Abstract
This review provides a short overview of the most significant biologically oriented theories of human personality. Personality concepts of Eysenck, Gray and McNaughton, Cloninger and Panksepp will be introduced and the focal evidence for the heritability of personality will be summarized. In this context, a synopsis of a large number of COMT genetic association studies (with a focus on the COMT Val158Met polymorphism) in the framework of the introduced biologically oriented personality theories will be given. In line with the theory of a continuum model between healthy anxious behavior and related psychopathological behavior, the role of the COMT gene in anxiety disorders will be discussed. A final outlook considers new research strategies such as genetic imaging and epigenetics for a better understanding of human personality.
Keywords: COMT Val158Met, personality, genetic imaging, anxiety, anxiety disorders, catechol-o-methyltransferase, dopamine
1. WHAT IS PERSONALITY?
Many definitions for personality exist in the literature and an important aspect of most definitions refers to the “characteristic patterns of behavior, thoughts and feelings” of a person over time [1]. A traditional branch of psychology called personality psychology is mainly concerned with explaining individual differences to better understand and even predict human behavior. Two major concepts of personality psychology are known as traits and states [2]. Whereas traits attempt to grasp the stable characteristics of a person over time, states more aptly describe a person’s experience at a given moment [3]. If one sets out to assess trait anxiety by questionnaire, one would administer an item such as: “In general, I am an anxious person”. In contrast, the measurement of the anxious state could be achieved by asking the item: “Right now, I feel anxious”. Clearly, the concepts of traits and states are not easy to distinguish from each other, because a trait anxious human is more likely to also feel anxious at any given point in time. In general, there is still a debate on how traits and states relate to each other and how their influence on personality should be weighted [2].
In the end, individual differences in personality mirror dispositions in how humans react to their environment and deal with challenges and hardships in their lives. This makes personality an important research area, because extreme forms of personality (such as being extremely anxious), are related to the development of psychopathological disorders [4, 5]. Mental disorders such as generalized anxiety or panic disorders bear high costs for society and great pain for the patients afflicted. Successful and rapid treatment is one of society’s great challenges. Biologically oriented personality psychology represents an important part in this endeavor.
2. BIOLOGICALLY ORIENTED PERSONALITY PSYCHOLOGISTS - FROM EYSENCK TO PANKSEPP
2.1. Eysenck’s Personality Theory
There is a long-standing tradition in personality psychology to attempt an explanation of human personality by examining the human brain. One of the founders of biologically oriented psychology - the famous Hans-Jürgen Eysenck - put forth the idea that individual differences in neuronal circuits of the brain must be responsible for differences in human personality. Eysenck [6] postulated that major processes of the human brain can be explained by excitatory and inhibitory mechanisms. The excitatory mechanisms help to keep the individual active and alert, while the inhibitory mechanisms downregulate brain activity and cause the person to be lethargic and inactive [7]. The ascending reticular activation system (ARAS), arising from the brain stem with projections to the hypothalamus, thalamus and several areas of the cortex, aims to keep the excitatory and inhibitory mechanisms in equilibrium. Differences in the neuroanatomy and functionality of the ARAS are associated with Eysenck’s personality dimensions of extraversion and introversion. The personality trait extraversion is linked to positive emotionality and describes persons who are outgoing, fun loving, and who love to socialize. Introverts prefer to stay alone and seek calmer activities such as reading instead of attending a party or participating in extreme sports like skydiving. Extraversion and introversion compose a bipolar unidimensional construct wherein high scorers on extraversion are simultaneously low scorers on introversion. The same is true for Eysenck's other personality dimension called neuroticism. Low scorers on this personality trait are able to manage their emotions in complicated life circumstances and are characterized by a stable, robust personality. In contrast, emotionally unstable persons (called neurotic) are shy, moody, anxious and have problems managing their emotions. Several studies have demonstrated that neuroticism represents a major risk factor for psychopathological disorders such as depression [4] and even suicide [8]. Therefore, the investigation of the biological underpinnings of neuroticism represents a huge focus in personality research. As already stated above, individual differences in the dimension extraversion/introversion are characterized by individual differences in the purported neuronal circuitry of the ARAS modulating excitatory and inhibitory mechanisms of the brain, which was further expanded upon by Eysenck’s arousal theory. Individual differences in the personality dimension neuroticism are hypothesized to be anchored in individual differences in the arousability of the limbic system of the human brain. High neurotics have been hypothesized to react with higher limbic activity to emotionally salient stimuli, whereas emotionally stable persons are hypothesized to show lower activity in this brain region. Although the ideas of Eysenck are exciting and introduced biology into the field of personality research, they are still a matter of debate and have found only partial support (e.g. [9–11]). In an attempt to measure Eysenck’s personality dimensions by self-report, researchers usually distribute Eysenck’s personality questionnaire [12].
2.2. Gray’s Personality Theory
Another important biologically oriented personality psychologist was Jeffrey Gray. Gray began as a student of Eysenck and based much of his work on the thoughts and ideas of Eysenck’s theories. One of Gray’s main research interests was the emotion of anxiety in mammals. In the early 1970’s he founded his now famous reinforcement sensitivity theory (RST), with which he tried to explain individual differences in human behavior by individual differences in the basic neuronal circuitry of the brain [13]. By applying lesion and electrical stimulation studies in combination with pharmacological challenges in rodent brains, he was able to identify two neuronal circuits that are relevant for basic approach and avoidance behaviors. Based on thirty years of work on the RST, Gray & McNaughton [14] published a revised version of their theory. This theory is summarized as follows: Approach behavior (such as exploration of the environment) of mammals can be triggered by initiation of the behavioral activation system (BAS) whereas orienting behavior is initiated in situations of high uncertainty (reflecting the emotion of anxiety) by the behavioral inhibition system (BIS). The neuroanatomical correlate of the BAS has been hypothesized to be the mesolimbic dopaminergic system, which is activated when rodents explore the environment in search for food or a mating partner. The BIS system is triggered by uncertain situations, followed by careful approach behavior to a potentially dangerous situation. This careful approach behavior is necessary in order to obtain more information on the level of danger present in the given situation. If the danger is real, the fight flight freezing system (FFFS) is activated (reflecting the emotion of fear). If there is no danger present, the initially inhibited exploratory behavior can be resumed. The combined firing of neurons in the hippocampus and the amygdala comprise the core of BIS activation [14]. FFFS activation is characterized by brain stem activity projecting to diverse regions of the limbic brain. If personality researchers aim to measure individual differences in the BIS and BAS system by self-report data, they usually choose the Carver and White BIS/BAS scales [15]. We would like to point out the important fact that this scale is based on the ideas of the older RST. Several workgroups have tried to establish a questionnaire for the newer, revised RST (e.g. [16]). Gray and McNaughton's theory probably represents the most significant and elaborate theory on the neuropsychology of anxiety and fear. A meta-analysis [17] revealed empirical support for the revised version of the RST. Empirical evidence for both Eysenck’s and Gray’s personality theory (the old RST) has been reviewed by Matthews and Gilliland [18].
2.3. Cloninger’s Personality Theory
US psychiatrist Robert Claude Cloninger developed another important biologically oriented personality theory. Cloninger et al. [19] based their conceptualization of human personality on data stemming from family studies, psychometric data, and biological variables (data from pharmacological challenges and neuroanatomy). Cloninger put forward the idea that personality can be described as a combination of temperament and character traits. Temperaments are anchored in phylogenetically older parts of the brain, develop early in life, and as a consequence should show higher heritability estimates. Character traits are shaped more by the environment and develop in late adolescence and should be associated with lower heritability estimates. The temperament traits of Cloninger's theory include novelty seeking, harm avoidance, reward dependence, and persistence. The character traits include cooperativeness, self-directedness and self-transcendence. As with the theories by Eysenck [6] and Gray [13]/Gray and McNaughton [14], Cloninger postulated biological correlates for his temperament traits, enabling the search for empirical evidence to support of his theory. Cloninger hypothesized that low levels of the neurotransmitter dopamine are associated with higher novelty seeking scores. Novelty seeking describes humans who are curious, impulsive, and show lower levels of discipline. Harm avoidance is related to trait anxiety. Anxious people in Cloninger's theory ruminate about future outcomes, show fear of uncertainty, and are shy. High scores on this temperament dimension have been hypothesized to be related to higher levels of serotonin. Novelty seeking is more reflective of the positive side of emotionality, whereas harm avoidance tends to reflect the negative side of emotionality. This is of course a simplified view, because without emotions such as anxiety and fear, evolutionary development towards homo sapiens would have been impossible. The remaining temperaments and characters are explained in detail in the paper by Cloninger et al. [19]. A good review can also be found in Kose [20]. The most important self-report measure for Cloninger’s temperament and character traits is the temperament and character inventory (TCI) [19]. Some studies utilize the tridimensional personality questionnaire (TPQ), measuring only the before-mentioned temperament traits. Cloninger’s theory has had a strong influence on research in the field. His ideas are of great importance, although some of them have not found empirical support. For example, twin studies have demonstrated that character traits such as self-directedness show high heritability estimates, comparable to heritability estimates of temperament traits [21, 22]. Moreover, genetic association studies have shown that diverse neurotransmitter systems (not limited to one system per temperament) are involved in the foundation of temperaments and character [23].
2.4. Panksepp’s Personality Theory
A rather new but very promising biologically oriented personality theory was proposed by Panksepp [24] and Davis et al. [25]. Jaak Panksepp can likely be considered the founder of the research field of “affective neuroscience”. Through application of electrical stimulation to the mammalian brain, he observed six emotional circuits which show resemblance across species. By electrically stimulating distinct mammalian neuronal circuits, Panksepp could elicit “seek” and “withdrawal” behaviors (see similarities with Gray 2.2.). All in all, Panksepp demonstrated the existence of emotional circuits for SEEK, PLAY, FEAR, SADNESS, ANGER and CARE1 behaviors. In contrast to the classical lexical approaches to personality (such as by Costa and McCrae [26]) and very much in line with the before-mentioned biologically oriented personality psychologists, Panksepp attempts to explain human personality by examining individual differences in neuronal emotional circuits. Together with Davis et al. [25], Panksepp developed an affective neuroscience personality scale (ANPS), which should reflect individual differences in his six proposed emotional circuits of the mammalian brain. A recent review [27] presented evidence from several studies that validate the ANPS with biological data from molecular genetics and brain imaging (e.g. [28, 29]).
2.5. Assessment of Biologically-Oriented Personality Theories
Although the before-mentioned biologically oriented personality theories all have particular specifications and limitations, they share the idea that individual differences in the human brain are responsible for individual differences in human personality. Moreover, it becomes clear that these theories describe the emotional aspect of personality. Of course cognition and intelligence represent another important aspect of personality [30], but this will not be discussed in the present review article. The focus of this review will lie on the molecular genetics (in particular the gene coding for catechol-O-methyltransferase) related to the emotional aspects of personality. In terms of the studies reviewed later in this article, we would like to provide the reader with an idea regarding the overlap between questionnaires measuring the different personality constructs of the various personality theories described to this point. In Table 1, we included correlations of the most important questionnaire measures from our own personality data bank consisting of more than 1500 participants. Table 1 demonstrates that the personality dimensions of interest show considerable overlap. In addition, several personality traits related to positive and negative emotionality are negatively correlated. This supports the idea that a considerable overlap of human personality traits is to be expected not only on a statistical level but also on a neuronal level. In Table 2 we summarize the most important concepts of the biologically oriented personality psychology theories.
Table 1.
Correlations Between the Most Important Personality Measures of this Review Study
| NS (TCI) |
HA (TCI) |
N (NEO-FFI) |
E (NEO-FFI) |
N (EPQ-R) |
E (EPQ-R) |
||
|---|---|---|---|---|---|---|---|
| NS (TCI) | correlation | 1 | −0,34 | −0,13 | 0,37 | −0,09 | 0,51 |
| p-value | p < .001 | p < .001 | p < .001 | p < .001 | p < .001 | ||
| N | 1754 | 1754 | 1555 | 1555 | 1551 | 1551 | |
| HA (TCI) | correlation | 1,00 | 0,72 | −0,52 | 0,66 | −0,54 | |
| p-value | p < .001 | p < .001 | p < .001 | p < .001 | |||
| N | 1754 | 1555 | 1555 | 1551 | 1551 | ||
| N (NEO-FFI) | correlation | 1,00 | −0,41 | 0,79 | −0,32 | ||
| p-value | p < .001 | p < .001 | p < .001 | ||||
| N | 1565 | 1565 | 1549 | 1549 | |||
| E (NEO-FFI) | correlation | 1,00 | −0,32 | 0,73 | |||
| p-value | p < .001 | p < .001 | |||||
| N | 1565 | 1549 | 1549 | ||||
| N (EPQ-R) | correlation | 1,00 | −0,23 | ||||
| p-value | p < .001 | ||||||
| N | 1557 | 1557 | |||||
| E (EPQ-R) | correlation | 1 | |||||
| p-value | |||||||
| N | 1557 | ||||||
The BIS/BAS dimensions and Panksepp’s dimensions are discussed in the review. Because these measures have been used less often in the COMT literature, we only show correlations for the presented dimensions shown above.
NS = novelty seeking, HA = harm avoidance, N = neuroticism, E = extraversion.
Table 2.
Biologically Oriented Personality Psychology Theories: A Short Overview
| Personality Dimension | Biological Correlate | Questionnaire Measure | |
|---|---|---|---|
| Eysenck | Neuroticism/Emotional Stability | Limbic System | Eysenck’s Personality Questionnaire |
| Extraversion/Introversion | Ascending reticular activation system | ||
| Gray & McNaughton | BIS | Hippocampus & Amygdala | Carver & White’s BIS/BAS |
| BAS | Mesolimbic dopaminergic pathways | ||
| FFFS | Brain stem areas, amygdala, etc. | ||
| Cloninger | Novelty Seeking | Low dopamine | Temperament and Character Inventory |
| Harm Avoidance | High Serotonin | ||
| Reward Dependence | Low Norepipherine | ||
| Panksepp | SEEK, FEAR, PLAY, ANGER, CARE, SADNESS | See Davis et al. (2003) and Panksepp (1998) | Affective Neuroscience Personality Scales |
Another important questionnaire used in the COMT literature is the NEO-Personality-Inventory (NEO-PI-R) and its short form (NEO-FFI) measuring the BIG5 of personality. The BIG5 have been derived by lexical approach and describe the dimensions neuroticism, extraversion, openness to experience, conscientiousness and agreeableness. As it is has no theoretical biological basis, it is not further discussed in this review.
EPQ: Eysenck's Personality Questionnaire (EPQ); TCI: Temperament and Character Inventory (TCI).
2.6. Measuring Personality with a Questionnaire?
There are many methods of measuring individual differences in human personality. Although we are of the opinion that questionnaires represent an important part in the assessment of human personality, we think it is of major importance to add (if possible) behavioral measures and recordings of the peripheral or central nervous system in order to obtain a deeper understanding of the biological underpinnings of personality. As will be explained later in this text, large sample sizes are required to identify the relatively small effects of a genetic variant on a certain personality trait. When hundreds of participants are needed for molecular genetic association studies, it is often too costly to collect further behavioral measures of personality - and so one must forgo measures that might better characterize a certain personality endophenotype when compared with a questionnaire. This explains why genetic association studies often measure personality “only” via self-report.
3. IS HUMAN PERSONALITY HERITABLE?
Investigation of the question whether nature or nurture influences human personality had long been a main focus of personality psychology. However, this heated debate is over because quantitative genetics demonstrated with twin designs that both genetics and the environment contribute to the genesis of personality (e.g. [31]). Given this understanding, it is now of higher importance to investigate the interaction of genetics and environment - this concept will be discussed in more detail later on in the text.
One of the simplest study designs for the investigation of heritability issues is the study of monozygotic (MZ) twins (sharing 100% of their genetic make-up) who have been reared apart. If researchers aim to answer the question whether preference for free-climbing is influenced by genetics, they could ask adult twins who have been reared separately about their sport activities. If a high preference for free-climbing is found in both monozygotic twins, a genetic influence is very likely. However, the prerequisite of this design that the twins have grown up in completely different environments is often not warranted. Therefore, twin designs comparing MZ and dizygotic (DZ) twins are the golden standard in quantitative genetics. Due to the fact that DZ twins share only 50% of their genes, a higher concordance with respect to a certain phenotype in monozygotic twins is an evidence for heritability. Twin studies using the MZ-DZ design revealed high heritability estimates for personality traits. Riemann et al. [32] showed heritability estimates for the BIG 52 of personality self-report data ranging from 0.42 to 0.56 and even higher measures when basing these estimates on peer report or a combination of self-report and peer-report. Investigation of the molecular genetics of human personality in healthy humans also sheds light on the biological basis of psychopathological disorders as is the case with a recent study [4] which demonstrated that a large set of genes influencing neuroticism also play an important part in the development of depression.
3.1. How Many Genes Influence Human Personality?
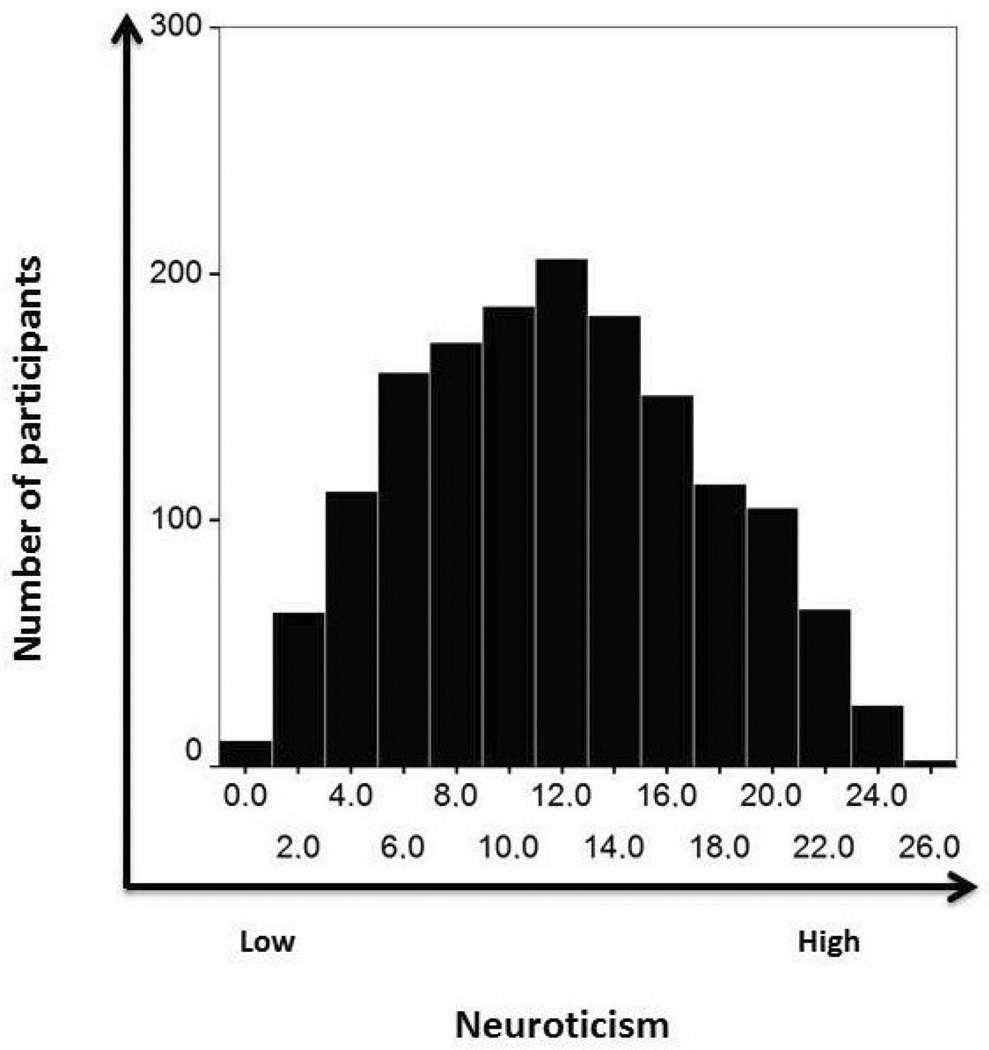
This question cannot be answered with precision due to the complexity and interactive nature of the relationship between molecular genetics and environment in the development of human personality. Nevertheless, it is possible to provide a rough approximation in an attempt to answer to this important question. Fig. (1) depicts individual differences in Eysenck’s personality dimension neuroticism assessed from a large sample consisting of more than 1500 participants collected in our laboratory.
Fig. (1).
A normal distribution of neuroticism scores from N = 1557 participants completing Eysenck’s personality questionnaire (mean = 11.4 (SD = 5.57)). Due to the normal distribution of the personality data, a large number of genetic variants (not just one) must build the molecular genetic basis of personality.
As can be clearly seen, the personality data in Fig. (1) are normally distributed. If a single genetic variant would be responsible for the total variation in trait anxiety, we would not expect the normal distribution seen in Fig. (1), but we would rather see two graphs with either high or low anxiety scores. Given that genes and environment both influence personality with a contribution of about 0.50 each (again, this is only a rough estimate3) and one genetic variant explains about 1% of the observed variance in personality (e.g. [29, 33]), it would follow that 50 genetic variants are required to explain 50% of the phenotypic variance (i.e. 100% of the genetic variance). However, this answer cannot ultimately be correct due to several lines of argument. First of all, this estimate considers only additive genetic effects. In addition, certain genetic variants do not seem to have an influence on personality or related psychopathological disorders without the occurrence of adverse environmental influences; for example, effects on a phenotype such as depression are only observed when a given genetic risk variant meets a negative environmental influence such as child abuse [34]. In sum, personality (like many other phenotypes) represents a quantitative trait, with a continuum of scores for each personality dimension in the population [35]. This has important consequences and implications for the understanding of the molecular genetics of personality.
3.2. Where in the Genome does the Search for Personality Genes Begin?
Given the fact that a large number of genes underlie the foundation of human personality, one might ask where to start the search for the "personality genes" on the extensive genome. There are two strategies that one can follow for the identification of candidate genes of importance to human personality. First, it is possible to conduct genome wide association studies (GWAS), where up to one million SNPs are simultaneously genotyped by means of a micro-array technique (e.g. [36]). Second, it is possible to derive hypotheses from pharmacological studies or animal studies using gene-knock-in [37, 38] or gene-knock-out models [39]. The advantage of the explorative GWAS approach can be found in the identification of new gene candidates linked to personality without the need for a theoretical background. In subsequent steps, the gene candidates identified through GWAS need to be tested with additional experiments to further describe their influence on human behavior. It is also of importance to demonstrate the functionality of these candidate gene variants in order to learn more about their influence on the biochemical pathways of the human brain. If a single nucleotide polymorphism (SNP) is located in an exonic region (exons constitute the blueprint for constructing the gene product) and leads to an amino acid exchange, then functionality is very likely. SNPs could also be found in promoter regions upstream of the gene and so could affect mRNA expression of the gene; SNPs could also be found in regulatory regions such as the 3’UTR of a gene and could affect the binding of miRNAs and so can affect the amount of mRNA/protein. Also, miRNA genes are sometimes located within intronic and intergenic regions and SNPs could influence their expression, which in turn affects the expression of a number of genes. Also, SNPs in intronic regions can lead to alternative splice variants. The discrepancy between the countless number of SNPs that have been associated with certain phenotypes and the small number of SNPs for which functionality is proven is striking. This phenomenon can be explained by the fact that cloning and proteomics studies that demonstrate the functionality of a given polymorphism are time and cost consuming, whereas sole genotyping procedures have become industrialized and cheaper to conduct. Other problems with genomewide polymorphism scans include limitations in finding genetic interaction effects and the need for a conservative correction for multiple testing. Due to these limitations, genomewide scans fail to identify a number of genetic variants that might impact human personality. As previously mentioned, another approach for discovery of novel candidate personality genes is the study of animal literature and human pharmacological studies (e.g. [29]). If a given neurotransmitter is shown to play an important role in a certain behavior, the gene encoding this neurotransmitter becomes an interesting target for a genetic association study. The gene becomes even more interesting if a polymorphism can be observed in an exonic region making it more likely yet that functionality of the polymorphism can be shown. As a consequence, it is possible not only to associate a genetic variant with personality, but to explain its impact on the biochemical pathways in the human brain. Both approaches are of importance and have already yielded new insights into the biological underpinnings of human personality.
4. DOPAMINE AND PERSONALITY
The neurotransmitter dopamine is one of the key molecular players influencing human behavior. In a seminal review, Wise [40] demonstrated that dopamine plays an important role in learning and motivation. In the context of personality, dopamine has been considered important for a long time. Although theories are somewhat contradictory with respect to the levels of dopamine (low or high) linked to positive emotionality [19, 41, 42], it has become clear that dopamine plays an essential part in most of these theories. Another controversy arises from the debate on the orthogonality of several personality traits, as several dimensions measuring positive emotionality show significant inverse correlation with personality traits linked to negative emotionality. Therefore, dopamine could not only play a role in individual differences in positive but also in negative emotionality. Dopamine might represent two sides of the same coin called personality [23, 43].
4.1. The Regulation of Dopaminergic Neurotransmission
Dopamine levels in the neuronal synaptic cleft can be modulated in diverse ways. Dopaminergic pathways are influenced by the rate of dopamine synthesis, depending on the availability of the amino acid tyrosine and enzymes such as tyrosine-hydroxylase and dopa-decarboxylase, which work to convert tyrosine to DOPA and then into the neurotransmitter dopamine [44, 45]. Dopamine can be catabolized and inactivated by the enzymes monoamine oxidase-B (MAO-B; [46]) or COMT [47]. Moreover, five dopamine receptors have been observed (D1–D5), which also regulate dopaminergic neurotransmission. Activation of D1 or D5 receptors leads to an increase of cAMP in the neuron, whereas activation of the D2, D3 or D4 receptors is associated with a down-regulation of cAMP [48]. Another important structure is the dopamine transporter (DAT) which regulates the re-uptake of dopamine in the presynapse. Taking this simplified view of dopaminergic neurotransmission into account (without even considering regulatory processes on dopamine by other neurotransmitters), several genes encoding the above-mentioned elements of the dopaminergic system become of great interest for genetic association studies with human personality. An informative review on the dopaminergic pathways described here can be found in Opmeer et al. [49].
4.2. The COMT Gene
The gene coding for the enzyme COMT is located on the q11 band of human chromosome 22. The COMT gene contains six exons and spans 28 kb [50]. Codon 158 of the COMT gene contains the Val158Met polymorphism (rs4680), which is one of the most investigated polymorphisms in the human neurosciences. The significant research efforts with respect to this SNP can be explained by the fact that it has been shown to have tremendous impact on dopaminergic neurotransmission. Carriers of the homozygous Met/Met variant catabolize three to four times less dopamine than carriers of the homozygous Val/Val variant [51]. The heterozygote Val/Met carriers fall in between and the alleles are co-dominant. Differences in enzyme activity between the ancestral Val and the younger Met allele can be explained by a higher thermostability at 37 °Celsius in Val compared to Met allele carriers [51]. The COMT enzyme has a strong impact on the prefrontal cortex (PFC) due to a paucity of dopamine transporter (DAT) in the PFC [52]. The resulting increase in the amount of dopamine available in the synaptic cleft provides increased substrate to be catabolized by COMT. In contrast, abundant DAT can be observed in several subcortical areas, and thus dopamine levels are largely regulated through presynaptic reuptake by DAT in striatal regions. Two isoforms of COMT (a membrane bound and a soluble form) are present in the human body. The membrane-bound form has been of particular importance to the human neurosciences while the soluble form has been mainly observed in the kidney and the liver [53].
5. THE COMT VAL158MET POLYMORPHISM AND PERSONALITY
The significant effect of the COMT Val158Met SNP on dopaminergic neurotransmission makes it a promising target for personality psychologists. It is nearly impossible to provide a complete overview of all COMT Val158Met studies to date because, at the time of writing this review, nearly 400 papers were found in Pubmed.gov investigating this prominent polymorphism. It becomes more manageable, but still a challenging task upon narrowing the review to COMT Val158Met personality studies. Using the keywords "COMT Val158Met" and "personality" as search criteria, 56 articles were returned in Pubmed. In addition to the above search strategy, we also searched "Google Scholar" with the same keywords. Given the abundant literature in the field, we decided to take the following approach to reviewing the COMT literature: We focused on the papers dealing with COMT in the context of the above mentioned biologically oriented personality theories, because we believe that these papers have a strong theoretical background. This of course does not suggest that the other studies are not properly conducted, but rather that different approaches to personality can lead to different results. Given the issues in replicating findings from genetic association studies, we believe that this approach is legitimate. Moreover, we’d like to begin with a review of COMT papers that used the same questionnaires (ergo have the same theoretical backgrounds). The correlations presented in Table 1 support this research strategy since the observed correlations range from moderate to strong between the different personality measures, and they are far from being perfectly correlated. In addition, we acknowledge the fact that genetic effects are small and can target both the shared and non-shared variance of the measured personality constructs. Further influences on the results of genetic association studies stem from different sample sizes of the conducted studies. Smaller sample sizes bear larger risks of false positive findings. Since allele frequencies can differ depending on the ethnicity, another factor influencing the results of molecular genetic studies is the ethnic background of the samples investigated. Both the Val and Met allele of the COMT SNP have a population frequency of 0.50 in the Caucasian population. In Asian countries such as Japan, the evolutionarily younger Met allele only occurs with frequencies about 0.30. An interesting research direction will explore whether the differences in allele frequencies observed across ethnicities have an impact on personality traits in the given populations in cross-cultural designs. A study by Terracciano et al. [54] began to investigate this question by examining a prominent SNP in the gene encoding BDNF.
5.1. COMT Val158Met Polymorphism and Cloninger’s TCI
Since most studies in the field administered Cloninger’s TCI (or its pre-version TPQ) to measure temperament and character traits, a review of COMT associations with these questionnaires will be presented first. Most of these studies focused on the traits novelty seeking and harm avoidance. Although the samples investigated differ markedly in their size and ethnic background, one can observe a slight overweighting of studies associating the Met allele or the Met/Met genotype with higher harm avoidance [55–57] and the Val/Val genotype with novelty seeking [58, 59]. This summary of the data is of course oversimplified, because the COMT-personality associations are influenced by several additional variables. According to the findings of Chen et al. [60], the association between COMT Val158Met and personality is strongly influenced by sex. This study showed that male Han Chinese carrying the Val/Val variant showed the highest harm avoidance scores in contrast to the abovementioned studies. No effect could be observed for females. Although novelty seeking and harm avoidance are negatively correlated (see Table 1), only one COMT-TCI study demonstrated an effect of COMT Val158Met on the shared variance of novelty seeking and harm avoidance. This effect is seen in the study by Chen et al. [60], who provide evidence for the yin and yang hypothesis of COMT in the context of personality. Males carrying the Val/Val genotype simultaneously showed both elevated harm avoidance and lower novelty seeking. This kind of effect could not be observed in the studies which investigated associations between the Val/Val genotype and novelty seeking [58, 59].
Although the Met allele-negative emotionality link is generally supported by the data summarized in Table 3, some additional inconsistencies merit discussion. The study by Hosak et al. [61] reported higher novelty seeking scores in carriers of the Met/Met genotype, in line with Chen et al. [60]. The inconsistency of the results from the Hosak et al. [61] study when compared to results from the remaining studies could be explained by the study’s small sample size (n = 37) and/or the type of sample investigated - namely methamphetamine dependent patients. Most of the other reported studies investigated healthy participants. Nevertheless, the Val allele was associated with higher negative emotionality in healthy participants [62]. No association between COMT and personality could be found by Ishii et al. [63], although the sample size was sufficient (N = 478). Three studies are not discussed further, because they deal either with the character trait cooperativeness [64, 65] or lack comparability (an fMRI experiment by Drabant et al. [66]).
Table 3.
Genetic Association Studies Between COMT Val158Met and the TCI and TPQ
| Authors | Sample Size | Ethnic Background and Type of Sample |
Personality Measure | Results |
|---|---|---|---|---|
| Chen et al. (2011) | 556 (250 m/306 f) | Healthy Han Chinese | TCI-rev. (aswell BIS BAS/ Beck Depression/Beck Anxiety) | Males with Val/Val genotype show higher negative emotionality than males with Met/Met genotype. Furthermore, the opposite effect can be shown for positive emotionality. No effect was observed for females. |
| Reuter et al. (2011) | 101 (26 m/74 f) | Healthy Caucasians | TCI | No association with cooperativeness could be observed, but carriers of the Val allele donate more money. |
| Mikołajczyk et al. (2010) | 211 (211 f) | Eating disorder patients (103) and control persons (108) | TCI | Carriers of the Val/Val genotype show lowest cooperation scores. |
| Enoch et al. (2008) | 249 (108 m/141 f) | Caucasians (233) and African Amercians (16) Healthy participants and participants with anxiety disorder |
TPQ, (aswell EPQ-R) | Met allele is associated with higher harm avoidance (combination study with BDNF Val66Met). |
| Hashimoto et al. (2007) | 139 (47 m/92 f) | Healthy Japanese | TCI | Met allele is associated with harm avoidance (highest scores in Met/Met carriers). |
| Light et al. (2007) | Two samples (195/177) | Depressed Patients (mainly Caucasians) | TCI | No associations with COMT on novelty seeking or harm avoidance could be observed. |
| Ishii et al. (2007) | 478 (246 m/232 f) | Healthy Japanese | TCI | No association with any of the scales. |
| Hosak et al. (2006) | 37 (27 m/10 f) | Czech methamphetamine dependent patients | TCI | Met allele carriers show highest novelty seeking scores. |
| Drabant et al. (2006)* | 101 (51 m/50 f) | Healthy Caucasians | TPQ (only available in N = 79) | Negative correlation between novelty seeking scores and functional connectivity between amygdala and OFC in Met/Met carriers can be observed. No association observed for Val/Val carriers. |
| Kim et al. (2006) | 286 (138 m/148 f) | Healthy Korean subjects | TCI | Val allele is associated with highest harm avoidance scores in females (Val/Val carriers have the highest scores). |
| Reuter et al. (2005) | 363 (101 m/262 f) | Healthy Caucasian subjects | TCI (aswell NEO-FFI) | Carriers of the Val/Val genotype show highest novelty seeking (only NS1 "exploratory excitability") scores. |
| Tsai et al. (2004) | 120 (120f) | Healthy Chinese | TPQ | Female Val/Val carrier show highest novelty seeking and carriers of at least one Val allele show the highest reward dependence scores. |
| Enoch et al. (2003) | 401 (160 m/241 f) | Caucasians (149) and Native Americans (252) | TPQ | Caucasian carriers of the Met/Met genotype show highest scores in the harm avoidance subscales: "anticipatory worry" (HA1) and "fear of uncertainty" (HA2). Native Indian Americans show same effect only for HA2. |
This study is a brain imaging study and therefore not directly comparable with the remaining questionnaire studies.
5.2. COMT Val158Met Polymorphism and Eysenck’s
Extraversion/Neuroticism
Both the EPQ-R and the NEO-PI-R (and its short version NEO-FFI) are frequently used in "personality-genetic association studies" with a focus on the COMT Val158Met polymorphism. Due to the high correlation between the extraversion and neuroticism dimensions of both questionnaires (ranging from 0.73 for extraversion to 0.79 for neuroticism, see Table 1), we discuss the results of these studies in one section. The same pattern is observed for the association presented for COMT Val158Met and Cloninger’s TCI. The studies show that either the Met allele or Met/Met genotype is associated with elevated neuroticism scores [56] and higher extraversion scores are observed for the Val/Val genotype [58, 67]. As with the TCI questionnaires, several null findings can be observed here as well. We note that some of the studies discussed under 5.1 and 5.2 appear in both sections because both the TCI/TPQ and EPQ-R/NEOPI- R questionnaires were applied (Table 4).
Table 4.
Genetic Association Studies Between COMT Val158Met and the NEO-PI-R, NEO-FFI and EPQ-R
| Authors | Sample Size | Ethnic background and Type of Sample |
Personality Measure |
Results |
|---|---|---|---|---|
| Aoki et al. (2011) | 143 (40 m/103 f) | Healthy Japanese | NEO-FFI | No effect of COMT with extraversion or neuroticism, but among others with agreeableness (Val/Val lowest scores). |
| Hamidovic et al. (2010) | 161 (89 m/72 f) | Healthy Caucasians | MPQ* | Carriers of the Val/Val genotype show highest extraversion scores. |
| Wray et al. (2008) | 2045 | Healthy Australians and depressed/anxious patients | EPQ-R (only n = 1968 with EPQ-R) | No association with COMT Val158Met and the EPQ-R can be observed. |
| Sheldrick et al. (2008) | 522 (267 m/255 f) | Healthy Caucasians | NEO-FFI (also SPQ-B) | No effect of COMT on the NEO-FFI dimensions. |
| Enoch et al. (2008) | 249 (108 m/141 f) | Caucasians (233) and African Amercians (16) Healthy participants and participants with anxiety disorder |
TPQ, EPQ-R | Met allele is associated with higher neuroticism (combination study with BDNF Val66Met). |
| Hoth et al. (2006) | 486 (53% male; 258 m/228 f) | Healthy Caucasians (83.1%, rest is mixed) | NEO-FFI | Carriers of the Met/Met show significantly lower extraversion compared to carriers of the Val/Val genotype. Moreover carriers of the Met/Met genotype show a trend towards highest neuroticism scores. |
| Tochigi et al. (2006) | 256 (64 m/192 f) | Healthy Japanese subjects | NEO-PI-R | No effect of COMT on the NEO-PI-R dimensions. |
| Reuter et al. (2005) | 363 (101 m/262 f) | Healthy Caucasian subjects | NEO-FFI (aswell TCI) | Carriers of the Val/Val genotype show highest extraversion scores. |
| Stein et al. (2005) | 497 (154 m/343 f) | Mixed ethnic participants | NEO-PI-R | Female carriers of the Met/Met genotype show highest neuroticism and lowest extraversion scores. |
| Olsson et al. (2005) | 962 | Australian panel study | NEO-PI-R | No effect of COMT SNP on neuroticism, but Met allele is associated with higher episodic anxiety in females. |
| Eley et al. (2003) | 119 (41 m/78 f) | Extreme group design (57 neuroticism high scorers vs. 62 neuroticism low scores) | NEO-FFI (self & peer-report) | Met allele occurs with higher frequency in neurotic females. |
As the Multidimensional Personality Questionnaire (MPQ) also measures extraversion, this study is also included in this table.
5.3. COMT Val158Met and Gray’s BIS/BAS
Only a few studies have examined the association between the COMT Val158Met polymorphism and Carver and White’s BIS/BAS scales. Aside from the abovementioned study by Chen et al. [60], only studies by Reuter et al. [58] and Montag et al. [68] included this questionnaire measure in their COMT studies. The latter study used an affective startle reflex paradigm to investigate the influence of the COMT Val158Met polymorphism on fear processing. Female carriers of the Met/Met genotype showed the most pronounced startle reflex upon hearing a loud burst while viewing unpleasant images. Aside from this finding, Montag et al. [68] showed a significant trend for carriers of the Met/Met variant and higher BIS scores.4 Reuter et al. [58] investigated the interaction between the COMT Val158Met polymorphism and the DRD2/ANKK1 Taq Ia polymorphism (rs1800497) on the BIS and BAS dimensions. The DRD2/ANKK1 Taq Ia polymorphism influences D2 receptor density in the striatum. Carriers of at least one A1 allele show lower gray matter volume in dopaminergic midbrain [69, 70] and lower D2 receptor density in the striatum (e.g. [71]). Reuter et al. [58] reported an interaction effect wherein carriers of the Val−/A1− variant (low enzyme activity of COMT and high D2 receptor density) and the Val+/A1+ variant (high enzyme activity of COMT and low D2 receptor density) show the highest BAS scores and the remaining constellations show low BAS scores. Interaction effects are of importance towards a better understanding of human personality and demonstrate the importance of considering multiple gene loci for explanation of individual differences in human behavior. This interaction effect on personality could not be replicated by Light et al. [72] investigating depressed patients with the TCI (see also Table 3).
5.4. COMT Val158Met and Panksepp’s Affective Neuroscience Personality Scales
A new study by Felten et al. [43] investigated the SADNESS dimension of Panksepp’s ANPS in the context of the COMT Val158Met polymorphism and the prominent DAT1 VNTR polymorphism. In line with several studies mentioned in sections 5.1 to 5.3, carriers of the Val/Val variant reported lowest SADNESS scores, but only when they were simultaneous carriers of the homozygous 9R/9R variant of the DAT1 VNTR polymorphism. A study by Heinz et al. [73] showed that carriers of at least one 9R allele are associated with lower DAT density in the striatum. In this context it is important to note that a recent meta-analysis [74] provided no evidence for individual differences depending on this DAT1 VNTR though. The personality dimension SADNESS describes individual differences in coping with the loss of a loved one and dealing with separation distress.
6. THE ROLE OF THE COMT VAL158MET POLYMORPHISM IN ANXIETY DISORDERS
In section 3.1 we discussed how personality is normally distributed in the population. When assessing trait anxiety with a questionnaire, most of the individuals in a population will achieve a medium anxiety score. Although heterogeneous findings exist, the majority of the literature on COMT Val158Met and personality suggests that the Met allele is associated with negative emotionality and the Val allele with positive emotionality. This supports Goldman's worrier/warrior model on the COMT Val158Met polymorphism [75, 76]. Whereas Goldman proposes that the Val allele is associated with robustness and an ability to handle stress - ergo reflecting the warrior - the Met allele is hypothesized to characterize the anxious worrier. This model is supported by Zubieta et al. [77], who showed that the Met allele is associated with lower pain thresholds and problems in activating the pain reducing opioid system of the human brain after being confronted with a pain inducing stimulus. What does this mean for anxiety disorders? Since personality and anxiety disorders can be described by a continuum-model (with a continuum of behavior ranging from healthy to psychopathological), it is very likely that the Met allele is not only associated with higher trait anxiety, but also a vulnerability for anxiety disorders. This idea was pursued in an early study [5], where only the extreme high and low neuroticism scorers were selected out of a large pool of participants for their COMT genetic analysis. In that study, Eley et al. [5] found partial support for the described continuum model between healthy and psychopathological behavior, because the Met allele was seen more frequently in the group of high female neurotics.
Just as sections 5.1 to 5.4 synthesized the studies associating COMT Val158Met and personality, the following section will review the literature on COMT Val158Met and anxiety-related psychopathological disorders in an effort to illuminate its role in anxiety disorders. In this endeavor, we encounter largely the same problems as with the COMT association studies on biologically oriented personality psychology traits. There is a large number of anxiety-related disorders, such as generalized anxiety disorders, panic disorders, post-traumatic stress disorders, obsessive compulsive disorders, etc., which show some overlap in their conceptualizations according to the diagnostic criteria in the DSM-IV or ICD-10. Nevertheless, each disorder has unique, differentiating characteristics, and the influence of COMT Val158Met could tap into the shared or non-shared variance of these diseases.
Table 5 provides an overview of the diverse studies dealing with COMT and anxiety-related disorders. Despite of the large number of anxiety disorders, the pattern describing the Met allele or the Met/Met genotype as the risk allele/genotype surfaces again (e.g. [78, 79]). As a consequence, COMT Val158Met seems to influence the shared characteristics of all anxiety disorders - namely being overly anxious. Nevertheless, we have to again point out some null findings [80, 81] and further inconsistencies, complicating the overall picture. A meta-analysis [82] showed that the Met allele is associated with panic disorders in female Asians, whereas the Val allele is associated with panic disorders in Caucasians. These results underline the importance of considering both the ethnicity and sex of the samples under investigation as important variables crucially influencing the results. As outlined by Goldman [83] in a comment on the COMT-PTSD work by Kolassa et al. [79], the effects of COMT could become especially pronounced when the role of COMT is investigated in humans in extreme situations such as the investigated Rwandese refugees in that study. In order to characterize the effects of COMT on human behavior, Goldman [83] underlines the importance of “building a telescope” and pointing it “in the right direction”. Therefore, some study designs are more likely than others to clarify the effects of COMT.
Table 5.
Genetic Association Studies Between COMT Val158Met and Anxiety/Panic Disorders
| Authors | Sample Size | Ethnic Background and Type of Sample |
Psychopathological Disorder |
Results |
|---|---|---|---|---|
| Valente et al. (2011) | 99 | Brazilian participants (Case (65) - Control study (34)) | Post Traumatic Stress Disorder (PTSD) | Met allele is associated with PTSD |
| Kolassa et al. (2010) | 424 (226 m/198 f) | Rwandese Refugees | PTSD | Met/Met carriers showed higher risk for PTSD independent of the severity of traumtic experiences |
| Gadow et al. (2009) | 67 | Children with autism spectrum disorder | Social Phobia | Teachers rated children with at least one Met allele higher on social phobia ((finding is barely significant) |
| Katerberg et al. (2010) | 835 | Mainly Caucasians (Case (373) -Control study (462) OCD (151 males, 222 females) -Controls (235 males, 227 females) | Obsessive Compulsive Disorder (OCD) | Trend significance for association of the Met allele with OCD in males; furthermore, a sex by COMT effect on somatic and sensory phenomena |
| Maron et al. (2008) | 110 (47 m/63 f) | Healthy Estonians | Inducing of panic attacks | COMT did not predict the experience of panic attacks after cholecystokinin-tetrapeptide challenge |
| Zalsman et al. (2008) | 605 | Caucasians (Case (486) - Controls (119) | Mood disorders | No association between COMT and suicdal behavior or diagnosis could be observed. |
| Baekken et al. (2008) | 5531 | Random population sample from Norway | Anxiety and depression | Met allele appeared at lower frequency in male patients with depression. |
| Hettema et al. (2008) | 1128 | Caucasians (Cases (589) - Controls (539) Case (350 men, 239 women) -Controls (343 men, 196 women) | Anxiety spectrum phenotypes | Haplotype with COMT gene predicts anxiety better than COMT Val158Met alone, contradictory findings with different analyses. |
| Lochner et al. (2008) | 261 (131 m/130 f) | Cases | OCD | Met/Met genotype associated with OCD factor "Obessional Checking" |
| Domschke et al. (2007)* | Metaanalysis of six Cases and Control studies In total 557 cases vs. 763 controls | Panic Disorder | Val allele is associated with panic disorder in Caucasians and the Met allele with panic disorder in female Asians | |
| Funke et al. (2005) | 861 (35.7% females/64.3% males) | Caucasian Cases (394) and controls (467) | Schizophrenia (196), schizoaffective disorder (62) bipolar disorder (82) depression (30) | G allele (Val) protective for suffering from schizophrenia or affective disorders |
| Poyurovski et al (2005) | 363 | OCD (79), Schizophrenia-OCD (113) and control persons (171) | OCD | Met allele occured more often in OCD patients compared with healthy controls and patients with schizophrenia-OCD. This effect does not hold after correction for multiple testing though |
| Samochowiec et al. (2004) | 303 | Phobia disorders (101) and Controls (202) | Anxiety disorders of the phobic spectrum. | No effect of COMT SNP on anxiety disorders could be observed. |
The six studies included in this meta-analysis are not shown separately in this table.
As anxiety disorders and depression share a common genetic factor, COMT has been hypothesized to also play a pivotal role in depression. A recent review article [49] indicated the large number of null-findings in the investigation of the link between COMT Val158Met and depression, as well as the heterogeneity of findings in this research field. Due to the current review’s focus on trait anxiety and anxiety disorders, we suggest the article by Opmeer et al. [49] for further details on the link with depression.
7. COMT VAL158MET AND BRAIN IMAGING
Genetic association studies provide the researcher with the opportunity to observe a link between a certain genetic variant and a chosen phenotype of interest (e.g. personality). Although such studies are a good starting point for a better understanding of genetic influences on personality, genetic associations with personality measures alone cannot give insights into the exact neuroanatomical targets of gene activity in the human brain [84]. Genetic imaging of the human brain represents a new, powerful, non-invasive tool to approach this kind of question [85]. Two approaches are generally followed in genetic imaging: structural and functional genetic imaging. Structural genetic imaging examines the effect of genetic variants on brain anatomy. Functional genetic imaging aims to investigate the influence of gene variants on the functionality of the human brain when the participant follows the instructions of an experiment in a functional MRI (fMRI) scanner.
A large number of studies have already been performed in order to investigate the role of COMT Val158Met on brain function. A recent meta-analysis [86] synthesized the relevant COMT fMRI studies to show that the Met allele was associated with better performance in terms of efficient brain activity in cognitive experimental paradigms. Carriers of the Met allele showed lower activation of the PFC when working on diverse cognitive tasks. In contrast, Met allele carriers showed stronger activations of the PFC in emotional tasks, reflecting higher efforts to control the experimentally induced emotions. Met allele carriers might need to invest more PFC activity than Val allele carriers when confronted with subcortical activity triggered by emotional stimuli. This idea found support in a study by Rasch et al. [87], who demonstrated that carriers of the homozygous Met variant reacted with higher amygdala activity of the right hemisphere in response to unpleasant images. Over-activity of subcortical limbic regions in response to unpleasant images could therefore create a necessity for stronger top-down regulation in Met allele carriers. This is once again supported by the Rasch et al. [87] study, as stronger connectivity between amygdala and orbitofrontal cortex could be observed in carriers of the Met allele. The review by Heinz and Smolka [88] on the relevant fMRI data supports this conclusion as well.
Several studies also investigated the effect of COMT Val158Met on the structure of the human brain (e.g. [89]). Among other findings, the authors report that Val allele carriers tend to have lower hippocampal gray matter volume when compared to homozygous Met carriers. Gray matter volume of the dorsolateral PFC was modulated by a Val allele dosage effect in the other direction: here, carriers of the Val/Val variant were associated with highest gray matter volume (this finding was trend significant). Moreover, this study was able to demonstrate an interaction effect between the COMT Val158Met polymorphism and a SNP in the promoter region of the COMT gene on hippocampal gray matter volume. Earlier studies investigating healthy participants either could not identify effects of COMT Val158Met on gray matter volume [90] or found that COMT effects on the temporal lobe regions of the brain were modulated by age and sex [91]. Future research efforts will need to combine structural and functional MRI data to answer the question of how both measures relate to each other.
8. THE COMT LITERATURE ON ANXIETY - A SHORT SYNOPSIS
A summary of the COMT Val158Met studies on personality research and related psychopathological disorders (in particular anxiety disorders) supports the link between the Met allele and negative emotionality. Once again, we reiterate that this rule of thumb represents an oversimplification, because several factors such as ethnicity/sex of the sample and the nature of the investigated dependent variables complicate the overall picture.
Given the simplified heuristic wherein the "Met allele associates with anxiety", one could speculate about the resulting biochemical processes underlying anxiety. As demonstrated by Lachman et al. [51], the Met allele has been associated with lower catabolism rates of dopamine, therefore putatively leading to higher dopamine levels in the synaptic cleft in the prefrontal cortex. The COMT effect on the PFC compared to other brain areas is regarded to be especially strong, because only a limited amount of dopamine in the synaptic cleft can be transferred back into the pre-synapse due to the paucity of DAT in this region of the brain [52]. Although one might ask how higher dopamine levels relate to increased anxiety (because higher dopamine could also be associated with higher positive emotionality (e.g. [41, 42]), we refer towards the importance of considering the specific area of the brain in which high dopamine levels are observed. The study by Depue et al. [41] challenged participants with bromocriptine, which mainly targets dopaminergic neurotransmission in striatal brain areas (bromocriptine is a D2 receptor agonist). High dopamine levels in the striatum might be associated with positive emotionality, whereas high dopamine levels in the PFC might be associated with negative emotionality. In line with the ideas of Bilder et al. [92], one could assume that higher dopamine levels in the PFC might be associated with a more stable representation of an unpleasant stimulus processed by the human organism. This stable representation goes along with inflexible attentive behavior and the consequence of "sticking" to the unpleasant stimulus. In addition, higher dopamine levels in the PFC of Met allele carriers could reflect higher top-down-regulation costs, when attenuating the effects of emotions elicited by the limbic system.
Finally, we would like to stress that it is misleading to refer to the Met allele as the "bad" allele. The term might have value in terms of anxiety-related disorders, because the Met allele might indeed represent a vulnerability factor. However, as outlined by Caspi et al. [34], it is of importance to include environmental influences into one's model in an attempt to explain molecular genetic influences on anxious behavior and negative emotionality. Anxious behavior is of value to each human being because it promotes survival in dangerous and uncertain environments. Therefore, linkage of the Met allele to "a healthy degree" of trait anxiety might represent something positive, because it helps the individual to stay alert. The Met allele could shift from the "good" to the "bad" allele when adverse environmental influences such as being beaten by an alcoholic parent during childhood; this traumatic incident could lead to altered gene activity, disturbing the homeostasis of the biochemistry in the brain. Therefore, future studies need to account for the influence of environmental factors (which indeed can have a clear positive or negative connotation) in order to understand the complex epigenetic mechanisms underlying human behavior. The investigation of methylation patterns reflecting gene activity will be one of the next fascinating research goals in this field [93]. Last but not least, personalized medicine represents another important research lead. Given the influence of COMT Val158Met on dopaminergic neurotransmission, it might be possible to adjust the level of therapeutic drugs according to the COMT Val158Met genotype in the context of anxiety-related psychopathological disorders [94]. This in turn could help by reducing suffering from anxiety disorders as well as decreasing costs for society.
9. LIMITATIONS OF THE PRESENT REVIEW
The present review focused primarily on genetic association studies between the prominent COMT Val158Met polymorphism and personality traits linked to positive and negative emotionality. We are aware of the fact that this represents a rather narrow approach, because COMT Val158Met has been abundantly researched in the context of schizophrenia (see review [95]) and schizotypal personality traits (e.g. [96, 97]). Moreover, it is of importance to also consider this prominent SNP in the context of the molecular genetics of cognition (see again [95]). These two interesting branches of COMT research are not highlighted in the present review. Moreover, dopaminergic neurotransmission is influenced by a large number of molecular factors (see also 2.1.), and therefore a focus on solely one element of the system naturally brings limitations. As stated in 3.1. one SNP alone can only explain a small proportion of a personality trait. Therefore we need to consider multiple genetic targets to disentangle the molecular genetic basis of personality. We hope that this review demonstrated that the COMT-anxiety research field contains many heterogeneous findings, and that at this time only meta-analysis can reveal the effects of COMT on each distinct endophenotype of anxiety.
ACKNOWLEDGEMENT
Molecular genetics of personality represent a vital and promising field in personality research. It is fantastic to see how many researchers worldwide have joined this fascinating research field. We did our best to include all relevant "anxiety-COMT studies" in the field. We apologize if relevant studies in the field are missing from the current manuscript.
ABBREVIATIONS
- ANPS
Affective neuroscience personality scale
- ARAS
Ascending reticular activation system
- BAS
Behavioral activation system
- BDNF
Brain derived neurotrophic factor
- BIS
Behavioral inhibition system
- COMT
Catechol-O-methyltransferase
- DAT
Dopamine transporter
- DZ
Dizygotic
- EPQ
Eysenck's personality questionnaire
- FFFS
Fight flight freezing system
- GWAS
Genome wide association studies
- MAO
Monoamine oxidase
- Met
Methionine
- MZ
Monozygotic
- OCD
Obsessive compulsive disorder
- PFC
Prefrontal cortex
- PTSD
Post traumtic stress disorder
- RST
Reinforcement sensitivity theory
- SNP
Single nucleotide polymorphism
- TCI
Temperament and character inventory
- TPQ
Tridimensional personality questionnaire
- Val
Valine
Footnotes
Letters are written in upper case as in Panksepp’s work.
In contrast to the above introduced biologically oriented personality theories, the BIG 5 have been derived by a lexical approach and describe a five factor model of personality [26]. The personality dimensions of this personality concept are called openness for experience, conscientiousness, extraversion, agreeableness and neuroticism.
It is far more complicated than this estimation suggests, because of shared and non-shared environmental influences and errors in the measurement of personality.
Quednow et al. [75] also investigated the effect of COMT Val158Met on the startle response, but without using emotional pictures. Here no significant effect could be observed.
CONFLICT OF INTEREST
Declared none.
REFERENCES
- 1.Allport GW. Pattern and growth in personality. New York: Holt, Rinehart and Winston; 1961. (cited after Maltby, J.; Day, L.; and Macaskill, A. Personality, Individual Differences and Intelligence. Pearson: Essex, 2007, p. 7.) [Google Scholar]
- 2.Steyer R, Schmitt M, Eid M. Latent state-trait theory and research in personality and individual differences. Eur. J. Pers. 1999;13(5):389–408. [Google Scholar]
- 3.Cattell RB. Personality: A systematic, theoretical, and factual study. New York: McGraw-Hill; 1950. [Google Scholar]
- 4.Kendler KS, Gatz M, Gardner CO, Pedersen NL. Personality and major depression: a Swedish longitudinal, population-based twin study. Arch. Gen. Psychiatry. 2006;63(10):1113–1120. doi: 10.1001/archpsyc.63.10.1113. [DOI] [PubMed] [Google Scholar]
- 5.Eley TC, Tahir E, Angleitner A, Harriss K, McClay J, Plomin R, Riemann R, Spinath F, Craig I. Association analysis of MAOA and COMT with neuroticism assessed by peers. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003;120B(1):90–96. doi: 10.1002/ajmg.b.20046. [DOI] [PubMed] [Google Scholar]
- 6.Eysenck HJ. The biological basis of personality. Springfield, Ill: Charles C. Thomas; 1967. [Google Scholar]
- 7.Maltby J, Day L, Macaskill A. Personality, Individual Differences and Intelligence. Pearson: Essex; 2007. p. 200. [Google Scholar]
- 8.McCann SJ. Suicide, big five personality factors, and depression at the American state level. Arch. Suicide Res. 2010;14(4):368–374. doi: 10.1080/13811118.2010.524070. [DOI] [PubMed] [Google Scholar]
- 9.Eysenck MW. Extraversion, activation and the recall of prose. Br. J. Psychol. 1976;67(1):53–61. doi: 10.1111/j.2044-8295.1976.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 10.Vogel F, Schalt E. The electroencephalogram (EEG) as a research tool in human behavior genetics: psychological examinations in healthy males with various inherited EEG variants. III. Interpretation of the results. Hum. Genet. 1979;47(1):81–111. doi: 10.1007/BF00295571. [DOI] [PubMed] [Google Scholar]
- 11.Beauducel A, Brocke B, Leue A. Energetical bases of extraversion: effort, arousal, EEG, and performance. Int. J. Psychophysiol. 2006;62(2):212–223. doi: 10.1016/j.ijpsycho.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Eysenck HJ, Eysenck MW. The manual to the Eysenck-Personality Inventory. San Diego: Educational and Industrial Testing Service; 1968. [Google Scholar]
- 13.Gray JA. The psychophysiological basis of introversion-extraversion. Behav. Res. Ther. 1970;8(3):249–266. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- 14.Gray J, McNaughton N. The Neuropsychology of Anxiety. Oxford, UK: Oxford Press; 2000. [Google Scholar]
- 15.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J. Pers. Soc. Psychol. 1994;67(2):319–333. [Google Scholar]
- 16.Jackson CJ. Jackson-5 Scales of revised Reinforcement Sensitivity Theory (r-RST) and their application to dysfunctional real world outcomes. J. Res. Pers. 2009;43(4):556–569. [Google Scholar]
- 17.Leue A, Beauducel A. A Meta-Analysis of Reinforcement Sensitivity Theory: On performance parameters in reinforcement tasks. Pers. Soc. Psychol. Rev. 2008;12(4):353–369. doi: 10.1177/1088868308316891. [DOI] [PubMed] [Google Scholar]
- 18.Matthews G, Gilliland K. The personality theories of H.J. Eysenck and J.A. Gray: a comparative review. Pers. Ind. Diff. 1999;26(4):583–626. [Google Scholar]
- 19.Cloninger CR, Svrakic DM, Przybeck TR. A psychobiological model of temperament and character. Arch. Gen. Psychiatry. 1993;50(12):975–990. doi: 10.1001/archpsyc.1993.01820240059008. [DOI] [PubMed] [Google Scholar]
- 20.Kose S. Psychobiological Model of Temperament and Character. Yeni Symposium. 2003;41(2):86–97. [Google Scholar]
- 21.Ando J, Ono Y, Yoshimura K, Onoda N, Shinohara M, Kanba S, Asai M. The genetic structure of Cloninger's seven-factor model of temperament and character in a Japanese sample. J. Pers. 2002;70(5):583–609. doi: 10.1111/1467-6494.05018. [DOI] [PubMed] [Google Scholar]
- 22.Ando J, Suzuki A, Yamagata S, Kijima N, Maekawa H, Ono Y, Jang KL. Genetic and environmental structure of Cloninger's temperament and character dimensions. J. Pers. Disord. 2004;18(4):379–393. doi: 10.1521/pedi.18.4.379.40345. [DOI] [PubMed] [Google Scholar]
- 23.Montag C, Markett S, Basten U, Stelzel C, Fiebach C, Canli T, Reuter M. Epistasis of the DRD2/ANKK1 Taq Ia and the BDNF Val66Met polymorphism impacts Novelty Seeking and Harm Avoidance. Neuropsychopharmacology. 2010;35(9):1860–1867. doi: 10.1038/npp.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panksepp J. Affective Neuroscience. London: Oxford University Press; 1998. pp. 187–205. [Google Scholar]
- 25.Davis KL, Panksepp J, Normansell L. The affective neuroscience personality scales: normative data and implications. Neuropsychoanalysis. 2003;5(1):57–69. [Google Scholar]
- 26.Costa PT, Jr, McCrae RR. Normal personality assessment in clinical practice: The NEO Personality Inventory. Psychol. Assess. 1992;4(1):5–13. [Google Scholar]
- 27.Davis KL, Panksepp J. The brain's emotional foundations of human personality and the Affective Neuroscience Personality Scales. Neurosci. Biobehav. Rev. 2011;35(9):1946–1958. doi: 10.1016/j.neubiorev.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Reuter M, Weber B, Fiebach CJ, Elger C, Montag C. The biological basis of anger: associations with the gene coding for DARPP-32 (PPP1R1B) and with amygdala volume. Behav. Brain Res. 2009;202(2):179–183. doi: 10.1016/j.bbr.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 29.Montag C, Fiebach CF, Kirsch P, Reuter M. Interaction of 5-HTTLPR and a variation on the oxytocin receptor gene OXTR influences negative emotionality. Biol. Psychiatry. 2011;69(6):601–603. doi: 10.1016/j.biopsych.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 30.Ackerman PL, Heggestad ED. Intelligence, personality, and interests: Evidence for overlapping traits. Psychol. Bull. 1997;121(2):219–245. doi: 10.1037/0033-2909.121.2.219. [DOI] [PubMed] [Google Scholar]
- 31.Borkenau P, Riemann R, Angleitner A, Spinath FM. Genetic and environmental influences on observed personality: evidence from the German Observational Study of Adult Twins. J. Pers. Soc. Psychol. 2001;80(4):655–668. doi: 10.1037//0022-3514.80.4.655. [DOI] [PubMed] [Google Scholar]
- 32.Rieman R, Angleitner A, Strelau J. Genetic and environmental influences on personality: a study of twins reared together using the self- and peer report NEO-FFI scales. J. Pers. 1997;65(3):449–475. [Google Scholar]
- 33.Markett S, Montag C, Reuter M. The nicotinic acetylcholine receptor gene CHRNA4 is associated with negative emotionality. Emotion. 2011;11(2):450–455. doi: 10.1037/a0021784. [DOI] [PubMed] [Google Scholar]
- 34.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 35.Plomin R, Haworth CM, Davis OS. Common disorders are quantitative traits. Nat. Rev. Genet. 2009;10(12):872–878. doi: 10.1038/nrg2670. [DOI] [PubMed] [Google Scholar]
- 36.Terracciano A, Sanna S, Uda M, Deiana B, Usala G, Busonero F, Maschio A, Scally M, Patriciu N, Chen WM, Distel MA, Slagboom EP, Boomsma DI, Villafuerte S, Sliwerska E, Burmeister M, Amin N, Janssens AC, van Duijn CM, Schlessinger D, Abecasis GR, Costa PT., Jr Genome-wide association scan for five major dimensions of personality. Mol. Psychiatry. 2010;15(6):647–656. doi: 10.1038/mp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. The BDNF Val66Met polymorphism and anxiety: Support for animal knock-in-studies from a genetic association study in humans. Psychiatry Res. 2010;179(1):86–90. doi: 10.1016/j.psychres.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Ross SA, Wong JY, Clifford JJ, Kinsella A, Massalas JS, Horne MK, Scheffer IE, Kola I, Waddington JL, Berkovic SF, Drago J. Phenotypic characterization of an alpha 4 neuronal nicotinic acetylcholine receptor subunit knock-out mouse. J. Neurosci. 2000;20(17):6431–6441. doi: 10.1523/JNEUROSCI.20-17-06431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise RA. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 41.Depue RA, Luciana M, Arbisi P, Collins P, Leon A. Dopamine and the structure of personality: relation of agonist-induced dopamine activity to positive emotionality. J. Pers. Soc. Psychol. 1994;67(3):485–498. doi: 10.1037//0022-3514.67.3.485. [DOI] [PubMed] [Google Scholar]
- 42.Reuter M, Schmitz A, Corr P, Hennig J. Molecular genetics support Gray's personality theory: the interaction of COMT and DRD2 polymorphisms predicts the behavioural approach system. Int. J. Neuropsychopharmacol. 2006;9(2):155–166. doi: 10.1017/S1461145705005419. [DOI] [PubMed] [Google Scholar]
- 43.Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 1(2):109–118. doi: 10.1002/brb3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagatsu T, Levitt M, Udenfriend S. Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J. Biol. Chem. 1964;239:2910–2917. [PubMed] [Google Scholar]
- 45.Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J. Biol. Chem. 1962;237:89–93. [PubMed] [Google Scholar]
- 46.Rosengren E. On the role of monoamine oxidase for the inactivation of dopamine in brain. Acta Physiol. Scand. 1960;49(4):370–375. doi: 10.1111/j.1748-1716.1960.tb01959.x. [DOI] [PubMed] [Google Scholar]
- 47.Axelrod J, Senoh S, Witkop B. O-methylation of catechol amines in vivo. J. Biol. Chem. 1958;233:697–701. [PubMed] [Google Scholar]
- 48.Hennig J, Netter P. Biologische Grundlagen der Persönlichkeit. Heidelberg: Elsevier; 2005. pp. 248–249. [Google Scholar]
- 49.Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Prog. Neurobiol. 2010;92(2):112–133. doi: 10.1016/j.pneurobio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 50.Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol-O-methyltransferase gene to 22q11.1-q11.2. Genomics. 1992;12(4):822–825. doi: 10.1016/0888-7543(92)90316-k. [DOI] [PubMed] [Google Scholar]
- 51.Lachman HM, Papolos DF, Saito T, You Y-M, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunioreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J. Comp. Neurol. 2001;432(1):119–136. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 53.Tenhunen J, Salminen M, Lundström K, Kiviluoto T, Savolainen R, Ulmanan I. Genomic organization of the human catechol-o-methyltransferase gene and its expression from two distinct promoters. Eur. J. Biochem. 1994;223(3):1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- 54.Terracciano A, Tanaka T, Sutin AR, Deiana B, Balaci L, Sanna S, Olla N, Maschio A, Uda M, Ferrucci L, Schlessinger D, Costa PT., Jr BDNF Val66Met is associated with introversion and interacts with 5-HTTLPR to influence neuroticism. Neuropsychopharmacology. 2010;35(5):1083–1089. doi: 10.1038/npp.2009.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Enoch MA, Schuckit MA, Johnson BA, Goldman D. Genetics of alcoholism using intermediate phenotypes. Alcohol Clin. Exp. Res. 2003;27(2):169–176. doi: 10.1097/01.ALC.0000052702.77807.8C. [DOI] [PubMed] [Google Scholar]
- 56.Enoch MA, White KV, Waheed J, Goldman D. Neurophysiological and genetic distinctions between pure and comorbid anxiety disorders. Depress. Anxiety. 2008;25(5):383–392. doi: 10.1002/da.20378. [DOI] [PubMed] [Google Scholar]
- 57.Hashimoto R, Noguchi H, Hori H, Ohi K, Yasuda Y, Takeda M, Kunugi H. A possible association between the Val158Met polymorphism of the catechol-O-methyl transferase gene and the personality trait of harm avoidance in Japanese healthy subjects. Neurosci. Lett. 2007;428(1):17–20. doi: 10.1016/j.neulet.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 58.Reuter M, Hennig J. Association of the functional catechol-O-methyltransferase VAL158MET polymorphism with the personality trait of extraversion. Neuroreport. 2005;16(10):1135–1138. doi: 10.1097/00001756-200507130-00020. [DOI] [PubMed] [Google Scholar]
- 59.Tsai SJ, Hong CJ, Yu YW, Chen TJ. Association study of catechol-O-methyltransferase gene and dopamine D4 receptor gene polymorphisms and personality traits in healthy young chinese females. Neuropsychobiology. 2004;50(2):153–156. doi: 10.1159/000079107. [DOI] [PubMed] [Google Scholar]
- 60.Chen C, Chen C, Moyzis R, Dong Q, He Q, Zhu B, Li J, Li H, Li J, Lessard J. Sex Modulates the Associations Between the COMT Gene and Personality Traits. Neuropsychopharmacology. 2011;36(8):1593–1598. doi: 10.1038/npp.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hosák L, Libiger J, Cizek J, Beránek M, Cermáková E. The COMT Val158Met polymorphism is associated with novelty seeking in Czech methamphetamine abusers: preliminary results. Neuroendocrinol. Lett. 2006;27(6):799–802. [PubMed] [Google Scholar]
- 62.Kim SJ, Kim YS, Kim SY, Lee HS, Kim CH. An association study of catechol-O-methyltransferase and monoamine oxidase A polymorphisms and personality traits in Koreans. Neurosci. Lett. 2006;401(1–2):154–158. doi: 10.1016/j.neulet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 63.Ishii G, Suzuki A, Oshino S, Shiraishi H, Matsumoto Y, Otani K, Goto K. Association study of catechol-O-methyltransferase Val158Met polymorphism with personality traits in Japanese healthy volunteers. Eur. Psychiatry. 2007;22(7):462–465. doi: 10.1016/j.eurpsy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 64.Mikołajczyk E, Grzywacz A, Samochowiec J. The association of catechol-O-methyltransferase genotype with the phenotype of women with eating disorders. Brain Res. 2010;1307:142–148. doi: 10.1016/j.brainres.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 65.Reuter M, Frenzel C, Walter NT, Markett S, Montag C. Investigating the genetic basis of altruism: the role of the COMT Val158Met polymorphism. Soc. Cogn. Affect. Neurosci. 2011;6(5):662–668. doi: 10.1093/scan/nsq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch. Gen. Psychiatry. 2006;63(12):1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- 67.Hamidovic A, Dlugos A, Palmer AA, de Wit H. Catechol-O-methyltransferase val158met genotype modulates sustained attention in both the drug-free state and in response to amphetamine. Psychiatry Genet. 2010;20(3):85–92. doi: 10.1097/YPG.0b013e32833a1f3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, Reuter M. COMT genetic variation affects fear processing: psychophysiological evidence. Behav. Neurosci. 2008;122(4):901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- 69.Cerasa A, Gioia MC, Tarantino P, Labate A, Arabia G, Annesi G, Lanza P, Di Palma G, Blasi V, Quattrone A. The DRD2 TaqIA polymorphism associated with changed midbrain volumes in healthy individuals. Genes Brain Behav. 2009;8(4):459–463. doi: 10.1111/j.1601-183X.2009.00492.x. [DOI] [PubMed] [Google Scholar]
- 70.Montag C, Weber B, Jentgens E, Elger C, Reuter M. An epistasis effect of functional variants on the BDNF and DRD2 genes modulates gray matter volume of the anterior cingulate cortex in healthy humans. Neuropsychologia. 2010c;48(4):1016–1021. doi: 10.1016/j.neuropsychologia.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 71.Jönsson EG, Nöthen MM, Grünhage F, Farde L, Nakashima Y, Propping P, Sedvall GC. Polymorphisms in the dopamine D2 receptor gene and their relationships to striatal dopamine receptor density of healthy volunteers. Mol. Psychiatry. 1999;4(3):290–296. doi: 10.1038/sj.mp.4000532. [DOI] [PubMed] [Google Scholar]
- 72.Light KJ, Joyce PR, Luty SE, Mulder RT, Carter JD, Frampton CM, Miller AL, Kennedy MA. An association study of DRD2 and COMT polymorphisms with novelty seeking and harm avoidance scores, in two independent samples of depressed patients. Behav. Brain Funct. 2007;3:3. doi: 10.1186/1744-9081-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, Lee KS, Linnoila M, Weinberger DR. Genotype influences in vivo DAT availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 74.Costa A, Riedel M, Müller U, Möller HJ, Ettinger U. Relationship between SLC6A3 genotype and striatal dopamine transporter availability: a meta-analysis of human single photon emission computed tomography studies. Synapse. 2011;65(10):998–1005. doi: 10.1002/syn.20927. [DOI] [PubMed] [Google Scholar]
- 75.Quednow BB, Schmechtig A, Ettinger U, Petrovsky N, Collier DA, Vollenweider FX, Wagner M, Kumari V. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study. Biol. Psychiatry. 2009;66(6):614–620. doi: 10.1016/j.biopsych.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 77.Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299(5610):1240–1243. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]
- 78.Valente NL, Vallada H, Cordeiro Q, Bressan RA, Andreoli SB, Mari JJ, Mello MF. Catechol-O-methyltransferase (COMT) val158met polymorphism as a risk factor for PTSD after urban violence. J. Mol. Neurosci. 2011;43(3):516–523. doi: 10.1007/s12031-010-9474-2. [DOI] [PubMed] [Google Scholar]
- 79.Kolassa IT, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val (158)Met polymorphism. Biol. Psychiatry. 2010;67(4):304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Samochowiec J, Hajduk A, Samochowiec A, Horodnicki J, Stepień G, Grzywacz A, Kucharska-Mazur J. Association studies of MAO-A, COMT, and 5-HTT genes polymorphisms in patients with anxiety disorders of the phobic spectrum. Psychiatry Res. 2004;128(1):21–26. doi: 10.1016/j.psychres.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 81.Zalsman G, Huang YY, Oquendo MA, Brent DA, Giner L, Haghighi F, Burke AK, Ellis SP, Currier D, Mann JJ. No association of COMT Val158Met polymorphism with suicidal behavior or CSF monoamine metabolites in mood disorders. Arch. Suicide Res. 2008;12(4):327–335. doi: 10.1080/13811110802324912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Domschke K, Ohrmann P, Braun M, Suslow T, Bauer J, Hohoff C, Kersting A, Engelien A, Arolt V, Heindel W, Deckert J, Kugel H. Influence of the catechol-O-methyltransferase val158met genotype on amygdala and prefrontal cortex emotional processing in panic disorder. Psychiatry Res. 2008;163(1):13–20. doi: 10.1016/j.pscychresns.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 83.Goldman D. Gene × environment interactions in complex behavior: First, build a telescope. Biol. Psychiatry. 2010;67(4):295–296. doi: 10.1016/j.biopsych.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buckholtz JW, Meyer-Lindenberg A. MAOA and the neurogenetic architecture of human aggression. Trends Neurosci. 2008;31(3):120–129. doi: 10.1016/j.tins.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 85.De Geus E, Goldberg T, Boomsma DI, Posthuma D. Imaging the genetics of brain structure and function. Biol. Psychol. 2008;79(1):1–8. doi: 10.1016/j.biopsycho.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 86.Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: a meta-analysis. Mol. Psychiatry. 2010;15(9):918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- 87.Rasch B, Spalek K, Buholzer S, Luechinger R, Boesiger P, de Quervain DJ, Papassotiropoulos A. Aversive stimuli lead to differential amygdala activation and connectivity patterns depending on catechol-O-methyltransferase Val158Met genotype. Neuroimage. 2010;52(4):1712–1719. doi: 10.1016/j.neuroimage.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 88.Heinz A, Smolka MN. The effects of catechol O-methyltransferase genotype on brain activation elicited by affective stimuli and cognitive tasks. Rev. Neurosci. 2006;17(3):359–367. doi: 10.1515/revneuro.2006.17.3.359. [DOI] [PubMed] [Google Scholar]
- 89.Honea R, Verchinski BA, Pezawas L, Kolachana BS, Callicott JH, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Impact of interacting functional variants in COMT on regional gray matter volume in human brain. Neuroimage. 2009;45(1):44–51. doi: 10.1016/j.neuroimage.2008.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol. Psychiatry. 2005;10(3):229, 287–298. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- 91.Zinkstok J, Schmitz N, van Amelsvoort T, de Win M, van den Brink W, Baas F, Linszen D. The COMT val158met polymorphism and brain morphometry in healthy young adults. Neurosci. Lett. 2006;405(1–2):34–39. doi: 10.1016/j.neulet.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 92.Bilder RM, Volavka J, Lachman HM, Grace AA. The catechol-O-methyltransferase polymorphism: relations to the tonic-phasic dopamine hypothesis and neuropsychiatric phenotypes. Neuropsychopharmacology. 2004;29(11):1943–1961. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 93.Meaney MJ, Ferguson-Smith AC. Epigenetic regulation of the neural transcriptome: the meaning of the marks. Nat. Neurosci. 2010;13(11):1313–1318. doi: 10.1038/nn1110-1313. [DOI] [PubMed] [Google Scholar]
- 94.Apud JA, Weinberger DR. Treatment of cognitive deficits associated with schizophrenia: potential role of catechol-O-methyltransferase inhibitors. CNS Drugs. 2007;21(7):535–557. doi: 10.2165/00023210-200721070-00002. [DOI] [PubMed] [Google Scholar]
- 95.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry. 2006;60(2):141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 96.Schürhoff F, Szöke A, Chevalier F, Roy I, Méary A, Bellivier F, Giros B, Leboyer M. Schizotypal dimensions: an intermediate phenotype associated with the COMT high activity allele. Am. J. Med. Genet.B. Neuropsychiatr. Genet. 2007;144B(1):64–68. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- 97.Docherty AR, Sponheim SR. Anhedonia as a phenotype for the Val158Met COMT polymorphism in relatives of patients with schizophrenia. J. Abnorm. Psychol. 2008;117(4):788–798. doi: 10.1037/a0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aoki J, Iwahashi K, Ishigooka J, Ikeda K. Association study on catechol-O-methyltransferase (COMT) Val158Met gene polymorphism and NEO-FFI. Psychiatry Res. 2011;187(1–2):312–313. doi: 10.1016/j.psychres.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 99.Wray NR, James MR, Dumenil T, Handoko HY, Lind PA, Montgomery GW, Martin NG. Association study of candidate variants of COMT with neuroticism, anxiety and depression. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2008;147B(7):1314–1318. doi: 10.1002/ajmg.b.30744. [DOI] [PubMed] [Google Scholar]
- 100.Sheldrick AJ, Krug A, Markov V, Leube D, Michel TM, Zerres K, Eggermann T, Kircher T. Effect of COMT val158met genotype on cognition and personality. Eur. Psychiatry. 2008;23(6):385–389. doi: 10.1016/j.eurpsy.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 101.Hoth KF, Paul RH, Williams LM, Dobson-Stone C, Todd E, Schofield PR, Gunstad J, Cohen RA, Gordon E. Associations between the COMT Val/Met polymorphism, early life stress, and personality among healthy adults. Neuropsychiatr. Dis. Treat. 2006;2(2):219–225. doi: 10.2147/nedt.2006.2.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tochigi M, Otowa T, Hibino H, Kato C, Otani T, Umekage T, Utsumi T, Kato N, Sasaki T. Combined analysis of association between personality traits and three functional polymorphisms in the tyrosine hydroxylase, monoamine oxidase A, and catechol-O-methyltransferase genes. Neurosci. Res. 2006;54(3):180–185. doi: 10.1016/j.neures.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30(11):2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- 104.Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatr. Genet. 2005;15(2):109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 105.Gadow KD, Roohi J, DeVincent CJ, Kirsch S, Hatchwell E. Association of COMT (Val158Met) and BDNF (Val66Met) gene polymorphisms with anxiety, ADHD and tics in children with autism spectrum disorder. J. Autism Dev. Disord. 2009;39(11):1542–1551. doi: 10.1007/s10803-009-0794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katerberg H, Cath DC, Denys DA, Heutink P, Polman A, van Nieuwerburgh FC, Deforce DL, Bochdanovits Z, van Balkom AJ, den Boer JA. The role of the COMT Val(158)Met polymorphism in the phenotypic expression of obsessive-compulsive disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2010;153B(1):167–176. doi: 10.1002/ajmg.b.30971. [DOI] [PubMed] [Google Scholar]
- 107.Maron E, Tõru I, Tasa G, Must A, Toover E, Lang A, Vasar V, Shlik J. Association testing of panic disorder candidate genes using CCK-4 challenge in healthy volunteers. Neurosci. Lett. 2008;446(2–3):88–92. doi: 10.1016/j.neulet.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 108.Baekken PM, Skorpen F, Stordal E, Zwart JA, Hagen K. Depression and anxiety in relation to catechol-O-methyltransferase Val158Met genotype in the general population: the Nord-Trøndelag Health Study (HUNT) BMC Psychiatry. 2008;8:48. doi: 10.1186/1471-244X-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hettema JM, An SS, Bukszar J, van den Oord EJ, Neale MC, Kendler KS, Chen X. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biol. Psychiatry. 2008;64(4):302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lochner C, Hemmings SM, Kinnear CJ, Nel D, Hemmings SM, Seedat S, Moolman-Smook JC, Stein DJ. Cluster analysis of obsessive-compulsive symptomatology: identifying obsessive-compulsive disorder subtypes. Isr. J. Psychiatry Relat. Sci. 2008;45(3):164–176. [PubMed] [Google Scholar]
- 111.Funke B, Malhotra AK, Finn CT, Plocik AM, Lake SL, Lencz T, DeRosse P, Kane JM, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behav. Brain Funct. 2005;1:19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Poyurovsky M, Michaelovsky E, Frisch A, Knoll G, Amir I, Finkel B, Buniak F, Hermesh H, Weizman R. COMT Val158Met polymorphism in schizophrenia with obsessive-compulsive disorder: a case-control study. Neurosci. Lett. 2005;389(1):21–24. doi: 10.1016/j.neulet.2005.06.064. [DOI] [PubMed] [Google Scholar]