Haootia quadriformis was described from the lower Fermeuse Formation of the Bonavista Peninsula of Newfoundland (approx. 560 Ma) and its numerous regularly aligned impressions were interpreted as evidence of muscular tissue ([1, figs 1a and 3b]). Consequently, this fossil could represent the earliest preservation of metazoan musculature in the geological record.
Although Liu et al. [1] identified H. quadriformis as a cnidarian, the species was not decisively assigned to any particular class, but potentially within Medusozoa [1]. The fossil was predominantly compared to staurozoans (Cnidaria) based on an assertion of consistent ‘positioning of muscular fibres in the calyx of modern Staurozoa’ [1, p. 6]. Indeed, the hypothesized general morphology of the body of H. quadriformis ([1, fig. 3b]) is similar to that of extant stauromedusae: a calyx with marginal branches (arms) and a peduncle with pedal disc (figure 1a; [2–5]). Other possible correspondence not exploited by Liu et al. [1] is the presence of an invagination of the epidermis of the pedal disc resulting in an axial canal at the base of the peduncle of some stauromedusae (figure 1b; [3]), similar to the impression found at the attachment area of H. quadriformis ([1, fig. 1e]). Putative dissonant characters [1, p. 6] do not contradict the staurozoan hypothesis because they are encompassed within inter- and intraspecific variation in Staurozoa, like the supplementary number of arms [6], and the absence/presence of morphological features such as anchors, gonads and nematocyst clusters [2,4,7–9]. By contrast, detailed comparison of the reconstruction of the muscular organization in the calyx of H. quadriformis ([1, fig. 3b]) to that of living stauromedusae provides evidence against a close relationship between Haootia and Staurozoa.
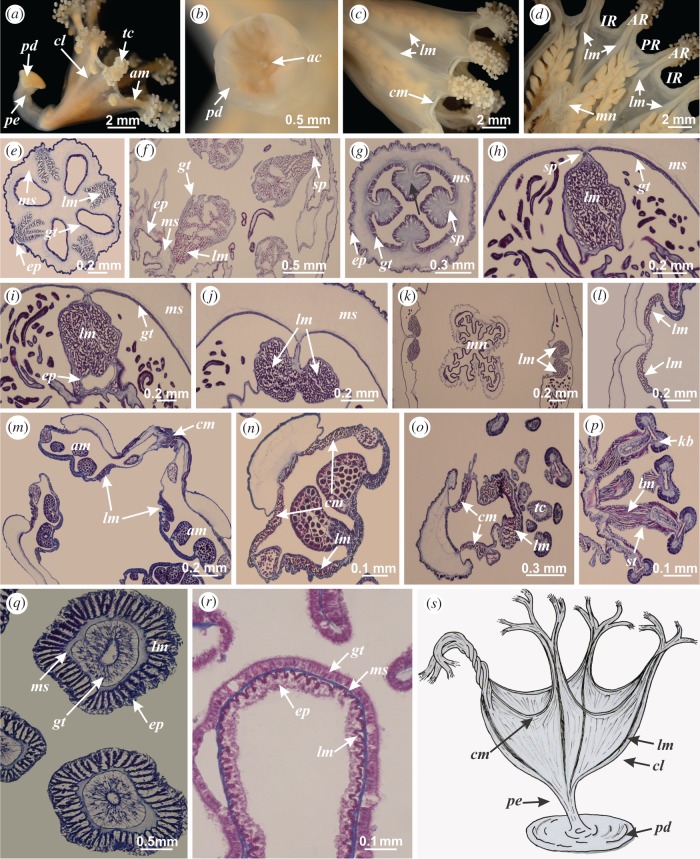
Figure 1.
(a) Haliclystus tenuis, general organization of stauromedusa. (b) Kishinouyea nagatensis, pedal disc with axial canal. (c) Craterolophus convolvulus, organization of musculature, exumbrellar view. (d) Craterolophus convolvulus, organization of musculature, subumbrellar view. (e) Haliclystus tenuis, intramesogleal interradial longitudinal muscles in peduncle. (f) Lucernaria quadricornis, interradial longitudinal muscles associated with septa. (g) Kishinouyea corbini, absence of interradial longitudinal muscles in peduncle (indicated by black arrow). (h) Kishinouyea corbini, interradial longitudinal muscle associated with septa at peduncle/calyx connection. (i) Kishinouyea corbini, interradial longitudinal muscle associated with septa at base of calyx. (j,k) Kishinouyea corbini, interradial longitudinal muscles divided into two sections at manubrium region. (l) Haliclystus tenuis, interradial longitudinal muscle divided into two adradial bands at calyx margin. (m) Haliclystus tenuis, muscular organization at calyx/arms connection. (n) Haliclystus tenuis, muscular organization of arm. (o) Kishinouyea corbini, longitudinal muscle of arm associated with secondary tentacles. (p,q) Kishinouyea corbini, longitudinal muscle of stem of secondary tentacles. (r) Craterolophus convolvulus, fibres of longitudinal muscle associated with manubrium. (s) Haootia quadriformis, alternative reconstruction of impressions interpreted as muscular fibres (mainly radially oriented, with coronal muscle restricted to a thin layer at calyx margin, modified from [1]). Histological sections: (e–o,q,r) cross sections; (p) longitudinal section (see methods in [5]). ac, axial canal; am, arm; AR, adradii; cl, calyx; cm, coronal muscle; ep, epidermis; gt, gastrodermis; IR, interradii; kb, knob of secondary tentacle; lm, longitudinal muscle; mn, manubrium; ms, mesoglea; pd, pedal disc; pe, peduncle; PR, perradii; sp, septum; st, stem of secondary tentacle; tc, secondary tentacle. (Online version in colour.)
The musculature of stauromedusae is organized in two muscular arrangements: coronal (circular) and longitudinal (radial) muscles (figure 1c,d; [3,4]), similar to Scyphozoa and Cubozoa [10,11]. The main muscular arrangement in Staurozoa is radial, and not circular as generally observed in active and free-swimming scyphomedusae and cubomedusae ([1, fig. S6]; [10–12]), consistent with the benthic habit of staurozoans [12,13]. The coronal muscle of a stauromedusa is a narrow band, restricted to the calyx margin, either entire or regionally divided at the arms, depending on the species (figure 1c; [7]). Therefore, the hypothetical reconstruction of a series of muscles parallel to the calyx margin in H. quadriformis ([1, fig. 3b]) is not present in staurozoans, nor would it be consistent with the behaviour of benthic stauromedusae, which do not vigorously open and close their calices (swimming movement).
Longitudinal muscles can be present or absent in the peduncle of stauromedusae, depending on species (figure 1e–g; [3,7]). When present, the longitudinal muscles of the peduncle are organized into four distinct interradial bands (intramesogleal or associated with gastric septa) (figure 1e,f) and are continuous with the four interradial muscle bands at the base of the calyx (figure 1h,i). While longitudinal striations along the peduncle of H. quadriformis ([1, fig. 1f]) could be interpreted as peduncular muscles, there is no clear evidence that there are four.
Longitudinal muscles from the base of the calyx to the stem of secondary tentacles have been observed in all studied species of stauromedusae (irrespective of the presence/absence of muscles in the peduncle) [3,4]. Gradually and towards the arms, the four interradial longitudinal bands of the calyx divide into two main sections (figure 1j–l), becoming eight adradial bands (figure 1l). At the calyx/arm connection, each one of these eight bands runs towards an adradial arm (figure 1m,n). Therefore, a cross section of the arm of an animal with divided coronal muscle is composed of three main bands: two bands of coronal muscle (perradial and interradial) and one band of longitudinal muscle (figure 1n,o). The longitudinal muscle band is progressively divided into diffuse bundles, running towards the stem of secondary tentacles (figure 1o–q). There are also thin fibres of longitudinal muscle associated with manubrium and perradii (figure 1r). Therefore, reconstruction of longitudinal muscles of the branches of H. quadriformis ([1, fig. 1d]) is consistent with that of modern stauromedusae.
In short, the hypothetical reconstruction of the muscular system of H. quadriformis contains two elements that are consistent with those of staurozoans and one that is not, thereby casting a staurozoan interpretation for H. quadriformis in doubt. The hypothetical coronal muscular arrangement in Haootia ([1, fig. 3b]) is not consistent with that of extant stauromedusae, but the reconstruction of longitudinal muscles in the branches and peduncle of H. quadriformis is similar to the organization observed in stauromedusae. A benthic lifestyle was proposed for H. quadriformis based on the evidence of a peduncle with pedal disc [1]. However, a benthic lifestyle does not seem to be consistent with the very well-developed coronal muscle organization hypothesized for the species ([1, fig. 3b]; [10]).
Fossil muscular impressions of Cambrian cnidarians, such as those of scyphomedusae, have an organization strikingly clear and similar to extant animals [14,15]. The impressions of H. quadriformis are considerably more complicated, probably because of its plane of preservation and possible superposition of morphological structures, making it difficult to reliably reconstruct muscular organization. Therefore, the proposed reconstruction of the muscular organization of H. quadriformis ([1, fig. 3b]) could be misinterpreted, perhaps biased by a more clear understanding of the muscular arrangement in Scyphozoa and Cubozoa, which is predominantly circular ([1, fig. S6]; [10,11]). Interestingly, a major radial muscular arrangement, with coronal muscle restricted to the margin, is not inconsistent with the body fossil impressions of H. quadriformis. Therefore, we provide an alternative interpretation, with mainly radially oriented fibres, for consideration (figure 1s). This reconstruction must be properly assessed with direct comparison to the fossils because of their important status as the earliest tangible evidence of an animal muscular system. If the hypothetical muscular impressions are reconstructed accurately in the original paper [1], a different life habit—benthic with vigorous pulsation, perhaps as part of a feeding process—should be considered. This could have been a state from which a swimming detached descendant [15] could plausibly have arisen.
Acknowledgement
We are grateful for the excellent suggestions made by Alexander Liu and two other reviewers.
Footnotes
The accompanying reply can be viewed at http://dx.doi.org/doi:10.1098/rspb.2014.2949.
Funding statement
This work was supported by FAPESP (grant no. 2010/07362-7 to L.S.M., grant no. 2011/50242-5 to A.C.M.), by CNPq (grant nos 142270/2010-5 and 165066/2014-8 to L.S.M., grant nos 562143/2010-6 and 305805/2013-4 to A.C.M.) and by CAPES/PDSE (grant no. 16499/12-3 to L.S.M.). This paper is a contribution of the NPBioMar, USP.
References
- 1.Liu AG, Matthews JJ, Menon LR, McIlroy D, Brasier MD. 2014. Haootia quadriformis n. gen., n. sp., interpreted as a muscular cnidarian impression from the Late Ediacaran period (approx. 560 Ma). Proc. R. Soc. B 281, 20141202 ( 10.1098/rspb.2014.1202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayer AG. 1910. Medusae of the world, vol. III. Scyphomedusae, pp. 499–735. Washington, DC: Carnegie Institution Publishing. [Google Scholar]
- 3.Uchida T. 1929. Studies on the Stauromedusae and Cubomedusae, with special reference to their metamorphosis. Jpn J. Zool. 2, 103–193. [Google Scholar]
- 4.Berrill M. 1963. Comparative functional morphology of the Stauromedusae. Can. J. Zool. 41, 741–752. ( 10.1139/z63-046) [DOI] [Google Scholar]
- 5.Miranda LS, Collins AG, Marques AC. 2013. Internal anatomy of Haliclystus antarcticus (Cnidaria, Staurozoa) with a discussion on histological features used in staurozoan taxonomy. J. Morphol. 274, 1365–1383. ( 10.1002/jmor.20185) [DOI] [PubMed] [Google Scholar]
- 6.Zagal CJ. 2008. Morphological abnormalities in the stauromedusa Haliclystus auricula (Cnidaria) and their possible causes. J. Mar. Biol. Assoc. UK 88, 259–262. ( 10.1017/S0025315408000179) [DOI] [Google Scholar]
- 7.Kramp PL. 1961. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. UK 40, 292–303. ( 10.1017/S0025315400007347) [DOI] [Google Scholar]
- 8.Hirano YM. 1997. A review of a supposedly circumboreal species of stauromedusa, Haliclystus auricula (Rathke, 1806). In Proc. 6th Int. Conf. on Coelenterate Biology, Leiden, The Netherlands, 16–21 July 1995 (ed. den Hartog JC.), pp. 247–252. Noordwijkerhout, The Netherlands: Nationaal Naturhistorisch Museum. [Google Scholar]
- 9.Miranda LS, Morandini AC, Marques AC. 2009. Taxonomic review of Haliclystus antarcticus Pfeffer, 1889 (Stauromedusae, Staurozoa, Cnidaria), with remarks on the genus Haliclystus Clark, 1863. Polar Biol. 32, 1507–1519. ( 10.1007/s00300-009-0648-8) [DOI] [Google Scholar]
- 10.Arai MN. 1997. A functional biology of Scyphozoa. London, UK: Chapman and Hall. [Google Scholar]
- 11.Satterlie RA, Thomas KS, Gray GC. 2005. Muscle organization of the cubozoan jellyfish Tripedalia cystophora Conant 1897. Biol. Bull. 209, 154–163. ( 10.2307/3593133) [DOI] [PubMed] [Google Scholar]
- 12.Gwilliam GF. 1960. Neuromuscular physiology of a sessile scyphozoan. Biol. Bull. 119, 454–473. ( 10.2307/1539262) [DOI] [Google Scholar]
- 13.Mills CE, Hirano YM. 2007. Stauromedusae. In Encyclopedia of tidepools and rocky shores (eds Denny MW, Gaines SD.), pp. 541–543. Berkeley, CA: University of California Press. [Google Scholar]
- 14.Cartwright P, Halgedahl SL, Hendricks JR, Jarrard RD, Marques AC, Collins AG, Lieberman BS. 2007. Exceptionally preserved jellyfishes from the Middle Cambrian. PLoS ONE 2, e1121 ( 10.1371/journal.pone.0001121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Iten H, Marques AC, Leme JM, Pacheco MLAF, Simões MG. 2014. Origin and early diversification of the phylum Cnidaria Verrill: major developments in the analysis of the taxon's Proterozoic and earliest Cambrian history. Palaeontology 4, 677–690. ( 10.1111/pala.12116) [DOI] [Google Scholar]