Abstract
The alleles that are detrimental to health, especially in older age, are thought to persist in populations because they also confer some benefits for individuals (through antagonistic pleiotropy). The ApoE4 allele at the ApoE locus, encoding apolipoprotein E (ApoE), significantly increases risk of poor health, and yet it is present in many populations at relatively high frequencies. Why has it not been replaced by natural selection with the health-beneficial ApoE3 allele? ApoE is a major supplier of cholesterol precursor for the production of ovarian oestrogen and progesterone, thus ApoE has been suggested as the potential candidate gene that may cause variation in reproductive performance. Our results support this hypothesis showing that in 117 regularly menstruating women those with genotypes with at least one ApoE4 allele had significantly higher levels of mean luteal progesterone (144.21 pmol l−1) than women with genotypes without ApoE4 (120.49 pmol l−1), which indicates higher potential fertility. The hormonal profiles were based on daily data for entire menstrual cycles. We suggest that the finding of higher progesterone in women with ApoE4 allele could provide first strong evidence for an evolutionary mechanism of maintaining the ancestral and health-worsening ApoE4 allele in human populations.
Keywords: antagonistic pleiotropy, apolipoprotein polymorphism, fertility, progesterone, ApoE
1. Introduction
An evolutionary approach to human health, comprising both evolutionary medicine and evolutionary public health, attempts to explain susceptibility of the human organism to diseases, especially those that influence ageing and lifespan [1–3]. Moreover, it is focused on individual variation in disease risks, which is due to the complex interaction of genetic, epigenetic and environmental factors [4].
Alleles that encode traits that increase risk of diseases segregate at many polymorphic loci in human populations, but one could expect their selective removal from populations. It is suggested that the alleles that are detrimental to health persist in populations because they also confer some benefits for individuals. Such a phenomenon, known as antagonistic pleiotropy (for a review see [5]) works, for example, through improving survival of allele carriers in young age or increasing their reproductive potential at maturity.
More generally, the presence of phenotypic trade-offs between fitness-related traits (such as survival and fecundity) does not by itself indicate antagonistic pleiotropy, unless there is also negative genetic correlation between the traits involved in the trade-off (see [6]). Unfortunately, the observed phenotypic trade-offs in life-history traits often lack known genetic mechanisms underlying them [7]. The first step towards documenting such pleiotropy is to show that genotypes at the locus in question differ phenotypically in some fitness components. Genetic trade-offs have been described for many species, including bacteria, yeast, nematodes, flies, birds and mice [8].
Polymorphism of gene ApoE, encoding apolipoprotein E (ApoE) that is involved in cholesterol metabolism, is discussed in the literature as a classic example of such antagonistic pleiotropic effects [9,10]. In humans, the ApoE locus has three commonly occurring alleles: apoE4Arg112/Arg158, ApoE3Cys112/Arg158 and ApoE2Cys112/Cys158 [11]. ApoE4 is considered the ancestral allele, while the ApoE3 allele is suggested to have appeared within the last 220 000 years, and ApoE2 was derived from ApoE3 more recently [12].
Carriers of ApoE4 allele have higher levels of cholesterol, owing to more efficient absorption from the intestine, and, consequently, face a higher risk of hypertension and of cardiovascular and Alzheimer's diseases [13–16]. By contrast, the ApoE3 allele is related to lower levels of cholesterol and a lower disease risk. Since the ApoE4 allele significantly increases the risk of poor health, it needs to be explained why it is present in many populations at relatively high frequencies [17] and why natural selection has not replaced it with the health-neutral or health-beneficial ApoE3 allele.
Carrying the ApoE4 allele may confer some advantages, however. It may be related to better development of the cognitive function [18,19], protection against infectious diseases [20–23] or to reproductive advantage [24], but the results of studies conducted in all these areas have been inconclusive [9,25,26]. We suggest that the ApoE4 allele has persisted in the human population because it significantly increases reproductive potential in women. We show that those women who are carriers of the ApoE4 allele have higher levels of progesterone in their menstrual cycles, which implies higher potential fertility.
2. Population and methods
(a). Subjects
One hundred and eighty-six Polish women between 24 and 37 years of age (mean age 29.9, s.d. = 3.54), who had regular menstrual cycles, did not take any hormonal medication or contraception, had no fertility problems and had not been pregnant or lactating during the six months before recruitment, collected daily, morning saliva samples for one entire menstrual cycle, that is from the first day of menstrual bleeding to the first day of bleeding of the next menstruation. Women received detailed written and oral instructions for collecting samples immediately after waking up, before eating, drinking or brushing their teeth. The women recorded duration and intensity of their daily physical activity, collected dietary, reproductive history and demographic data by questionnaires, and had anthropometric measurements taken by a trained researcher. A detailed description of the study was published elsewhere [27]. Mean length of menstrual cycle was 28.4 days (s.d. 3.46, range 22–38 days). Of the 186 participants who provided salivary specimens, 118 agreed to give a blood sample for genetic analysis.
(b). Genotyping
The ApoE is encoded by the polymorphic gene with three most common isoforms E2, E3 and E4, based on 2 SNPs (single-nucleotide polymorphisms) at positions 2059T/C (codon 112 Cys/Arg) and 2197 C/T (codon 158 Arg/Cys). For the polymorphic discrimination of ApoE genotypes, the method of sequence-specific primers PCR was used [28]. For each DNA sample, three PCR mixtures specific for each ApoE allele were prepared. All mixtures contained PCR buffer ((NH4)2SO4), MgCl2, dNTPs, Taq DNA polymerase (Thermoscientific) and reference primers (primers 5 and 6) for HLA-DR locus in chromosome 6 as the control of positive PCR amplification. Reaction mixture for ApoE2 contained specific primers 1 and 3. Reaction mixture for amplification of ApoE3 isoform contained primers 1 and 2, and for ApoE4 primers 4 and 2.
Primer sequences: primer 5: TGC CAA GTG GAG CAC CCA A; primer 6: GCA TCT TGC TCT GTG CAG AT; primer 1: CGG ACA TGG AGG ACG TGT (Cys112 specific); primer 2: CTG GTA CAC TGC CAG GCG (Arg158 specific); primer 3: CTG GTA CAC TGC CAG GCA (Cys158 specific); primer 4: CGG ACA TGG AGG ACG TGC (Arg112 specific). The PCR cycling condition were: initial denaturation for 1 min at 96°C; followed by 5 cycles of 20 s at 96°C, 45 s at 70°C, and 25 s at 72°C; 21 cycles of 25 s at 96°C, 50 s at 65°C, and 30 s at 72°C; 4 cycles of 30 s at 96°C, 60 s at 55°C, and 120 s at 72°C. The PCR products were visualized on 2% agarose gel with Midori Green in TBE buffer. The three specific PCR products of each sample were run in the neighbouring rows.
The results were verified by the standard PCR/RFLP method using HhaI restrictase [29]. HhaI enzyme cleaves the GCGC sequence that encodes Arg112 (E4) and Arg158 (E3, E4), but does not cut the GTGC sequence that encodes Cys112 (E2 and E3) and Cys158 (E2). The E2/E2 genotype was cut to 91 and 83 bp HhaI fragments. The E3/E3 genotype was cut to 91, 48 and 35 bp bands. The E4/E4 sample had 48, 35 and 72 bp bands. The heterozygotes had the proper combination of cleaved fragments.
(c). Hormone assays
Saliva samples from the last 14 days (reverse cycle day −1 to −14) of each cycle were analysed for the concentration of progesterone (P) and saliva samples from 20 days (reverse cycle day −5 to −24) of each cycle were analysed for the concentration of 17-b-oestradiol (referred to simply as oestradiol) by radioimmunoassay. The details of laboratory analysis are described elsewhere [30]. Prior to statistical analyses of the oestradiol levels, the cycles were aligned based on the identification of the day of the midcycle oestradiol drop (day 0), which provides a reasonable estimate of the day of ovulation [31]. Oestradiol values from 18 consecutive days of each cycle aligned on day 0 were used in analyses (n = 90). Because of missing samples, a reliable identification of the day of the midcycle oestradiol drop could not be done for 11 subjects (10 for genotypes without ApoE4 and 1 for genotypes with ApoE4), and they were excluded from oestradiol analyses. Data from all 117 cycles were used in the P analyses.
(d). Statistical analysis
Differences in hormonal indices between two genotypic classes of women were tested in a factorial repeated-measures analysis of variance, using JMP 9 (SAS Institute, Cary, NC, USA 2011). Genotypic categories (i.e. genotypes with at least one ApoE4 allele and genotypes without ApoE4 alleles) were the first factor. Since energy expenditure is known to have a strong effect on reducing levels of reproductive hormones in women, the second factor comprised three groups that were established based on the tertiles of the distribution of the mean daily physical activity level (low, moderate and high) assessed for each woman. Both between-subjects factors were entered into the model in a full interaction design.
The within-subjects factor was formed either by 14 consecutive measurements of the progesterone concentrations on days from −14 to −1 or by 18 consecutive measurements of the oestradiol concentrations on days from −9 to +8. We analysed the log-transformed cumulative curves created for each woman from individual measurements. Missing data were imputed with interpolated values, calculating arithmetic means of two neighbouring or two consecutive measurements.
Since the sphericity requirements were violated in both analyses (Mauchly criterion close to zero, p < 0.0001), which is not surprising considering that the analysed profiles are temporal variables [32], adjusted univariate significance tests were carried out. We used the Huyhn–Feldt correction (H-F), which is recommended when the compound symmetry assumption is not necessary [33]. The use of univariate tests was further necessitated by the fact that the number of within-subject variables (14 or 18 days of the hormonal profiles) was greater than the number of subjects in the smallest group [32].
Several lifestyle and reproductive factors are known to influence levels of ovarian steroid hormones and fertility in healthy women [27,34–39]. Therefore, we analysed differences between genotypes with at least one ApoE4 allele and without ApoE4 alleles in age, reproductive characteristics (menarcheal age, number of children, age at first reproduction, length of menstrual cycle), body size (at birth and in adulthood), levels of daily physical activity and of education.
The hormonal analyses in our study were based on the daily collected samples for the entire menstrual cycle, which assured high reliability of the hormonal assessment for each participant [40]. However, owing to the fact that the ApoE4 allele is not present in most populations with high frequency, our sample size did not allow for multivariate analyses. Such analyses should be conducted in future studies in order to properly control for the confounding effects of the environmental and genetic factors [4,41].
3. Results
Frequencies of ApoE alleles in the studied sample are presented in table 1. In the group of 118 participating women, 99 did not have any ApoE4 alleles (89 were E3E3 homozygotes and 10 E2E3 heterozygotes), and 19 had at least one ApoE4 allele. Out of these 19 women, three were ApoE4 homozygotes, 15 were E3E4 heterozygotes and one was of the E2E4 genotype. Genotype frequencies did not differ significantly from those expected under the Hardy–Weinberg equilibrium (χ2-test with Yates's correction 1.07, d.f. = 4, p = 0.899, confirmed by Fisher's exact test, p = 0.862). We also compared genotype frequencies observed in our study with the frequency reported in a study on a randomly selected sample of 170 women from the Polish population [42], and these two samples did not differ significantly in the genotype frequencies (χ2-test with Yates's correction 2.649, d.f. = 5, p = 0.754, confirmed by Fisher's exact test, p = 0.337).
Table 1.
In 117 regularly menstruating women, those with genotypes with at least one ApoE4 allele had higher levels of mean luteal progesterone (144.21 pmol l−1) than women with genotypes without ApoE4 (120.49 pmol l−1). The mean progesterone values in the subsample of 100 women for whom both progesterone and physical activity data were available were 141.82 pmol l−1 for genotypes with ApoE4 (n = 18) and 115.73 pmol l−1 for genotypes without ApoE4 (n = 82)—the former were significantly (by over 20%) higher (between-subjects test, F1,94 = 4.273, p = 0.042; figure 1). The cumulative progesterone profiles across 14 days were similar in both genotypic categories, as shown by the insignificant time–genotype interaction term (F2,228 = 1.037, p = 0.367, with H-F correction). The overall effects of physical activity (F2,94 = 0.032, p = 0.968) were not detectable, and the interaction between genotypic category and physical activity was also not statistically significant (F2,94 = 1.078, p = 0.345). Finally, there was no significant variation among cumulative profiles of progesterone within six genotype–activity combinations (F5,228 = 0.548, p = 0.734, with H-F correction).
Figure 1.
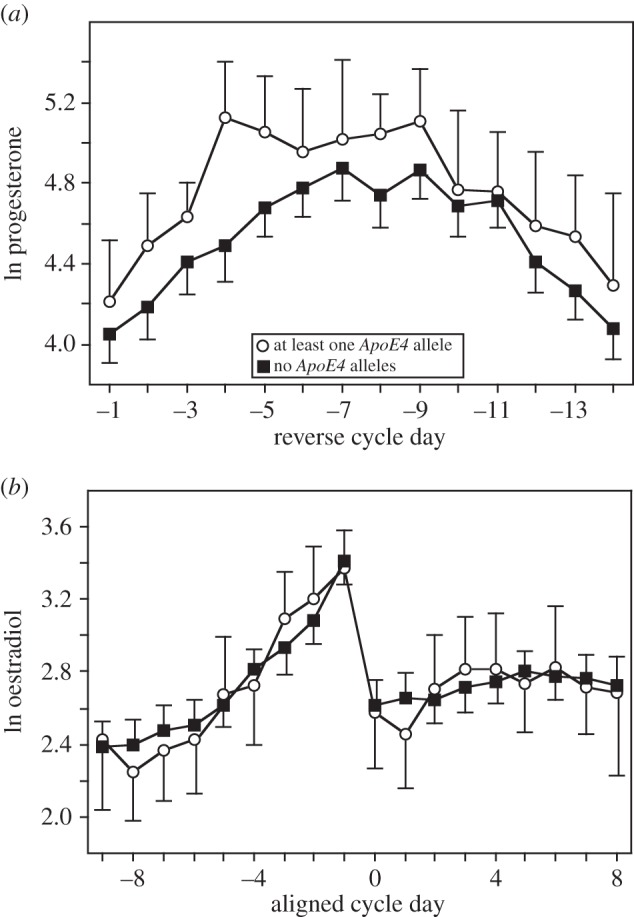
Profiles of progesterone and 17-b-oestradiol in groups of reproductive-age women with genotypes with at least one ApoE4 allele and genotypes without ApoE4 alleles.
Women of genotypes with at least one ApoE4 allele had similar levels of oestradiol in their menstrual cycles to women who did not have the ApoE4 allele (between-subjects test, F1,84 = 0.779, p = 0.380; figure 1). There was no statistically significant difference between these two genotypic classes in oestradiol levels: mean oestradiol values were 17.78 and 19.14 pmol l−1 (in women with ApoE4 allele and without, n =17 and n = 73, respectively). The cumulative oestradiol profiles across 18 days were similar in both genotypic categories, as shown by the insignificant time–genotype interaction term (F2,202 = 0.307, p = 0.776, with H-F correction). The effects of physical activity (F2,84 = 0.429, p = 0.652) and the interaction between genotypic category and physical activity were not statistically significant (F2,84 = 1.521, p = 0.224). There was a marginally significant variation among the cumulative profiles of oestradiol within six genotype–activity combinations (F5,202 = 2.304, p = 0.048, with H-F correction). However, small sample sizes within the ApoE4 genotypic category (5, 5 and 7 women in, respectively, low, moderate and high physical activity groups) suggest that further biological interpretations of this result may be premature.
Groups of women with at least one ApoE4 allele and women who did not have the ApoE4 allele did not differ significantly in the average values of characteristics that could potentially influence levels of steroid hormones in their cycles, and they did not differ in the number of children or age at first reproduction (table 2). However, women participating in our study originated from a controlled fertility population and were, on average, 30 years old, thus far from completing their lifetime reproduction.
Table 2.
Characteristics of groups of women with and without the ApoE4 allele. None of the differences (analysed by t-tests) was statistically significant. Numbers varied (from 118) due to missing observations.
| genotypes with ApoE4 n = 19 | genotypes without ApoE4 n = 99 |
|||
|---|---|---|---|---|
| mean (s.d.) | mean (s.d.) | t | p-value | |
| age (years) | 30.4 (3.02) | 29.4 (3.38) | 1.17 | 0.24 |
| age at menarche (years) | 13.4 (1.07) | 13.3 (1.23) | 0.35 | 0.73 |
| age at first birth (for parous women; years) | 22.2 (8.65) | 22.9 (2.68) | 0.48 | 0.63 |
| number of children | 0.68 (0.82) | 1.01 (1.23) | 1.10 | 0.27 |
| body weight (kg) | 58.2 (8.61) | 61.3 (10.36) | 1.23 | 0.22 |
| body height (cm) | 162.7 (5.39) | 163.5 (6.38) | 0.53 | 0.59 |
| BMI (kg m–2) | 22.1 (2.83) | 23.0 (3.79) | 1.08 | 0.28 |
| body fat (%) | 25.6 (4.69) | 26.5 (7.59) | 0.53 | 0.60 |
| birth weight (gr) | 3197.0 (788.68) | 3310.2 (600.0) | 0.67 | 0.50 |
| Ponderal index (kg m–3) | 19.96 (6.14) | 21.40 (4.96) | 0.99 | 0.32 |
| physical activity (MET/day) | 43.5 (14.61) | 42.8 (12.10) | 0.22 | 0.83 |
| length of menstrual cycle (days) | 28.5 (3.49) | 28.6 (3.60) | 0.14 | 0.89 |
| education (number of years) | 16.3 (3.0) | 15.3 (3.29) | 1.21 | 0.23 |
4. Discussion
We show that women with the ApoE4 allele have higher levels of progesterone during the menstrual cycle, and this finding suggests, albeit indirectly, an evolutionary reason for maintaining the ancestral ApoE4 allele in the human populations. The presence of the ApoE4 allele is related to several health problems, especially in older age. A meta-analysis showed that carriers of the ApoE4 allele have over 40% higher risk for coronary heart diseases compared with individuals with the ApoE3/3 genotype [16]. Another meta-analysis documented that the ApoE4 allele was a significant risk factor for Alzheimer's disease in all studied ethnic groups, across all ages, and in both men and women [14], and a recent meta-analysis showed that the ApoE4 allele significantly increased the risk of progression from mild cognitive impairment to Alzheimer's disease [44].
Because ApoE is a major supplier of cholesterol precursor for the production of steroid hormones, including ovarian oestrogen and progesterone, ApoE has been suggested as the potential candidate gene that may cause variation in reproductive performance [45]. Our results support this hypothesis—women with genotypes that include at least one ApoE4 allele have approximately 20% higher progesterone during their menstrual cycles than women without the ApoE4 allele, that is of genotypes E3/E3 or E3/E2 (none of our participants had the E2/E2 genotype).
Progesterone is essential for the establishment of pregnancy and its adequate production is critical to the maintenance of pregnancy until the placenta takes over this function [46]. Progesterone is involved in endometrial maturation and high levels of this hormone allow for complete transformation of the endometrium [47]. Progesterone levels in the luteal phase are positively correlated with the chance of successful conception [48,49] and negatively correlated with uterine arterial pulsatility, which was found to be associated with recurrent pregnancy loss [50]. In women undergoing embryo transfer, live birth rates were greater for those receiving progesterone, compared with those who did not take progesterone [51]. Also, in non-human mammals, there is increasing evidence of an association between the concentration of the systemic progesterone and an early embryo loss [52]. Progesterone is a physiological regulator of sperm motility and hyperactivation [53], and has also an immunomodulatory role and is involved in processes related to the maternal immuno-tolerance of the fetus [54,55].
Optimal steroid hormone production in the ovary critically depends on the availability of cholesterol [56]. Even though the ovary can synthesize cholesterol de novo, it preferentially uses the cholesterol supplied from LDL and HDL circulating in the plasma [57,58], thus higher levels of the circulating cholesterol present in ApoE4 carriers may support a higher production of steroid hormones, including progesterone. The conversion of cholesterol to pregnenolone, which is the initial step in the synthesis of steroid hormones, is considered a limiting stage in steroid production [56]. This limitation results from a limited availability of cholesterol at the substrate site of P450 (enzyme CYP11A1 converting cholesterol to pregnenolone—a rate-limiting, non-reversible step in the initiation of steroid biosynthesis), rather than from an insufficient activity of the P450 enzyme itself.
It is therefore not surprising that our study documented differences in progesterone levels, but not in oestradiol levels, in relation to the ApoE genotype. Progesterone is just a few metabolic steps away from cholesterol in the steroid production pathway. By contrast, the production of 17-beta oestradiol includes many steps with several different enzymes involved thus many more factors than just the availability of cholesterol constitute limiting steps in oestradiol production.
Several hypotheses have been proposed to account for the rise of the new ApoE3 allele during the course of human evolution [17,59–61]. Less interest, however, has been attracted by the issue of why ApoE4, the ancestral and health-worsening allele, is still present in human populations. There are at least two possible explanations. The first relies on the fact that diseases affect ApoE4 carriers mostly in older age. Therefore, the efficacy of natural selection as an evolutionary agent removing detrimental alleles from the gene pool is low, compared with the mutational pressure which continually restores them. Alternatively, the presence of alleles that significantly increase the risk of old-age diseases can be explained through the phenomenon of antagonistic pleiotropy [5]. Carrying the ApoE4 allele may improve fitness at a younger age, and any allele that increases survival at a young age or improves reproductive success is expected to be favoured by natural selection even if it reduces survival at an older age.
We are unable to determine whether the ApoE4 allele is simply being driven to extinction, but it may be observed in the population because the process is very slow. Alternatively, it may be maintained in the population in a balanced polymorphism at the ApoE locus. It would be premature to suggest that we are dealing with the latter scenario, because the conditions for the maintenance of such stable polymorphisms are sometimes quite stringent (as shown, for example, by Curtsinger et al. [6] for the case of antagonistic pleiotropy).
Evidence for the antagonistic effects of ApoE4 have been suggested, but not proved, for three different areas: cognitive benefits during childhood development, protection against infectious disease and reproductive advantage. Support for the hypothesis that young ApoE4 carriers have an advantage in developing cognitive function comes from studies documenting better memory and neural development [62], greater hippocampal activation when performing some tasks [19], advantage on tasks mediated by the frontal lobe [18], and a greater activation in medial frontal and anterior cingulate areas when using a working memory paradigm [63]. However, a recent meta-analysis of 20 studies shows that the ApoE4 allele is not associated with statistically significant cognitive benefits in young adults, adolescents or children. Thus, the pleiotropic effect of ApoE4 in cognitive abilities across the lifespan has not been proved [25].
The evidence for an increased protection against infectious diseases in ApoE4 carriers is also inconclusive. The most convincing evidence comes from a small sample of Brazilian shanty-town children, where those with a lower diarrhoea burden had a higher frequency of the ApoE4 allele, which suggests a potential protective effect of this allele [20]. A recent review pointed out that ApoE4 seems to be protective against a chronic hepatitis C virus infection, is associated with less severe liver disease in patients with chronic hepatitis B and C and HBV than in ApoE3 carriers [21,22], and reduces progression of fibrosis [23].
However, ApoE4 is clearly detrimental in cases of other infectious diseases. In infections with the human immunodeficiency virus, having ApoE4 accelerates cell entry and is related to a faster disease progression [64]. Similarly, in the herpes simplex virus infection, ApoE4 intensifies latency of the virus and is related to an increased oxidative damage to the central nervous system [23] and it also increases the susceptibility to Chlamydia pneumoniae [65]. The evidence that having ApoE4 protects against malaria parasites [66] is also inconclusive and, for example, the ApoE4 allele was associated with higher malaria parasite densities in Gabonese children, most probably due to a higher concentration of cholesterol, which is needed for parasite growth and replication [26].
Reproductive benefits associated with carrying the ApoE4 allele have been suggested by a study on two natural fertility populations from Ecuador, where the frequency of the ApoE4 allele was higher among women with high fertility (9–17 children) than among those with lower fertility [24]. By contrast, in Italy the ApoE4 allele was found to be associated with a lower fertility than was the ApoE3 allele [9], but it is important to emphasize that the result was probably spurious: the women in the study group had low fertility in general and came from a controlled fertility population. Further, the number of children in modern populations is not a good measure of reproductive potential since it is affected by many socio-cultural factors [2].
Why would ApoE4 carriers have higher fertility in natural fertility populations? Other mechanisms, in addition to the one of the higher progesterone levels observed in our study, could be suggested. The presence of the ApoE4 allele was related to a later age at menopause and a longer reproductive lifespan in Chinese women [67]. Having the ApoE4 allele was also related to less frequently occurring spontaneous abortions, suggesting some protective effects during embryogenesis [68,69].
Our results provide support, although indirect, for the hypothesis that carriers of ApoE4 have a reproductive advantage. Higher levels of progesterone in the menstrual cycle are related to higher fecundity (potential fertility) and thus contribute to higher reproductive success in women who are ApoE4 carriers. Finally, our results also suggest another (potentially important) pleiotropic effect of ApoE polymorphism, namely that of a trade-off between higher reproductive potential at a younger age and an increased breast cancer risk in the post-reproductive years. High lifetime levels of reproductive steroid hormones, including progesterone, are a major risk factor for breast cancer [70–72]. Evidence that women with ApoE4 have higher levels of progesterone suggests a causal link to the observed increased breast cancer risk in ApoE4 carriers [73–75]. Consequently, we believe that the reported results document conditions for antagonistic pleiotropy as an explanation for the persistence of the deleterious ApoE4 allele in the human population.
Acknowledgements
We are grateful to the women who participated in our study and to our assistants.
Ethics statement
The research protocol was approved by the Bioethical Committee of Jagiellonian University and informed written consent was obtained from each participant.
Data accessibility
Datasets supporting this article can be found at the Dryad data repository (doi:10.5061/dryad.vn63n).
Funding statement
This study was supported by grants from the Ministry of Science and Higher Education (grant no. IdP2011 000161 to G.J.), Salus Publica Foundation and Yale University Program in Reproductive Ecology.
References
- 1.Williams GC, Nesse RM. 1991. The dawn of Darwinian medicine. Q. Rev. Biol. 66, 1–22. ( 10.1086/417048) [DOI] [PubMed] [Google Scholar]
- 2.Jasienska G. 2013. The fragile wisdom: an evolutionary view on women's biology and health. Cambridge, MA: Harvard University Press. [Google Scholar]
- 3.Stearns SC. 2005. Issues in evolutionary medicine. Am. J. Hum. Biol. 17, 131–140. ( 10.1002/ajhb.20105) [DOI] [PubMed] [Google Scholar]
- 4.Jasienski M, Jasienska G. 2014. Nature–nurture interaction is ubiquitous, essential, but elusive. Curr. Anthropol. 55, 541–542. [Google Scholar]
- 5.Carter AJR, Nguyen AQ. 2011. Antagonistic pleiotropy as a widespread mechanism for the maintenance of polymorphic disease alleles. BMC Med. Genet. 12, 160–173. ( 10.1186/1471-2350-12-160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curtsinger JW, Service PM, Prout T. 1994. Antagonistic pleiotropy, reversal of dominance, and genetic-polymorphism. Am. Nat. 144, 210–228. ( 10.1086/285671) [DOI] [Google Scholar]
- 7.Zhang JZ, Wagner GP. 2013. On the definition and measurement of pleiotropy. Trends Genet. 29, 383–384. ( 10.1016/j.tig.2013.05.002) [DOI] [PubMed] [Google Scholar]
- 8.Delaney JR, Murakami CJ, Olsen B, Kennedy BK, Kaeberlein M. 2011. Quantitative evidence for early life fitness defects from 32 longevity-associated alleles in yeast. Cell Cycle 10, 156–165. ( 10.4161/cc.10.1.14457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbo RM, Ulizzi L, Piombo L, Scacchi R. 2008. Study on a possible effect of four longevity candidate genes (ACE, PON1, PPAR-gamma, and APOE) on human fertility. Biogerontology 9, 317–323. ( 10.1007/s10522-008-9143-9) [DOI] [PubMed] [Google Scholar]
- 10.Finch CE. 2010. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl Acad. Sci. USA 107, 1718–1724. ( 10.1073/pnas.0909606106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zannis VI, et al. 1982. Proposed nomenclature of APOE-isoproteins, APOE-genotypes, and phenotypes. J. Lipid Res. 23, 911–914. [PubMed] [Google Scholar]
- 12.Fullerton SM, et al. 2000. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am. J. Hum. Genet. 67, 881–900. ( 10.1086/303070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda M, Martinez R, Kudo T, Tanaka T, Okochi M, Tagami S, Morihara T, Hashimoto R, Cacabelos R. 2010. Apolipoprotein E and central nervous system disorders: reviews of clinical findings. Psychiatry Clin. Neurosci. 64, 592–607. ( 10.1111/j.1440-1819.2010.02148.x) [DOI] [PubMed] [Google Scholar]
- 14.Farrer LA, et al. 1997. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease—a meta-analysis. JAMA 278, 1349–1356. ( 10.1001/jama.278.16.1349) [DOI] [PubMed] [Google Scholar]
- 15.Niu WQ, Qi Y, Qian YS, Gao PJ, Zhu DL. 2009. The relationship between apolipoprotein E epsilon 2/epsilon 3/epsilon 4 polymorphisms and hypertension: a meta-analysis of six studies comprising 1812 cases and 1762 controls. Hypertens. Res. 32, 1060–1066. ( 10.1038/hr.2009.164) [DOI] [PubMed] [Google Scholar]
- 16.Song YQ, Stampfer MJ, Liu SM. 2004. Meta-analysis: Apolipoprotein E genotypes and risk for coronary heart disease. Ann. Intern. Med. 141, 137–147. ( 10.7326/0003-4819-141-2-200407200-00013) [DOI] [PubMed] [Google Scholar]
- 17.Corbo RM, Scacchi R. 1999. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann. Hum. Genet. 63, 301–310. ( 10.1046/j.1469-1809.1999.6340301.x) [DOI] [PubMed] [Google Scholar]
- 18.Marchant NL, King SL, Tabet N, Rusted JM. 2010. Positive effects of cholinergic stimulation favor young APOE epsilon 4 carriers. Neuropsychopharmacology 35, 1090–1096. ( 10.1038/npp.2009.214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. 2009. Distinct patterns of brain activity in young carriers of the APOE-epsilon 4 allele. Proc. Natl Acad. Sci. USA 106, 7209–7214. ( 10.1073/pnas.0811879106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oria RB, Patrick PD, Zhang H, Lorntz B, Costa CMD, Brito GAC, Barrett LJ, Lima AAM, Guerrant RL. 2005. APOE4 protects the cognitive development in children with heavy diarrhea burdens in northeast Brazil. Pediatr. Res. 57, 310–316. ( 10.1203/01.pdr.00000148719.82468.ca) [DOI] [PubMed] [Google Scholar]
- 21.Wozniak MA, Itzhaki RF, Faragher EB, James MW, Ryder SD, Irving WL. 2002. Apolipoprotein E-epsilon 4 protects against severe liver disease caused by hepatitis C virus. Hepatology 36, 456–463. ( 10.1053/jhep.2002.34745) [DOI] [PubMed] [Google Scholar]
- 22.Toniutto P, et al. 2010. Genetic polymorphism at the apolipoprotein E locus affects the outcome of chronic hepatitis B. J. Med. Virol. 82, 224–231. ( 10.1002/jmv.21642) [DOI] [PubMed] [Google Scholar]
- 23.Kuhlmann I, Minihane AM, Huebbe P, Nebel A, Rimbach G. 2010. Apolipoprotein E genotype and hepatitis C, HIV and herpes simplex disease risk: a literature review. Lipids Health Dis. 9, 8–22. ( 10.1186/1476-511x-9-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corbo RM, Ulizzi L, Scacchi R, Martinez-Labarga C, De Stefano GF. 2004. Apolipoprotein E polymorphism and fertility: a study in pre-industrial populations. Mol. Hum. Reprod. 10, 617–620. ( 10.1093/molehr/gah082) [DOI] [PubMed] [Google Scholar]
- 25.Ihle A, Bunce D, Kliegel M. 2012. APOE epsilon 4 and cognitive function in early life: a meta-analysis. Neuropsychology 26, 267–277. ( 10.1037/a0026769) [DOI] [PubMed] [Google Scholar]
- 26.Rougeron V, Woods CM, Tiedje KE, Bodeau-Livinec F, Migot-Nabias F, Deloron P, Luty AJF, Fowkes FJI, Day KP. 2013. Epistatic interactions between apolipoprotein E and hemoglobin S genes in regulation of malaria parasitemia. PLoS ONE 8, e76924 ( 10.1371/journal.pone.0076924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jasienska G, Ziomkiewicz A, Thune I, Lipson SF, Ellison PT. 2006. Habitual physical activity and estradiol levels in women of reproductive age. Eur. J. Cancer Prev. 15, 439–445. ( 10.1097/00008469-200610000-00009) [DOI] [PubMed] [Google Scholar]
- 28.Pantelidis P, Lambert-Hammill M, Wierzbicki AS. 2003. Simple sequence-specific-primer-PCR method to identify the three main apolipoprotein E haplotypes. Clin. Chem. 49, 1945–1948. ( 10.1373/clinchem.2003.021683) [DOI] [PubMed] [Google Scholar]
- 29.Hixson JE, Vernier DT. 1990. Restriction isotyping of human Apolipoprotein-E by gene amplification and cleavage with HhaI J. Lipid Res. 31, 545–548. [PubMed] [Google Scholar]
- 30.Jasienska G, Ziomkiewicz A, Ellison PT, Lipson SF, Thune I. 2004. Large breasts and narrow waists indicate high reproductive potential in women. Proc. R. Soc. Lond. B 271, 1213–1217. ( 10.1098/rspb.2004.2712) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lipson SF, Ellison PT. 1996. Comparison of salivary steroid profiles in naturally occurring conception and non-conception cycles. Hum. Reprod. 11, 2090–2096. ( 10.1093/oxfordjournals.humrep.a019055) [DOI] [PubMed] [Google Scholar]
- 32.Tabachnick BG, Fidell LS. 2013. Using multivariate statistics, 6th edn Boston, MA: Pearson. [Google Scholar]
- 33.Wilkinson L, Blank G, Gruber C. 1996. Desktop data analysis with SYSTAT. Upper Saddle River, NJ: Prentice Hall. [Google Scholar]
- 34.Jasienska G, Ellison PT. 1998. Physical work causes suppression of ovarian function in women. Proc. R. Soc. Lond. B 265, 1847–1851. ( 10.1098/rspb.1998.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jasienska G, Thune I, Ellison PT. 2006. Fatness at birth predicts adult susceptibility to ovarian suppression: an empirical test of the predictive adaptive response hypothesis. Proc. Natl Acad. Sci. USA 103, 12 759–12 762. ( 10.1073/pnas.0605488103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ellison PT. 2003. Energetics and reproductive effort. Am. J. Hum. Biol. 15, 342–351. ( 10.1002/ajhb.10152). [DOI] [PubMed] [Google Scholar]
- 37.Nunez-de la Mora A, Chatterton RT, Choudhury OA, Napolitano DA, Bentley GR. 2007. Childhood conditions influence adult progesterone levels. PLoS Med. 4, e167 ( 10.1371/journal.pmed.0040167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colleran H, Jasienska G, Nenko I, Galbarczyk A, Mace R. 2014. Community-level education accelerates the cultural evolution of fertility decline. Proc. R. Soc. B 281, 20132732 ( 10.1098/rspb.2013.2732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziomkiewicz A, Ellison PT, Lipson SF, Thune I, Jasienska G. 2008. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Hum. Reprod. 23, 2555–2563. ( 10.1093/humrep/den213) [DOI] [PubMed] [Google Scholar]
- 40.Jasienska G, Jasienski M. 2008. Interpopulation, interindividual, intercycle, and intracycle natural variation in progesterone levels: a quantitative assessment and implications for population studies. Am. J. Hum. Biol. 20, 35–42. ( 10.1002/ajhb.20686) [DOI] [PubMed] [Google Scholar]
- 41.McGuigan K. 2006. Studying phenotypic evolution using multivariate quantitative genetics. Mol. Ecol. 15, 883–896. ( 10.1111/j.1365-294X.2006.02809.x) [DOI] [PubMed] [Google Scholar]
- 42.Bednarska-Makaruk M, Broda G, Kurjata P, Rodo M, Roszczynko M, Rywik S, Wehr H. 2001. Apolipoprotein E genotype, lipid levels and coronary heart disease in a Polish population group. Eur. J. Epidemiol. 17, 789–792. ( 10.1023/A:1015657709060) [DOI] [PubMed] [Google Scholar]
- 43.Kowalska A, Wiechmann I, Walter H. 1998. Genetic variability of apolipoprotein E in a Polish population. Hum. Biol. 70, 1093–1099. [PubMed] [Google Scholar]
- 44.Ma F, Wang JH. 2013. Apolipoprotein epsilon 4-allele as a significant risk factor for conversion from mild cognitive impairment to Alzheimer's disease: a meta-analysis of prospective studies. J. Mol. Neurosci. 50, 257–263. ( 10.1007/s12031-012-9934-y) [DOI] [PubMed] [Google Scholar]
- 45.Corbo RM, Scacchi R, Cresta M. 2004. Differential reproductive efficiency associated with common apolipoprotein E alleles in postreproductive-aged subjects. Fertil. Steril. 81, 104–107. ( 10.1016/j.fertnstert.2003.05.029) [DOI] [PubMed] [Google Scholar]
- 46.Norwitz ER, Schust DJ, Fisher SJ. 2001. Mechanisms of disease—implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408. ( 10.1056/NEJMra000763) [DOI] [PubMed] [Google Scholar]
- 47.Santoro N, et al. 2000. Luteal progesterone relates to histological endometrial maturation in fertile women. J. Clin. Endocrinol. Metab. 85, 4207–4211. ( 10.1210/jcem.85.11.6974) [DOI] [PubMed] [Google Scholar]
- 48.Lu YC, Bentley GR, Gann PH, Hodges KR, Chatterton RT. 1999. Salivary estradiol and progesterone levels in conception and nonconception cycles in women: evaluation of a new assay for salivary estradiol. Fertil. Steril. 71, 863–868. ( 10.1016/S0015-0282(99)00093-X) [DOI] [PubMed] [Google Scholar]
- 49.Ioannidis G, Sacks G, Reddy N, Seyani L, Margara R, Lavery S, Trew G. 2005. Day 14 maternal serum progesterone levels predict pregnancy outcome in IVF/ICSI treatment cycles: a prospective study. Hum. Reprod. 20, 741–746. ( 10.1093/humrep/deh644) [DOI] [PubMed] [Google Scholar]
- 50.Habara T, Nakatsuka M, Konishi H, Asagiri K, Noguchi S, Kudo T. 2002. Elevated blood flow resistance in uterine arteries of women with unexplained recurrent pregnancy loss. Hum. Reprod. 17, 190–194. ( 10.1093/humrep/17.1.190) [DOI] [PubMed] [Google Scholar]
- 51.Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A. 2011. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil. Steril. 95, 534–537. ( 10.1016/j.fertnstert.2010.05.019) [DOI] [PubMed] [Google Scholar]
- 52.Morris D, Diskin M. 2008. Effect of progesterone on embryo survival. Animal 2, 1112–1119. ( 10.1017/s1751731108002474) [DOI] [PubMed] [Google Scholar]
- 53.Sagare-Patil V, Vernekar M, Galvankar M, Modi D. 2013. Progesterone utilizes the PI3K-AKT pathway in human spermatozoa to regulate motility and hyperactivation but not acrosome reaction. Mol. Cell. Endocrinol. 374, 82–91. ( 10.1016/j.mce.2013.04.005) [DOI] [PubMed] [Google Scholar]
- 54.Hughes GC, Clark EA, Wong AH. 2013. The intracellular progesterone receptor regulates CD4(+) T cells and T cell-dependent antibody responses. J. Leukoc. Biol. 93, 369–375. ( 10.1189/jlb.1012491) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arck P, Solano ME, Walecki M, Meinhardt A. 2014. The immune privilege of testis and gravid uterus: same difference? Mol. Cell. Endocrinol. 382, 509–520. ( 10.1016/j.mce.2013.09.022) [DOI] [PubMed] [Google Scholar]
- 56.Hu J, Zhang ZH, Shen WJ, Azhar S. 2010. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutr. Metab. 7, 47 ( 10.1186/1743-7075-7-47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azhar S, Leers-Sucheta S, Reaven E. 2003. Cholesterol uptake in adrenal and gonadal tissues: the SR-BI and ‘selective’ pathway connection. Front. Biosci. 8, S998–S1029. ( 10.2741/1165) [DOI] [PubMed] [Google Scholar]
- 58.Azhar S, Reaven E. 2002. Scavenger receptor class BI and selective cholesteryl ester uptake: partners in the regulation steroidogenesis. Mol. Cell. Endocrinol. 195, 1–26. ( 10.1016/s0303-7207(02)00222-8) [DOI] [PubMed] [Google Scholar]
- 59.Finch CE, Sapolsky RM. 1999. The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol. Aging 20, 407–428. ( 10.1016/s0197-4580(99)00053-6) [DOI] [PubMed] [Google Scholar]
- 60.Finch CE, Stanford CB. 2004. Meat-adaptive genes and the evolution of slower aging in humans. Q. Rev. Biol. 79, 3–50. ( 10.1086/381662) [DOI] [PubMed] [Google Scholar]
- 61.Hales CN, Barker DJP. 1992. Type-2 (non-insulin-dependent) diabetes mellitus—the thrifty phenotype hypothesis. Diabetologia 35, 595–601. ( 10.1007/BF00400248) [DOI] [PubMed] [Google Scholar]
- 62.Mondadori CRA, et al. 2007. Better memory and neural efficiency in young apolipoprotein E epsilon 4 carriers. Cereb. Cortex 17, 1934–1947. ( 10.1093/cercor/bhl103) [DOI] [PubMed] [Google Scholar]
- 63.Filbey FM, Slack KJ, Sunderland TP, Cohen RM. 2006. Functional magnetic resonance imaging and magnetoencephalography differences associated with APOE epsilon 4 in young healthy adults. Neuroreport 17, 1585–1590. ( 10.1097/01.wnr.0000234745.27571.d1) [DOI] [PubMed] [Google Scholar]
- 64.Burt TD, et al. 2008. Apolipoprotein (apo) E4 enhances HIV-1 cell entry in vitro, and the APOE epsilon 4/epsilon 4 genotype accelerates HIV disease progression. Proc. Natl Acad. Sci. USA 105, 8718–8723. ( 10.1073/pnas.0803526105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shima K, Kuhlenbaumer G, Rupp J. 2010. Chlamydia pneumoniae infection and Alzheimer's disease: a connection to remember? Med. Microbiol. Immunol. (Berl). 199, 283–289. ( 10.1007/s00430-010-0162-1) [DOI] [PubMed] [Google Scholar]
- 66.Fujioka H, Phelix CF, Friedland RP, Zhu XW, Perry EA, Castellani RJ, Perry G. 2013. Alipoprotein E4 prevents growth of malaria at the intraerythrocyte stage: implications for differences in racial susceptibility to Alzheimer's disease. J. Health Care Poor Underserved 24, 70–78. ( 10.1353/hpu.2014.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meng FT, Wang YL, Liu J, Zhao J, Liu RY, Zhou JN. 2012. ApoE genotypes are associated with age at natural menopause in Chinese females. Age 34, 1023–1032. ( 10.1007/s11357-011-9287-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zetterberg H, et al. 2002. Influence of the apolipoprotein E epsilon 4 allele on human embryonic development. Neurosci. Lett. 324, 189–192. ( 10.1016/s0304-3940(02)00198-2) [DOI] [PubMed] [Google Scholar]
- 69.Collazo MS, Porrata-Doria T, Flores I, Acevedo SF. 2012. Apolipoprotein E polymorphisms and spontaneous pregnancy loss in patients with endometriosis. Mol. Hum. Reprod. 18, 372–377. ( 10.1093/molehr/gas004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Henderson BE, Feigelson HS. 2000. Hormonal carcinogenesis. Carcinogenesis 21, 427–433. ( 10.1093/carcin/21.3.427) [DOI] [PubMed] [Google Scholar]
- 71.Wiebe JP. 2006. Progesterone metabolites in breast cancer. Endocrine-Related Cancer 13, 717–738. ( 10.1677/erc.1.01010). [DOI] [PubMed] [Google Scholar]
- 72.Jasienska G, Thune I, Ellison PT. 2000. Energetic factors, ovarian steroids and the risk of breast cancer. Eur. J. Cancer Prev. 9, 231–239. ( 10.1097/00008469-200008000-00003) [DOI] [PubMed] [Google Scholar]
- 73.Surekha D, Vishnupriya S, Sailaja K, Rao DN, Raghtinadharao D. 2008. Influence of apolipoprotein E gene polymorphism on the risk for breast cancer. Int. J. Hum. Genet. 8, 277–282. [Google Scholar]
- 74.Chang NW, Chen DR, Wu CT, Aouizerat BE, Chen FN, Hung SJ, Wang SH, Wei MF, Chang CS. 2005. Influences of apolipoprotein E polymorphism on the risk for breast cancer and HER2/neu status in Taiwan. Breast Cancer Res. Treat. 90, 257–261. ( 10.1007/s10549-004-4656-7) [DOI] [PubMed] [Google Scholar]
- 75.Saadat M. 2012. Apolipoprotein E (APOE) polymorphisms and susceptibility to breast cancer: a meta-analysis. Cancer Res. Treat. 44, 121–126. ( 10.4143/crt.2012.44.2.121) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets supporting this article can be found at the Dryad data repository (doi:10.5061/dryad.vn63n).


