Abstract
BACKGROUND
There is worldwide interest in newborn screening for lysosomal storage diseases because of the development of treatment options that give better results when carried out early in life. Screens with high differentiation between affected and nonaffected individuals are critical because of the large number of potential false positives.
CONTENT
This review summarizes 3 screening methods: (a) direct assay of enzymatic activities using tandem mass spectrometry or fluorometry, (b) immunocapture-based measurement of lysosomal enzyme abundance, and (c) measurement of biomarkers. Assay performance is compared on the basis of small-scale studies as well as on large-scale pilot studies of mass spectrometric and fluorometric screens.
SUMMARY
Tandem mass spectrometry and fluorometry techniques for direct assay of lysosomal enzymatic activity in dried blood spots have emerged as the most studied approaches. Comparative mass spectrometry vs fluorometry studies show that the former better differentiates between nonaffected vs affected individuals. This in turn leads to a manageable number of screen positives that can be further evaluated with second-tier methods.
Classical lysosomal storage diseases (LSDs)4 are a collection of at least 50 inborn errors of metabolism resulting from a deficiency in the function of lysosomal enzymes and transporters (1). Buildup of one or more cellular components by a defective lysosomal enzyme may lead to cell death and associated tissue dysfunction. Diseases that involve aberrant lysosome biogenesis and endosomal–lysosomal trafficking may sometimes lead to a storage disorder and may be classified as an LSD under an expanded definition (2). Like with many genetic diseases, LSDs can present as a continuum of severity ranging from infant- to adult-onset variants.
Newborn screening (NBS) of LSDs has become a topic of intense interest because of the development of treatment options for a subset of these disorders and the demonstration that initiation of treatment shortly after birth often leads to a better outcome. Treatment options include enzyme replacement therapy (3), hematopoietic stem cell transplantation (4), small molecular weight drugs (5), and gene therapy combined with hematopoietic stem cell gene therapy (6). This review focuses on recent advances in the technologies used for NBS of LSDs.
Overall Strategy for NBS of LSDS
Four methods have been considered for NBS of LSDs: (a) direct measurement of lysosomal enzymatic activity; (b) direct measurement of lysosomal enzyme abundance; (c) LSD biomarker quantification; and (d) sequencing of the gene encoding the lysosomal protein. Option (d) can be immediately discounted because we do not have a complete list of LSD pathogenic mutations, and technology is still too slow and costly for high-throughput NBS. Pathogenic mutation databases for LSDs are available and updated, i.e., for Pompe disease (7), but the clinical phenotype of individual mutations is often not known with certainty, in large part because most LSD patients are complex heterozygotes, and the pathogenicity of many alleles is unknown. For example, the Pompe disease database (7) contains 501 mutations with the following annotations: 155 as very severe, 137 as potentially less severe, 5 as presumably nonpathogenic, and 57 as unknown pathogenic significance. The New York State NBS laboratory has sequenced the coding regions of the galactosylceramidase (GALC) gene in several hundred screen-positive samples out of about 2 million DBS tested for Krabbe disease. More than half of these sequences reveal DNA changes of unknown pathogenic significance, again showing that DNA sequencing is not appropriate for first-tier NBS of LSDs. Sequencing of the coding regions of the causative gene does play a useful role in helping to confirm LSD diagnosis (8, 9).
Direct enzyme activity analysis in dried blood spots (DBS) (option 1) has been extensively developed in recent years and has been studied in recent large-scale pilot studies. Protein abundance measurements (option 2) have also been developed, and the analytical phase of a pilot study in Minnesota has been completed (D. Matern, Mayo Clinic, personal communication, October 20, 2014). Biomarker analysis for newborn screening of LSDs (option 3) is at a relatively early stage of development and holds promise, especially for second-tier analyses of samples that are screen positive in enzyme activity screens.
NBS for LSDs by Direct Measurement of Enzymatic Activities in DBS: Methods Based on Tandem Mass Spectrometry
Chamoles and coworkers were the first to show that several lysosomal enzymatic activities could be assayed after rehydration of DBS punches with aqueous buffers (10– 15). These pioneering studies were done with fluorometric and radiometric assays.
The studies by Chamoles and coworkers were followed by studies from Gelb, Scott, Turecek, and coworkers, who used tandem mass spectrometry (MS/MS) and internal standards to detect multiple enzymatic products in a multiplex analysis of Fabry, Gaucher, Krabbe, mucopolysaccharidosis-I (MPS-I), Niemann-Pick-A/B, and Pompe diseases (16–18). The work was derived from the well-established use of MS/MS for NBS of amino acid, fatty acid, and organic acid inborn errors of metabolism (19). The method was modified slightly to make it more compatible with NBS laboratories (20, 21). The necessary MS/MS reagents were manufactured by Genzyme Corp. and distributed to NBS laboratories worldwide by the CDC along with QC DBS standards (22).
Assay of acid α-glucosidase for the NBS of Pompe disease is problematic because of the presence of the interfering enzyme maltase-glucoamylase, which, like the Pompe enzyme, displays activity in the low pH range and is present in DBS. The glycohydrolase inhibitor acarbose has been shown to selectively inhibit maltase-glucoamylase (16). Millimolar concentrations of maltose can also be used to selectively block maltase-glucoamylase, but low micromolar concentrations of acarbose give slightly better discrimination among nonaffected, Pompe heterozygote, and Pompe homozygote samples (23). An international panel of experts, without the participation of the laboratory that reported the results with acarbose, has recommended the use of acarbose for NBS of Pompe disease (24). The MS/MS method with acarbose was successfully implemented in the first small-scale pilot studies of Fabry and Pompe diseases carried out by Bodamer and coworkers in Europe (25, 26) and is used byHwuet al. in Taiwan in the first-ever live NBS program for Pompe disease (27).
The initial versions of the MS/MS methods made use of solid-phase extraction using plugs of silica gel to clean up the samples before flow injection into the triple quadrupole MS/MS instrument (16, 17, 20, 21). Because of the need to evaporate 4–5 mL of solvent, some NBS laboratories expressed concern about the length of time needed for the solid-phase extraction step. Variations of the MS/MS method were subsequently developed to facilitate sample processing. The groups of la Marca et al. and of Kasper et al. injected the samples onto an ultra– high-pressure liquid chromatography (UHPLC) column interfaced directly with the MS/MS (28–31). The Kasper team also carried out a seminal newborn screening pilot study in Austria, which showed the feasibility of NBS for several LSDs using enzymatic activity analysis in DBS based on MS/MS (discussed below) (30). Subsequently, the University of Washington team developed a UHPLC-MS/MS assay of a 3-plex LSD assay (32) and a 9-plex LSD assay (the above-described 6-plex plus MPS-II, -IVA, and -VI) using a single 3-mm DBS punch incubated with 9 substrates and internal standards in a single buffer (33). This nicely highlights the multiplexing capacity of MS/MS. A variation of this assay has been piloted in the Illinois NBS laboratory with plans to initiate testing for 6 LSDs in October 2014 (http://www.idph.state.il.us/HealthWellness/fs/lysosomal.htm). A recent study on the use of UHPLC/MS/MS was also successfully carried out for MPS-I (34).
Despite the successful implementation of the UHPLC/MS-MS methods in NBS laboratories, there has been some pushback about the added complexity of using UHPLC. It was found that the solid phase extraction step could be replaced by a simple liquid–liquid extraction using the nontoxic solvent ethyl acetate. This led to the current 6-plex assay being developed by the University of Washington team in collaboration with PerkinElmer Corp. The 6-plex is for Fabry, Gaucher, Krabbe, MPS-I, Niemann-Pick-A/B, and Pompe diseases, in which a single 3-mm DBS punch is incubated with a mixture of 6 substrates and internal standards in a single buffer. After incubation, the reaction is subjected to liquid–liquid extraction followed by flow-injection MS/MS (inject-to-inject time of 115 s). This is the final version of the 6-plex LSD assay being commercialized by PerkinElmer and will replace the Genzyme/CDC reagent distribution program and associated method. A pilot study of this 6-plex carried out on 100000 random newborns is currently ongoing in the WA state NBS laboratory. A 3-plex version of this assay for Fabry, Pompe, and MPS-I was carried out on 111000 DBS, showing the feasibility of enzyme activity– based NBS of LSDs by MS/MS (9, 32) (discussed below).
A key advantage of the MS/MS multiplex assay is based on the fact that multiple enzymes are analyzed in a single DBS punch. If all enzymes in the multiplex read low it would flag a mishandled DBS so that the assay could be repeated. Furthermore, normalization schemes are possible to help control for the variability in the white cell count per DBS punch, i.e., low activity for all enzymes in a properly handled DBS punch would signify a low white cell count. Such a normalization protocol will be evaluated in the upcoming pilot studies.
NBS for LSDs by Direct Measurement of Enzymatic Activites in DBS: Methods Based on Fluorescence
Many, but not all, lysosomal enzymes can be assayed using fluorogenic substrates (10–15, 35–40). For example, acid α-glucosidase for Pompe disease is assayed with the α-glycoside between glucose and 4-methylumbelliferone. The assay is quenched with high pH buffer, and the fluorescence of the conjugate base of 4-methylumbelliferone is measured.
Hwu and coworkers have carried out seminal studies on the use of a standard 96-well plate fluorometer for NBS of Pompe disease in Taiwan (27, 35). Because of the high prevalence of pseudodeficiency alleles in Taiwan, the assay is more complex and involves measurement of both acid and neutral α-glucosidase activities in separate fluorometric assays. The latter is used in a normalization scheme to help separate Pompe-positive from pseudodeficiency samples. An MS/MS version of this duplex assay can be carried out in a single assay with the use of a pair of differentially mass-encoded substrates. The use of standard fluorometry provides a viable alternative to MS/MS and digital microfluidic fluorescence assays (see below) in cases in which 1–2 LSDs are being analyzed. However, the lack of multiplexing of standard fluorescence assays will be problematic as more LSDs are analyzed. Because it is clear that the number of LSDs entering the NBS arena is increasing, standard fluorometric assays provide only a temporary solution, because they cannot be multiplexed. Other limitations of fluorometric assays are noted below. Also, homebrew assays are not always possible for LSDs because assay reagents are not available from vendors that supply generic reagents. Reagent preparation for fluorometric and MS/MS assays often require more than 10 synthetic steps and must be carefully analyzed for trace impurities and isomeric mixtures that can have a large negative effect on NBS.
Advanced Liquid Logic Inc. (now Baebis, Inc.) has developed a novel fluorometric lysosomal enzyme assay platform based on digital microfluidics in which submicroliter droplets are moved on an electrode-plate chip under a layer of oil to prevent evaporation, by a process known as electrowetting (41–43). The latest published version of this technology includes a digital microfluidics chip that accepts 48 assay samples and 5 reagent reservoirs so that 5 lysosomal enzymes can be assayed by mixing a portion of each sample with the 5 reagents, followed by incubation, quenching, and fluorometry (43). This latest version measures the activity of enzymes relevant to MPS-I, MPS-II, Pompe, Fabry, and Gaucher diseases and is discussed further below.
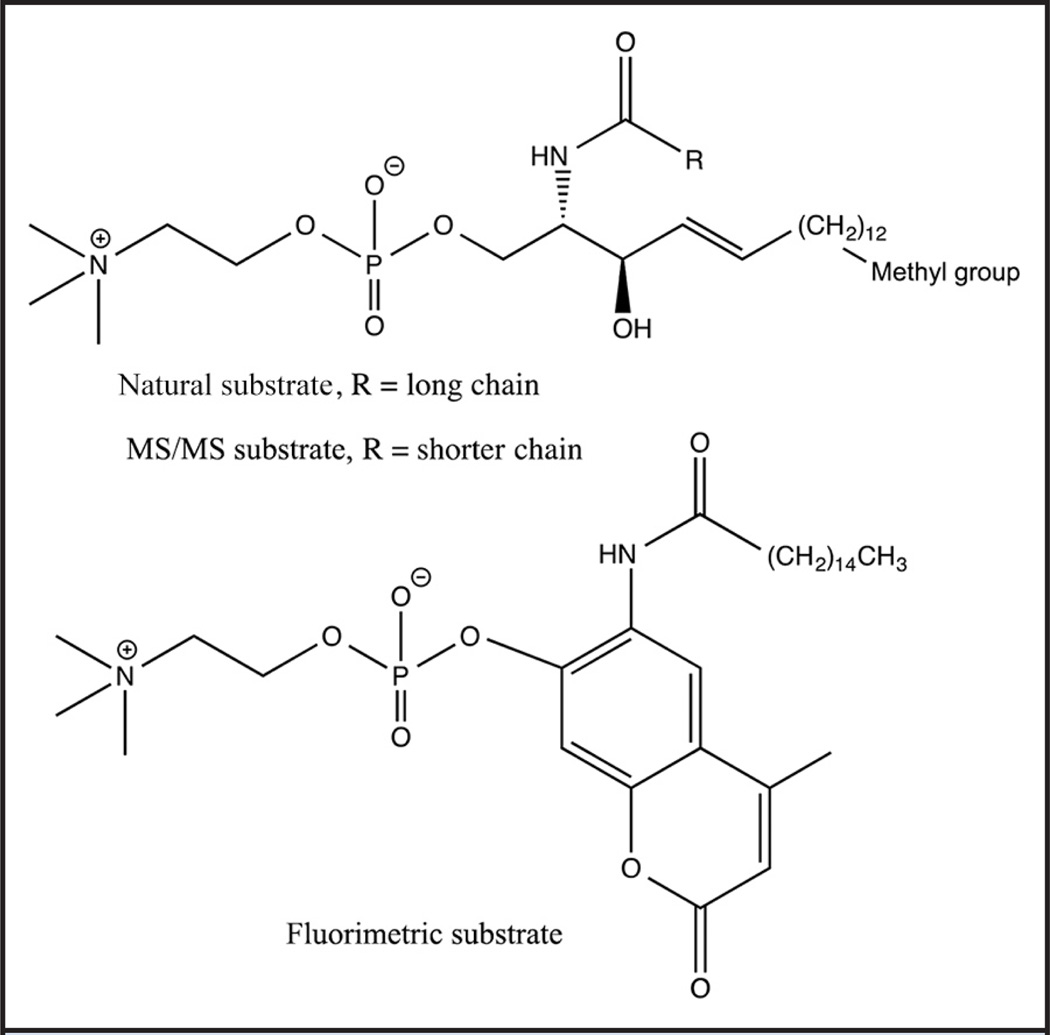
Not all lysosomal enzymes can be assayed by fluorometric methods. It has been well described that all known fluorometric substrates for Niemann-Pick-A/B diseases lead to a significant false-negative problem due to a common mutation (p.Gln292Lys) in the population that causes an anomalous high reading of enzyme activity with the fluorogenic substrates compared to the natural sphingomyelin substrate (38, 44). The structure of natural sphingomyelin, the fluorometric substrate, and the MS/MS substrate are shown in Fig. 1. The false-negative problem with the fluorogenic substrates is due to the fact that some pathogenic mutants are properly folded but contain active site mutations that greatly weaken binding of the natural substrate sphingomyelin to the enzyme but have little or no effect on the binding of the artificial fluorogenic substrates (38). Van Diggelen and coworkers have shown that this type of mutation can be revealed if the assay with the fluorogenic substrate is carried out in the presence and absence of sphingomyelin; the wild-type enzyme shows stronger inhibition by the latter than the mutant enzyme (38). Thus, at least 2 assays for every newborn would have to be done with the fluorometric method. The MS/MS substrate is nearly identical in structure to natural sphingomyelin, the only difference is the length of the fatty acyl chain. Recent studies have shown that the p.Gln292Lys mutant that leads to the false-negative problem displays similarly low enzymatic activity on sphingomyelin and the MS/MS substrate, yet the fluorogenic substrate gives an activity that is approximately 1.3-fold higher than that from the wild-type enzyme (Gelb laboratory, unpublished observations). False negatives must be minimized in NBS screening, and a novel method will be required if fluorescence is to be used for NBS of Niemann-Pick-A/B disease. Fluorometric assay for acid sphingomyelinase by analysis of assay of liberated choline with choline oxidases is not a viable solution because the plasma concentration of free choline (45) is well above the amount of choline generated in the sphingomyelinase assay [calculated based on the level of acid sphingomyelinase in DBSs measured with the MS/MS method (16)].
Fig. 1.
Natural, MS/MS and fluorimetric substrates for Niemann-Pick-A/B disease.
Immunological Assays of Lysosomal Enzyme Abundance and Activity
Hopwood and coworkers have developed elegant antibody-based protein detection methods for multiplex quantification of the abundance of several lysosomal proteins in plasma, purified cells, and DBS (46, 47). This method is based on the fact that many LSDs are caused by mutations that destabilize the lysosomal protein so that it is degraded in the protein-folding QC checkpoints. The same group also demonstrated the use of immunocapture to assay the activity of lysosomal enzymes in the immunopurified fraction. Typically, fluorometric substrates are used. The methods have been applied to plasma, cells, and DBS, and our discussion is limited to the latter because of our focus on NBS. Immunoquantification of acid α-glucosidase and its precursor protein in 1951 DBS and in 17 DBS from Pompe-affected patients showed that 16 of 17 Pompe-affected samples had protein concentrations below the range for the normal controls (48). In a follow-up study, Pompe enzyme abundance and activity were measured after immunocapture (49). Two of 17 Pompe patients had protein abundance in the range of normal adults, and all 17 had enzymatic activity well below the adult range. It was suggested that 2 of the 17 Pompe patients produced mutant acid α-glucosidases that were not degraded, and thus not unstable, but displayed low enzymatic activity on the fluorometric substrate.
Immunoquantification of α-galactosidase in DBS for the analysis of Fabry disease showed that all 13 Fabry hemizygotes fell below the range of protein concentration for 71 control DBS (50). Assay of α-galactosidase activity after immunocapture also showed complete separation between the 2 groups. Immunoquantification of α-glucosidase activity in 20 DBS from control patients and 9 from Gaucher patients showed significant overlap between the 2 groups, whereas the groups were completely separated when the enzymatic activity was measured after immunocapture (51). For MPS-II, immunoquantification of iduronate-2-sulfatase in DBS showed that 11 of 12 MPS-II patients had essentially no detectable protein, and 1 had a concentration of protein just below the low end of the range for non–MPS-II samples (52). In a study of MPS-VI using DBS, all 7 MPS-VI patients displayed arylsulfatase B protein and activity after immunocapture below the values for 32 controls (53). The method performed well to help diagnose patients referred to a metabolic clinic for LSDs, including the analysis of LSDs not mentioned above (47).
Detailed Comparison of MS/MS vs Digital Microfluidic Fluorometric Assay of Lysosomal Enzymes for NBS of LSDs
There is now sufficient data available to begin to compare MS/MS vs fluorescence methods to assay lysosomal enzymes for NBS of LSDs. In this section we provide this comparison as well as the outcome of pilot studies published to date. A large-scale pilot studies of NBS based on immunocapture has recently been completed at the Mayo clinic, and published results are expected shortly (see above). For the MS/MS assays we focus our discussion on Fabry, Gaucher, Krabbe, MPS-I, Niemann-Pick-A/B, and Pompe diseases, and for the fluorescence assays we focus on Fabry, Gaucher, MPS-I, MPS-II, and Pompe diseases because extensive data are available only for these assays. For a summary of the available assays, space and manpower requirements, and approximate costs, see Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol61/issue2.
All methods start with the punching of an approximately 3-mm DBS punch into a well of a 96-well microtiter plate. In the case of the MS/MS method, the assay cocktail is added to the same well that received the punch. In the case of the standard and digital microfluidics fluorometric protocols, the well with the punch is first charged with extraction buffer, followed by a brief centrifugation and finally transfer of an aliquot of supernatant to the assay well or chip. In the case of the MS/MS method the assay cocktail is added to the punch without prior extraction, and centrifugation is also used, but this is to separate the aqueous and organic solvent layers for liquid–liquid extraction. The total number of liquid transfers (additions and transfers by manually operated multichannel pipet) is 5 for the MS/MS with a minimum volume transferred of 30 µL and 9 for the fluorometric methods with a minimum volume transferred of 1.6 µL (see online Supplemental Material). None of the methods are fully automated.
The variation in the amount of enzyme from the DBS is based on the following: (a) for MS/MS it comes down to the size of the DBS punch; (b) for microfluidics fluorometry it comes to the size of the DBS punch, the amount of enzyme extracted, and the amount of extract applied to the chip.
A single MS/MS instrument has the throughput of approximately 7.8 digital microfluidics plate readers (see online Supplemental Material). The space requirements for both platforms are nearly the same (see online Supplemental Material). Some NBS laboratories have backup MS/MS instruments to handle the load if an instrument requires servicing. Essentially all laboratories doing MS/MS-based NBS will already have at least 1 backup instrument and will probably not opt to obtain an additional backup for LSDs. It is reasonable to expect that laboratories running digital microfluidics workstations would also have a backup instrument. Manpower requirements for the 2 methods appear to be similar (see the online Supplemental Material).
A detailed cost analysis of the 2 methods is not possible at the present time, but some useful estimates can be provided. The full costs for flow injection analysis–MS/MS (FIA-MS/MS) instrumentation, including instrument purchase, nitrogen generation, servicing, installation, and electricity, comes to 4.7 US cents per newborn per LSD analyzed (see the online Supplemental Material). The equipment costs for the digital microfluidics analyzers are not known; however, the important point is that the overall costs per sample per LSD analyzed is expected to be similar with both methods (~$1 per sample per LSD analyzed) (see online Supplemental Table 1).
The data above show that cost and ease of analysis are not major factors in selection between the MS/MS and digital microfluidics fluorometric methods. We now turn our attention to a discussion of the reproducibility and nonaffected/affected enzymatic activity ratio data. The CV for multiple punches of the low- and high-activity QC DBS are similar for the 2 methods or slightly better for the MS/MS method (Table 1). A critical parameter is the rate of false positives. However, comparison of false-positive rates is complicated by the fact that the values depend on the chosen cutoffs for screen-positive samples and by the uncertainty in establishing a positive sample based on follow-up gene sequencing (given the large numbers of DNA variations of unknown pathogenic significance). Probably the most important metric is the measured ratio of mean enzymatic activity of random newborns to that of LSD-affected samples. Table 1 shows that this ratio for MS/MS is 5- to 23-fold higher than that for digital microfluidics. This substantially higher ratio is expected to lead to a lower false-positive rate for the MS/MS method. Table 1 also shows the ratio of mean activity in random newborns to that measured with filter paper blanks (no blood); published data for digital microfluidics are not available. For MS/MS, this ratio is >85-fold for all assays. It is fully expected that the MS/MS method will do a better job of spreading the enzymatic activity values, which should prove useful for stratifying babies for follow-up diagnostic studies and for minimizing false positives.
Table 1.
Reproducibility and nonaffected/affected ratio data for MS/MS and digital microfluidics fluorimetry assays of LSD enzymes.
| % CV CDC high/low QC samplesa |
Mean normal activity/ mean LSD-affected activity |
Mean normal activity/ mean activity of the no-blood control |
|||
|---|---|---|---|---|---|
| MS/MSb | Digital microfluidicsc |
MS/MSb | Digital microfluidicsc |
MS/MSb | |
| Fabry | 6/16 | 9/12 | 28 | 6.1 | 109 |
| Gaucher | 12/14 | 8/12 | 67 | 3.7 | 216 |
| MPS-I | 9/11 | 9/17 | 168 | 7.4 | 230 |
| MPS-II | 6/8 | 21/12 | 60 | 3.9 | 90 |
| Pompe | 9/10 | 6.0/13.2 | 63 | 5.0 | 367 (240)d |
| Krabbe | 7/12 | No data | 27 | No data | 85 (150)d |
| Niemann-Pick-A/B | 11/11 | No data | 26 | No data | 104 |
Percent CV for DBS from nonaffected patients [100 × (SD/mean)].
The CVs are for multiple punches from the high and low QC sample from the CDC analyzed on the same MS/MS instrument (unpublished observations obtained in the authors’ laboratory).
The CVs are for multiple punches from the high and low QC sample from the CDC analyzed on different digital microfluidics instruments [Sista et al. (43)].
Data from J. Orsini (Wadsworth Center, NY) for their MS/MS assays.
NBS Pilot Studies of LSDs
Here we describe pilot studies of NBS for LSDs that were carried out, in most, on tens of thousands of DBS so that estimates of screen-positive and false-positive values could be obtained. False-negative values are typically not calculated, for obvious reasons. Kasper and coworkers carried out the first large-scale, enzymatic activity– based NBS pilot for LSDs (30), which was followed by a more recent study by our laboratory. Data for both studies are summarized in Table 2. Kasper used LC-MS/MS and we used flow injection–MS/MS. False-positive rates for both studies were acceptably low. For example, in Washington state, with approximately 80000 births per year, only approximately 11, approximately 12, and approximately 6 babies would need to be followed up for the potential to develop Pompe, Fabry, and MPS-I diseases, respectively.
Table 2.
Pilot studies of LSD NBS.a
| Method | Lab (reference) | LSDs | Number of DBS | Screen cutoff (% of mean) |
Estimated no. of screen positives (per 105 DBS)b |
Estimated no. of true positives (per 105 DBS)c |
|---|---|---|---|---|---|---|
| Flow injection MS/MS (UW/PE) | WA state NBS Lab [Scott et al. (9)] | Fabry MPS-I Pompe |
111000 | 19% (Fabry) 32% (MPS-I) 15% (Pompe) |
15 (Fabry) 7 (MPS-I) 14 (Pompe) |
6 (Fabry) 2 (MPS-I) 4 (Pompe) |
| WA state NBS Lab [Gelb et al. (79)] | Fabry Gaucher Krabbe MPS-I Niemann-Pick-A/B Pompe |
4300 | 15% (Fabry) 15% (Gaucher) 10% (Krabbe) 20% (MPS-I) 20% (NP-AB) 15% (Pompe) |
Out of 4300 0 (Fabry) 1 (Gaucher) 1 (Krabbe) 3 (MPS-I) 1 (NP-AB) 0 (Pompe) |
2 (MPS-I) No DNA sequencing data yet on the others |
|
| Flow injection MS/MS (Genzyme/CDC) | Taiwan (multiple labs) [Liao et al. (80)] | Fabry Gaucher Pompe |
191767 (Fabry, Pompe) 108658 (Gaucher) | 15% (Fabry) 33% (Gaucher) 6% (Pompe) |
41 (Fabry) 5 (Gaucher) 117 (Pompe) |
33 (Fabry) 3 (Gaucher) 8 (Pompe) |
| Europe (multiple labs) [Whittmann et al. (54)] | Fabry Gaucher Niemann-Pick-A/B Pompe |
40024 | 22% (Fabry) 20% (Gaucher) 22% (NP-A/B) 20% (Pompe) |
85 (Fabry) 42 (Gaucher) 12 (NP-A/B) 160 (Pompe) |
7 (Fabry) 7 (Gaucher) 5 (NP-A/B) 22 (Pompe) |
|
| LC-MS/MS | Austrian National NBS lab [Mechtler et al. (30)] | Fabry Gaucher Pompe Niemann-Pick-A/B |
34736 | 35% (Fabry) 25% (Gaucher) 22% (NP-A/B) 16% (Pompe) |
80 (Fabry) 11 (Gaucher) 3 (NP-A/B) 14 (Pompe) |
26 (Fabry) 6 (Gaucher) 0 (NP-A/B) 11 (Pompe) |
| IL state NBS lab [Basheeruddin et al. (81)] | Fabry Gaucher MPS-I Niemann-Pick-A/B Pompe |
12000 | 15% (Fabry) 15% (Gaucher) 20% (MPS-I) 20% (NP-AB) 15% (Pompe) |
Out of 12000 4 (Fabry) 2 (Gaucher) 4 (MPS-I) 2 (NP-AB) 2 (Pompe) |
No DNA sequencing data yet | |
| Fluorimetry with digital microfluidics | MO state NBS lab [Hopkins et al. (82)] | Fabry Gaucher MPS-I Pompe |
118000 | Unpublished | 158 total (4 LSDs) disease breakdown not published |
30 of 158 total (4 LSDs) disease breakdown not published |
| IL state NBS lab [Burton et al. (55)] | Fabry Gaucher Pompe |
8012 | Unpublished | 137 (Fabry) 274 (Gaucher) 25 (Pompe) |
87 (Fabry) 25 (Gaucher) 0 (Pompe) |
Pilot studies of Pompe disease in Taiwan are discussed in the main text only because the methodology does not conform to the categories in this table.
Equal to the number of samples below the screen cutoff normalized to 100 000 samples except as noted (the pilot studies started recently, and only 12 000 and 4300 samples have been analyzed in IL and WA, respectively).
Equal to the number of screen-positive samples that contained pathogenic mutations on both chromosomes normalized to 100 000 samples.
The relatively high false-positive rates in the European study on 40000 samples (Table 2) (54) is probably due to the fact that the original MS/MS assay was used (16, 20, 21). When the optimized MS/MS assay is used in the 3-plex study in Washington state, the false-positive rates are much lower (9) (Table 2), and these values should be taken as the new predictive values because they are based on the most recent assay developments. Furthermore, the original MS/MS assay based on CDC-distributed reagents will cease to exist at the end of 2015.
A pilot study of the new FIA-MS/MS 6-plex (being commercialized by PerkinElmer and described above) started in June 2014 in the Washington NBS laboratory. Initial data on 4300 samples show a very low number of screen positives, as does the pilot study using LC-MS/MS in the Illinois NBS laboratory on 12 000 samples (Table 2).
A pilot study with 118000 samples in the Missouri NBS laboratory using the digital microfluidics fluorometry platform has shown a much higher number of screen positives, 158 out of 100000 (for Fabry, Gaucher, MPS-I, and Pompe) compared to 36 out of 100000 for the 3-plex flow injection MS/MS study in the Washington NBS laboratory (Table 2). The initial 6-plex flow injection MS/MS data in Table 2 show that addition of Gaucher to the 3-plex would not increase the number of screen positives by more than approximately 20. These results on estimated false-positives rates are consistent with the data in Table 1 showing that the ratio of mean enzymatic activity for nonaffected to affected individuals is much higher for the MS/MS method compared to the digital microfluidics fluorometric method. Relatively high numbers of screen positives using digital microfluidics fluorometry were also reported in a small-scale pilot study in the Illinois NBS laboratory (55) (Table 2), consistent with the large-scale study in the Missouri NBS laboratory.
Extensive pilot studies have been reported by multiple NBS centers in Taiwan. Hwu and coworkers used standard fluorometric assays to test every newborn for acid α-glucosidase activity at low pH in the presence of acarbose and for neutral α-glucosidase activity at neutral pH without acarbose, and calculate the neutral-to-acid ratio (27). After screening 473738 babies, 31 had a neutral-to-acid ratio of >100, and DNA sequencing revealed 17 late-onset, 5 infantile-onset, and 5 non-Pompe patients. A total of 2210 samples were submitted to a second-tier enzyme assay if the neutral-to-acid ratio had an intermediate value. Of these samples, 219 retested with a high neutral-to-acid ratio, and DNA analysis of these led to 2 late-onset and 207 non-Pompe patients, and 10 patients who refused to undergo follow-up analysis. These results again underscore the challenge of screening for Pompe disease in Taiwan because of the prevalence of pseudodeficiency alleles.
A second laboratory in Taiwan reported on a NBS study of 402281 newborns for Pompe enzyme activity, first using a fluorometric assay and then switching to an MS/MS assay (56). It is not possible to compare the fluorometric to the MS/MS data from this report because all the data are compiled together. Pompe patients were identified after second-tier analyses to sort through the large number of pseudodeficiencies. Patients with Pompe enzyme activity below the screen cutoff underwent a panel of second-tier assays focused on analysis of muscle function as a way to help categorize the pseudodeficiency vs affected patients.
False-positive rates for Gaucher and Fabry in 191000 babies screened by FIA-MS/MS in Taiwan gave acceptable follow-up rates for all but Pompe (Table 2). Again, the high rate of pseudodeficiency mutations in Taiwan argues for a more complex algorithm developed by Hwu and colleagues (27) (described above).
Extensive postpilot NBS data are available for Krabbe disease from the New York NBS program and will be reported by the state program elsewhere. A retrospective study of 100000 DBS started at the Mayo Clinic to compare the Genzyme/CDC FIA-MS/MS, the immunoquantification, and the digital microfluidics fluorometric methods (57), and a pilot study of 100000 DBS using the most updated FIA-MS/MS method for MPS-II and -VI started in the WA NBS laboratory in July 2014. Data from these studies have not been disclosed yet.
BIOMARKERS FOR ANALYSIS OF LSDS
A number of biomarkers have been proposed for the analysis of LSDs. Here we focus on biomarkers that tend to accumulate because they are substrates for the deficient lysosomal enzyme and because these are the most promising candidates for NBS.
POMPE DISEASE
Glucose tetrasaccharide has been studied as a potential biomarker for Pompe disease (58). It is generally accepted that this biomarker is not specific enough to Pompe disease to warrant its use for first-tier NBS (S. Heales, University College London, personal communication, October 14, 2014). More recently a liquid chromatographic assay has been developed to monitor glucose tetrasaccharine in urine samples (59). The tetrasaccharide was undetectable in nonaffected patients, all 9 confirmed Pompe patients showed increased biomarker, and concentrations in a Pompe pseudodeficiency patient were increased but below the concentrations for the Pompe patients. This study shows that glucose tetrasaccharide is a useful biomarker for second-tier sorting of samples after first-tier enzymatic activity– based NBS and for monitoring enzyme replacement therapy. More work is needed to determine if it can be used to distinguish between early- and late-onset Pompe patients. A study using the improved FIA-MS/MS described above on early- and late-onset Pompe DBS is also warranted.
GAUCHER DISEASE
Glucosylceramide, the substrate for the relevant enzyme, is reported to be on average a 3-fold increase in type I Gaucher patients compared to healthy individuals (60). However, even when a small number of DBSs are analyzed, overlap between affected and nonaffected patients is apparent (60). Very recently, Rolfs and coworkers analyzed the blood concentrations of glucosylsphingosine by LC-MS/MS in 148 healthy non-Jewish white individuals, 98 genetically-confirmed Gaucher patients, and 262 patients with other LSDs (61). The concentration of glucosylsphingosine in the Gaucher samples was about 100-fold higher than in the healthy patients and in patients with other LSDs, and there were no significant differences between males and females. With a cutoff for glucosylsphingosine of 12 ng/mL, this team was able to identify the Gaucher patients with 100% specificity. In the same study it was found that glucosylsphingosine was a more reliable biomarker of Gaucher disease than were chitotriosidase and CCL18, 2 other previously proposed biomarkers. The team also provided data to suggest that monitoring glucosylsphingosine is useful in monitoring the response of Gaucher patients to enzyme replacement therapy. These are very exciting developments, and further studies of this biomarker on a larger population are warranted. It remains unclear if monitoring glucosylsphingosine is best done as a first-tier NBS for Gaucher disease or whether it should be done after first-tier enzyme assay analysis of DBS as a way to stratify the results from the screen. Glucosylsphingosine analysis will probably require LC coupled to MS/MS. The authors did not provide data to show if their LC-MS/MS method could resolve glucosylsphingosine from psychosine (galactosylsphingosine). This seems unlikely with their LC setup, on the basis of the results presented below for psychosine analysis in Krabbe patients. Should it be necessary to resolve these 2 blood components, it may be difficult to do this in <2–3 min per sample, and then it would probably be too slow to support the high throughput needed to use this assay as a first-tier NBS method.
KRABBE DISEASE
Galactosylsphingosine (psychosine) concentrations were studied by LC-MS/MS in a detailed study of Krabbe disease by Chuang et al. (61). This study is particularly informative because the DBS examined were from the New York NBS program for Krabbe disease, which is based on the analysis of GALC activity in DBS by FIA-MS/MS (8, 17). Out of approximately 1.2 million newborns screened, 10 were found to have very low GALC activity of ≤0.15 nmol · mg−1 · h−1 protein, and 4 of these contained mutations consistent with infantile Krabbe disease. The psychosine concentrations in DBS from these 4 babies were all increased (23–73 ng/mL) compared to those in random newborns (approximately 2.5 ng/mL). Six babies also had low GALC activity of ≤0.15 nmol · mg−1 · h−1 protein but had genotypes consistent with late onset Krabbe disease and were asymptomatic at the time of the study. Psychosine concentrations were closer to those in controls in these samples (1–8 ng/mL). Babies with higher GALC activity (0.16–0.50 nmol · mg−1 · h−1 protein) were considered to be of lower risk for developing Krabbe disease, remained asymptomatic, and had psychosine concentrations of undetectable to 3 ng/mL. This study suggests that quantification of psychosine by LC-MS/MS offers a way to stratify patients that are revealed to have low GALC activity in the primary screen. The data also show that genotyping the low GALC activity samples can help stratify the patients, and that the babies with the lowest GALC activity are at higher risk for developing Krabbe disease. It is doubtful that psychosine analysis can be used as a first-tier NBS for Krabbe disease given that the separation of psychosine from glucosylsphingosine required a tandem set of UHPLC columns and a run time of >5 min per sample (62). It may not be necessary to separate psychosine from glucosylsphingosine. Presumably the former would be increased in Krabbe patients, and the latter would be increased in Gaucher patients (60). Thus, one algorithm to consider is that a high concentration of unresolved psychosine plus glucosylsphingosine combined with low GALC activity would suggest Krabbe disease, whereas the same LC-MS/MS result with a low acid β-d-glucosidase activity would suggest Gaucher disease. Further studies of psychosine as a biomarker of Krabbe disease are warranted.
NIEMANN-PICK-A/B DISEASE
It was recently shown that lysosphingomyelin is increased in DBS from Niemann-Pick-B patients (63). This study included 27 affected patients and 20 nonaffected patients. The mean lysosphingomyelin concentration in affected patients was approximately 5-fold higher than in nonaffected patients. Concentrations of sphingomyelin were similar in nonaffected vs Niemann-Pick-B patient DBS. These results warrant an expanded study of this potential biomarker on a larger population. Given that the Niemann-Pick-B patient with the lowest lysosphingomyelin concentration and the nonaffected patient with the highest concentration differed only by 1.8-fold, it seems that lysosphingomyelin analysis will be most useful as a component of a Niemann-Pick diagnosis panel and to follow therapy rather than as a first-tier NBS method.
MUCOPOLYSACCHARIDOSES
There have been relatively large numbers of recent studies on the analysis of glycosaminoglycan fragments by MS/MS as potential biomarkers for mucopolysaccharidoses. This topic has been recently reviewed (64, 65), and we limit our discussion to a few points. Tomatsu and coworkers studied the enzymatic degradation of glycosaminoglycans in DBS and measured a disaccharide derived from chondroitin sulfate, and 2 disaccharides derived from heparan sulfate (65). DBS from 6 MPS patients were studied (4 MPS-I, 1 MPS-II, and 1 MPSVII) along with 326 nonaffected controls. There were no false negatives in this analysis, but unfortunately the false-positive rates were high (0.3%–1.5%). It is clear that this method alone would not be suitable for first-tier NBS of MPS; however, if used on top of a first-tier NBS method based on enzymatic activity analysis, it could prove to be a useful supplement.
de Ruijter et al. studied heparan sulfate– and dermatan sulfate– derived disaccharides in DBS among MPS-I, -II, and -III patients (66). The method starts by incubation of the DBS punch in extraction buffer for 10 min followed by sonication for 15 min. The extract is treated for 2 h with chondroitinase B followed by addition of disaccharide internal standard, boiling, and centrifugation. The supernatant is transferred to a centrifugal ultrafilter, and the filtrate is analyzed by LC-MS/MS with a total run time of 7.1 min. The analysis focused on the most abundant disaccharide derived from heparan sulfate and dermatan sulfate, because minor fragments were not sufficiently detected for quantification. All MPS patients (10 MPS-I, 1 MPS-II, and 6 MPS-III) showed increased concentrations of the heparan sulfate-derived disaccharide in the range of approximately 4–10-fold above the mean concentration in the set of nonaffected samples. The dermatan sulfate– derived disaccharide was approximately 8- to 20-fold higher in the MPS-I patients compared to the mean for the control group. This marker was not increased in the MPS-II and MPS-III patients as expected. This approach does not allow the 4 types of MPS-III syndrome to be dissected. One concern is that the level of biomarker elevation may not be sufficient to allow this method to be used for first-tier NBS. Additional factors are the high cost of the ultrafilters and the long LC time of 7.1 min. This method would not be appropriate for first-tier NBS.
A new approach called the Sensi-Pro assay examines the nonreducing ends of glycosaminoglycan fragments (67). Glycosaminoglycan chains are first degraded with bacterial lyases to give disaccharides. The reducing end of the latter are derivatized with heavy isotopic aniline by reductive amination, and the derivatives are analyzed by LC-MS/MS (65). Preliminary studies were carried out on DBS from a panel of MPS patients (MPS-I, -II, -IIIA, and -IIIB), and in all cases the concentrations of specific biomarkers were increased compared to DBS from nonaffected controls (65). Because numerical data were not provided in this report, the relative abundance of these biomarkers cannot be commented on. It is thus too early to know if this type of method will be useful for NBS of the MPSs. One concern is that the current method requires a relatively large number of steps before LC-MS/MS: (a) digestion of proteins in the DBS with a protease; (b) ion exchange enrichment of glycosaminoglycans and desalting; (c) digestion of carbohydrate polymers with bacterial lyases; (e) reductive amination tagging with isotopic aniline; (f) addition of synthetic standards; and (g) MS/MS coupled with UHPLC.
It is too early to decide if enzyme activity assay or glycosaminoglycan fragment analysis is the best method for first-tier NBS of LSDs. Given the more involved and lengthy procedures for MPS biomarker quantification, it seems likely that biomarkers will be used mainly as a second-tier analysis and to monitor the efficacy of therapies.
Assays of Other Lysosomal Enzymes
MS/MS assays of the sulfatases relevant to the LSDs MPS-II, -IIIA-D, -IVA, and -VI have been reported (68–72). Fluorometric assays rely on the use of a second enzyme that releases the 4MU fluorophore only after the sulfate is removed from the monosaccharide and are available for MPS-II, MPS-IIIA-D and MPS-IVA but not for MPS-VI (36, 37, 39, 40, 73, 74). MPS-VI is often analyzed using fluorometric substrates that bear essentially no structural resemblance to the natural substrates (sulfated 4MU isomers), and this is the basis for the name arylsulfatase B for the MPS-VI enzyme. Although these substrates are useful in second-tier diagnostic studies in which an LSD patient is suspected, they are probably not appropriate for first-tier NBS because there are approximately 17 sulfatases encoded by the human genome, and the specificity of these enzymes on the4MU substrates has not been fully studied.
A fluorometric assay for lysosomal acid lipase using DBS has been developed based on a 4MU fatty acid substrate and a covalent inactivator of the lysosomal acid lipase, which enables the activity of this enzyme to be teased out of the total lipase activity from a collection of enzymes in blood (75). This assay may not be useful for NBS because the activity of the lipase is calculated as the difference between 2 large numbers, and thus the residual is expected to have high error. Fluorometric assays for protein palmitoyl thioesterase-1 (76) and tripeptidyl peptidase 1 (several reports) are reported, as are MS/MS assays for both (77).
Hexosaminidase fluorometric assays for diagnosis of Tay-Sachs disease have been around for many years and make use of a pair of assays with heat treatment to tease out HexA and HexB activities. An MS/MS variation of this assay has been recently developed that is done in a 2-plex fashion with a pair of differentially mass-encoded substrates (78).
A subset of these additional lysosomal enzyme assays may enter the NBS arena if treatment options for the diseases are developed (MPS-II NBS pilot studies have started in Illinois, Missouri, Washington state, and Taiwan).
Supplementary Material
Acknowledgments
Research Funding: Grants to University of Washington from National Institutes of Health (DK67859), Genzyme Corp., BioMarin Corp., PerkinElmer, and Shire.
Footnotes
Nonstandard abbreviations: LSD, lysosomal storage disease; NBS, newborn screening; DBS, dried blood spot on filter paper; MS/MS, tandem mass spectrometry; MPS-I, mucopolysaccharidosis-I; UHPLC, ultra–high-pressure liquid chromatography; FIA-MS/MS, flow injection–MS/MS.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Employment or Leadership: None declared.
Consultant or Advisory Role: M.H. Gelb, PerkinElmer; C.R. Scott, Genzyme.
Stock Ownership: None declared.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: F. Turecek, patent number PCT/US2012/064205.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
References
- 1.Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. 8th ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 2.Boustany RM. Lysosomal storage diseases: the horizon expands. Nature Rev Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- 3.Ohashi T. Enzyme replacement therapy for lysosomal storage diseases. Pediatr Endocrinol Rev. 2012;10(Suppl 1):26–34. [PubMed] [Google Scholar]
- 4.Lund TC. Hematopoietic stem cell transplant for lysosomal storage diseases. Pediatr Endocrinol Rev. 2013;11(Suppl 1):91–98. [PubMed] [Google Scholar]
- 5.Weinreb NJ. Oral small molecule therapy for lysosomal storage diseases. Pediatr Endocrinol Rev. 2013;11(Suppl 1):77–90. [PubMed] [Google Scholar]
- 6.Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341(6148):1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 7.Kroos M, Hoobeveen-Westerveld M, Michelakakis H, Pomponio R, Vad de Ploeg A, Halley D, Reuser A. Update of the Pompe disease mutation database with 60 novel GAA sequence variants and additional studies on the functional effect of 34 previously reported variants. Hum Mutat. 2012;33:1161–1165. doi: 10.1002/humu.22108. [DOI] [PubMed] [Google Scholar]
- 8.Duffner PK, Caggana M, Orsini JJ, Wenger DA, Patterson MC, Crosley CJ, et al. Newborn screening for Krabbe disease: the New York State model. Pediatr Neurol. 2009;40:245–252. doi: 10.1016/j.pediatrneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Scott CR, Elliott S, Buroker N, Thomas LI, Keutzer J, Glass M, et al. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J Pediatr. 2013;163:498–503. doi: 10.1016/j.jpeds.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–196. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- 11.Chamoles NA, Blanco M, Gaggioli D. Diagnosis of alpha-l-iduronidase deficiency in dried blood spots on filter paper: the possibility of newborn diagnosis. Clin Chem. 2001;47:780–781. [PubMed] [Google Scholar]
- 12.Chamoles NA, Blanco M, Gaggioli D, Casentini C. Tay-Sachs and Sandhoff diseases: enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;318:133–137. doi: 10.1016/s0009-8981(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 13.Chamoles NA, Blanco M, Gaggioli D, Casentini C. Gaucher and Niemann-Pick diseases– enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;317:191–197. doi: 10.1016/s0009-8981(01)00798-7. [DOI] [PubMed] [Google Scholar]
- 14.Chamoles NA, Blanco MB, Gaggioli D, Casentini C. Hurler-like phenotype: enzymatic diagnosis in dried blood spots on filter paper. Clin Chem. 2001;47:2098–2102. [PubMed] [Google Scholar]
- 15.Chamoles NA, Niizawa G, Blanco M, Gaggioli D, Casentini C. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin Chim Acta. 2004;347:97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Brockman K, Turecek F, Scott CR, Gelb MH. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for Krabbe disease. Clin Chem. 2004;50:638–640. doi: 10.1373/clinchem.2003.028381. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–2070. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–1817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, Keutzer JM. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008;54:1725–1728. doi: 10.1373/clinchem.2008.104711. [DOI] [PubMed] [Google Scholar]
- 21.Zhang XK, Elbin CS, Turecek F, Scott CR, Chuang WL, Keutzer J, Gelb MH. Multiplex lysosomal enzyme activity assay on dried blood spots using tandem mass spectrometry. Methods Mol Biol. 2010;603:339–350. doi: 10.1007/978-1-60761-459-3_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Jesus VR, Zhang XK, Keutzer J, Bodamer O, Muhl A, Orsini JJ, et al. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin Chem. 2009;55:158–164. doi: 10.1373/clinchem.2008.111864. [DOI] [PubMed] [Google Scholar]
- 23.Zhang H, Kallwass H, Young SP, Carr C, Dai J, Kishnani PS, et al. Comparison of maltose and acarbose as inhibitors of maltase-glucoamylase activity in assaying acid alpha-glucosidase activity in dried blood spots for the diagnosis of infantile Pompe disease. Genet Med. 2006;8:302–306. doi: 10.1097/01.gim.0000217781.66786.9b. [DOI] [PubMed] [Google Scholar]
- 24.Winchester B, Bali D, Bodamer O, Caillaud C, Christensen E, Cooper A, et al. Methods for a prompt and reliable laboratory diagnosis of Pompe disease: report from an international consensus meeting. Molec Genet Metabol. 2007;93:275–281. doi: 10.1016/j.ymgme.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Dajnoki A, Fekete G, Keutzer J, Orsini JJ, De Jesus VR, Chien YH, et al. Newborn screening for Fabry disease by measuring GLA activity using tandem mass spectrometry. Clin Chim Acta. 2010;411:1428–1431. doi: 10.1016/j.cca.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Dajnoki A, Muhl A, Fekete G, Keutzer J, Orsini J, DeJesus V, et al. Newborn screening for Pompe disease by measuring acid alpha-glucosidase activity using tandem mass spectrometry. Clin Chem. 2008;54:1624–1629. doi: 10.1373/clinchem.2008.107722. [DOI] [PubMed] [Google Scholar]
- 27.Chiang SC, Hwu WL, Lee NC, Hsu LW, Chien YH. Algorithm for Pompe disease newborn screening: results from the Taiwan screening program. Mol Genet Metab. 2012;106:281–286. doi: 10.1016/j.ymgme.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 28.la Marca G, Casetta B, Malvagia S, Guerrini R, Zammarchi E. New strategy for the screening of lysosomal storage disorders: the use of online trapping-and-cleanup liquid chromatography/mass spectrometry. Anal Chem. 2009;81:6113–6121. doi: 10.1021/ac900504s. [DOI] [PubMed] [Google Scholar]
- 29.Kasper DC, Herman JL, De Jesus VR, Mechtler TP, Metz TF, Shushan B. The application of multiplexed, multidimensional ultra-high-performance liquid chromatography/tandem mass spectrometry to the high-throughput screening of lysosomal storage disorders in newborn dried bloodspots. Rapid Commun Mass Spectrom. 2010;24:989–994. doi: 10.1002/rcm.4496. [DOI] [PubMed] [Google Scholar]
- 30.Mechtler TP, Stary S, Metz TF, De Jesus VR, Greber- Platzer S, Pollak A, et al. Neonatal screening for lysosomal storage disorders: feasibility and incidence from a nationwide study in Austria. Lancet. 2012:335–341. doi: 10.1016/S0140-6736(11)61266-X. [DOI] [PubMed] [Google Scholar]
- 31.Metz TF, Mechtler TP, Orsini JJ, Martin M, Shushan B, Herman JL, et al. Simplified newborn screening protocol for lysosomal storage disorders. Clin Chem. 2011;57:1286–1294. doi: 10.1373/clinchem.2011.164640. [DOI] [PubMed] [Google Scholar]
- 32.Spáčil Z, Elliott S, Reeber SL, Gelb MH, Scott CR, Tureček F. Comparative triplex tandem mass spectrometry assays of lysosomal enzyme activities in dried blood spots using fast liquid chromatography: application to newborn screening of Pompe, Fabry, and Hurler diseases. Anal Chem. 2011;83:4822–4828. doi: 10.1021/ac200417u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spacil Z, Tatipaka HB, Marcenas M, Scott CR, Turecek F, Gelb MH. High throughput assay of nine lysosomal enzymes for newborn screening. Clin Chem. 2013;59:502–511. doi: 10.1373/clinchem.2012.189936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gucciardi A, E L, Di Gangi IM, Corbetta C, Tomanin R, Scarpa M, Giordano G. A column-switching HPLC-MS/MS method for mucopolysaccharidosis type I analysis in a multiplex assay for the simultaneous newborn screening of six lysosomal storage disorders. Biomed Chromatogr. 2014;28:1131–1139. doi: 10.1002/bmc.3133. [DOI] [PubMed] [Google Scholar]
- 35.Chien YH, Chiang SC, Zhang XK, Keutzer J, Lee NC, Huang AC, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39–e45. doi: 10.1542/peds.2007-2222. [DOI] [PubMed] [Google Scholar]
- 36.He W, Voznyi Y, Boer AM, Kleijer WJ, van Diggelen OP. A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease type D (MPS IIID) J Inherit Metab Dis. 1993;1993:935–941. doi: 10.1007/BF00711508. [DOI] [PubMed] [Google Scholar]
- 37.Karpova EA, Voznyi YV, Keulemans JL, Hoogeveen AT, Winchester B, Tsvetkova IV, van Diggelen OP. A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease type A (MPS IIIA) J Inherit Metab Dis. 1996;19:278–285. doi: 10.1007/BF01799255. [DOI] [PubMed] [Google Scholar]
- 38.van Diggelen OP, Voznyi YV, Keulemans JL, Schooderwoerd K, Ledvinova J, Mengel E, et al. A new fluorimetric enzyme assay for the diagnosis of Niemann-Pick A/B, with specificity of natural sphingomyelinase substrate. J Inherit Metab Dis. 2005;28:733–741. doi: 10.1007/s10545-005-0105-y. [DOI] [PubMed] [Google Scholar]
- 39.Voznyi YV, Karpova EA, Dudukina TV, Tsvetkova IV, Boer AM, Janse HC, van Diggelen OP. A fluorimetric enzyme assay for the diagnosis of Sanfilippo disease C (MPS III C) J Inherit Metab Dis. 1993;16:465–472. doi: 10.1007/BF00710299. [DOI] [PubMed] [Google Scholar]
- 40.Voznyi YV, Keulemans JL, van Diggelen OP. A fluorometric assay for diagnosis of MPS II (Hunter syndrome) J Inherit Metab Dis. 2001;24:675–680. doi: 10.1023/a:1012763026526. [DOI] [PubMed] [Google Scholar]
- 41.Sista RS, Eckhardt AE, Wang T, Grahman C, Rouse JL, Norton SM, et al. Digital microfluidic platform for multiplexing enzyme assays: implications for lysosomal storage disease screening in newborns. Clin Chem. 2011;57:1444–1451. doi: 10.1373/clinchem.2011.163139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sista R, Eckhardt AE, Wang T, Sellos-Moura M, Pamula VK. Rapid, single-step assay for Hunter syndrome in dried blood spots using digital microfluidics. Clin Chim Acta. 2011;412:1895–1897. doi: 10.1016/j.cca.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Sista R, Wang T, Wu N, Graham C, Eckhardt AE, Winger T, et al. Multiplex newborn screening for Pompe, Fabry, Hunter, Gaucher, and Hurler diseases using a digital microfluidic platform. Clin Chim Acta. 2013;424:12–18. doi: 10.1016/j.cca.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harzer K, Rolfs A, Bauer P, Zschiesche M, Mengel E, Backes J, et al. Niemann-pick disease type A and B are clinically but also enzymatically heterogenous: pitfall in the laboratory diagnosis of sphingomyelinase deficiency associated with the mutation q292k. Neuropediatrics. 2003;34:301–306. doi: 10.1055/s-2003-44668. [DOI] [PubMed] [Google Scholar]
- 45.Zeisel SH, Epstein MF, Kurtman RJ. Elevated choline concentration in neonatal plasma. Life Sci. 1980;26:1827–1831. doi: 10.1016/0024-3205(80)90585-8. [DOI] [PubMed] [Google Scholar]
- 46.Meikle PJ, Grasby DJ, Dean CJ, Lang DL, Bockmann M, Whittle AM, et al. Newborn screening for lysosomal storage disorders. Molec Genet Metabol. 2006;87:307–314. doi: 10.1016/j.ymgme.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Fuller M, Tucker JN, Lang DL, Dean CJ, Fietz MJ, Meikle PJ, Hopwood JJ. Screening patients referred to a metabolic clinic for lysosomal storage disorders. J Med Genet. 48:422–425. doi: 10.1136/jmg.2010.088096. [DOI] [PubMed] [Google Scholar]
- 48.Umapathysivam K, Whittle AM, Ranieri E, Bindloss C, Ravenscroft EM, van Diggelen OP, et al. Determination of acid alpha-glucosidase protein: evaluation as a screening marker for Pompe disease and other lysosomal storage disorders. Clin Chem. 2000;46:1318–1325. [PubMed] [Google Scholar]
- 49.Umapathysivam K, Hopwood JJ, Meikle PJ. Determination of acid alpha-glucosidase activity in blood spots as a diagnostic test for Pompe disease. Clin Chem. 2001;47:1378–1383. [PubMed] [Google Scholar]
- 50.Fuller M, Loevjoy M, Brooks DA, Harkin ML, Hopwood JJ, Meikle PJ. Immunoquantification of alpha-galactosidase: evaluation for the diagnosis of Fabry disease. Clin Chem. 2004;50:1979–1985. doi: 10.1373/clinchem.2004.037937. [DOI] [PubMed] [Google Scholar]
- 51.Fuller M, Lovejoy M, Hopwood JJ, Meikle PJ. Immunoquantification of beta-glucosidase: diagnosis and prediction of severity in Gaucher disease. Clin Chem. 2005;51:2200–2202. doi: 10.1373/clinchem.2005.053538. [DOI] [PubMed] [Google Scholar]
- 52.Dean CJ, Brockmann MR, Hopwood JJ, Brooks DA, Meikle PJ. Detection of mucopolysaccharidosis type II by measurement of iduronate-2-sulfatase in dried blood spots and plasma samples. Clin Chem. 2006;52:643–649. doi: 10.1373/clinchem.2005.061838. [DOI] [PubMed] [Google Scholar]
- 53.Hein LK, Meikle PJ, Dean CJ, Bockmann M, Auclair D, Hopwood JJ, Brooks DA. Development of an assay for the detection of mucopolysaccharidosis type VI patients using dried blood spots. Clin Chim Acta. 2005;353:67–74. doi: 10.1016/j.cccn.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Whittmann J, Karg E, Turi S, Legnini E, Wittmann G, Iese AK, et al. Newborn screening for lysosomal storage disorders in Hungary. JIMD Rep. 2012;6:117–125. doi: 10.1007/8904_2012_130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burton B, Charrow J, Angle B, Widera B, Waggoner D. A pilot newborn screening program for lysosomal storage disorders in Illinois. Molec Genet Metabol. 2012;105:S23–S24. [Google Scholar]
- 56.Yang CF, Liu HC, Hsu TR, Tsai FC, Chiang SF, Chiang CC, et al. A large scale nationwide newborn screening program for Pompe disease in Taiwan: towards effective diagnosis and treatment. Am J Med Genet A. 2014;164A:54–61. doi: 10.1002/ajmg.a.36197. [DOI] [PubMed] [Google Scholar]
- 57.Matern D, Oglesbee D, Tortorelli S. Newborn screening for lysosomal storage disorders and other neuronopathic conditions. Dev Disabil Res Rev. 2013;17:247–253. doi: 10.1002/ddrr.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An Y, Young SP, Hillman SL, Van Hove JL, Chen YT, Millington DS. Liquid chromatographic assay for a glucose tetrasaccharide, a putative biomarker for the diagnosis of Pompe disease. Anal Biochem. 2000;287:136–143. doi: 10.1006/abio.2000.4838. [DOI] [PubMed] [Google Scholar]
- 59.Manwaring V, Prunty H, Bainbridge K, Burke D, Finnegan N, Franses R, et al. Urinary analysis of glucose tetrasaccharide by HPLC; a useful marker for the investigation of patients with Pompe and other glycogen storage diseases. J Inherit Metabol Dis. 2012;35:311–316. doi: 10.1007/s10545-011-9360-2. [DOI] [PubMed] [Google Scholar]
- 60.Groener JE, Poorthuis BJ, Kuiper S, Hollak CE, Aerts JM. Plasma glucosylceramide and ceramide in type 1 Gaucher disease patients: correlations with disease severity and response to therapeutic intervention. Biochim Biophys Acta. 2008;1781:72–78. doi: 10.1016/j.bbalip.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Rolfs A, Giese AK, Grittner U, Mascher D, Elstein D, Zimran A, et al. Glucosylsphingosine is a highly sensitive and specific biomarker for primary diagnostic and follow-up monitoring in Gaucher disease in a non- Jewish, Caucasian cohort of Gaucher disease patients. PLoS One. 2013;8:e79732. doi: 10.1371/journal.pone.0079732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chuang WL, Pacheco J, Zhang XK, MartinMM,Biski CK, Keutzer JM, et al. Determination of psychosine concentration in dried blood spots from newborns that were identified via newborn screening to be at risk for Krabbe disease. Clin Chim Acta. 2013;18:73–76. doi: 10.1016/j.cca.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 63.Chuang WL, Pacheco J, Cooper S, McGovern MM, Cox GF, Keutzer J, Zhang XK. Lyso-sphingomyelin is elevated in dried blood spots of Niemann-Pick B patients. Molec Genet Metabol. 2014;111:209–211. doi: 10.1016/j.ymgme.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shiada T, Maeda M, et al. Newborn screening and diagnosis of mucopolysaccharidoses. Molec Genet Metabol. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawrence R, Brown E, Lowry F, Dickson PI, Crawford BE, Esko JD. Glycan-based biomarkers for mucopolysaccharidoses. Molec Genet Metabol. 2014;111:73–83. doi: 10.1016/j.ymgme.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Ruijter J, Ru MH, Wagemans T, Ijist L, Lund AM, Orchard PJ, et al. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses types I, II and III. Mol Genetc Metab. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 67.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons G-J, et al. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat Chem Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolfe BJ, Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter Syndrome) Anal Chem. 2011;83:1152–1156. doi: 10.1021/ac102777s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem. 2011;57:128–131. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolfe BJ, Ghomashchi F, Abam CA, Sadilek M, Thompson JR, Scott CR, et al. New substrates and enzyme assays for the detection of mucopolysaccharidosis III (Sanfilippo Syndrome) types A, B, C, and D by tandem mass spectrometry. Bioconjug Chem. 2012;23:557–564. doi: 10.1021/bc200609x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis VI (Maroteaux-Lamy Syndrome) Anal Chem. 2010;82:9587–9591. doi: 10.1021/ac102090v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chennamaneni NK, Kumar AB, Barcenas M, Spacil Z, Scott CR, Turecek F, Scott CR. Improved reagents for newborn screening of mucopolysaccharidosis types I, II, and VI by tandem mass spectrometry. Anal Chem. 86:4508–4514. doi: 10.1021/ac5004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camelier MV, Burin MG, de Mari J, Vieira TA, Marasca G, Giugliana R. Practical and reliable enzyme test for the detection of mucopolysaccharidosis IVA (Morquio Syndrome type A) in dried blood samples. Clin Chim Acta. 2011;412:1805–1808. doi: 10.1016/j.cca.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 74.van Diggelen OP, Zhao H, Kleijer WJ, Janse HC, Poorthuis BJ, van Pelt J, et al. A fluorimetric enzyme assay for the diagnosis of Morquio disease type A (MPS IV A) Clin Chim Acta. 1990;187:131–139. doi: 10.1016/0009-8981(90)90339-t. [DOI] [PubMed] [Google Scholar]
- 75.Hamilton J, Jones I, Srivastava R, Galloway P. A new method for measurement of lysosomal acid lipase in dried blood spots using the inhibitor Lalistat 2. Clin Chim Acta. 2012;413:1207–1210. doi: 10.1016/j.cca.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 76.van Diggelen OP, Keulemans JL, Winchester B, Hofman IL, Vanhanen SL, Santavuori P, Voznyi YV. A rapid fluorogenic palmitoyl-protein thioesterase assay: preand postnatal diagnosis of INCL. Mol Genet Metab. 1999;66:240–244. doi: 10.1006/mgme.1999.2809. [DOI] [PubMed] [Google Scholar]
- 77.Barcenas M, Xue C, Marushchak-Vlaskin T, Scott CR, Gelb MH, Turecek F. Tandem mass spectrometry assays of palmitoyl protein thioesterase 1 and tripeptidyl peptidase activity in dried blood spots for the detection of neuronal ceroid lipofuscinoses in newborns. Anal Chem. 2014;86:7962–7968. doi: 10.1021/ac501994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flitterer B, Hall P, Antonishyn N, Desikan R, Gelb MH, Lehotay D. Incidence and carrier frequency of Sandhoff disease in Saskatchewan determined using a novel substrate with detection by tandem mass spectrometry and molecular genetic analysis. Molec Genet Metabol. 2014;111:382–389. doi: 10.1016/j.ymgme.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gelb MH, Barcenas M, Chennamaneni NK, Kumar A, Masi S. Multiplex mass spectrometric newborn screening and diagnosis of lysosomal storage disease; Oral session presented at: 2014 Newborn Screening and Genetic Testing Symposium; Oct 27–30; Anaheim, CA. 2014. [Accessed December 2014]. http://www.aphl.org/conferences/2014_newborn_screening_and_genetic_testing_symposium/pages/default.aspx. [Google Scholar]
- 80.Liao HC, Chiang CC, Niu DM, Wang CH, Kao SM, Tsai FJ, et al. Detecting multiple lysosomal storage diseases by tandem mass spectrometry: a national newborn screening program in Taiwan. Clin Chim Acta. 2014;431:80–86. doi: 10.1016/j.cca.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 81.Basheeruddin K, Shao R, Gelb MH, Gardley P. A high-throughput multiplex newborn screening assay for six lysosomal storage disorders (LSDs) using dried blood spots and UPLC-tandem mass spectrometry [Abstract]; Poster session presented at: 2014 Newborn Screening and Genetic Testing Symposium; Oct 27–30; Anaheim, CA. 2014. [Accessed December 2014]. http://www.aphl.org/conferences/2014_newborn_screening_and_genetic_testing_symposium/pages/default.aspx. [Google Scholar]
- 82.Hopkins P. Missouri’s experience with full population pilot screening for Pompe, Gaucher, Fabry and Hurler disorders using digital microfluidics methodology; Oral session presented at: 2014 Newborn Screening and Genetic Testing Symposium; Oct 27–30; Anaheim, CA. 2014. [Accessed December 2014]. http://www.aphl.org/conferences/2014_newborn_screening_and_genetic_testing_symposium/pages/default.aspx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.