|
| E. coli strains | ||
| EC100D pir+ | F− mcrA Δ(mrr-hsdRMS-mcrBC) ϕ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ− rpsL nupG pir+ (DHFR) | Epicentre |
| S17-1 | hsdR17(rK− mK−) recA RP4-2 (Tcr::Mu-Kmr::Tn7 Strr) | 49 |
| DH5α | F− ϕ80dlacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Clontech |
| E. anophelis strains | ||
| MSU001 | Isolated from Anopheles gambiae at Michigan State University | This study |
| SCH814 | Strain carrying expression cassette PompA+nluc | This study |
| SCH837 | Strain carrying expression cassette PompA+gfp | This study |
| Plasmids | ||
| pGEM-T Easy | Cloning vector; Ampr | Promega |
| pHimarEm1 | mariner transposon functional in flavobacteria; Kmr (Emr) | 34 |
| pSCH760 | Modified pHimarEm1 with MCS site SmaI-BamHI-SacII; Kmr (Emr) | This study |
| pSCH722 | PompA+nluc on pCP29; Ampr (Emr) | 38 |
| pFj29 | PompA+gfp on pCP29; Ampr (Emr) | 50 |
| pSCH770 | PompA+gfp on pGEM-T Easy with SmaI and SacII restriction sites; Ampr | This study |
| pSCH773 | PompA+gfp inserted at SmaI and SacII sites on pSCH760; Kmr (Emr) | This study |
| pFD1146 | Shuttle plasmid between Bacteroides and E. coli; Spr (Emr) | 51 |
| pNJR5 | IncQ, E. coli-Bacteroides shuttle vector; Kmr (Emr) | 52 |
| pSCH791 | PompA+nluc on pGEM-T Easy; Ampr | This study |
| pSCH801 | PompA+nluc on pSCH760; Kmr (Emr) | This study |
Unless indicated otherwise, antibiotic resistance phenotypes are those expressed in E. coli. Antibiotic resistance phenotypes listed in parentheses are those expressed in E. anophelis strains but not in E. coli. DHFR, dihydrofolate reductase.
Mosquito rearing.
Anopheles stephensi Liston Johns Hopkins strain, Aedes triseriatus Say MSU strain, and Anopheles gambiae sensu stricto Giles KISUMU strain mosquitoes were used in this study. Mosquito adults were confined in 60- by 60- by 60-cm insect cages. Cages were held in a chamber (Percival, IA) maintained at 28°C ± 1°C (mean ± standard deviation) and 50% ± 10% relative humidity under a light/dark 12:12-h photoperiod without dawn/dusk transitions. Sucrose solution (10%) was placed in cages with wicks and reservoirs to provide carbohydrate. Sodium-heparinated bovine blood (Hemostat Lab, Dixon, CA) was fed to adult mosquitoes via an artificial membrane feeder for around 30 min, twice per week. After 2 days, mosquito eggs were collected on the wet filter paper which was then supported by a water-statured cotton ball in a petri dish. Filter papers containing eggs were transferred into plastic containers with distilled water for hatching. Either First Bite (Kyorin, Himeji, Japan) or Tetramin tropical fish food flakes (Tetra, Blacksburg, VA) were provided for Anopheles and Aedes larval mosquitoes in the first instar stage, respectively. After that stage, pet food (Purina Cat Chow; Nestlé) was given once per day.
Molecular manipulation methods.
Genomic DNA was prepared using a Wizard genomic DNA purification kit (Promega, Madison, WI), and plasmid DNA was purified with the QIAprep spin miniprep kit (Qiagen, Germantown, MD). Restriction and modification enzymes were purchased from Promega (Madison, WI) or New England BioLabs (Beverly, MA). PCR amplifications were done with the Failsafe PCR system (Epicenter Technology, Madison, WI). Amplicons were separated in 0.7 to 1.0% (wt/vol) agarose gels, and DNA fragments were purified with the QIAquick gel extraction system (Qiagen). Ligation mixtures were transformed into E. coli cells, and transformants were plated onto LB plates with appropriate antibiotic selection. Resistant colonies were isolated and then screened for the acquisition of plasmids. All constructs were sequenced to verify structure.
The transposon pHimarEm1 was modified to introduce unique SmaI-BamHI-SacII restriction sites and to insert the reporter expression cassette PompA+gfp (Table 1). PCR was done with pHimarEm1 DNA as the template and using forward primer Walker142 (CGCGGATCCGCGTCCCCCGGGGGACTTGACAACCACCCGACTTTGAACTACG) and reverse primer Walker143 (CGCGGATCCGCGTCCCCGCGGGGAGCTGCCGCATAACGGCTGGCAAATTGG). The amplicon was digested with BamHI, self-ligated, and transformed into E. coli S17(λ pir)(pSCH760) (Table 1). The reporter expression cassette PompA+gfp was amplified with primers Walker146 (CCGCGGCCCAGGCTTTACACTTTATGCTTCCG) and Walker147 (CCCGGGATTATAGGGAATTCCGGACCGGTACC) and using pFj29 as the template according to standard procedures (20). The PCR product (PompA+gfp) was first inserted into a T-easy vector (pSCH770) (Table 1). The insert was released from pSCH770 by using SmaI and SacII enzymes and inserted into the same sites on plasmid pSCH760, creating the chromosome-tagging reporter construct PompA+gfp on pHimarEm1(pSCH773) (Table 1).
The nluc gene, encoding NanoLuc luciferase, was amplified with plasmid pSCH722 (Table 1) as a template by using forward primer Walker156 (ACCCGGGAACACTTAGACAAGGCAATAGAAGC) and reverse primer Walker157 (ACCGCGGTTAGACGTTGATGCGAGCTGAAGCAC) and cloned into the T-easy vector (pSCH791). The gene nluc was next released from pSCH791 with SmaI and SacII and inserted into the sites on pSCH760, leading to a transposon with a NanoLuc reporter (pSCH801) (Table 1). To investigate the insertion site for the transposon, genomic DNA was extracted, digested with XbaI, self-ligated, and introduced into the E. coli(λ pir) strain. The plasmid was sequenced with primers Walker85 (TGGGAATCATTTGAAGGTTGG) and Walker86 (TCGGGTATCGCTCTTGAAGGG).
For bacterial conjugation, both donor and recipient cells were cultured to mid-log phase, concentrated by centrifugation (4,500 × g, 15 min), washed once with LB, and resuspended in either LB (recipient cells) or a 1:1 mixture of LB and 10 mM MgSO4 (donor cells). The mixture was spotted on an LB agar plate and incubated overnight at 30°C. Following incubation, the cells were scraped off the agar and resuspended in LB broth. The homogenized suspension was spread on LB plates containing 200 μg/ml of Em to select for transconjugants. Em-resistant colonies were selected and purified after 48 h of incubation at 30°C. Following the above procedures, plasmid pSCH773 or pSCH801 in E. coli S17(λ pir) was separately conjugated into E. anophelis, leading to the green fluorescent protein (GFP) reporter strain SCH837 or NanoLuc reporter strain SCH814, respectively (Table 1). Em-resistant transconjugants were screened by using the primers Walker140 (TTCCTTGCGCAGCTGTGCTCGAC) and Walker141 (CGCTCAGAAGAACTCGTCAAGAAG). Detection of the respective transposase gene in transconjugants was conducted using primers Walker186 (GCAAAATTCAAGCGTGGTGAAATGAGC) and Walker187 (CGAGCATCCTTTTGAGGTCTGAGAAC).
Epifluorescence microscopy.
SCH837 (GFP-tagged) cells were visualized with an Olympus Provis AX70 microscope, equipped with appropriate filters, a mercury lamp for UV light source, and a DP-50 digital camera linked to an external PC. Second-instar larval mosquitoes were fed a suspension of the appropriate strains of E. anophelis at room temperature for 2 h, killed by 100% ethanol, transferred onto a 0.1% thin layer of agarose on the microscope slide, and observed using a UV filter.
Determination of GFP and NanoLuc reporter activity in E. anophelis.
Quantitative analysis of GFP or NanoLuc production was performed using a SpectraMax M5 (Molecular Devices, CA) or EnVision automated microplate reader (PerkinElmer, MA). Aliquots of cultures were adjusted to an optical density at 600 nm (OD600) of 0.4 and subjected to fluorescence determination in a 96-well microtiter plate (Costar, Corning, NY). GFP fluorescence was determined at an excitation wavelength of 490 nm, an emission wavelength of 530 nm, and a cutoff of 515 nm. The MSU001 strain without a gfp gene was used as the blank for calculation of the relative fluorescence units (17).
For determination of NanoLuc reporter activity, the cells or mosquitoes were sampled, homogenized, diluted, and immediately added to an equal volume of NanoLuc assay buffer (Promega, Madison, WI), and light intensity was quantified in 96-well microtiter plates by using a plate reader according to the manufacturer's protocol. If necessary, E. anophelis cells were first lysed with passive lysis buffer (PLB; Promega) and lysozyme, and then the lysate was mixed with an equal volume of NanoLuc assay buffer as described above. Standard curves were established to quantify the relationship between bacterial density and luminescence.
Analysis of digestion.
Third-instar larval mosquitoes (Aedes triseriatus, A. gambiae, and A. stephensi) were starved for 2 h in sterile water at room temperature before incubation with reporter strain SCH814 at the log phase of growth for 2 h. Next, larvae were extensively rinsed with water and immediately transferred into a 6-well plate (four larvae in 2 ml of sterile H2O per well). Bacteria in the larvae and the incubation solution were sampled at time points 0, 1, 1.5, 2, and 2.5 h. The four larvae in each well were pooled, homogenized with a sterile pestle, centrifuged, washed with phosphate-buffered saline (PBS), resuspended in PBS, and subjected to NanoLuc reporter analysis as described above.
Bacterial infection tests.
Reporter-tagged strains (SCH814 and SCH837) were fed to larval and adult mosquitoes to track their fates. For infection tests in the larval stage, larval mosquitoes (second instar) were incubated overnight with SCH814 or SCH837 with a final concentration at ∼2.4 × 108 CFU/ml, during which time larvae were actively feeding on the suspension. The exposed larvae were extensively washed and reared in 300 ml of distilled water until they molted to the pupal stage, a nonfeeding stage. Pupae were collected, washed extensively in sterile water, and placed in a container for adult mosquito emergence. Adult mosquitoes were randomly collected, processed by homogenization using a sterile pestle, and subjected to the luciferase assay. To infect adult A. stephensi mosquitoes, SCH814 was cultured overnight at 30°C, pelleted, washed, and adjusted to ∼2.4 × 108 CFU/ml in sterile 10% sucrose solution. After feeding for 16 to 24 h, the bacterial solution was replaced with fresh sterile 10% sucrose. At specific times (days), mosquitoes were sampled randomly and subjected to the luciferase assay.
Statistical analyses.
Statistical analyses were performed using SAS (version 9.2; SAS Institute, Cary, NC).
Nucleotide sequence accession number.
The GenBank accession number of 16S rRNA sequence for E. anophelis strain MSU001 is KP125493.
RESULTS
Phylogenetic placement of E. anophelis strain MSU001.
Genomic DNA was extracted from strain MSU001, and the 16S rRNA gene was amplified using primers 63f and 1387r (21). Both strands of the amplified fragment were sequenced. Sequence analysis showed that it was 99% identical to the 16S rRNA genes of E. anophelis R26 and E. meningoseptica, 93% identical to that of Riemerella anatipestifer, and 84% identical to that of Flavobacterium johnsoniae. Placement of the sequence into a phylogenetic tree using MEGA (22) revealed a close relationship to several Elizabethkingia isolates from Anopheles mosquitoes (see Fig. S1 in the supplemental material).
Construction of mariner-based transposons carrying reporter expression cassettes.
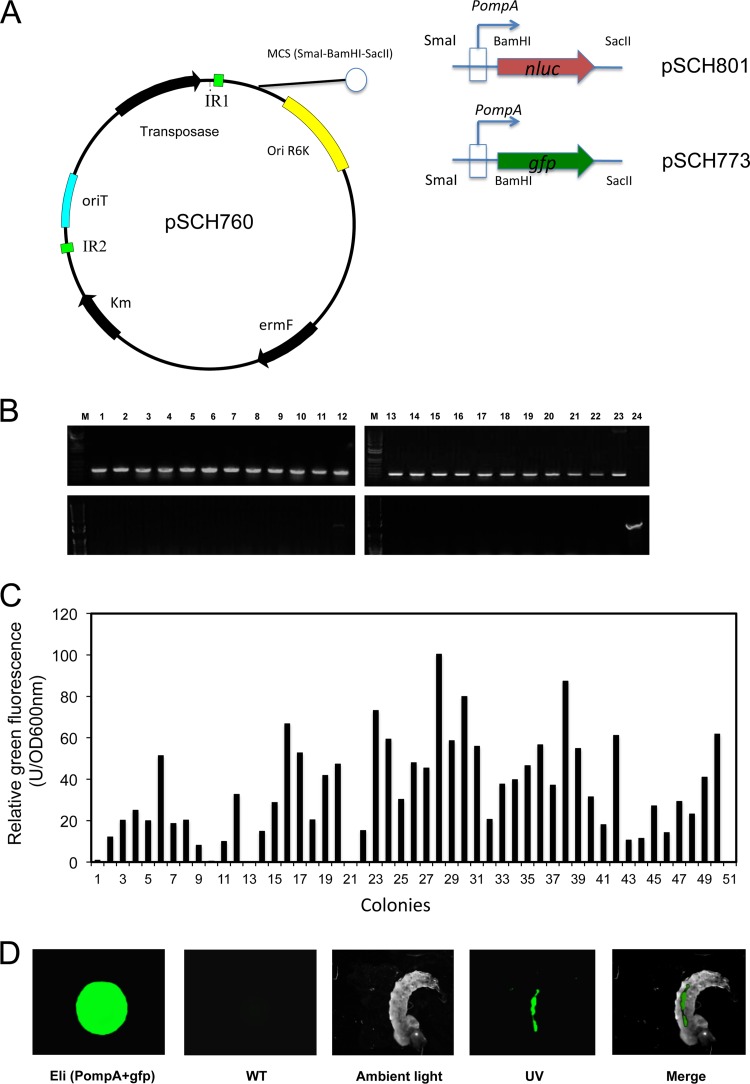
We attempted to introduce Bacteroidetes-E. coli shuttle plasmids (pFj29 and pFD1146) into E. anophelis MSU001 cells, but stable transformants were not obtained (data not shown), indicating that these plasmids were not replicable or that the host cells resisted transformation. Instead, we successfully obtained Em-resistant transformants when the mini-mariner transposon pHimarEm1 was conjugatively transferred into MSU001 cells, showing that introduction of foreign genetic elements was not problematic in E. anophelis wild-type strains. The transposon pHimarEm1 was modified with a multiple-cloning site (SmaI-BamHI-SacII) to facilitate the insertion of genes of interest (Fig. 1A). The PompA+gfp gene expression cassette was cloned into the transposon and successfully introduced into strain E. anophelis MSU001; the transposition frequency was estimated at 1.5 × 10−7. Fifty fluorescent colonies were counted using fluorimetry after being first screened from several thousand Em-resistant colonies (Fig. 1C). The colonies showed fluorescence at various intensities, ranging from 10 to 100 U/OD600 (Fig. 1C). The brightest-fluorescing colony (Eli28) was designated E. anophelis strain SCH837 and was selected for further study (Fig. 1C). SCH837 cells were readily detected, with obvious fluorescence in the foregut and midgut but much less in the hindgut of larval mosquitoes after 2 h of feeding, indicating that some of SCH837 cells were digested in A. gambiae (Fig. 1D). In reporter strain SCH837, a transposon carrying expression cassette PompA+gfp was inserted in a hypothetical protein gene encoding a putative TonB-dependent receptor (WP_024563967). Similarly, in reporter strain SCH814, a transposon carrying PompA+nluc was inserted in a hypothetical protein gene showing homology with cell envelope biogenesis protein AsmA (WP_009087586). Similar growth rates were observed in MSU001, SCH837, and SCH814 strains (see Fig. S2 in the supplemental material); thus, integration of the reporter on the mariner transposon in the E. anophelis chromosome at these sites did not affect bacterial growth. Furthermore, we validated cell growth and NanoLuc luciferase activity (see Fig. S3 in the supplemental material). The NanoLuc activity increased with cell growth during log phase (between 2 and 7 h) and remained stable in the stationary phase (between 10 and 12 h) (see Fig. S3B), indicating that expression of the nluc gene driven by the constitutive promoter PompA was not significantly affected by growth phase (see Fig. S3C). It was therefore appropriate for estimation of cell density by determination of luciferase activity, due to the linear relationship between luciferase activity and viable cell number (see Fig. S3D).
FIG 1.
Reporter strain construction and demonstration of E. anophelis cells tagged with GFP and their ingestion by mosquito larvae. (A) Diagram of the pSCH760 construct. pHimarEm1(MCS) was modified with a multiple-cloning site (SmaI-BamHI-SacII); the expression cassettes PompA+gfp or PompA+nluc were inserted into SmaI and SacII sites on pSCH760 to generate pSCH773 and pSCH801, respectively. (B) The transposon incorporated into the E. anophelis MSU001 chromosome. (Upper panel) PCR screening results for the Em-resistant transconjugants, using the primers Walker140 and Walker141. Lane M, molecular marker; lanes 1 to 22, DNA fragments amplified from Em-resistant transconjugants; lane 23, positive control (pSCH760 as the amplification template); lane 24, negative control (E. anophelis MSU001). (Lower panel) Presence of transposase, determined using primers Walker186 and Walker187. Lanes 1 to 22, the same transconjugants as in the upper panel; lane 23, negative control (E. anophelis MSU001); lane 24, positive control (pSCH760 as the amplification template). (C) Quantitative analysis of E. anophelis emitting GFP fluorescence. Transconjugants were first screened under UV, and the fluorescent colonies were next quantified using fluorometry. The brightest colony was chosen for further study. (D) The cultures carrying the GFP reporter were incubated with Anopheles mosquito larvae for 2 h, and the larvae were observed by using epifluorescence microscopy. The control was E. anophelis MSU001 cells.
Comparison of E. anophelis digestibility in mosquitoes.
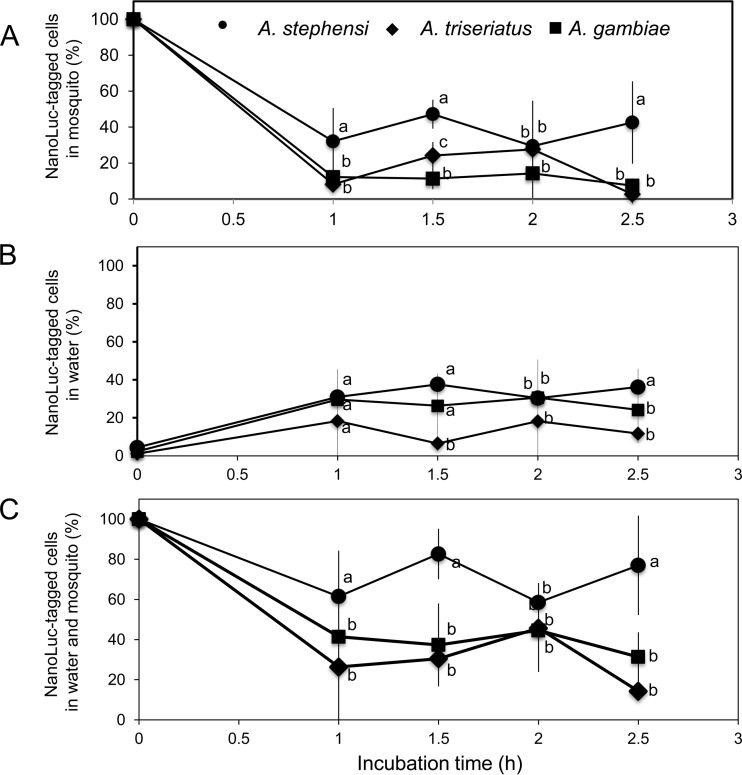
Ingested E. anophelis cells can be, alternatively, (i) preserved alive in larval mosquitoes, (ii) ejected into the surrounding solution by egestion from defecation, or (iii) digested. To track the fate after ingestion, we determined the density of E. anophelis SCH814 cells in larval mosquitoes and incubation solution (sterile water). Compared to initial cell densities at time zero, 30%, 46%, 30%, and 43% of initial E. anophelis cells were detected in A. stephensi larval guts after being transferred into sterile water for 1 h, 1.5 h, 2 h, and 2.5 h, respectively (Fig. 2A). At these same sampling times and compared to time zero, 32%, 39%, 32%, and 37% of SCH814 were detected in the incubation solution. From these findings, overall, about 23% of SCH814 cells in A. stephensi were estimated to have been digested after 2.5 h of incubation in water (Fig. 2C). However, under the same conditions, a low level of residual SCH814 cells (ranging from 6.2% to 15.7% of the initial number of cells) were found in larval A. gambiae after being transferred within 2.5 h (Fig. 2A), while ca. 22% to 27% of ingested SCH814 cells were excreted into water (Fig. 2B). The digestion rate of SCH814 in A. gambiae larvae was estimated to be 71% after 2.5 h of incubation in sterile water (Fig. 2C). For Aedes triseriatus, the NanoLuc activity of SCH814 showed great variation (Fig. 2A) in larvae during the incubation time (ranging from 3% to 41%). However, only a small portion of the ingested E. anophelis cells (5% to 12% of replicates) were detected in water (i.e., excreted) during the incubation period (Fig. 2B). The digestion rate in Aedes triseriatus was estimated to be 85% after 2.5 h (Fig. 2C). Collectively, E. anophelis cells were more resistant to digestion in larval A. stephensi than in A. gambiae and Aedes triseriatus during the 2.5-h incubation period in water (Fig. 2C).
FIG 2.
Digestibility analysis of E. anophelis by Aedes triseriatus, A. gambiae, and A. stephensi larvae. (A) Larval mosquitoes fed SCH814 cells were pooled (4 at each time point), homogenized, washed, and subjected to the NanoLuc activity assay. Cell densities at the different time points were normalized to the initial cell densities in corresponding mosquitoes at time zero. (B) Cells in the water were sampled, washed with PBS by centrifuging, resuspended in PBS, and subjected to the NanoLuc activity assay. Cell densities at the different time points were normalized to the initial cell densities at time zero. (C) The NanoLuc-tagged cells recovered from mosquitoes and water samples were quantified and normalized to those at time zero. Values are means ± standard deviations; triplicate experiments were performed. Significant differences among Aedes triseriatus, A. gambiae, and A. stephensi samples at each time point were determined by using PROC GLM. Different letters (a, b, and c) indicate significant differences in NanoLuc-tagged cell densities among these samples at each time point (P < 0.05). Means with the same letters indicate that no statistically significant difference was observed for these samples (P > 0.05).
Association of E. anophelis with selected mosquitoes.
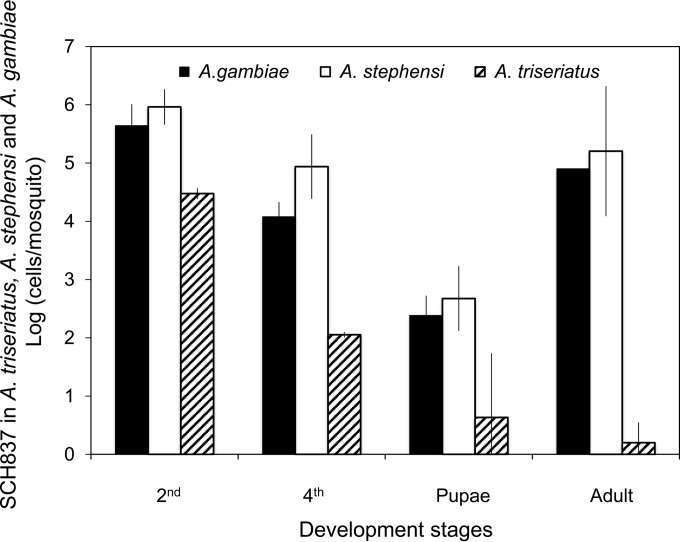
Patterns of colonization of the host by E. anophelis were investigated in A. gambiae, A. stephensi, and Aedes triseriatus individuals by using GFP-labeled E. anophelis strain SCH837. After initial incubation of bacteria in second-instar larval mosquitoes at the concentration of 5 × 108 cells/ml for 24 h, the bacterial density in second-instar Anopheles was estimated at 5.7 × 105 cells/larva. Approximately 1.8 × 104 and 4.2 × 102 GFP-tagged E. anophelis cells were detected in A. gambiae fourth-instar larvae and pupae, respectively (Fig. 3). The adult mosquitoes retained up to 8 × 104 cells/mosquito, indicating some of the introduced E. anophelis survived digestion and propagated in A. gambiae (Fig. 3). Similar results were observed for A. stephensi (Fig. 3). For Aedes triseriatus, we only detected 3.0 × 104 and 1.1 × 102 GFP-tagged E. anophelis cells in larval mosquitoes at the second and fourth instar stages (Fig. 3). On average, less than 10 SCH837 cells could be detected in pupae and adult stages (Fig. 3).
FIG 3.
Association of introduced E. anophelis with mosquitoes. Cells tagged with GFP were introduced to A. gambiae, A. stephensi, and Aedes triseriatus second-instar mosquito larvae. The numbers of CFU were counted and calculated by plating homogenized mosquito samples (pools of 5 mosquitoes) on LB plates containing Em. Values are means ± standard deviations; triplicate experiments were performed.
When NanoLuc E. anophelis cells (SCH814) were introduced in the larval stage (second instar), they were readily detected in adult mosquitoes, with an 82% infection rate in A. stephensi (27/33 adults at 3 days postemergence) (Table 2). Our data further indicated there was no significant difference between female and male adult mosquitoes in retention of E. anophelis cells when feeding with 10% sucrose (see Fig. S4 in the supplemental material). The infection ranges of mosquitoes were next evaluated by introducing strain SCH814 into A. gambiae and Aedes triseriatus (second-instar larvae). The infection rate was ∼71% (37/52) for A. gambiae. Only one was detected with introduced E. anophelis infection among the 30 adult Aedes triseriatus mosquitoes (Table 2). When SCH814 was introduced into mosquitoes in the adult stage (fed 10% sucrose), up to 96% (48/50) and 98% (54/55) of adult A. stephensi and A. gambiae mosquitoes were found to be carrying SCH814, respectively; for Aedes triseriatus, we detected luciferase activity in 88 of 99 randomly sampled mosquitoes.
TABLE 2.
Infection rates of SCH814 in A. gambiae, Aedes triseriatus, and A. stephensi mosquitoes
| Introduced stagea | Infection rate (no. infected/total) |
||
|---|---|---|---|
| A. gambiae | Aedes triseriatus | A. stephensi | |
| Larvae | 71% (37/52) | 3% (1/30) | 82% (27/33) |
| Adult | 98% (54/55) | 89% (88/99) | 96% (48/50) |
To introduce E. anophelis for the larvae infection study, NanoLuc reporter bacteria were fed to second-instar larval mosquitoes (see Materials and Methods). Pupae were transferred into sterile water for adult emergence. Once adult mosquitoes emerged, they were randomly sampled and subjected to the NanoLuc reporter assay. For the adult infection study, mosquitoes were fed a suspension of E. anophelis in 10% sucrose overnight. The adults were subjected to the NanoLuc reporter assay after 3 days.
Effects of sugars and amino acids on growth of E. anophelis in vitro and in vivo.
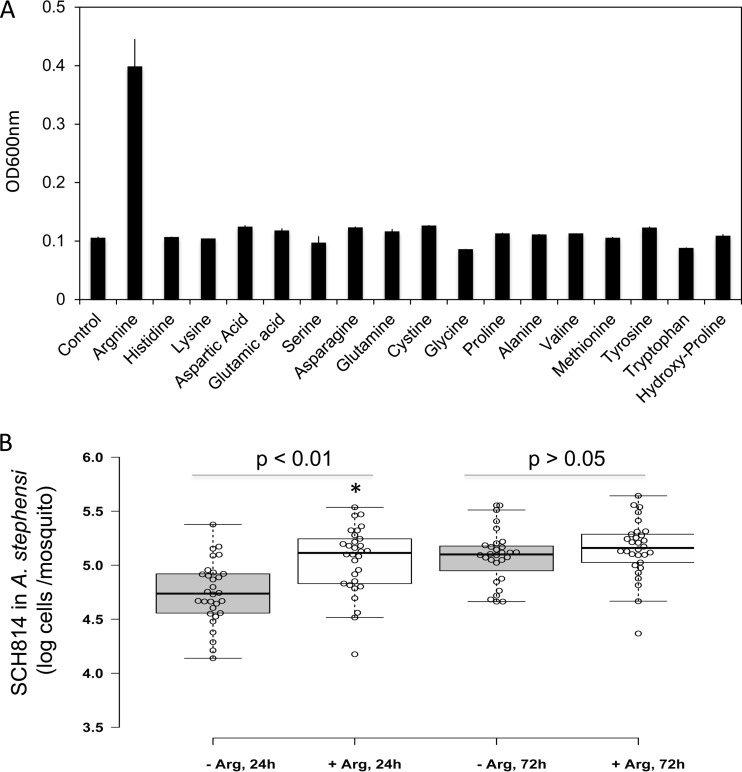
Very little information is available about the nutrient requirements for flavobacteria in mosquitoes. Effects of physiological factors, including various nitrogen (amino acid) and carbon sources simulating the mosquito gut environment on flavobacterial growth, were investigated (Fig. 4 and 5). No significant E. anophelis growth was detected when cultured in M9 or SD (data not shown). To determine if E. anophelis needs specific amino acids for cell growth, 17 selected amino acids were individually tested in M9 medium (Fig. 4). Arginine (4 mM) was the only amino acid that significantly increased cell density (≥3-fold), indicating it is a critical amino acid for E. anophelis. A. stephensi mosquitoes were fed 10 mM arginine in 10% sucrose sugar meal to test if arginine affected E. anophelis growth in vivo. Compared to controls (fed 10% sucrose without arginine), NanoLuc-tagged E. anophelis cells significantly increased up to 100% (P < 0.001) in A. stephensi mosquitoes at 24 h when the sucrose was supplemented with 10 mM arginine (Fig. 4). However, there was no significant difference (P > 0.05) in density of NanoLuc-tagged bacteria between mosquitoes fed with arginine and fed sucrose without arginine after 72 h (Fig. 4).
FIG 4.
Effects of amino acids on SCH814 growth in vivo and in vitro. (A) Selected amino acids at 4 mM (final concentration) were individually added to M9 medium with glucose. After 24 h of incubation at 30°C, the cells were subjected to optical density determinations at 600 nm. (B) A. stephensi mosquitoes were fed 10% sucrose supplemented with SCH814 for 24 h (NanoLuc reporter strain). After the adult mosquitoes emerged, they were fed 10% sucrose with 10 mM arginine or 10% sucrose without arginine. After 24 h and 72 h, 30 mosquitoes were randomly sampled from sucrose without arginine treatment at each time point. Under the same conditions, 28 and 30 mosquitoes were sampled from sucrose with 10 mM arginine treatment, respectively. Mosquitoes were homogenized and subjected to NanoLuc assays. Significant differences between arginine addition and no-arginine addition samples were determined by using PROC GLM; significantly different cell densities are denoted by an asterisk (P < 0.05).
FIG 5.
Effects of carbon source on SCH814 growth in vivo and in vitro. (A) Selected carbon sources at a 0.5% (wt/vol) final concentration were added to M9 medium. After 24 h of incubation at 30°C, the cells were subjected to optical density determinations at 600 nm. (B) Second-instar larvae (A. stephensi) were inoculated with SCH814 (NanoLuc reporter strain). After the adult mosquitoes emerged, they were fed 10% glucose or 10% sucrose. Thirty mosquitoes from each treatment group were randomly sampled, homogenized, and subjected to NanoLuc assays. Significant differences between glucose and sucrose samples were determined by using PROC GLM.
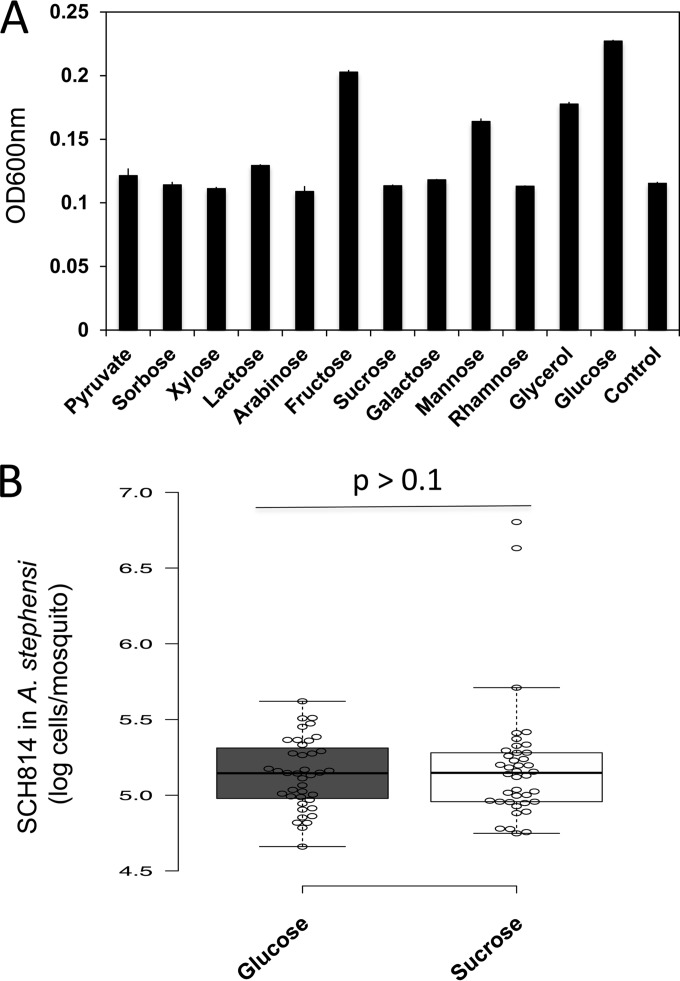
Several sugars, including those possibly present in the normal diet of larval and/or adult mosquitoes (such as plant or animal detritus in water, sediments, or plant saps) were amended to investigate their effects on bacterial cell growth in vitro on M9 medium. The highest cell growth was observed in cultures supplemented with glucose, fructose, mannose, or glycerol, with glucose as the best carbon source. However, there was no significant difference for growth of NanoLuc-tagged E. anophelis (around 1.3 × 105 cells/mosquito in A. stephensi) in vivo between the mosquitoes fed with glucose (10%) and those fed with sucrose (10%) (Fig. 5).
Effects of animal blood on E. anophelis growth in vivo and in vitro.
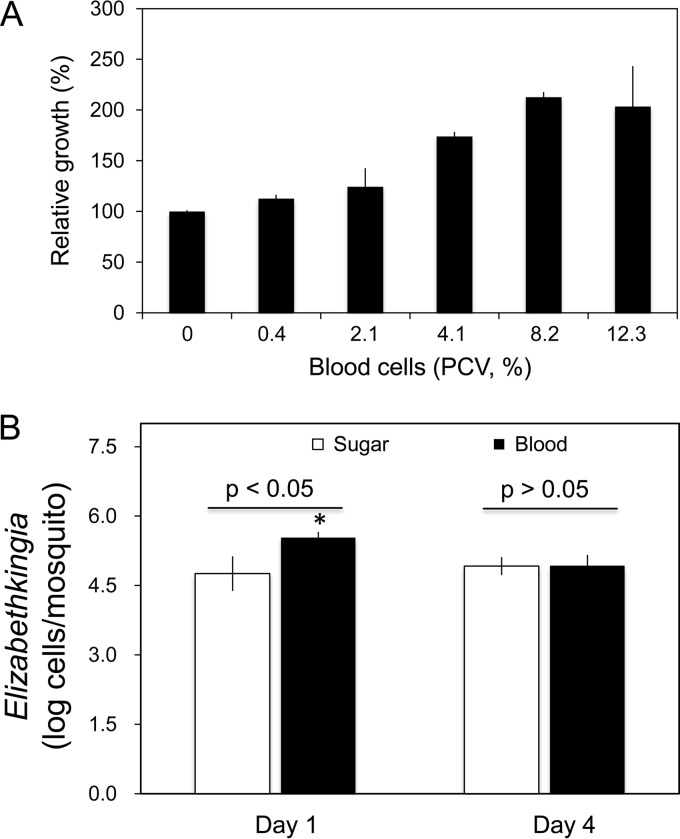
Different concentrations of horse blood (calculated packed cell volume [PCV], 0, 0.4%, 2.1%, 4.1%, 8.2%, and 12.3%) were tested for effects on E. anophelis SCH814 cell growth in modified M9 medium (Fig. 6A). Cell growth significantly increased with concentration of blood. Compared to controls, the number of cells doubled when the culture was supplemented with 8.2% PCV horse blood. The growth of E. anophelis SCH814 in vivo was also evaluated in mosquitoes fed a blood versus sugar meal. As shown in Fig. 6B, the number of introduced E. anophelis cells in blood-fed mosquitoes (3.4 × 105 cells/mosquito) was significantly higher than that in sugar-fed ones (5.7 × 104 cells/mosquito) after a 24-hour feeding (P < 0.01). However, at 4 days post-blood meal, E. anophelis cells decreased to a density of 8.5 × 104 cells/mosquito, similar to that in sugar-fed mosquitoes (Fig. 6B).
FIG 6.
Effects of animal blood on SCH814 growth in vivo and in vitro. (A) Effect of different concentrations of animal blood cells on SCH814 growth in M9 medium in vitro. SCH814 cells were cultured in M9 medium (with glucose) supplemented with various concentrations of horse blood cells. After incubation at 30°C for 24 h, SCH814 cells were estimated by the determination of NanoLuc activity; the relative growth was expressed as the percentage relative to the control (without supplementation with horse blood cells, set as 100%). Values are means of single measurements from triplicate experiments (± standard deviations). (B) Effect of sugar and blood meals on SCH814 growth in mosquitoes. A suspension of SCH814 cells in 10% sucrose was fed to A. stephensi mosquitoes for 24 h in order to introduce NanoLuc-tagged bacteria. The mosquitoes were then given sugar meal (10% sucrose) or blood meal via a membrane apparatus (see Materials and Methods). Four mosquitoes from each treatment group were sampled for assay of NanoLuc activity on day 1. Under the same testing conditions, 8 mosquitoes from each treatment group were sampled on day 4. Significant differences between sugar and blood meals were determined by using PROC GLM and are denoted by an asterisk (P < 0.05).
DISCUSSION
Mechanistic studies on bacterial colonization into the gut of mosquito hosts have emphasized microbial community analysis and adaptation of the bacteria to the gut environment (4, 23–25). The microbiota in mosquito guts has been revealed to be diverse and dynamic and greatly dependent on the host species, mosquito habitat, developmental stages, diet, and immune status (24). However, despite the diversity and dynamics of the assemblage of the gut microbiome, a set of “core taxa” are present and mostly consist of Actinobacteria, Bacteroidetes, and Proteobacteria (1, 25). Among them, bacteria of the phylum Bacteroidetes are ubiquitously and predominantly distributed in several vector mosquitoes, such as A. gambiae (up to 86%), Aedes aegypti (up to 40%), and A. stephensi (up to 33%) (7, 8). Yellow or orange colonies were the most dominant ones observed when we plated the mosquito midgut contents from lab-reared A. stephensi and A. gambiae, and these colonies provided isolates of culturable microbes for our studies. The 16S rRNA analysis showed that representative isolates were Elizabethkingia spp. and Chryseobacterium spp. We focused on one of the isolates, E. anophelis MSU001, because it grew well in LB medium and predominated for adult A. gambiae and A. stephensi. Molecular phylogenetic analysis of this primary isolate showed 99% identity with those from E. anophelis or E. meningoseptica, demonstrating that this strain isolated from the MSU insectary was similar to those isolated from mosquitoes in Europe and Africa (see Fig. S1 in the supplemental material). Due to their close phylogenetic associations with, as well as their dominance in, vector mosquitoes, Elizabethkingia and related bacteria have been proposed to play important physiological roles in mosquito biology and, further, might serve as potential control agents for intervening in malaria parasite development and subsequent transmission (10, 25). Generally, the invertebrate animal-associated microbiota have been demonstrated to profoundly affect their hosts in a wide variety of ways, such as metabolism and immunity (26, 27). Despite their ubiquity, our understanding of the physiological functions of Elizabethkingia species and related bacteria in vector mosquitoes is extremely limited. Further investigation of these commensals in mosquitoes could allow us to elucidate their physiological functions and explore their potential as paratransgenic tools for control of parasite transmission (7, 13). However, effective tools for molecular manipulation of these bacteria had heretofore been lacking, hampering investigations of meaningful interactions.
The superactive mariner transposon (pHimar) is widely used for mediating random mutagenesis of Gram-negative bacteria, such as Haemophilus, Shewanella, and Delftia species (28–30), and Gram-positive bacteria, such as Listeria, Mycobacterium, and Bacillus species (31–33). Once this transposon was equipped with the selective markers functional in Bacteroidetes, it was successfully utilized for mutagenesis analysis of various bacteria in the phylum Bacteroidetes, such as Flavobacterium, Bacteroides, Riemerella, and Cytophaga (34–37). In this study, we extended its utilization to the insertion of gene cassettes of interest into E. anophelis strains, including genes expressing the sensitive reporters GFP and NanoLuc. The expression cassettes PompA+gfp and PompA+nluc were assembled into the pHimarEm1 transposon and integrated into the E. anophelis chromosome successfully. Compared to the cell-tagging strategy that entails integration of reporters on plasmids, delivery of the gene by the transposon mechanism has several advantages. First, the chromosomal insertion of reporters avoids gene loss after propagation for generations in the host without selection pressure. Second, unlike other transposons with instability in host chromosomes, the transposase of pHimarEm1 (positioned outside the insertion sites) is not introduced into the chromosome, thus conferring considerable stability through immobilization (34). Third, when the PompA expression system, a strong constitutive promoter in various flavobacteria, was used to promote expression of exogenous genes, it caused cell toxicity because of overexpression of the genes in multiple-copy plasmids (38). Single insertion of this expression system alleviated this problem. Fourth, the insertion sites of pSCH801 (NanoLuc) or pSCH773 (GFP) in SCH814 or SCH837, respectively, characterized by sequencing gene fragments on the recovered transposon, did not interrupt critical metabolism, such that cell growth was normal compared to the wild type. The GFP-tagged cells allowed us to qualitatively investigate the localization of the ingested bacteria in situ (Fig. 1) and quantitatively study bacterial colonization in mosquitoes using culture-based assays (Fig. 3). The NanoLuc reporter, functional in F. johnsoniae and Flavobacterium hibernum, has proven to be the most sensitive reporter so far in Bacteroidetes (38). Here, we have demonstrated that it is an excellent reporter in E. anophelis. NanoLuc reporter activity in E. anophelis varied negligibly in the different growth phases; detection for target cells based on the NanoLuc determination ranged from 5 × 102 to 5 × 108 cells/ml with good linearity, providing a reliable standard curve (see Fig. S3 in the supplemental material). The NanoLuc reporter activity representative of living E. anophelis was detected in mosquitoes at up to 14 days (see Fig. S4 in the supplemental material). These attributes are important when research requires that reporters in living organisms be sensitive, stable in expression, and not unduly affected by environmental factors (39, 40).
Even though E. anophelis has been described as either an endosymbiont or commensal in A. gambiae, the infection range and fitness of E. anophelis in this species or, comparatively, in other mosquito species, remained unclear (5, 8). Multiple pathways (vertical, horizontal, and transstadial) have been demonstrated for Elizabethkingia species transmission in mosquitoes (5, 8, 41). For example, E. meningoseptica bacterial cells were detected in A. gambiae ovarian tissues and embryos, and they successfully colonized the F1 generation, indicating vertical transmission (5). These experiments involved axenic mosquitoes (i.e., those treated by antibiotics) and did not consider the difference in competency between introduced bacteria and indigenous ones. Our studies here demonstrated that E. anophelis efficiently spread to A. gambiae and A. stephensi populations with high rates of infection (71% and 82%, respectively) when introduced to the larval stages by the feeding route. Introduction of E. anophelis cells in both larval and adult stages was feasible for infection of A. stephensi because the infection rate in adult A. stephensi mosquitoes (96%) was comparable to that in the larval stages (82%). Further, colonization and perpetuation of E. anophelis in guts of conventionally reared mosquitoes without either prior antibiotic treatment or concurrent antibiotic selection pressure suggest that it is a promising bacterial species for paratransgenesis applications.
The dynamics of E. anophelis infection and survival in Aedes triseriatus were quite different from those in the two Anopheles species (Fig. 2 and 3). Infection reached a high rate when SCH814 cells were provided to the adult stages in sugar or blood meals, but the infection rate in Aedes triseriatus was substantially lower than those in A. gambiae and A. stephensi when the bacteria were fed to larvae, indicating that E. anophelis varies in survival by stage across these three mosquito species (Table 2 and Fig. 3). Flavobacteria are well known to spread ubiquitously in nature and widely reside in mosquito habitats (42). Among the few groups of bacteria common in Aedes and Anopheles guts of their various development stages, some flavobacteria (such as E. anophelis) seem to have evolved mechanisms to adapt to the harsh midgut environment of mosquitoes, despite a certain degree of digestibility (8). However, surveys of the microbial community in Culex species mosquito guts showed flavobacteria were not associated with them (43). The same observation was also reported for the mosquito Georgecraigius atropalpus, even though the species was reared under similar conditions as those for A. gambiae and Aedes aegypti (8). Microbial community structure in the mosquito gut is regulated by many complicated factors, such as mosquito species, developmental stages, immune status, and diet (27). However, very few studies have investigated whether the dominant members of the gut microbiota could be digested by larval mosquitoes. Flavobacterium hibernum cells were quickly and thoroughly digested by larval Aedes triseriatus, showing that they were a food source for mosquitoes, rather than a gut commensal or symbiont (38). Some E. anophelis cells were digested in larval mosquitoes in our experiments, and indeed digestion varied among the three species, with a high rate in Aedes triseriatus and A. gambiae. Different digestibilities may explain why E. anophelis has differential persistence in various mosquito species, which raises questions regarding survival and proliferation of different flavobacteria in the mosquito midgut environment (8, 25). However, as demonstrated in Fig. 3, a residual portion (i.e., undigested and GFP-tagged E. anophelis) passed via transstadial transmission from larvae through pupae to the adults in A. stephensi and A. gambiae, indicating that E. anophelis can adapt to the A. stephensi and A. gambiae midgut environments and survive the several molting events during larval stages, as well as hydrolytic processes during metamorphosis, during which most bacteria are typically eliminated (8). The bacterial digestibility by mosquitoes should be one of the important factors affecting their durability in the insect midgut (44). However, further experiments should be conducted to examine how different mosquitoes selectively preserve their symbionts and transmit them to future generations (25).
The dominant flavobacteria in the mosquito gut may have a beneficial role for insects, e.g., Chryseobacterium rescued axenic larval mosquito development (8). Like a few other Gram-negative bacteria, Chryseobacterium species have been suggested to provide unknown signaling molecules that are critical for regulating larval growth processes (8). In addition, such bacteria could possibly supply necessary nutrients that are important to larvae, such as a nitrogen source, or vitamins or other unknown factors. On the other hand, for commensal E. anophelis cells to live in the adult mosquito, it might also interact with host and other midgut microbes to maintain metabolic activities. Elizabethkingia have been assumed to have a good ability to use the sugars obtained by mosquitoes, compared to the less-dominant bacteria (2). However, E. anophelis did not utilize sucrose as a sole carbon in M9, but it propagated in the mosquitoes when fed sucrose (Fig. 5) (1). Sucrose is one of the most common sugars ingested by mosquitoes from plant sap or floral nectar and is widely used for rearing mosquitoes in the lab (45). It is unclear why E. anophelis did not grow well when sucrose was used as the sole carbon source, though there are some genes encoding α-glucosidases which can hydrolyze sucrose to release α-glucose (46). However, the mosquito hosts may directly participate in this process, because they secrete several α-glucosidases into the midgut (47). E. anophelis may lack transporters for sucrose.
E. anophelis did not utilize most of the selected amino acids to support cell growth (Fig. 4). Arginine was identified as a critical amino acid for E. anophelis for metabolism when cultured in defined medium. Supplementation of arginine in the sugar diet significantly increased E. anophelis growth in Anopheles mosquito midgut after 24 h, indicating that arginine is also an important amino acid for supporting E. anophelis cell growth in vivo. Even though we did not generate experimental evidence that indicated the specific nitrogen source for E. anophelis growth in the mosquito midgut, we assume amino acids may partially come from other microbes or from the mosquito host. E. anophelis could lyse a wide range of Gram-positive and Gram-negative bacteria, which may allow E. anophelis to more efficiently recycle necessary nutrients, such as amino acids (41). It has been reported that bacterial symbionts in insects obtain necessary nutrients (nitrogen or carbon sources) from hosts or other microbes (48). For example, bacilli predominant in the honey bee gut need to take up amino acids from diet or from biosynthesis by microflora (49). Lee et al. proposed that Gammaproteobacteria and Actinobacteria provide all essential and other nonessential amino acids for bacilli (49). E. anophelis could also obtain necessary nutrients (such as amino acids) from lysed animal erythrocytes in mosquito midguts (Fig. 6). The finding of a 6.0-fold-higher level of E. anophelis cells after the blood meal agrees with previous observations that flavobacteria dramatically increase proportionately after blood ingestion (1). Kukutla et al. demonstrated that E. anophelis has hemolytic activity in vitro (2). Many genes encoding putative hemolysins and heme-degrading proteins are present in the E. anophelis genome. Data presented here and by others support the idea that E. anophelis is involved in digestion of erythrocytes, which could influence mosquito fecundity. It should be noted that E. anophelis cell growth was not significantly inhibited when erythrocytes were added to M9 medium, indicating that this bacterium has evolved mechanisms to tolerate high oxidative pressures related to blood meal utilization (2).
We successfully developed the techniques for integrating foreign DNA into the chromosome and expressing genes of interest in commensal Elizabethkingia. This development will provide alternative avenues to develop novel biocontrol agents for mosquito-borne diseases. The reporter strains developed in this study will allow us to understand bacterial infection, fitness, and fates in various vector mosquitoes. The NanoLuc-based or GFP-based reporter construct will also facilitate studies of gene regulation and in vivo cell localization. In summary, the availability of the culture conditions, sensitive reporters, and transposons described in this study will deepen our understanding of the interactions between mosquitoes and bacteria or between bacterial species under complex conditions. Future studies should focus on identifying effective effector molecules to use in the expression system and developing methods to increase the stability of transgene expression over time.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mark McBride (University of Wisconsin—Milwaukee) for generously providing several bacterial strains.
This project was funded by NIH grant R37AI21884.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03733-14.
REFERENCES
- 1.Wang Y, Gilbreath TM, Kukutla P, Yan G, Xu J. 2011. Dynamic gut microbiome across life history of the malaria mosquito Anopheles gambiae in Kenya. PLoS One 6:e24767. doi: 10.1371/journal.pone.0024767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kukutla P, Lindberg BG, Pei D, Rayl M, Yu W, Steritz M, Faye I, Xu J. 2014. Insights from the genome annotation of Elizabethkingia anophelis from the malaria vector Anopheles gambiae. PLoS One 9:e97715. doi: 10.1371/journal.pone.0097715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boissière A, Tchioffo MT, Bachar D, Abate L, Marie A, Nsango SE, Shahbazkia HR, Awono-Ambene PH, Levashina EA, Christen R, Morlais I. 2012. Midgut microbiota of the malaria mosquito vector Anopheles gambiae and interactions with Plasmodium falciparum infection. PLoS Pathog 8:e1002742. doi: 10.1371/journal.ppat.1002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terenius O, Lindh JM, Eriksson-Gonzales K, Bussière L, Laugen AT, Bergquist H, Titanji K, Faye I. 2012. Midgut bacterial dynamics in Aedes aegypti. FEMS Microbiol Ecol 80:556–565. doi: 10.1111/j.1574-6941.2012.01317.x. [DOI] [PubMed] [Google Scholar]
- 5.Akhouayri IG, Habtewold T, Christophides GK. 2013. Melanotic pathology and vertical transmission of the gut commensal Elizabethkingia meningoseptica in the major malaria vector Anopheles gambiae. PLoS One 8:e77619. doi: 10.1371/journal.pone.0077619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kämpfer P, Matthews H, Glaeser SP, Martin K, Lodders N, Faye I. 2011. Elizabethkingia anophelis sp. nov., isolated from the midgut of the mosquito Anopheles gambiae. Int J Syst Evol Microbiol 61:2670–2675. doi: 10.1099/ijs.0.026393-0. [DOI] [PubMed] [Google Scholar]
- 7.Ngwa CJ, Glöckner V, Abdelmohsen UR, Scheuermayer M, Fischer R, Hentschel U, Pradel G. 2013. 16S rRNA gene-based identification of Elizabethkingia meningoseptica (Flavobacteriales: Flavobacteriaceae) as a dominant midgut bacterium of the Asian malaria vector Anopheles stephensi (Dipteria: Culicidae) with antimicrobial activities. J Med Entomol 50:404–412. doi: 10.1603/ME12180. [DOI] [PubMed] [Google Scholar]
- 8.Coon KL, Vogel KJ, Brown MR, Strand MR. 2014. Mosquitoes rely on their gut microbiota for development. Mol Ecol 23:2727–2739. doi: 10.1111/mec.12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosokawa T, Kikuchi Y, Shimada M, Fukatsu T. 2007. Obligate symbiont involved in pest status of host insect. Proc Biol Sci 274:1979–1984. doi: 10.1098/rspb.2007.0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y, Manfredini F, Dimopoulos G. 2009. Implication of the mosquito midgut microbiota in the defense against malaria parasites. PLoS Pathog 5:e1000423. doi: 10.1371/journal.ppat.1000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cirimotich CM, Ramirez JL, Dimopoulos G. 2011. Native microbiota shape insect vector competence for human pathogens. Cell Host Microbe 10:307–310. doi: 10.1016/j.chom.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ricci I, Damiani C, Rossi P, Capone A, Scuppa P, Cappelli A, Ulissi U, Mosca M, Valzano M, Epis S, Crotti E, Daffonchio D, Alma A, Sacchi L, Mandrioli M, Bandi C, Favia G. 2011. Mosquito symbioses: from basic research to the paratransgenic control of mosquito-borne diseases. J Appl Entomol 135:487–493. doi: 10.1111/j.1439-0418.2011.01613.x. [DOI] [Google Scholar]
- 13.Bahia AC, Dong Y, Blumberg BJ, Mlambo G, Tripathi A, BenMarzouk-Hidalgo OJ, Chandra R, Dimopoulos G. 2014. Exploring Anopheles gut bacteria for Plasmodium blocking activity. Environ Microbiol 16:2980–2994. doi: 10.1111/1462-2920.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quick J, Constantinidou C, Pallen MJ, Oppenheim B, Loman NJ. 2014. Draft genome sequence of Elizabethkingia meningoseptica isolated from a traumatic wound. Genome Announc 2(3):e00355-14. doi: 10.1128/genomeA.00355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teo J, Tan SY-Y, Liu Y, Tay M, Ding Y, Li Y, Kjelleberg S, Givskov M, Lin RTP, Yang L. 2014. Comparative genomic analysis of malaria mosquito vector-associated novel pathogen Elizabethkingia anophelis. Genome Biol Evol 6:1158–1165. doi: 10.1093/gbe/evu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Bagdasarian M, Kaufman M, Bates A, Walker E. 2007. Mutational analysis of the ompA promoter from Flavobacterium johnsoniae. J Bacteriol 189:5108–5118. doi: 10.1128/JB.00401-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen S, Bagdasarian M, Kaufman M, Walker E. 2007. Characterization of strong promoters from an environmental Flavobacterium hibernum strain by using a green fluorescent protein-based reporter system. Appl Environ Microbiol 73:1089–1100. doi: 10.1128/AEM.01577-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong W-R, Xiang L-X, Shao J-Z. 2010. Novel antibiotic-free plasmid selection system based on complementation of host auxotrophy in the NAD de novo synthesis pathway. Appl Environ Microbiol 76:2295–2303. doi: 10.1128/AEM.02462-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun T, McBride M. 2005. Flavobacterium johnsoniae GldJ is a lipoprotein that is required for gliding motility. J Bacteriol 187:2628–2637. doi: 10.1128/JB.187.8.2628-2637.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 21.Marchesi JR, Sato T, Weightman AJ, Martin TA, Fry JC, Hiom SJ, Wade WG. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl Environ Microbiol 64:795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osei-Poku J, Mbogo CM, Palmer WJ, Jiggins FM. 2012. Deep sequencing reveals extensive variation in the gut microbiota of wild mosquitoes from Kenya. Mol Ecol 21:5138–5150. doi: 10.1111/j.1365-294X.2012.05759.x. [DOI] [PubMed] [Google Scholar]
- 24.Ricci I, Damiani C, Capone A, DeFreece C, Rossi P, Favia G. 2012. Mosquito/microbiota interactions: from complex relationships to biotechnological perspectives. Curr Opin Microbiol 15:278–284. doi: 10.1016/j.mib.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Minard G, Mavingui P, Moro C. 2013. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit Vectors 6:146. doi: 10.1186/1756-3305-6-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broderick NA, Lemaitre B. 2012. Gut-associated microbes of Drosophila melanogaster. Gut Microbes 3:307–321. doi: 10.4161/gmic.19896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon R, Dillon V. 2004. The gut bacteria of insects: nonpathogenic interactions. Annu Rev Entomol 49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- 28.Lampe DJ, Akerley BJ, Rubin EJ, Mekalanos JJ, Robertson HM. 1999. Hyperactive transposase mutants of the Himar1 mariner transposon. Proc Natl Acad Sci U S A 96:11428–11433. doi: 10.1073/pnas.96.20.11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen S, Hickey WJ. 2011. Development of tools for genetic analysis of phenanthrene degradation and nanopod production by Delftia sp. Cs1-4. Front Microbiol 2:187. doi: 10.3369/fmicb.2011.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhenni R, Gehrke A, Saffarini D. 2005. Identification of genes involved in cytochrome c biogenesis in Shewanella oneidensis, using a modified mariner transposon. Appl Environ Microbiol 71:4935–4937. doi: 10.1128/AEM.71.8.4935-4937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zemansky J, Kline BC, Woodward JJ, Leber JH, Marquis H, Portnoy DA. 2009. Development of a mariner-based transposon and identification of Listeria monocytogenes determinants, including the peptidyl-prolyl isomerase PrsA2, that contribute to its hemolytic phenotype. J Bacteriol 191:3950–3964. doi: 10.1128/JB.00016-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sassetti CM, Boyd DH, Rubin EJ. 2001. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci U S A 98:12712–12717. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Breton Y, Mohapatra NP, Haldenwang WG. 2006. In vivo random mutagenesis of Bacillus subtilis by use of TnYLB-1, a mariner-based transposon. Appl Environ Microbiol 72:327–333. doi: 10.1128/AEM.72.1.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun TF, Khubbar MK, Saffarini DA, McBride MJ. 2005. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J Bacteriol 187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veeranagouda Y, Husain F, Wexler HM. 2013. Transposon mutagenesis of Bacteroides fragilis using a mariner transposon vector. Anaerobe 22:126–129. doi: 10.1016/j.anaerobe.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Q, Zhu Y, Tu J, Yin Y, Wang X, Han X, Ding C, Zhang B, Yu S. 2012. Identification of the genes involved in Riemerella anatipestifer biofilm formation by random transposon mutagenesis. PLoS One 7:e39805. doi: 10.1371/journal.pone.0039805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ji X, Bai X, Li Z, Wang S, Guan Z, Lu X. 2013. A novel locus essential for spreading of Cytophaga hutchinsonii colonies on agar. Appl Microbiol Biotechnol 97:7317–7324. doi: 10.1007/s00253-013-4820-2. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Kaufman MG, Korir ML, Walker ED. 2014. Ingestibility, digestibility, and engineered biological control potential of Flavobacterium hibernum, isolated from larval mosquito habitats. Appl Environ Microbiol 80:1150–1158. doi: 10.1128/AEM.03319-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waidmann MS, Bleichrodt FS, Laslo T, Riedel CU. 2011. Bacterial luciferase reporters: the Swiss army knife of molecular biology. Bioeng Bugs 2:8–16. doi: 10.4161/bbug.2.1.13566. [DOI] [PubMed] [Google Scholar]
- 40.Geoffroy M-C, Guyard C, Quatannens B, Pavan S, Lange M, Mercenier A. 2000. Use of green fluorescent protein to tag lactic acid bacterium strains under development as live vaccine vectors. Appl Environ Microbiol 66:383–391. doi: 10.1128/AEM.66.1.383-391.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngwa CJ, Glöckner V, Abdelmohsen UR, Scheuermayer M, Fischer R, Hentschel U, Pradel G. 2013. 16S rRNA gene-based identification of Elizabethkingia meningoseptica (Flavobacteriales: Flavobacteriaceae) as a dominant midgut bacterium of the Asian malaria vector Anopheles stephensi (Dipteria: Culicidae) with antimicrobial activities. J Med Entomol 50:404–414. doi: 10.1603/ME12180. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman MG, Chen S, Walker ED. 2008. Leaf-associated bacterial and fungal taxa shifts in response to larvae of the tree hole mosquito, Ochlerotatus triseriatus. Microb Ecol 55:673–684. doi: 10.1007/s00248-007-9310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pidiyar VJ, Jangid K, Patole MS, Shouche YS. 2004. Studies on cultured and uncultured microbiota of wild Culex quinquefasciatus mosquito midgut based on 16s ribosomal RNA gene analysis. Am J Trop Med Hyg 70:597–603. [PubMed] [Google Scholar]
- 44.Rossignol PA, Lueders AM. 1986. Bacteriolytic factor in the salivary glands of Aedes aegypti. Comp Biochem Physiol B 83:819–822. doi: 10.1016/0305-0491(86)90153-7. [DOI] [PubMed] [Google Scholar]
- 45.Gary RE, Foster WA. 2004. Anopheles gambiae feeding and survival on honeydew and extra-floral nectar of peridomestic plants. Med Vet Entomol 18:102–107. doi: 10.1111/j.0269-283X.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 46.Marinotti O, James AA. 1990. An α-glucosidase in the salivary glands of the vector mosquito, Aedes aegypti. Insect Biochem 20:619–623. doi: 10.1016/0020-1790(90)90074-5. [DOI] [Google Scholar]
- 47.Souza-Neto JA, Machado FP, Lima JB, Valle D, Ribolla PEM. 2007. Sugar digestion in mosquitoes: identification and characterization of three midgut α-glucosidases of the neo-tropical malaria vector Anopheles aquasalis (Diptera: Culicidae). Comp Biochem Physiol A Mol Integr Physiol 147:993–1000. doi: 10.1016/j.cbpa.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 48.Feldhaar H. 2011. Bacterial symbionts as mediators of ecologically important traits of insect hosts. Ecol Entomol 36:533–543. doi: 10.1111/j.1365-2311.2011.01318.x. [DOI] [Google Scholar]
- 49.Lee FJ, Rusch DB, Stewart FJ, Mattila HR, Newton ILG. 6 June 2014. Saccharide breakdown and fermentation by the honey bee gut microbiome. Environ Microbiol doi: 10.1111/1462-2920.12526. [DOI] [PubMed] [Google Scholar]
- 50.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology (NY) 1:784–791. doi: 10.1038/nbt1183-784. [DOI] [Google Scholar]
- 51.Chen S, Kaufman MG, Bagdasarian M, Bates AK, Walker ED. 2010. Development of an efficient expression system for Flavobacterium strains. Gene 458:1–10. doi: 10.1016/j.gene.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker AC, Jeffrey Smith C. 2012. Development of an IPTG inducible expression vector adapted for Bacteroides fragilis. Plasmid 68:86–92. doi: 10.1016/j.plasmid.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maley J, Shoemaker NB, Roberts IS. 1992. The introduction of colonic-Bacteriodes shuttle plasmids into Porphyromonas gingivalis: identification of a putative P. gingivalis insertion-sequence element. FEMS Microbiol Lett 93:75–81. doi: 10.1111/j.1574-6968.1992.tb05043.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.