Abstract
Brain-derived neurotrophic factor (BDNF) has been implicated in hippocampal-dependent learning processes, and carriers of the Met allele of the Val66Met BDNF genotype are characterized by reduced hippocampal structure and function. Recent nonhuman animal work suggests that BDNF is also crucial for amygdala-dependent associative learning. The present study sought to examine fear conditioning as a function of the BDNF polymorphism. Fifty-seven participants were genotyped for the BDNF polymorphism and took part in a differential-conditioning paradigm. Participants were shocked following a particular conditioned stimulus (CS+) and were also presented with stimuli that ranged in perceptual similarity to the CS+ (20, 40 or 60% smaller or larger than the CS+). The eye blink component of the startle response was measured to quantify fear conditioning; post-task shock likelihood ratings for each stimulus were also obtained. All participants reported that shock likelihood varied with perceptual similarity to the CS+ and showed potentiated startle in response to CS ± 20% stimuli. However, only the Val/Val group had potentiated startle responses to the CS+. Met allele carrying individuals were characterized by deficient fear conditioning – evidenced by an attenuated startle response to CS+ stimuli. Variation in the BDNF genotype appears related to abnormal fear conditioning, consistent with nonhuman animal work on the importance of BDNF in amygdala-dependent associative learning. The relation between genetic variation in BDNF and amygdala-dependent associative learning deficits is discussed in terms of potential mechanisms of risk for psychopathology.
Keywords: Anxiety, BDNF, fear, fear-potentiated startle, generalization, startle
A common single nucleotide polymorphism (SNP) in the human brain-derived neurotrophic factor (BDNF) gene has been identified that produces a functional valine (Val) to methionine (Met) substitution in the prodomain at codon 66 (Val66Met; Egan et al. 2003). The heterozygous Val/Met genotype occurs in approximately 20–30% of Caucasian populations; the Met/Met allele is much more infrequent (about 2–3%; Shimizu et al. 2004). Met substitution reduces BDNF trafficking and activity-dependent secretion (Chen et al. 2004; Egan et al. 2003). Consistent with the role of BDNF in hippocampal-dependent cognitive function in nonhuman animals (Figurov et al. 1996; Korte et al. 1995; Lu & Gottschalk 2000; Patterson et al. 1996; Poo 2001), individuals who carry a Met allele of the BDNF polymorphism have relatively poor memory, as well as reduced hippocampal activation during memory tasks, compared with individuals homozygous for the Val allele (Dempster et al. 2005; Egan et al. 2003; Hariri et al. 2003).
Recent work highlights the role of BDNF in other forms of associative learning, including amygdala-dependent fear conditioning (Monfils et al. 2007; Rattiner et al. 2005; Ressler & Davis 2003). BDNF is expressed in the amygdala during fear conditioning (Chhatwal et al. 2006; Conner et al. 1997; Jones et al. 2007; Yan et al. 1997) – and temporal patterns of BDNF expression in the basolateral amygdala suggest that BDNF is necessary for the acquisition of conditioned fear (Ou & Gean 2006; Rattiner et al. 2004a,b). Moreover, Chen et al. (2006) found that expression of a Met allele produced altered BDNF expression and increases in anxiety-related behaviors.
Fear conditioning in humans and nonhuman animals can be measured through potentiation of the startle response – a cross-species defensive reflex to an abrupt and intense stimulus (Davis 1984; Davis 2006; Grillon & Baas 2003). The startle response is larger when the eliciting stimulus is delivered in the presence of a cue previously paired with a shock – a phenomenon referred to as fear-potentiated startle (Brown et al. 1951; Davis 2006; Davis et al. 1993; Grillon & Baas 2003). Extensive neurobiological research in nonhuman animals has highlighted the central role of the amygdala during fear conditioning (Pare et al. 2004; Sigurdsson et al. 2007; Wilensky et al. 2006) and in the potentiation of the startle reflex (Davis 2006; Davis et al. 1993).
Consistent with this body of work, the human startle reflex is enhanced in the context of aversive stimuli and situations: startle magnitude is larger in the presence of conditioned stimuli (Grillon & Davis 1997) and in response to threat of shock (Grillon et al. 1991); moreover, startle magnitude tracks the association between conditioned and unconditioned stimuli across acquisition and extinction periods of fear conditioning (Vansteenwegen et al. 1998; Walker et al. 2002).
Both human and nonhuman research indicates that the startle response can be used to measure amygdala-dependent fear conditioning (LaBar et al. 1998; Phelps et al. 2001; Phillips & LeDoux 1992). In light of recent work highlighting the importance of BDNF during amygdala-dependent associative learning, the goal of the present study was to relate variation in the human BDNF genotype to the fear-potentiated startle response during a differential fear-conditioning paradigm in which stimuli varied in their perceptual similarity to the CS+.
Methods and materials
Participants and genotyping
Sixty-two college students (33 females) were genotyped for the Val66Met single nucleotide BNDF polymorphism (rs6265). [From the original sample of 62 participants, 3 (two females) were excluded because they did not produce quantifiable startle responses; another 2 (one female) were excluded because their sample did not yield adequate genetic material for genotyping. Three samples were initially selected as the expected genotypes (Val/Val, Val/Met and Met/Met) based on melt analyses and were confirmed by DNA sequencing. These samples were used on every polymerase chain reaction (PCR) plate for comparison.] All participants received course credit for participation. DNA was extracted from buccal cells using the Quick Extract DNA Extraction Solution (Epicentre Technologies, Madison, WI, USA). Genotype analysis was performed with high-resolution melt analysis. PCR was carried out in a 10-µl volume with forward (5′-TGGTCCTCATCCAACAGCTC-3′) and reverse (5′-CCCAAGGCAGGTTCAAGAG-3′) primers. Each amplification was overlaid with mineral oil and contained 2 µl of extracted buccal DNA, 0.25 µm of each primer and 1× Light Scanner Master Mix (Idaho Technology Inc., Salt Lake City, UT, USA). Reaction conditions began with a denaturation at 94°C for 2 min, followed by 40 cycles of 94°C for 30 seconds, 66.6°C for 30 seconds and 72°C for 30 seconds. Melt analysis was performed between 75 and 95°C (0.1°C/seconds) with a Light Scanner (Idaho Technology Inc.) and SNP status determined using the Small Amplicon Module. The average peaks for the Met/Met, Val/Met and Val/Val genotypes were obtained at 84.8, 84.5 and 85.3°C, respectively. One individual with each genotype was sequenced to confirm accuracy of the high-resolution melt analysis (data not shown).
Stimuli
To assess fear conditioning in the present study, a paradigm was employed in which participants were shocked following a specific CS+ but were presented with a range CS− stimuli that varied in perceptual similarity to the CS+. This design was employed to provide a richer representation of fearful responding to complex stimuli, more akin to real-world scenarios where danger and safety cues share perceptual similarities (cf. Lissek et al. 2008).
To this end, seven rectangles that were identical in height (56 pixels) but ranged from 112 to 448 pixels in width served as the stimuli and were presented in red against a white background on a 19-inch monitor set with a resolution of 1024 × 768 pixels. The middle-sized rectangle (218 pixels wide) was always the threat cue (CS+); six other stimuli differed by 20, 40 or 60% in width from the CS+ (hereafter CS+, CS ± 20%, CS ± 40% and CS ± 60%, respectively). At a viewing distance of 25 inches, each stimulus occupied approximately 1.5°of visual angle vertically and 4.0–15.0°of visual angle horizontally.
The startle probe was a 50-ms burst of white noise that was set to a volume of 105 dB and was delivered through headphones using a noise generator (Contact Precision Instruments, Cambridge, MA, USA). Electrical shocks were delivered to the participant’s left tricep using an electrical stimulator (Contact Precision Instruments) that produced 60 Hz constant AC stimulation between 0 and 5 mA for 500 ms. All stimuli and psychophysiological responses were presented and recorded using PSYLAB hardware and psylab 8 software (Contact Precision Instruments).
Procedure
The shock intensity for each participant was determined on an individual basis – participants initially received a mild shock, which was raised based on participant feedback. Participants were asked to choose a level of shock that would be uncomfortable but manageable.
A habituation phase (four trials) without any shocks was used to elicit initial extreme startle responses. Next, the experimenter informed the participant that they would always be shocked following the presentation of the middle length rectangle (i.e. the CS+) and that they would never be shocked following the presentation of all other rectangles. The experimenter showed a trial that consisted of the CS+ followed by a shock.
The remainder of the experiment consisted of three blocks of 12 trials (12 CS+, 8 CS ± 20%, 8 CS ± 40% and 8 CS ± 60% trials in total). The order of stimulus presentation was random; 4 CS+ stimuli were presented in each block. Stimuli were presented for 8 seconds with a 10–12 seconds intertrial interval (ITI); startle probes were delivered on every trial 5–7 seconds following stimulus onset. Startle probes were also presented six times during random ITI periods to reduce the predictability of the startle probes.
Last, all participants completed a self-report rating of shock likelihood. Each rectangle was rated using a 5-point Likert-type scale that ranged from “certainly not shocked” (1) to “certainly shocked” (5); “unsure” was the midpoint (3).
Data recording, reduction and analysis
Startle-elicited electromyographic (EMG) activity was recorded using a PSYLAB Stand Alone Monitor Unit and Bio Amplifier (Contact Precision Instruments). Two Ag–AgCl electrodes were positioned approximately 25 mm apart over the orbicularis oculi muscle beneath the left eye. A third electrode on the forehead served as an isolated ground. EMG activity was sampled at 500 Hz, and band-pass filtered between 30 and 500 Hz. Startle EMG response was rectified in a 200-ms window beginning 50 ms before the startle probe and smoothed using a 6-point running average. The startle amplitude was quantified as the peak response in a 150-ms post-probe window relative to the average activity in the 50 ms preprobe baseline period. Startle amplitude for each subject was converted to T scores to reduce between-subject variability unrelated to variables of interest. Comparable results, however, were obtained when raw scores were analyzed.
All measures were statistically evaluated through repeated measures anova with the Greenhouse-Geisser correction applied. Generalization effects were examined using a 2 (BDNF genotype: Val/Val, Met carrying) × 4 (stimulus type: CS+, CS ± 20%, CS ± 40% and CS ± 60%) repeated measures anova. To identify points on the stimulus continuum in which startle was reliably potentiated, paired sample t tests were performed relative to the CS ± 60% stimuli using Bonferroni’s correction for multiple comparisons (0.05/4 = 0.0125).
Results
BDNF genotypes
Of the 57 participants, 44 individuals carried the Val/Val BDNF allele (25 female), 10 carried the Val/Met allele (4 female) and 3 carried the Met/Met allele (1 female). Because of the relative infrequency of the Met/Met allele, and consistent with previous studies, individuals carrying at least one Met allele were grouped together and compared with individuals homozygous for the Val allele (Frodl et al. 2007; Hariri et al. 2003; Miyajima et al. 2008; Pezawas et al. 2004). The two groups did not differ in terms of gender [χ2(1, N = 57) = 1.36, P > 0.20] or ethnic composition [χ2(5, N = 57) = 3.08, P > 0.60].
Startle EMG
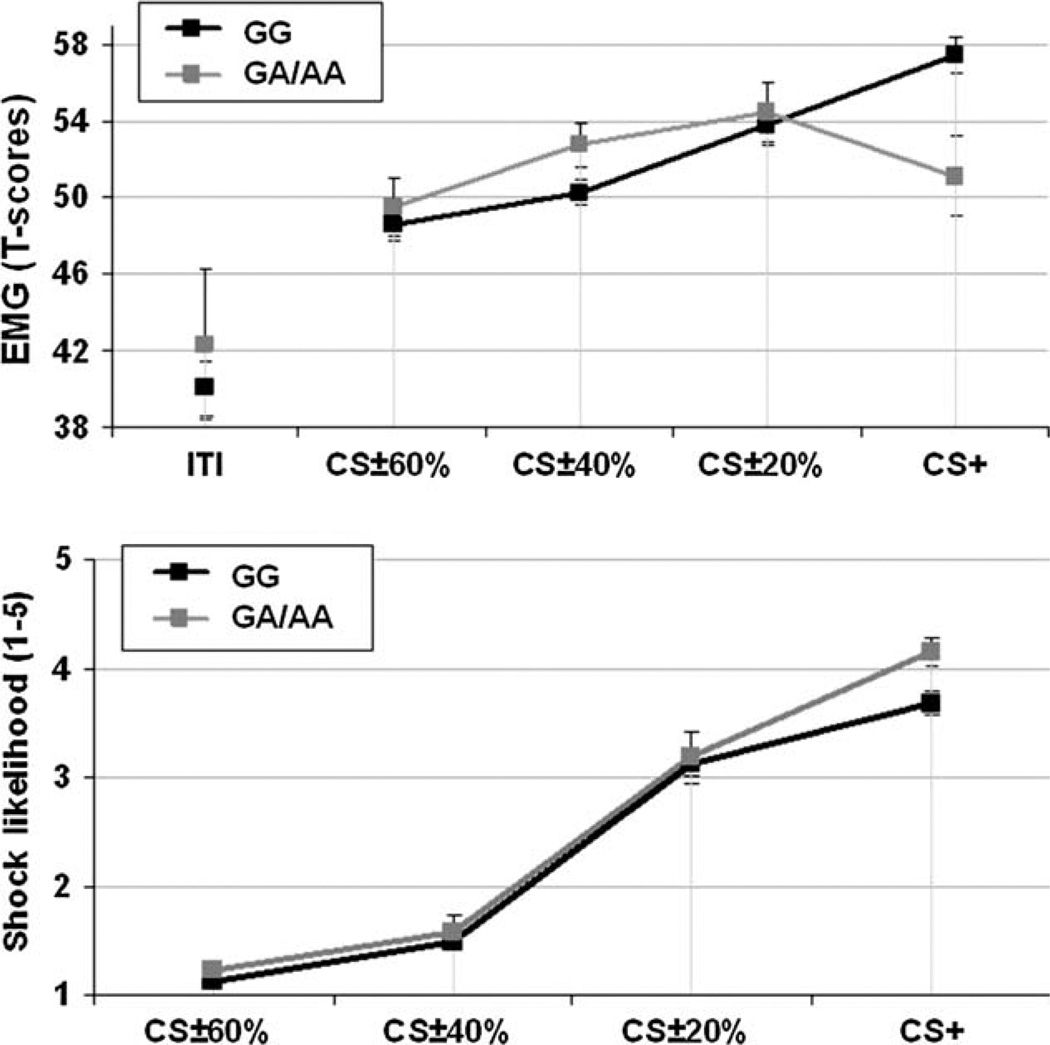
Figure 1 (top) presents startle T scores elicited during the ITI and presentation of all stimuli. Consistent with the impression from Fig. 1, startle magnitude varied as a function of stimulus type [F(3,165) = 6.39, P < 0.001]. However, this effect was qualified by a significant interaction with BDNF genotype [F(3,165) = 3.90, P < 0.05]; startle did not differ overall between BDNF genotypes [F(1,55) < 1]. [The Val/Val and Met-carrying groups (Val/Met and Met/Met) did not differ in terms of their ITI startle responses (mean = 40.01, SD = 9.33 and mean = 42.33, SD = 14.13, respectively, t(55) = 0.70, P > 0.45). Also, the Val/Val (mean = 128.64, SD = 54.08) and Met-carrying groups (mean = 131.92, SD = 45.16) did not differ in terms of selected shock intensity, t(55) = 0.20, P > 0.80. Moreover, we examined EMG activity elicited by the unconditioned stimulus itself: although Met-carrying individuals had numerically larger startle responses to the UCS (mean = 17.96, SD = 18.27) compared with the Val/Val individuals (mean = 10.92 mv, SD = 11.96), this difference did not reach significance t(55) = 1.63, P > 0.10. Finally, when EMG to the UCS was entered as a covariate in the repeated measures anova, the significant interaction between stimulus type and BDNF genotype remained robust, F(3,162) = 3.35, P < 0.05].
Figure 1. Startle response magnitude and post-task shock likelihood ratings as a function of stimulus type.
Standardized EMG activity elicited by startle probes (top) and behavioural ratings of shock likelihood (bottom; 1 = “certainly not shocked”, 5 = “certainly shocked”) for each stimulus type for both individuals carrying the Val/Val BDNF genotype (N = 44) and those carrying one or two Met alleles of the BDNF genotype (N = 13).
Among those participants homozygous for the Val allele, post hoc comparisons confirmed that startle magnitude was potentiated (relative to the CS ± 60% stimuli) for both CS+ [t(43) = 5.69, P < 0.001] and CS ± 20% [t(43) = 3.62, P < 0.001] stimuli; there was a trend for startle potentiation to CS ± 40% stimuli [t(43) = 1.91, P > 0.05]. Overall then, individuals with the Val/Val genotype showed potentiation of their startle response to CS+ stimuli and generalized defensive responding to perceptually similar stimuli (i.e. CS ± 20%).
Importantly, Met-carrying individuals did not show the same relation between startle and stimulus type. Rather, Met allele carriers only showed a potentiated startle response to CS ± 20% stimuli [t(12) = 3.30, P < 0.0125]; Met allele carriers did not show a potentiated startle response to either CS ± 40% [t(12) = 2.22, P < 0.05] or CS+ [t(12) = 0.54, P > 0.55] stimuli.
Self-reported shock likelihood
Figure 1 (bottom) presents post-task ratings of shock likelihood for both Val/Val and Met allele carriers as a function of stimulus type. Although shock likelihood ratings varied as a function of stimulus type [F(3,165) = 90.41, P < 0.001], ratings did not differ as a function of BDNF genotype [F(1,55) = 1.37, P > 0.20] and BDNF genotype did not interact with stimulus type [F(3,165) < 1]. Shock was rated as more likely following the CS+ stimuli relative to CS ± 60% [t(58) = 13.23, P < 0.001], CS ± 40% [t(58) = 10.69, P < 0.001] and CS ± 20% [t(58) = 3.43, P < 0.001] stimuli. Additionally, shock was rated as more likely following CS ± 20% compared with CS ± 40% [t(58) = 15.20, P < 0.001] and CS ± 60% [t(58) = 21.03, P < 0.001] stimuli; finally, shock was rated as more likely following CS ± 40% than CS ± 60% stimuli [t(58) = 4.65, P < 0.001]. Thus, shocks were perceived as being progressively likely as stimuli became more perceptually similar to the CS+.
Discussion
Participants in the current study reported that shock was more likely as stimuli resembled the CS+, despite the fact that only the CS+ was ever followed by an aversive shock. Indeed, individuals homozygous for the Val/Val BDNF polymorphism also showed an increase in startle response as stimuli were more perceptually similar to the CS+. That is, individuals with the Val/Val BDNF genotype showed a robust potentiation of the their startle response to both CS+ and CS ± 20% compared with CS ± 60% stimuli; indeed, there was a trend towards potentiation of the startle response to CS ± 40% stimuli as well. These results dovetail nicely with those reported by Lissek et al. (2008), who also found that perceived risk and startle potentiation were related to perceptual similarity of stimuli to a CS+.
However, a different pattern of startle potentiation was obtained among individuals carrying one or two Met alleles of the BDNF polymorphism. Specifically, Met allele carriers did not show potentiation of the startle response to CS+ stimuli. This group did show a potentiation of their startle response to CS ± 20% stimuli, suggesting that Met allele carriers were characterized by a specific deficit on CS+ trials. Thus, both Val/Val and Met-carrying groups showed comparable generalization of fear-potentiated startle to stimuli that were perceptually similar to the CS+; however, only the Val/Val allele carriers showed a robust potentiation of startle to the actual CS+. In fact, among Met allele carriers, startle response to the CS+ was most similar to the CS ± 60% stimuli – the most perceptually dissimilar stimuli to the CS+.
Importantly, BDNF groups did not differ in their post-task ratings of shock likelihood, chosen level of shock intensity or EMG response amplitude to the UCS itself. Thus, the observed deficits in startle potentiation to the CS+ do not simply reflect a lack of awareness of shock contingencies. Rather, the reduction in startle potentiation to the CS+ among Met allele carriers may reflect a specific abnormality in defensive responding – potentially reflecting abnormal amygdala-mediated learning. Indeed, work on transgenic mice suggests that the Met allele may specifically impair hippocampus-dependent context conditioning (Liu et al. 2004). The degree to which the generalization task employed here relies on functioning of the hippocampus is unknown. Future studies may wish to examine whether the observed pattern of results during a generalization paradigm would also be evident in a simpler CS+/CS− paradigm and in context-conditioning paradigms (cf. Liu et al. 2004).
These results add an important dimension to existing work on the Val66Met BDNF polymorphism. Although previous work has linked the Met allele to abnormal hippocampal structure and function (Dempster et al. 2005; Egan et al. 2003; Hariri et al. 2003; Pezawas et al. 2004), the current study suggests that BDNF Met allele carriers are also characterized by deficient fear conditioning in this type of generalization paradigm. These results are generally consistent with recent nonhuman animal work on the fundamental role of BDNF during fear and context conditioning (Chhatwal et al. 2006; Conner et al. 1997; Jones et al. 2007; Liu et al. 2004; Ou & Gean 2006; Rattiner et al. 2004a,b; Yan et al. 1997) and suggest that BDNF might play a similar role in the acquisition of fear in humans.
These results have potential implications regarding mechanisms linking genetic variation in BDNF to risk for psychopathology. Among depressed patients for instance, recent studies have found that individuals who carry the Met allele of the BDNF gene have significantly reduced hippocampal volume (Frodl et al. 2007) and greater hypothalamic–pituitary–adrenocortical response to dexamethasone challenge (Schule et al. 2006). Reduced hippocampal volume and function have also been implicated in risk for posttraumatic stress disorder (PTSD; Gilbertson et al. 2002, 2006).
The current study raises the possibility that the Met allele of the BDNF polymorphism may place individuals at risk for forms of psychopathology such as depression and PTSD – and may do so by altering processes relevant to fear conditioning. Specifically, a deficient ability to elicit defensive responses to appropriate stimuli may underlie generalization of fear in PTSD following a trauma. In the case of incorrectly learned CS–UCS contingencies, aversive stimuli and events may be more unexpected, and the situations might be more stressful overall. These possibilities are consistent with the fact that the UCS and ITI startle magnitudes in the Met group were higher than Val/Val participants; future studies might further examine the UCS and ITI responses during cue and context conditioning as a function of BDNF polymorphism. In addition, it will be important to determine whether Met-related startle deficits relate to differences in memory and behavior – for instance, whether Met allele carriers are less likely to return for a second testing session and, among those who do, whether they would continue to show a reduced fear-potentiated startle response to the CS+ (cf. Ameli et al. 2001; Grillon & Davis 1997).
In summary, the present data suggest that individuals homozygous for the Val allele of the Val66Met BDNF polymorphism show a potentiation of the defensive startle response to CS+ stimuli as well as perceptually similar stimuli that were never followed by an aversive shock (i.e. CS ± 20% stimuli). These results are consistent with a recent startle study on fear generalization by Lissek et al. (2008). Individuals carrying one or two Met alleles of the BDNF gene, however, were characterized by deficient fear-potentiated startle specifically to the CS+. This pattern of results was evident despite relatively normal generalization of potentiated startle to CS ± 20% stimuli and post-task ratings of shock likelihood comparable to the Val/Val group. These results are consistent with recent nonhuman animal work implicating BDNF in amygdala-based associative learning processes and suggest that the Met allele of the BDNF polymorphism relates to abnormal fear conditioning.
References
- Ameli R, Ip C, Grillon C. Contextual fear-potentiated startle conditioning in humans: replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- Brown JS, Kalish HI, Farber IE. Conditioned fear as revealed by magnitude of startle response to an auditory stimulus. J Exp Psychol. 1951;41:317–328. doi: 10.1037/h0060166. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The mammalian startle response. In: Eaton RC, editor. Neural Mechanisms of Startle Behavior. New York, NY: Plenum Press; 1984. pp. 287–351. [Google Scholar]
- Davis M. Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol. 2006;61:741–756. doi: 10.1037/0003-066X.61.8.741. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Dempster E, Toulopoulou T, McDonald C, Bramon E, Walshe M, Filbey F, Wickham H, Sham PC, Murray RM, Collier DA. Association between BDNF val66 met genotype and episodic memory. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:73–75. doi: 10.1002/ajmg.b.30150. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M, Moller H-J, Meisenzahl EM. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 2007;64:410–416. doi: 10.1001/archpsyc.64.4.410. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, Orr SP. Neurocognitive function in monozygotic twins discordant for combat exposure: relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Fear-potentiated startle in humans: effects of anticipatory anxiety on the acoustic blink reflex. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SV, Stanek-Rattiner L, Davis M, Ressler KJ. Differential regional expression of brain-derived neurotrophic factor following olfactory fear learning. Learn Mem. 2007;14:816–820. doi: 10.1101/lm.781507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Carroll P, Wolf E, Brem G, Thoenen H, Bonhoeffer T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, Cornwell BR, Alvarez RP, Pine DS, Grillon C. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav Res Ther. 2008;46:678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu IYC, Lyons WE, Mamounas LA, Thompson RF. Brain-derived neurotrophic factor plays a critical role in contextual fear conditioning. J Neurosci. 2004;24:7958–7963. doi: 10.1523/JNEUROSCI.1948-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Gottschalk W. Modulation of hippocampal synaptic transmission and plasticity by neurotrophins. Prog Brain Res. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Pendleton N, Horan M, Payton A. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav. 2008;7:411–417. doi: 10.1111/j.1601-183X.2007.00363.x. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, LeDoux JE. Brain-derived neurotrophic factor: linking fear learning to memory consolidation. Mol Pharmacol. 2007;72:235–237. doi: 10.1124/mol.107.038232. [DOI] [PubMed] [Google Scholar]
- Ou LC, Gean PW. Regulation of amygdala-dependent learning by brain-derived neurotrophic factor is mediated by extracellular signal-regulated kinase and phosphatidylinositol-3-kinase. Neuropsychopharmacology. 2006;31:287–296. doi: 10.1038/sj.npp.1300830. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, Ledoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102. doi: 10.1523/JNEUROSCI.2680-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci. 2001;4:437–441. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004a;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learn Mem. 2004b;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–333. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Ressler K, Davis M. Genetics of childhood disorders: L. Learning and memory, part 3: fear conditioning. J Am Acad Child Adolesc Psychiatry. 2003;42:612–615. doi: 10.1097/01.CHI.0000046835.90931.32. [DOI] [PubMed] [Google Scholar]
- Schule C, Zill P, Baghai TC, Eser D, Zwanzger P, Wenig N, Rupprecht R, Bondy B. Brain-derived neurotrophic factor Val66Met polymorphism and dexamethasone/CRH test results in depressed patients. Psychoneuroendocrinology. 2006;31:1019–1025. doi: 10.1016/j.psyneuen.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;B126:122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Longterm potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Vansteenwegen D, Crombez G, Baeyens F, Eelen P. Extinction in fear conditioning: effects on startle modulation and evaluative self-reports. Psychophysiology. 1998;35:729–736. [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intraamygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE. Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci. 2006;26:12387–12396. doi: 10.1523/JNEUROSCI.4316-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Rosenfeld RD, Matheson CR, Hawkins N, Lopez OT, Bennett L, Welcher AA. Expression of brain-derived neurotrophic factor protein in the adult rat central nervous system. Neuroscience. 1997;78:431–448. doi: 10.1016/s0306-4522(96)00613-6. [DOI] [PubMed] [Google Scholar]