Abstract
A novel α-amylase, AmyM, was purified from the culture supernatant of Corallococcus sp. strain EGB. AmyM is a maltohexaose-forming exoamylase with an apparent molecular mass of 43 kDa. Based on the results of matrix-assisted laser desorption ionization–time of flight mass spectrometry and peptide mass fingerprinting of AmyM and by comparison to the genome sequence of Corallococcus coralloides DSM 2259, the AmyM gene was identified and cloned into Escherichia coli. amyM encodes a secretory amylase with a predicted signal peptide of 23 amino acid residues, which showed no significant identity with known and functionally verified amylases. amyM was expressed in E. coli BL21(DE3) cells with a hexahistidine tag. The signal peptide efficiently induced the secretion of mature AmyM in E. coli. Recombinant AmyM (rAmyM) was purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography, with a specific activity of up to 14,000 U/mg. rAmyM was optimally active at 50°C in Tris-HCl buffer (50 mM; pH 7.0) and stable at temperatures of <50°C. rAmyM was stable over a wide range of pH values (from pH 5.0 to 10.0) and highly tolerant to high concentrations of salts, detergents, and various organic solvents. Its activity toward starch was independent of calcium ions. The Km and Vmax of recombinant AmyM for soluble starch were 6.61 mg ml−1 and 44,301.5 μmol min−1 mg−1, respectively. End product analysis showed that maltohexaose accounted for 59.4% of the maltooligosaccharides produced. These characteristics indicate that AmyM has great potential in industrial applications.
INTRODUCTION
Amylases are important industrial enzymes widely applied in sugar, animal nutrition, food, fermentation, textile, paper and pulp, and bioenergy industries (1), and they make up ∼25% of the world's enzyme market (2). Amylases are classified into four groups: (i) endoamylases, (ii) exoamylases, (iii) debranching enzymes, and (iv) transferases (3). Most of these amylases belong to the GH13 family of the glycoside hydrolase group of enzymes (4). The α-amylase family is the most extensively investigated group of enzymes that hydrolyze starch to yield diverse products, including dextrins and progressively smaller polymers composed of glucose units (5). Among them, α-amylase and pullulanase are the most important enzymes in the food and detergent industries (6). In order to meet the requirements of different industrial processes, the demand for novel α-amylases that have high activity and sufficient stability has increased. Various amylases with different characteristics have been isolated from different microorganisms: alkaliphilic amylase from Bacillus spp. (6), hyperthermotolerant amylases from thermophilic archaea (7) and Bacillus spp. (8, 9), acidic amylases from Aspergillus awamori (10), and salt- and detergent-resistant amylases from Nesterenkonia sp. and Saccharopolyspora sp. (11, 12).
In recent years, interest in the use of maltooligosaccharides as biopreservatives and functional foods has grown (13). The production of specific maltooligosaccharides originated in the discovery of related enzymes (14). Various maltooligosaccharide-producing exoamylases, such as the maltotriose-forming amylase from Natronococcus sp. strain Ah-36 (15), exomaltotetraohydrolase (EC 3.2.1.60) from Pseudomonas stutzeri (16), maltopentaose-producing amylase from Bacillus sp. strain JAMB-204 (16), and exomaltohexaohydrolase from Klebsiella pneumoniae (17), have been discovered. In addition to their use in the food industry, maltooligosaccharides have potential applications in pharmaceutical industries as well. They were found to reduce the levels of intestinal putrefactive bacteria such as Clostridium perfringens and Enterobacteriaceae, thus improving colonic conditions in humans (18). Researchers have also found that maltooligosaccharides associated with the trophoblast surface may exhibit immunoregulatory activities (19). Maltotetraose-forming α-amylase from Pseudomonas saccharophila has been commercially applied in the production of maltotetraose syrup and antistaling agents used in bread.
Myxobacteria are a type of solid-dwelling and Gram-negative soil bacteria that are characterized by a multicellular stage in their life cycle induced by nutritional starvation and changes in the host environment (20). Myxobacteria have been extensively studied for their ability to produce various natural products (21). However, very little research has been carried out to characterize the enzymes produced by myxobacteria. A few of these enzymes have been characterized, including proteases from Myxococcus virescens (22), α-amylases from Corallococcus coralloides D (23), N-acetyl-beta-d-glucosaminidases from Stigmatella aurantiaca (24), and lipases from Sorangium cellulosum (25). We previously isolated Corallococcus sp. strain EGB, which abundantly produces extracellular enzymes. Here, we report the purification of an exomaltohexaose-forming amylase and the cloning and expression of its gene in Escherichia coli.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
Corallococcus sp. strain EGB (China Center for Type Culture Collection [CCTCC] no. M2012528), which produces extracellular amylase with high activity, was isolated from soil samples. The EGB strain was cultivated in a culture medium (pH 7.0) consisting of 0.5% (wt/vol) yeast extract, 1% (wt/vol) tryptone, 1% (wt/vol) soluble starch, and 0.1% (wt/vol) CaCl2. To prepare the crude enzyme, the strain was incubated at 30°C on a rotary shaker at 180 rpm · min−1 for 3 to 4 days. The supernatant was collected by centrifugation at 15,000 × g for 20 min at 4°C and used for amylase purification.
E. coli DH5α and E. coli DH10B (Invitrogen Corporation, Shanghai, China) were employed for recombinant plasmid construction of the amylase. The pMD-19T vector (TakaRa Biotechnology, Dalian, China) was utilized for gene cloning and DNA sequencing, and the pET-29a plasmid was used as the expression vector for the overexpression of amylase in E. coli BL21(DE3) (Novagen Co., Shanghai, China).
Amylase activity assay.
An amylase activity assay was carried out by measuring the formation of reducing sugars released during starch hydrolysis. The enzyme reaction mixture containing 1 μl of appropriately diluted enzyme and 1 ml of 0.5% soluble starch in 50 mM Tris-HCl buffer (pH 7.0) was incubated at 50°C for 10 min. The amount of released reducing sugar was determined by the dinitrosalicylic (DNS) acid method (26). One unit of amylase-hydrolyzing activity is defined as the amount of enzyme needed to release 1 μmol of reducing sugar per minute. Protein concentrations were estimated according to the method of Bradford (27).
Purification of amylase from Corallococcus sp. strain EGB.
Amylase was purified according to a procedure described previously by Loyter and Schramm (28), with slight modifications, and all purification procedures were carried out at 4°C. The supernatant of the culture was used as a crude enzyme preparation and then fractionated at 40% to 60% saturation with ammonium sulfate. The precipitate was centrifuged at 15,000 × g for 20 min and dissolved in a minimum volume of 50 mM Tris-HCl (pH 7.0). The enzyme was further purified by amylase-glycogen complex precipitation followed by autodigestion of the complex. The released enzyme was precipitated by bringing the digestive juice to 60% saturation with ammonium sulfate. The precipitated amylase was suspended in a minimum volume of 50 mM Tris-HCl (pH 7.0). The purity and molecular mass of the amylase were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 12% acrylamide, as described previously by Laemmli (29).
After electrophoresis, the gel was cut into two strips for staining and zymogram analysis. The gel strip for staining was stained with 0.1% (wt/vol) Coomassie brilliant blue R250 in 10% (vol/vol) acetic acid–45% ethanol. Destaining was then performed with a mixture of 5% ethanol and 7.5% acetic acid. SDS was removed from the gel strip for zymogram analysis by incubating the gel twice with 2.5% (vol/vol) Triton X-100, each time for 30 min. Thereafter, the strip was incubated in 0.1 M Tris-HCl (pH 8.0) and then in 0.05 M Tris-HCl (pH 7.0) to renature the enzyme. The renatured gel was incubated on a 1.5% agar plate containing 0.5% soluble starch for 30 min at 37°C, and the plate was stained with Lugol's iodine until a transparent halo became visible (30). The renatured gel was compared to the stained gel, and the band on the stained gel corresponding to the position of the transparent halo was excised. The gel slice was then stored at −80°C for peptide mass fingerprinting (PMF) analysis.
Characterization of the purified amylase.
To detect the hydrolysis pattern of the purified amylase, the hydrolysis products of soluble starch caused by the enzyme were detected by thin-layer chromatography (TLC), as described previously (31). One microliter of appropriately diluted enzyme was incubated with 0.5 ml of 0.5% soluble starch in 50 mM Tris-HCl buffer (pH 7.0). The reaction was carried out at 50°C for different time intervals from 5 min to 1 h. Thin-layer chromatography was performed with a solvent system of n-butyl alcohol–carbinol–water (4:2:1), and the spots were visualized by spraying the silica gel plate (10 by 20 cm; Merck, Germany) with H2SO4-methanol (1:1, vol/vol), followed by heating at 90°C for 10 min (32).
Peptide mass fingerprinting of the enzyme.
The gel slice containing AmyM was analyzed by peptide mass fingerprinting (Boyuan Bio-Tech, Shanghai, China), and the results were analyzed based on searches of the Mascot database (Matrix Science).
Cloning of the α-amylase gene.
Genomic DNA was extracted from Corallococcus sp. strain EGB cells according to a method described previously by Kaiser et al. (33). According to the results of peptide mass fingerprinting and the genome sequence of C. coralloides DSM 2259 (34), the gene encoding glucan-1,4-alpha-maltohexaohydrolase was cloned by PCR amplification with the primer pair AmyF (5′-CATATGACGTTGAAGACCCGCCTG-3′) and AmyR (5′-CTCGAGGAAGCTGGCGGTGGC-3′). The amplified PCR fragment was sequenced by Invitrogen Corporation (Shanghai, China).
Expression of α-amylase in E. coli and purification of the recombinant enzyme.
The PCR products were digested with NdeI and XhoI and inserted into pET29a(+) digested with the same enzymes to produce plasmid pET29a-AmyM. The plasmids were then transformed into E. coli BL21(DE3). The transformants were grown in LB medium with 50 μg/ml kanamycin at 37°C to an optical density at 600 nm (OD600) of 0.5 to 0.6. Protein expression was induced at 18°C with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.2 mM. The cells were harvested, washed, and resuspended in an equilibration buffer (50 mM Tris-HCl buffer, pH 7.0) at 4°C and lysed by ultrasonication (Insonator M201; Kubota, Japan). The lysate was centrifuged at 15,000 × g for 20 min, and the supernatant was retained as crude AmyM. C-terminally His-tagged recombinant AmyM (rAmyM) was purified with Ni2+-nitrilotriacetic acid (NTA) resin (Qiagen, Valencia, CA, USA) (35) according to the manufacturer's instructions.
Biochemical characterization of effects of temperature, pH, and salts on α-amylase activity and stability of rAmyM.
Studies on the influence of temperature and pH were conducted with rAmyM. The effect of pH on the activity of the α-amylase at a concentration of 0.05 M was determined in various buffers with starch as the enzyme substrate (citrate buffer, pH 3.0 to 6.0; sodium phosphate buffer, pH 6.0 to 7.0; Tris-HCl buffer, pH 7.0 to 9.0; glycine-NaOH buffer, pH 9.0 to 10.0) by maintaining a constant temperature of 50°C. The enzyme activity was calculated at pH values ranging from pH 3.0 to 10.0. pH stability assays were performed by incubating aliquots of the enzyme at a pH range of pH 3.0 to 10.0 for 24 h at 4°C and then determining the enzyme activities as described above. The effect of temperature on enzymatic activity was determined by assays with starch at a temperature range of 20°C to 80°C for 1 h. Assays were performed as described above.
To determine the effect of the salt concentration on rAmyM activity, an enzyme assay was performed by using the standard assay described above with NaCl and KCl at final concentrations of between 1 and 4 M.
Effects of metal ions and chemicals on α-amylase activity.
The effect of metal ions and chemicals on the activity of rAmyM was determined at 50°C for 10 min with appropriate metal salts at final concentrations of 1 mM and 5 mM. The remaining activity was determined by using a standard enzyme assay. Activity in the absence of metal ions was taken as 100%. Purified rAmyM was treated with EDTA overnight at 4°C, and EDTA was then dialyzed away with 50 mM Tris-HCl buffer (pH 7.0), which was prepared with deionized water that was not contaminated by metal ions.
To determine the effects of EDTA (10 mM and 20 mM), dimethyl sulfoxide (DMSO), methanol, ethanol, acetonitrile (10% and 20%), and various concentrations of surfactants (Triton X-100 and SDS), the procedure used was the same as the one described above for metal ions. Activity in the absence of any additives was taken as 100%.
Substrate specificities of the α-amylase.
Alternative substrates were used to determine the substrate specificity of rAmyM. All assays were conducted for 10 min at 50°C. The enzyme assay was performed with a reaction mixture containing various carbohydrates (starch, amylose, amylopectin, glycogen, pullulan, dextran, dextrin, α-cyclodextrin, and β-cyclodextrin) at a concentration of 0.5% (wt/vol) in Tris-HCl (50 mM; pH 7.0).
Catalytic properties of the α-amylase.
The hydrolysis products of rAmyM oligosaccharides were detected by TLC as described previously (31). Oligosaccharides (G7, G6, G5, and G4) at a final concentration of 0.5% (wt/vol) were hydrolyzed by rAmyM. The reaction mixture was incubated at 50°C for 1 h. TLC was performed as described above.
Meanwhile, 10 μl of appropriately diluted enzyme was incubated with 0.5 ml of 0.5% soluble starch at 50°C for 1 h. To determine hydrolysate compositions, the sample was analyzed by high-performance liquid chromatography (HPLC) with a Cosmosil Sugar-D column (Nacalai Tesque, Kyoto, Japan) and a refractive index detector (RID) maintained at 30°C. The mobile phase was a mixture of acetonitrile and water (75:25, vol/vol) with a flow rate of 1 ml min−1 (36). Pure HPLC-grade glucose, maltose, maltotriose, maltotetraose, maltopentaose, maltohexaose, and maltoheptaose (Aladdin, USA) were used as standards for the identification and quantification of released products in the reaction mixture.
Hydrolysis of waxy maize starch.
The commercial bacterial amylase Novo BAN800 from Bacillus was used for comparison with AmyM. The activity of BAN800 was calibrated by the DNS acid method. A waxy maize starch dispersion (8%, wt/vol) was prepared according to methods described previously (37, 38), and the suspension was mechanically stirred with heating in a boiling bath until full gelatinization was reached and adjusted to pH 6.0 with phosphoric acid. The starch dispersion was hydrolyzed at 50°C for AmyM and at 70°C for BAN800 for 3 and 12 h, respectively. The amylases were added at a dose of 100 U per gram of dry starch. The enzyme reactions were stopped by boiling at 100°C for 20 min. The hydrolysis products from different times were analyzed by HPLC with a Cosmosil Sugar-D column (KS-803, 8 mm by 300 mm; Nacalai Tesque, Japan) and with the RID maintained at 50°C. The mobile phase consisted of ultrapure water with a flow rate of 0.8 ml min−1.
Sequence identities.
Computer-assisted protein sequence analyses were performed by using ClustalW version 2.0 (39). Protein sequence identity searches were performed by using the BLASTP algorithm at the National Center for Biotechnology Information (NCBI) server (40). Reference sequences utilized for phylogenetic analysis were retrieved from the NCBI database and aligned with ClustalW (39). The molecular mass and the isoelectric point (pI) were calculated with the ExPASy Proteomics server (41). The putative signal peptide was predicted by using the SignalP server (http://www.cbs.dtu.dk/services/SignalP).
Nucleotide sequence accession number.
The sequence for the novel α-amylase gene amyM, isolated from Corallococcus sp. EGB, has been deposited into the GenBank database under accession no. KM114206.
RESULTS
Purification of the α-amylase AmyM.
Purification of the amylase from Corallococcus sp. strain EGB was performed as described in Materials and Methods. Following ammonium sulfate precipitation, ethanol precipitation, and glycogen complex formation, a protein with amylase activity was purified, as shown by zymogram analysis. The amylase was designated AmyM (myxobacterium). A summary of the purification is shown in Table 1. The specific activity of AmyM increased 28-fold from 114.7 U/mg in the crude enzyme to 3,310 U/mg in digestive juice.
TABLE 1.
Summary of purification of α-amylase from Corallococcus sp. strain EGB
| Treatment | Total activity (U) | Protein concn (mg) | Sp act (U/mg) | Yield (%) | Purification (fold) |
|---|---|---|---|---|---|
| Crude enzyme | 25,300 | 221 | 114.7 | 100 | 1 |
| (NH4)2SO4 fractionation (40%–60%) | 24,035 | 83 | 289.7 | 95 | 2.52 |
| Enzyme-glycogen complex | 16,103 | 5 | 3,320 | 67 | 28 |
| Enzyme precipitate at 4°C (after glycogen digestion) | 9,821 | 3.05 | 3,310 | 39 | 28 |
As shown in Fig. 1, the band with amylase activity had an apparent molecular mass of ∼43 kDa. Several proteins binding to starch were also purified, but they showed no amylase activity. Since the amylase of strain EGB could bind to various protein purification matrices (DEAE/CM-Toyopearl and Sephadex serial gels), no further purification was carried out. The pattern of the end product of AmyM with soluble starch (0.5%, wt/vol) as a substrate was examined by TLC. The major product was maltohexaose (G6), with maltose (G2) to maltopentaose (G5) as minor products, as shown in Fig. 2. The results indicated that AmyM was a maltohexaose-producing α-amylase.
FIG 1.
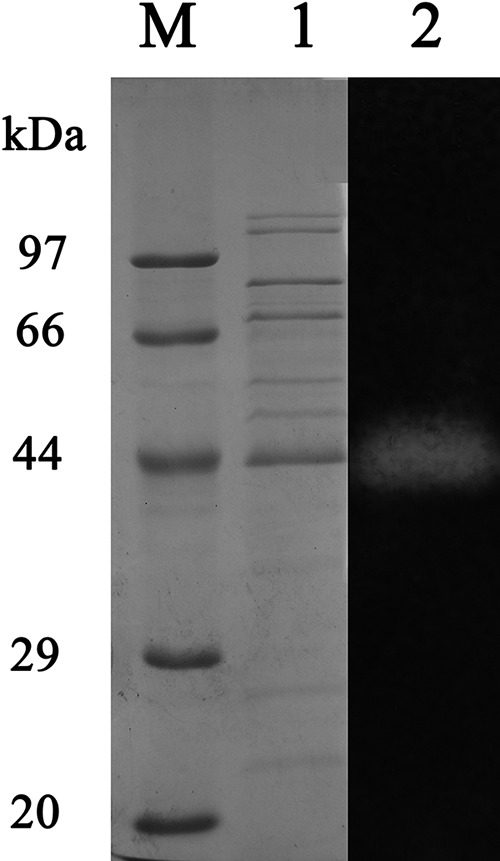
SDS-PAGE analysis of the purified amylase AmyM. SDS-PAGE analysis of AmyM using a 12% denaturing gradient acrylamide separating gel and a 5% stacking gel with SDS. Lane M, molecular mass markers; lane 1, SDS-PAGE analysis of purified AmyM visualized by Coomassie brilliant blue staining; lane 2, zymogram analysis of AmyM visualized by activity staining.
FIG 2.
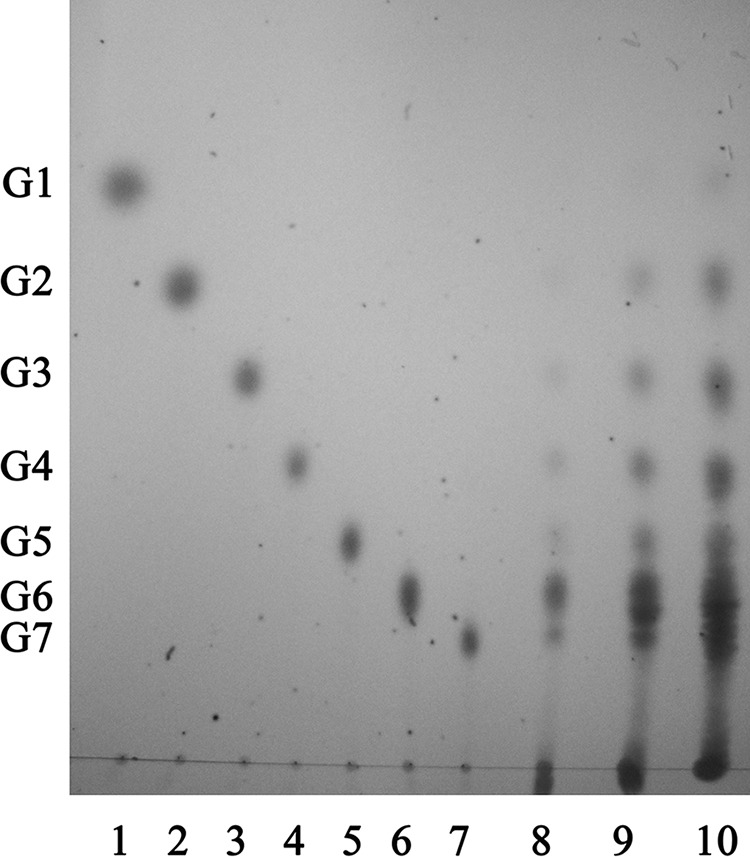
TLC analysis of the hydrolysis products of soluble starch from the purified α-amylase. Lanes 1 to 7, standard maltooligosaccharides (G1 to G7); lanes 8 to 10, starch hydrolyzed by recombinant α-amylase with reaction times of 5 min, 10 min, and 1 h. G1, glucose; G2, maltose; G3, maltotriose; G4, maltotetraose; G5, maltopentaose; G6, maltohexaose; G7, maltoheptaose.
The stained gel band was excised and analyzed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) peptide mass fingerprinting (PMF), and the results of PMF were interpreted by referencing the Mascot database. Proteins receiving the highest molecular weight search (MOWSE) scores were selected as the peptide fragments of AmyM (42). Three peptide fragments were detected, and the sequences of the three peptides were AESGLYAAIIGGK, GLLNDALTNNNYSR, and ASDGKPAGGIGWWASR, which shared 100% identity to the peptides from glucan-1,4-alpha-maltohexaohydrolase in the complete genome sequence of C. coralloides DSM 2259.
Cloning of the α-amylase gene from Corallococcus sp. EGB.
The α-amylase was cloned as described in Materials and Methods. The PCR primer pair was designed according to the genome of C. coralloides DSM 2259. As expected, amplification of the α-amylase resulted in a PCR product of ∼1.5 kb. Analysis of the nucleotide sequence of the PCR product revealed the presence of an open reading frame (ORF) of 1,569 bp that encodes a GHF13 α-amylase composed of 522 amino acid residues with a putative signal peptide of 23 amino acid residues. The calculated molecular mass of the AmyM protein was 55.46 kDa, and the isoelectric point (pI) was predicted to be 6.45.
BLASTP analysis showed that the amylase belongs to family 13 and shared the highest identity (94%) with the glucan-1,4-alpha-maltohexaohydrolase in the genome of C. coralloides DSM 2259 (34), followed by the α-amylases from Cellvibrio japonicus Ueda107 (59%) (43), Cystobacter fuscus DSM 2262 (59%), and Stigmatella aurantiaca DW4/3-1 (60%) (44). AmyM also shared identity with putative amylase or hypothetical proteins from the genome sequences of nine bacterial genera in the GenBank database at levels of ∼50%. All these proteins have not been functionally verified.
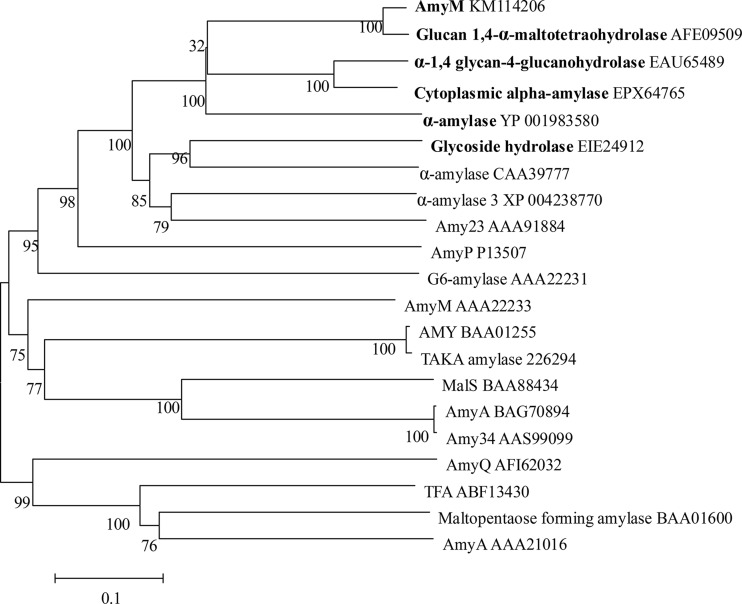
Since AmyM is a maltooligosaccharide-producing amylase, multiple-sequence alignments of AmyM with sequences retrieved from GenBank and verified maltooligosaccharide-producing amylases were performed to construct the phylogenic tree. The phylogenic tree showed that amylases from Corallococcus formed an independent branch. AmyM is completely different from other experimentally characterized bacterial and fungal α-amylases and maltooligosaccharide-producing amylases. Interestingly, AmyM was shown to be significantly homologous to putative α-amylases from various plants and algae at levels of identity of ∼40% (Fig. 3). These results indicated that AmyM is a novel member of the GH13 family.
FIG 3.
Multiple-sequence alignment. The phylogenetic tree shows the relationship of AmyM from Corallococcus sp. strain EGB with other α-amylases obtained from different sources. The sequence for AmyM was aligned with those of the following proteins: glucan-1,4-alpha-maltohexaohydrolase from C. coralloides DSM 2259 (GenBank accession no. AFE09509), α-amylase from Cellvibrio japonicus Ueda107 (accession no. EAU65489), cytoplasmic alpha-amylase from Cystobacter fuscus DSM 2262 (accession no. EPX64765), alpha-amylase from Stigmatella aurantiaca DW4/3-1 (accession no. YP_001983580), glycoside hydrolase from Coccomyxa subellipsoidea C-169 (accession no. EIE24912), alpha-amylase from the Oryza sativa Japonica group (accession no. CAA39777), alpha-amylase from Solanum lycopersicum (accession no. XP_004238770), Amy23 from potato (Solanum tuberosum) (accession no. AAA91884), AmyP from Pseudomonas stutzeri MO-19 (accession no. P13507), G6 amylase from alkaliphilic Bacillus sp. strain 707 (accession no. AAA22231), AmyM from Bacillus stearothermophilus (accession no. AAA22233), AMY from Aspergillus shirousamii (accession no. BAA01255), Taka from Aspergillus oryzae (accession no. 226294), MalS from Klebsiella pneumoniae (accession no. BAA88434), AmyA from Bacillus halodurans MS-2-5 (accession no. BAG70894), Amy34 from Bacillus halodurans LBK 34 (accession no. AAS99099), AmyQ from Bacillus subtilis JN16 (accession no. AFI62032), α-amylase from Thermobifida fusca (TFA) (accession no. ABF13430), maltopentaose-forming amylase from Pseudomonas sp. strain KO-8940 (accession no. BAA01600), and AmyA from Aeromonas hydrophila MCC-1 (accession no. AAA21016). (Boldface type denotes that the enzymes are not experimentally verified but were derived from the genome; the others are experimentally verified.)
Expression and purification of the recombinant α-amylase.
The full-length amylase gene (with the signal peptide sequence) was expressed in E. coli BL21(DE3) at low expression levels of 1.4 mg liter−1. However, >70% of the expressed amylase was secreted into the culture medium, indicating that the AmyM signal peptide was a strong signal for protein secretion. The recombinant amylase was purified from E. coli by Ni-NTA affinity chromatography. The expression and purification of recombinant α-amylase were analyzed by SDS-PAGE (Fig. 4).
FIG 4.
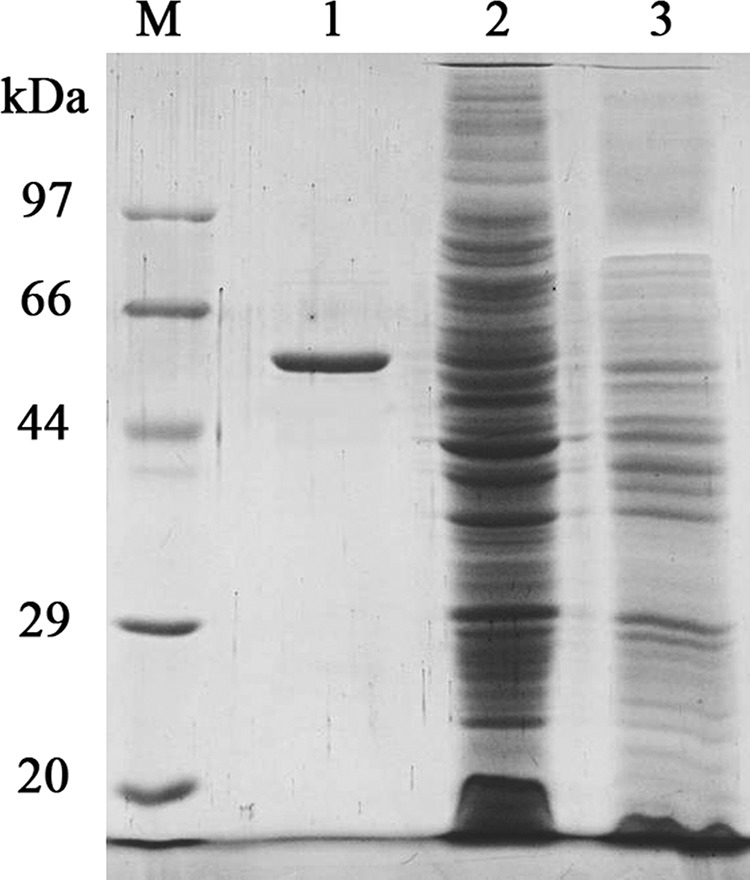
SDS-PAGE gel of rAmyM. Lane M, protein molecular mass markers; lane 1, purified rAmyM enzyme; lane 2, crude enzyme of recombinant E. coli strain BL21 with pET29a-AmyM; lane 3, crude enzyme of recombinant E. coli strain BL21 with pET29a(+) as a control.
Recombinant AmyM (rAmyM) was purified 89.8-fold with a yield of 75%. The purified enzyme had a specific activity of 14,000 U/mg. The kinetic parameters Km and Vmax were determined to be 6.61 mg ml−1 and 44,301.5 μmol min−1 mg−1, respectively.
Effect of temperature and pH on enzyme activity and stability.
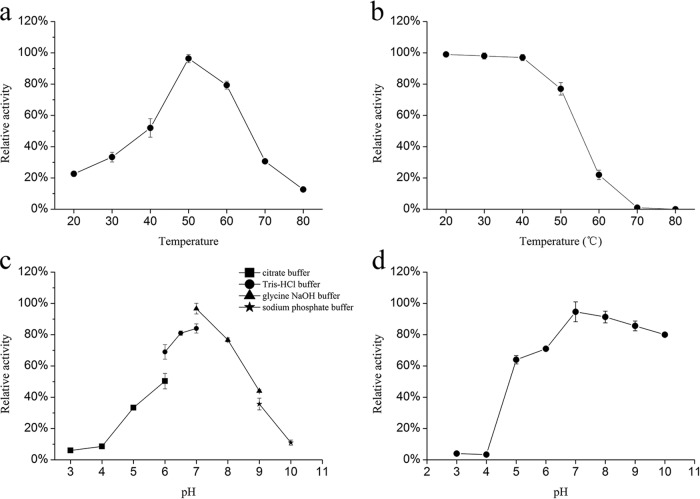
The temperature and pH profiles of amylase activity are shown in Fig. 5. The temperature and pH ranges at which recombinant α-amylase was active were determined by using soluble starch as the substrate. rAmyM was active at a broad temperature range (20°C to 80°C), and the highest activity was observed at 50°C. In terms of pH, rAmyM was active at a broad range of pH values (3.0 to 10.0), and the maximum enzyme activity was observed at pH 7.0. More than 60% of the maximum activity was retained at pH 6.0 to 8.0, and ∼40% was retained at pH 5.0 to 9.0.
FIG 5.
Effect of temperature and pH on the activity and stability of the novel rAmyM. (a) For determination of the optimal temperature, the recombinant enzyme was incubated in Tris-HCl buffer (50 mM; pH 7.0) for 5 min at different temperatures (20°C to 80°C). (b) Thermostability of the recombinant amylase. Activity was measured under optimal conditions after incubation of the enzyme at the indicated temperatures for 1 h. (c) For the determination of optimized pH, the recombinant enzyme was incubated for 10 min at 50°C in universal buffer (pH 3.0 to 10.0). (d) Stability of the recombinant amylase at different pH values. The enzyme activity was measured under optimal conditions in Tris-HCl buffer (50 mM at pH 7.0 and at 50°C for 10 min) after incubation of the purified enzyme with buffers with various pH values at 4°C for 24 h.
rAmyM retained full activity after 1 h of incubation at 40°C and retained ∼80% of the original activity after 1 h of incubation at 50°C, but it lost 80% of the original activity after 1 h of incubation at 60°C. rAmyM was most stable at pH 7.0, and it retained >60% of the original activity over a wide range of pH values, from pH 5.0 to 10.0.
Effect of metal ions and chemical reagents on α-amylase activity.
The effects of various metal ions on the activity of the recombinant α-amylase were tested (Table 2). Most tested metal ions showed a significant negative effect on the activity of rAmyM. Ca2+ at 1 mM had no effect on the activity of rAmyM, but 60% of rAmyM activity was inhibited at a concentration of 5 mM Ca2+, which indicated that the activity of rAmyM was independent of Ca2+. The activity of rAmyM was significantly inhibited by Cu+, Cu2+, Co2+, Fe2+, Zn2+, Ni2+, and Fe3+ at concentrations of 1 mM and 5 mM and by Cr3+ at a concentration of 5 mM, but no effect was caused by Mg2+ at concentrations of 1 mM and 5 mM. These results also showed that K+ is an effective activator of rAmyM, as it was able to stimulate the activity of rAmyM by ∼20%.
TABLE 2.
Effects of metal ions on α-amylase activitya
| Metal ion | Mean relative activity (%) at 1 mM concn ± SD | Mean relative activity (%) at 5 mM concn ± SD |
|---|---|---|
| K+ (KCl) | 126.6 ± 2.5 | 116.1 ± 1.9 |
| Cu+ (CuCl) | 16.9 ± 0.9 | 5 ± 0.5 |
| Mg2+ (MgCl2) | 116.5 ± 2.7 | 108.7 ± 1.2 |
| Mn2+ (MnCl2) | 64.8 ± 3.1 | 104.8 ± 2.5 |
| Co2+ (CoCl2) | 31.7 ± 2.7 | 35.5 ± 3.3 |
| Ca2+ (CaCl2) | 109.2 ± 1.2 | 38.7 ± 3.4 |
| Ni2+ (NiCl2) | 40.3 ± 0.8 | 11.7 ± 0.6 |
| Ba2+ (BaCl2) | 88.9 ± 3.3 | 33.1 ± 1.2 |
| Cu2+ (CuCl2) | 10.2 ± 0.4 | 0.6 ± 0.1 |
| Fe2+ (FeCl2) | 25.3 ± 1.6 | 15.8 ± 0.8 |
| Zn2+ (ZnCl2) | 8.1 ± 1.9 | 2.9 ± 0.2 |
| Fe3+ (FeCl3) | 14.1 ± 0.6 | 3.4 ± 0.15 |
| Cr3+ (CrCl3) | 89.9 ± 3.9 | 6.5 ± 0.3 |
Samples were preincubated with various metal ions (1 mM and 5 mM) for 10 min at 50°C, and the remaining activity was measured under standard assay conditions. Activity in the absence of any additives was taken as 100%. Mean values ± standard deviations from three independent experiments are shown.
Amylase activity in the presence of various chemical reagents was also studied (Table 3). The presence of the chelating agent EDTA inhibited the enzyme to 17% (10 mM and 20 mM) of its original activity. The enzyme was highly stable in the presence of nonionic surfactants, retaining full activity in the presence of 30 mg/ml Triton X-100. However, the enzyme lost 54% and 53% of its activity in the presence of 2 mg/ml and 5 mg/ml SDS, respectively. rAmyM was tolerant to various organic solvents. Low concentrations of methanol, ethanol, acetonitrile, and DMSO (10%) did not affect its activity.
TABLE 3.
Effects of chemical agents on α-amylase activitya
| Chemical agent | Low concn | Mean relative activity (%) ± SD at low concn | High concn | Mean relative activity (%) ± SD at high concn |
|---|---|---|---|---|
| EDTA | 10 mM | 17 ± 2.1 | 20 mM | 16.0 ± 2.0 |
| SDS | 2 mg/ml | 53.5 ± 0.8 | 5 mg/ml | 52.5 ± 1.8 |
| Triton X-100 | 30 mg/ml | 97.5 ± 3.8 | 40 mg/ml | 121.5 ± 2.8 |
| Methanol | 10% | 96.6 ± 0.8 | 20% | 77.6 ± 0.8 |
| Ethanol | 10% | 95.5 ± 1.5 | 20% | 70.5 ± 0.5 |
| Acetonitrile | 10% | 96.5 ± 2.1 | 20% | 61.4 ± 1.1 |
| DMSO | 5% | 94.0 ± 2.8 | 10% | 81.7 ± 1.8 |
The enzyme solution was incubated at 50°C in the presence or absence of different concentrations of organic solvents for 10 min. Residual activity was measured under standard assay conditions. Activity in the absence of any additives was taken as 100%. Mean values ± standard deviations from three independent experiments are shown.
Halotolerance of the α-amylase.
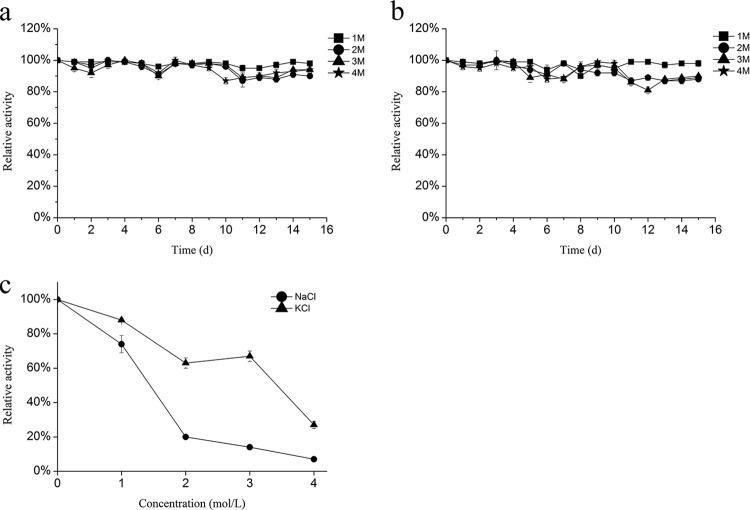
To determine the effect of salt concentrations on the activity of rAmyM, the α-amylase was incubated with 1 to 4 M KCl (Fig. 6a) and NaCl (Fig. 6b) for up to 15 days. More than 95% of its activity was retained for long-term treatment with both salts. The α-amylase was still active in the presence of high salt concentrations, with 74% and 88% of its activity being retained in 1 M NaCl and KCl solutions, respectively, and 67% of its activity being retained in a 3 M KCl solution. The enzymatic activity was reduced when the NaCl concentration was >2 M (Fig. 6c).
FIG 6.
Halotolerance of the α-amylase. (a and b) Halotolerance was determined by measuring residual activity under optimal conditions following preincubation of AmyM for up to 15 days with 1 to 4 M KCl (a) or 1 to 4 M NaCl (b). (c) Enzymatic activity was determined at final salt concentrations of 1 to 4 M NaCl or KCl.
Substrate specificity of rAmyM.
The recombinant α-amylase was examined for its ability to hydrolyze various carbohydrates under standard assay conditions (Table 4). Of the substrates tested, soluble starch was most effectively hydrolyzed by the enzyme. Amylose, amylopectin, glycogen, sweet potato starch, cornstarch, and dextrin were also hydrolyzed to a lesser extent. No reaction was observed for pullulan, dextran, and α- or β-cyclodextrins.
TABLE 4.
Substrate specificity of rAmyM from Corallococcus sp. strain EGBa
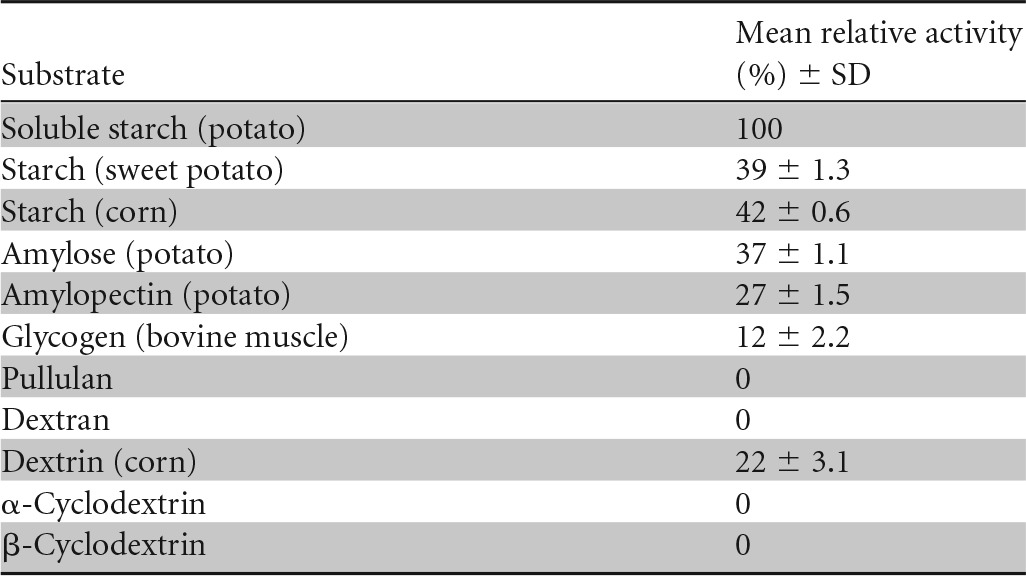
For the determination of substrate specificity, the recombinant enzyme was incubated in Tris-HCl buffer (50 mM; pH 7.0) for 10 min at 50°C with different substrates (0.5%, wt/vol).
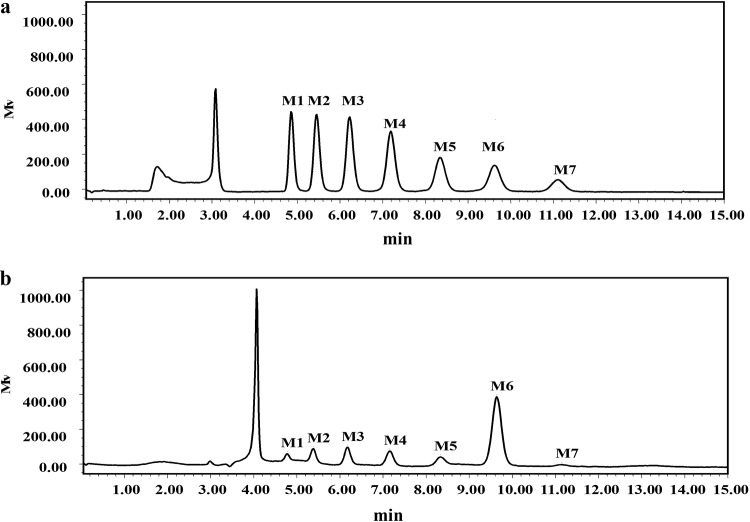
The reaction of rAmyM on various maltooligosaccharides was also tested (Fig. 7). rAmyM acted only on maltooligosaccharides larger than those with a degree of polymerization of 6 (DP6), making it a good catalyst for the production of maltohexaose. The end product of the action of rAmyM on soluble starch was analyzed by HPLC. About 60% of the produced maltooligosaccharides were maltohexaose, with intermediate products being glucose (G1; 3.9%), maltose (G2; 6.2%), maltotriose (G3; 8.1%), maltotetraose (G4; 8.3%), maltopentaose (G5; 8.8%), maltohexaose (G6; 59.4%), and maltoheptaose (G7; 5.3%) (Fig. 8).
FIG 7.
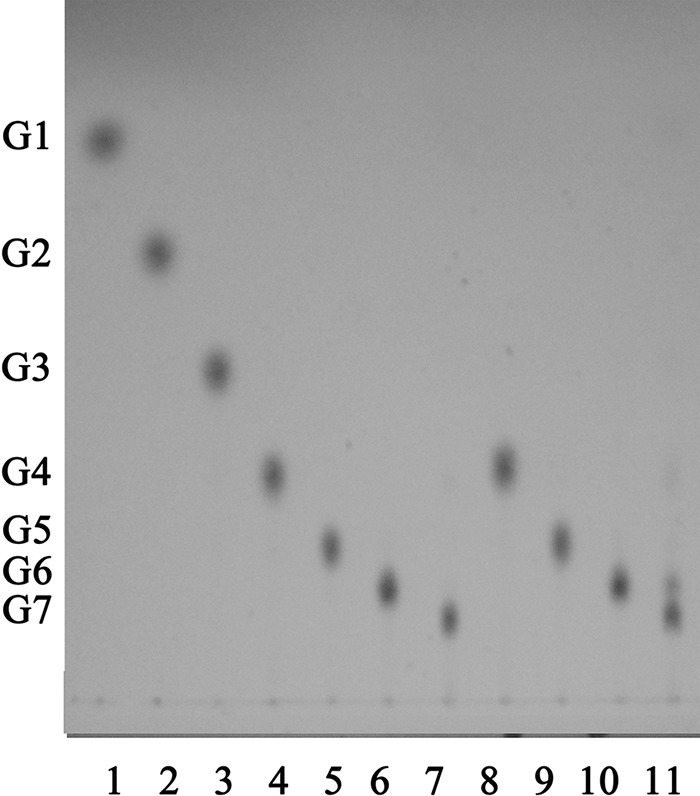
TLC analysis of the main hydrolysis products of maltooligosaccharide-producing recombinant α-amylase. Lanes 1 to 7 show standard maltooligosaccharides (G1 to G7). rAmyM was reacted with maltotetraose (lane 8), maltopentaose (lane 9), maltohexaose (lane 10), and maltoheptaose (lane 11) for 1 h at 50°C. G1, glucose; G2, maltose; G3, maltotriose; G4, maltotetraose; G5, maltopentaose; G6, maltohexaose; G7, maltoheptaose.
FIG 8.
HPLC analysis of the product of the action of rAmyM from Corallococcus sp. strain EGB on 1% starch for 1 h. (a) The standard maltooligosaccharides (G1 to G7) glucose (G1), maltose (G2), maltotriose (G3), maltotetraose (G4), maltopentaose (G5), maltohexaose (G6), and maltoheptaose (G7). (b) HPLC results showing the composition of the released hydrolytic products (G1, 3.9%; G2, 6.2%; G3, 8.1%; G4, 8.3%; G5, 8.8%; G6, 59.4%; G7, 5.3%).
Hydrolysis of waxy maize starch.
The hydrolysis products of the actions of rAmyM and BAN800 on waxy maize starch at different times were analyzed by HPLC (Fig. 9). Compared with Novo BAN800, AmyM showed high hydrolysis efficiency (Fig. 9a and b). The reaction of AmyM reached stability at ∼3 h, while that of BAN800 reached stability at 12 h. Maltohexaose composed nearly 54% of the total oligosaccharides produced from soluble starch by AmyM, and the composition remained stable during the entire hydrolysis process (Fig. 9a). Maltooligosaccharides with DP1 to DP6 were produced by BAN800 at 3 h; among them, only 14% were maltohexaose. The hydrolysis product of BAN800 was complicated compared to that of AmyM, and this phenomenon was maintained until 12 h (Fig. 9c).
FIG 9.
HPLC analysis of the hydrolysis products of AmyM and BAN800 on waxy maize starch. (a) HPLC results showing the hydrolysis product of AmyM on waxy maize starch at 1.5 h, 3 h, 5 h, and 12 h. RI, refractive index; nRIU, nano-refractive index units. (b) HPLC results showing the hydrolysis product of AmyM compared with that of BAN800 at 3 h with enzyme added at a dose of 100 U/g dry starch. (c) HPLC results showing the hydrolysis product of AmyM compared with that of BAN800 at 12 h with enzyme added at a dose of 100 U/g dry starch. MW, molecular weight.
DISCUSSION
The mining of amylase resources has attracted the interest of academic and industrial researchers because of their wide applications in the processing of starch materials. In this study, a highly active exoamylase, AmyM from Corallococcus sp. strain EGB, was heterologously expressed and purified in E. coli with a specific activity of ∼14,000 U/mg and without the addition of Ca2+. AmyM showed 94% identity with Mta (COCOR_02752) from C. coralloides DSM 2259. In the genome of DSM 2259, Mta was predicted to be a glucan-1,4-alpha-maltohexaohydrolase. In this research, we verified that AmyM hydrolyzed starch into maltooligosaccharides, with maltohexaose being the main product. However, sequence alignment showed that AmyM shared maximum identity with G4-amylase from Pseudomonas stutzeri (31.8% identity) and no similarity with known G6-amylases. Amylases that produce maltohexaose (G6-amylase) were first characterized in Aerobacter aerogenes (45). Since then, several maltohexaose enzymes have been isolated from Bacillus (6, 14, 46–50). AmyM shared no significant similarity with these maltohexaosidases (<15% identity), indicating that AmyM is a novel maltohexaose-producing α-amylase.
Similar to most maltooligosaccharide-producing amylases, AmyM is an intermediate-temperature-stable (ITS) amylase (51) that was stable at temperatures of <50°C. The ITS characteristic renders AmyM easily denatured when necessary. Maltohexaosidases from Gram-positive and Gram-negative strains have different pH adaptabilities. AmyM and maltohexaosidases from Aerobacter aerogenes (45) were active at neutral pH but stable in a wide pH range (5–10). Maltohexaosidases from Bacillus were all active under alkaline conditions (pH 9 or above). Amylases showed different responses to various metal ions (51). Ca2+ is usually an important metal ion for most α-amylases to react and maintain stability. The activity of AmyM was independent of Ca2+, and 5 mM Ca2+ significantly inhibited its activity. However, K+ showed a stimulating effect on AmyM of ∼20% at concentrations of 1 mM and 5 mM (Table 2). Also, K+ is an excellent stabilizer for long-term storage of liquid AmyM. Stimulation of amylases by K+ has been rarely reported (52). Although the activity of AmyM was independent of metal ions, 10 mM EDTA showed a severe inhibition of AmyM. Removal of EDTA could restore its activity to 90% of the original activity. Considering the loss of the enzyme during dialysis, we deduce that EDTA might itself be an inhibitor of the amylase and did not inhibit the enzyme by chelating the metal ions. These characteristics of AmyM indicate that the structure of AmyM is unique. AmyM is also highly resistant to various detergents, and high concentrations of Triton X-100 even improved its activity. These biochemical characteristics of AmyM make it potentially useful in the baking, paper, and detergent industries (6).
The enzymatic production of syrups with high contents of specific maltooligosaccharides has been industrialized with the discovery of amylases that produce specific oligosaccharides (45). This industrial process requires the amylases to be specific for substrates. Maltohexaosidases from Bacillus clausii BT-21 (53), Bacillus sp. strain KSM-1378 (6), and B. halodurans LBK 34 (50) first hydrolyze starch to maltohexaose in a limited time, and maltohexaose is then hydrolyzed to maltotetraose with an extension of the hydrolysis time. AmyM could not act on maltooligosaccharides shorter than G6 but hydrolyzed G7 to generate G1 and G6 as the major end products, along with G2 and G4 in trace amounts (Fig. 6). The reaction pattern of AmyM is different from those of previously reported maltohexaosidases. AmyM could be used to produce a large amount of maltohexaose from raw cornstarch with techniques and equipment compatible with those currently used in the starch industry (data not shown). Organic solvents were reported to increase the percentage of specific maltooligosaccharides in the end products during the hydrolysis of starch by amylases (54, 55). Compared with the commercial bacterial amylase Novo BAN800, the amylase AmyM showed high hydrolysis efficiency (Fig. 9) in a short time. Compared with the complicated hydrolysis products of BAN800, the hydrolysis products of AmyM were relatively unitary. AmyM endured high concentrations of organic solvents (Table 3), and it is possible to increase the percentage of maltohexaose by the addition of certain organic solvents in the processing of starch. Combined with pullulanases, the content of maltohexaose can be further increased.
Corallococcus is a myxobacterium that can utilize various polysaccharides (56, 57). The first amylase from Myxococcus (Corallococcus) coralloides D was reported by M. E. Fárez-Vidal et al. (23). The apparent molecular mass, specific activity, response to metal ions, and optimal reaction temperature and pH were different from those of AmyM produced by Corallococcus sp. EGB. Myxobacteria have attracted growing attention because of their ability in secondary metabolism and their importance as a model for developmental research. Valuable enzymes in myxobacteria have rarely been studied. Our results indicated that myxobacteria might be a good source of industrial enzymes.
In summary, a novel α-amylase, AmyM, was purified from Corallococcus sp. strain EGB. Its encoding gene was cloned and expressed in E. coli BL21(DE3). AmyM may find applications in starch processing technologies because of its highly specific activity, halotolerance, unique substrate specificity, and end product pattern.
ACKNOWLEDGMENTS
This work was supported by the Natural Science Foundation of Jiangsu Province, China (no. BK2012029); the Natural Science Foundation of Jiangsu Province (no. BK20140687); and the Project for National Basic Science personnel training fund (no. J1210056).
We are grateful to Jianming Ju of Nanjing TCM University for his help in the analysis of the composition of maltooligosaccharides in the end products.
REFERENCES
- 1.Jabbour D, Sorger A, Sahm K, Antranikian G. 2013. A highly thermoactive and salt-tolerant α-amylase isolated from a pilot-plant biogas reactor. Appl Microbiol Biotechnol 97:2971–2978. doi: 10.1007/s00253-012-4194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiran KK, Chandra T. 2008. Production of surfactant and detergent-stable, halophilic, and alkalitolerant alpha-amylase by a moderately halophilic Bacillus sp. strain TSCVKK. Appl Microbiol Biotechnol 77:1023–1031. doi: 10.1007/s00253-007-1250-z. [DOI] [PubMed] [Google Scholar]
- 3.Van Der Maarel MJ, Van Der Veen B, Uitdehaag J, Leemhuis H, Dijkhuizen L. 2002. Properties and applications of starch-converting enzymes of the α-amylase family. J Biotechnol 94:137–155. doi: 10.1016/S0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 4.Janeček Š, Svensson B, MacGregor EA. 2014. α-Amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 71:1149–1170. doi: 10.1007/s00018-013-1388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma S, Khan FG, Qazi GN. 2010. Molecular cloning and characterization of amylase from soil metagenomic library derived from Northwestern Himalayas. Appl Microbiol Biotechnol 86:1821–1828. doi: 10.1007/s00253-009-2404-y. [DOI] [PubMed] [Google Scholar]
- 6.Hagihara H, Igarashi K, Hayashi Y, Endo K, Ikawa-Kitayama K, Ozaki K, Kawai S, Ito S. 2001. Novel α-amylase that is highly resistant to chelating reagents and chemical oxidants from the alkaliphilic Bacillus isolate KSM-K38. Appl Environ Microbiol 67:1744–1750. doi: 10.1128/AEM.67.4.1744-1750.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieille C, Zeikus GJ. 2001. Hyperthermophilic enzymes: sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–43. doi: 10.1128/MMBR.65.1.1-43.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asgher M, Asad MJ, Rahman S, Legge R. 2007. A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J Food Eng 79:950–955. doi: 10.1016/j.jfoodeng.2005.12.053. [DOI] [Google Scholar]
- 9.Jana M, Maity C, Samanta S, Pati BR, Islam SS, Mohapatra PKD, Mondal KC. 2013. Salt-independent thermophilic α-amylase from Bacillus megaterium VUMB109: an efficacy testing for preparation of maltooligosaccharides. Ind Crops Prod 41:386–391. doi: 10.1016/j.indcrop.2012.04.048. [DOI] [Google Scholar]
- 10.Prakasham R, Subba Rao C, Sreenivas Rao R, Sarma P. 2007. Enhancement of acid amylase production by an isolated Aspergillus awamori. J Appl Microbiol 102:204–211. doi: 10.1111/j.1365-2672.2006.03058.x. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty S, Khopade A, Biao R, Jian W, Liu XY, Mahadik K, Chopade B, Zhang L, Kokare C. 2011. Characterization and stability studies on surfactant, detergent and oxidant stable α-amylase from marine haloalkaliphilic Saccharopolyspora sp. A9. J Mol Catal B Enzym 68:52–58. doi: 10.1016/j.molcatb.2010.09.009. [DOI] [Google Scholar]
- 12.Shafiei M, Ziaee AA, Amoozegar MA. 2011. Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. strain F. J Ind Microbiol Biotechnol 38:275–281. doi: 10.1007/s10295-010-0770-1. [DOI] [PubMed] [Google Scholar]
- 13.Barreteau H, Delattre C, Michaud P. 2006. Production of oligosaccharides as promising new food additive generation. Food Technol Biotechnol 44:323–333. [Google Scholar]
- 14.Fogarty W, Bourke A, Kelly C, Doyle E. 1994. A constitutive maltotetraose-producing amylase from Pseudomonas sp. IMD 353. Appl Microbiol Biotechnol 42:198–203. [Google Scholar]
- 15.Shida O, Takano T, Takagi H, Kadowaki K, Kobayashi S. 1992. Cloning and nucleotide sequence of the maltopentaose-forming amylase gene from Pseudomonas sp. KO-8940. Biosci Biotechnol Biochem 56:76–80. [DOI] [PubMed] [Google Scholar]
- 16.Harada H, Fujita T, Miyamoto M, Kimura Y, Maruyama M, Furia A, Miyata T, Taniguchi T. 1989. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58:729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- 17.Kainuma K, Kobayashi S, Ito T, Suzuki S. 1972. Isolation and action pattern of maltohexaose producing amylase from Aerobacter aerogenes. FEBS Lett 26:281–285. doi: 10.1016/0014-5793(72)80593-3. [DOI] [PubMed] [Google Scholar]
- 18.Crittenden R, Playne MJ. 1996. Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol 7:353–361. doi: 10.1016/S0924-2244(96)10038-8. [DOI] [Google Scholar]
- 19.Zhu LJ, Liu QQ, Wilson JD, Gu MH, Shi YC. 2011. Digestibility and physicochemical properties of rice (Oryza sativa) flours and starches differing in amylose content. Carbohydr Polym 86:1751–1759. doi: 10.1016/j.carbpol.2011.07.017. [DOI] [Google Scholar]
- 20.Reichenbach H. 1999. The ecology of the myxobacteria. Environ Microbiol 1:15–21. doi: 10.1046/j.1462-2920.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 21.Diez J, Martinez JP, Mestres J, Sasse F, Frank R, Meyerhans A. 2012. Myxobacteria: natural pharmaceutical factories. Microb Cell Fact 11:52. doi: 10.1186/1475-2859-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnosspelius G. 1978. Purification and properties of an extracellular protease from Myxococcus virescens. J Bacteriol 133:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fárez-Vidal ME, Fernández-Vivas A, Gonzalez F, Arias J. 1995. Properties and significance of an α-amylase produced by Myxococcus coralloides D. J Appl Microbiol 78:14–19. [Google Scholar]
- 24.Bourgerie S, Karamanos Y, Grard T, Julien R. 1994. Purification and characterization of an endo-N-acetyl-beta-d-glucosaminidase from the culture medium of Stigmatella aurantiaca DW4. J Bacteriol 176:6170–6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng YY, Qian YK, Li ZF, Wu ZH, Liu H, Li YZ. 2011. A novel cold-adapted lipase from Sorangium cellulosum strain So0157-2: gene cloning, expression, and enzymatic characterization. Int J Mol Sci 12:6765–6780. doi: 10.3390/ijms12106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller GL. 1959. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- 27.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 28.Loyter A, Schramm M. 1962. The glycogen-amylase complex as a means of obtaining highly purified α-amylases. Biochim Biophys Acta 65:200–206. [DOI] [PubMed] [Google Scholar]
- 29.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 30.Bano S, Qader SAU, Aman A, Syed MN, Azhar A. 2011. Purification and characterization of novel α-amylase from Bacillus subtilis KIBGE HAS. AAPS Pharmscitech 12:255–261. doi: 10.1208/s12249-011-9586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckert K, Zielinski F, Leggio LL, Schneider E. 2002. Gene cloning, sequencing, and characterization of a family 9 endoglucanase (CelA) with an unusual pattern of activity from the thermoacidophile Alicyclobacillus acidocaldarius ATCC27009. Appl Microbiol Biotechnol 60:428–436. doi: 10.1007/s00253-002-1131-4. [DOI] [PubMed] [Google Scholar]
- 32.Hmidet N, Bayoudh A, Berrin JG, Kanoun S, Juge N, Nasri M. 2008. Purification and biochemical characterization of a novel α-amylase from Bacillus licheniformis NH1: cloning, nucleotide sequence and expression of amyN gene in Escherichia coli. Process Biochem 43:499–510. doi: 10.1016/j.procbio.2008.01.017. [DOI] [Google Scholar]
- 33.Avery L, Kaiser D. 1983. In situ transposon replacement and isolation of a spontaneous tandem genetic duplication. Mol Gen Genet 191:99–109. [DOI] [PubMed] [Google Scholar]
- 34.Huntley S, Zhang Y, Treuner-Lange A, Kneip S, Sensen CW, Søgaard-Andersen L. 2012. Complete genome sequence of the fruiting myxobacterium Corallococcus coralloides DSM 2259. J Bacteriol 194:3012–3013. doi: 10.1128/JB.00397-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janknecht R, de Martynoff G, Lou J, Hipskind RA, Nordheim A, Stunnenberg HG. 1991. Rapid and efficient purification of native histidine-tagged protein expressed by recombinant vaccinia virus. Proc Natl Acad Sci U S A 88:8972–8976. doi: 10.1073/pnas.88.20.8972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajagopalan G, He J, Yang KL. 2014. Production, purification, and characterization of α-amylase from solventogenic Clostridium sp. BOH3. Bioenergy Res 7:132–141. doi: 10.1007/s12155-013-9356-x. [DOI] [Google Scholar]
- 37.Napaporn A, Jaylin J, Geoffrey H. 2006. Reaction pattern of a novel thermostable a-amylase. Carbohydr Polym 64:582–588. doi: 10.1016/j.carbpol.2005.11.014. [DOI] [Google Scholar]
- 38.Sang HY, Jaylin J. 2002. Molecular weights and gyration radii of amylopectins determined by high-performance size-exclusion chromatography equipped with multi-angel laser-light scattering and refractive index detectors. Carbohydr Polym 49:307–314. doi: 10.1016/S0144-8617(01)00339-3. [DOI] [Google Scholar]
- 39.Larkin M, Blackshields G, Brown N, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 40.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 41.Gasteiger E, Hoogland C, Gattiker A, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, p 571–607. In Walker JM. (ed), The proteomics protocols handbook. Humana Press, Totowa, NJ. [Google Scholar]
- 42.Goodin JL, Raab RW, McKown RL, Coffman GL, Powell BS, Enama JT, Ligon JA, Andrews GP. 2005. Yersinia pestis outer membrane type III secretion protein YscC: expression, purification, characterization, and induction of specific antiserum. Protein Expr Purif 40:152–163. doi: 10.1016/j.pep.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 43.DeBoy RT, Mongodin EF, Fouts DE, Tailford LE, Khouri H, Emerson JB, Mohamoud Y, Watkins K, Henrissat B, Gilbert HJ. 2008. Insights into plant cell wall degradation from the genome sequence of the soil bacterium Cellvibrio japonicus. J Bacteriol 190:5455–5463. doi: 10.1128/JB.01701-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huntley S, Hamann N, Wegener-Feldbrügge S, Treuner-Lange A, Kube M, Reinhardt R, Klages S, Müller R, Ronning CM, Nierman WC. 2011. Comparative genomic analysis of fruiting body formation in Myxococcales. Mol Biol Evol 28:1083–1097. doi: 10.1093/molbev/msq292. [DOI] [PubMed] [Google Scholar]
- 45.Nakakuki T. 1993. Oligosaccharides: production, properties, and applications, vol 3 Gordon and Breach, Basel, Switzerland. [Google Scholar]
- 46.Hayashi T, Akiba T, Horikoshi K. 1988. Properties of new alkaline maltohexaose-forming amylases. Appl Microbiol Biotechnol 28:281–285. [Google Scholar]
- 47.Kimura K, Tsukamoto A, Ishii Y, Takano T, Yamane K. 1988. Cloning of a gene for maltohexaose producing amylase of an alkalophilic Bacillus and hyper-production of the enzyme in Bacillus subtilis cells. Appl Microbiol Biotechnol 27:372–377. [Google Scholar]
- 48.Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, Uemura T, Ara K, Ozaki K, Kawai S, Kobayashi T. 1998. Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Appl Environ Microbiol 64:3282–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ali MB, Mezghani M, Bejar S. 1999. A thermostable α-amylase producing maltohexaose from a new isolated Bacillus sp. US100: study of activity and molecular cloning of the corresponding gene. Enzyme Microb Technol 24:584–589. [Google Scholar]
- 50.Hashim SO, Delgado OD, Martínez MA, Kaul RH, Mulaa FJ, Mattiasson B. 2005. Alkaline active maltohexaose-forming α-amylase from Bacillus halodurans LBK 34. Enzyme Microb Technol 36:139–146. doi: 10.1016/j.enzmictec.2004.07.017. [DOI] [Google Scholar]
- 51.Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B. 2003. Microbial α-amylases: a biotechnological perspective. Process Biochem 38:1599–1616. doi: 10.1016/S0032-9592(03)00053-0. [DOI] [Google Scholar]
- 52.Pandey A, Nigam P, Soccol C, Soccol V, Singh D, Mohan R. 2000. Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152. doi: 10.1042/BA19990073. [DOI] [PubMed] [Google Scholar]
- 53.Duedahl-Olesen L, Kragh KM, Zimmermann W. 2000. Purification and characterisation of a malto-oligosaccharide-forming amylase active at high pH from Bacillus clausii BT-21. Carbohydr Res 329:97–107. doi: 10.1016/S0008-6215(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 54.Doukyu N, Yamagishi W, Kuwahara H, Ogino H, Furuki N. 2007. Purification and characterization of a maltooligosaccharide-forming amylase that improves product selectivity in water-miscible organic solvents, from dimethylsulfoxide-tolerant Brachybacterium sp. strain LB25. Extremophiles 11:781–788. doi: 10.1007/s00792-007-0096-8. [DOI] [PubMed] [Google Scholar]
- 55.Kumar S, Khare S. 2012. Purification and characterization of maltooligosaccharide-forming α-amylase from moderately halophilic Marinobacter sp. EMB8. Bioresour Technol 116:247–251. doi: 10.1016/j.biortech.2011.11.109. [DOI] [PubMed] [Google Scholar]
- 56.Irschik H, Reichenbach H. 1985. An unusual pattern of carbohydrate utilization in Corallococcus (Myxococcus) coralloides (Myxobacterales). Arch Microbiol 142:40–44. doi: 10.1007/BF00409234. [DOI] [Google Scholar]
- 57.Gerth K, Pradella S, Perlova O, Beyer S, Müller R. 2003. Myxobacteria: proficient producers of novel natural products with various biological activities—past and future biotechnological aspects with the focus on the genus Sorangium. J Biotechnol 106:233–253. doi: 10.1016/j.jbiotec.2003.07.015. [DOI] [PubMed] [Google Scholar]