Abstract
The synthesis of secondary metabolites is a hallmark of plant defence against herbivores. These compounds may be detrimental to consumers, but can also protect herbivores against parasites. Floral nectar commonly contains secondary metabolites, but little is known about the impacts of nectar chemistry on pollinators, including bees. We hypothesized that nectar secondary metabolites could reduce bee parasite infection. We inoculated individual bumblebees with Crithidia bombi, an intestinal parasite, and tested effects of eight naturally occurring nectar chemicals on parasite population growth. Secondary metabolites strongly reduced parasite load, with significant effects of alkaloids, terpenoids and iridoid glycosides ranging from 61 to 81%. Using microcolonies, we also investigated costs and benefits of consuming anabasine, the compound with the strongest effect on parasites, in infected and uninfected bees. Anabasine increased time to egg laying, and Crithidia reduced bee survival. However, anabasine consumption did not mitigate the negative effects of Crithidia, and Crithidia infection did not alter anabasine consumption. Our novel results highlight that although secondary metabolites may not rescue survival in infected bees, they may play a vital role in mediating Crithidia transmission within and between colonies by reducing Crithidia infection intensities.
Keywords: tritrophic interactions, plant secondary metabolites, pollination, parasitism, bumblebees, Crithidia bombi
1. Introduction
Plant secondary metabolites play a fundamental role in structuring interactions between plants and herbivores, with consequences for population dynamics, community structure and trait evolution [1]. There is growing recognition that these compounds can mediate tritrophic interactions among plants, herbivores, and their natural enemies. For example, although secondary metabolites can reduce survivorship and reproduction, their consumption can also benefit herbivores by reducing parasitism or predation [2,3]. Similarly, plant secondary metabolite consumption slows the growth of some protozoan gut parasites of insects, including the causal agents of human diseases such as Chagas and leishmaniasis [4,5].
Plant secondary metabolites are not only found in leaves but are also common in floral rewards such as nectar [6]. For example, a broad survey revealed that virtually all plant species' floral nectar contained non-protein amino acids, 36% contained phenolics and 8% contained alkaloids [7]. Although bees are generally pollinating mutualists of plants, bees can also broadly be considered herbivores that consume nectar and pollen. As such, they are exposed to an extensive array of secondary metabolites as both larvae and adults. Like other herbivores, bees are integrated in trophic systems that include both host plants and natural enemies [8]. Parasites are strongly implicated in the decline of both managed and wild bees [9], but impacts of dietary secondary metabolites on bee-disease dynamics remains a largely unexplored frontier.
Similar to foliar herbivores, bees experience both negative and positive effects of consuming plant chemicals. There are numerous reports of bees poisoned by secondary metabolites in nectar and pollen [6,10], and bees can incur reproductive costs when they consume these compounds [11]. However, limited evidence demonstrates that secondary metabolites can benefit bees, for example by enhancing memory and foraging efficiency [12], reducing parasite infection [13] and controlling pathogenic fungi [14]. The potential therefore exists for plant secondary metabolites to play an important role in mediating tritrophic interactions among plants, pollinators and parasites, but how costs and benefits of chemical consumption affect bee survival and reproduction remains relatively unexplored.
The goals of this study were to assess the extent to which consumption of a diverse array of plant secondary metabolites affects bee resistance to parasites, and ascertain how secondary metabolites affect bee performance directly and indirectly via impacts on parasitism. We studied the common eastern bumblebee, Bombus impatiens, a generalist forager whose diet includes a variety of plant secondary metabolites [15], and its protozoan intestinal parasite, Crithidia bombi (hereafter Crithidia), which has negative fitness effects on bumblebees, including shortened individual and colony lifespan and reduced colony production of new queens [16]. We infected bees with Crithidia, then fed them sugar solutions containing either one of eight naturally occurring nectar chemicals (table 1) or a control (electronic supplementary material, figure S1). Given their medicinal and anti-trypanosome effects in other contexts [4,5], we predicted that secondary metabolites would lower bee parasite infection. Second, for the alkaloid anabasine, which most strongly reduced Crithidia counts in bees, we used replicate microcolonies to test the individual and combined effects of parasite infection and diet chemistry on bee survival and reproduction (electronic supplementary material, figure S1). We predicted we would observe a trade-off, where both parasitism and anabasine consumption would directly reduce reproduction and survival of bees, but anabasine would indirectly benefit bees by reducing parasite load. Given global concerns over pollinator declines [23], plant secondary metabolites could play key roles in maintaining pollinator health by reducing bee disease if such benefits outweigh the costs.
Table 1.
Experiment 1: plant secondary metabolites naturally occurring in floral nectar tested for effects on the gut pathogen Crithidia bombi when consumed by its host, Bombus impatiens. (Compound concentrations were chosen based on reports of occurrence in floral nectar, except gallic acid and thymol, for which we estimated values from concentrations reported in Apis mellifera honey, and aucubin and catalpol, which are unpublished data. We report main effects for ANCOVAs comparing parasite load of bees consuming each compound to those of controls. Statistically significant differences: *p ≤ 0.05; **p ≤ 0.01 and ***p ≤ 0.001.)
| compound | concentration (ppm) | exemplar plants | refs | treatment effect |
|---|---|---|---|---|
| alkaloids | ||||
| anabasine | 5.0 | Nicotiana glauca | [17] | F1,26 = 13.77, p = 0.001*** |
| caffeine | 98.0 | Citrus spp., Coffea spp., Camellia oleifera | [12,18,19] | F1,21 = 0.50, p = 0.49 |
| nicotine | 2.0 | Nicotiana glauca | [17] | F1,29 = 7.67, p = 0.01** |
| cyanogenic glycosides | ||||
| amygdalin | 50.0 | Prunus amygdalus | [20] | F1,53 = 0.19, p = 0.67 |
| iridoid glycosides | ||||
| aucubin | 1600.0 | Chelone glabra | M. D. Bowers 2012, pers. comm. | F1,44 = 2.15, p = 0.15 |
| catalpol | 1417.0 | Chelone glabra | M. D. Bowers 2012, pers. comm. | F1,44 = 3.89, p = 0.05* |
| phenolics | ||||
| gallic acid | 50.0 | Fagopyrum esculentum | [21] | F1,38 = 1.38, p = 0.25 |
| terpenoids | ||||
| thymol | 0.2 | Tilia europea | [22] | F1,38 = 7.89, p = 0.01** |
2. Material and methods
(a). Study system
Bombus impatiens was used for the experiments. It is a generalist pollinator species, common across eastern North America [15] and commercially available. Crithidia bombi (Kinetoplastea; Trypanosomatida) is a single-host protozoan intestinal parasite that infects many species of bumblebees, including B. impatiens [24]. Crithidia has been identified as one of numerous possible causal agents in the decline of North American bumblebees [25]. Crithidia is horizontally transmitted via contact between bees and faecal contamination of nest materials and flowers [26]. Infection rates vary greatly, with as many as 80% of individuals infected in some populations [24]. The parasite stimulates an immune response in bees [27], with cell numbers reaching an asymptote 7–10 days after infection [28]. Crithidia negatively affects bee fitness by shortening individual and colony lifespan, altering foraging behaviour and reducing the production of offspring [16].
(b). Experimental protocol
(i). Crithidia inoculum
We isolated Crithidia from wild B. impatiens collected in Massachusetts and Vermont, USA, and maintained it in commercial B. impatiens colonies (Biobest Canada, Leamington, Canada; Koppert Biological Systems, Howell, MI, USA). We prepared a fresh Crithidia inoculum each day to inoculate bees in experimental trials. To do so, we sacrificed 10 workers from infected colonies and ground each intestine with a glass rod in 300 µl of dH2O, after which we vortexed samples and allowed them to settle at room temperature for 6–8 h. We transferred 10 µl aliquots of clear solution to a haemocytometer and examined samples for Crithidia. We then transferred 50–150 µl of solution from samples containing Crithidia to a clean container and diluted to create approximately 25% sucrose solution containing 6000 Crithidia cells × 10 µl−1.
(ii). Experiment 1: effects of secondary metabolites on bee parasite infection
We tested how eight nectar secondary metabolites, including alkaloids (anabasine, caffeine and nicotine), iridoid glycosides (aucubin and catalpol), phenolics (gallic acid), terpenoids (thymol) and cyanogenic glycosides (amygdalin; table 1) affected Crithidia infection in B. impatiens, using a total of 539 worker-caste bees in experiments conducted January to December, 2012 (electronic supplementary material, figure S1). We chose to study secondary metabolites that are: (i) known to occur in nectar of plants growing within the distributional limits of Bombus species and likely to be visited by them; (ii) commercially available; and (iii) representative of a broad range of chemical classes.
In eight separate trials, we inoculated bees with Crithidia and then fed them a diet of control sucrose or sucrose plus secondary metabolites, using a protocol modified from Manson et al. [13]. Each trial included bees from two to eight Crithidia-free source colonies. We attempted to balance treatment and control sample sizes, and used a mean of 38 (±16.5 s.d.) bees per treatment per trial (range: 15–67 bees). To rule out possible effects of contact with nest-mates on experimental bees, we removed clumps of worker-caste pupae from colonies before emergence and placed them in separate plastic containers. Each day, we weighed newly emerged adult workers and moved them to individual 18.5 ml clear plastic vials with honeybee-collected pollen (Koppert Biological Systems; Linden Apiaries, Walpole, NH, USA) and wicking feeders for nectar solution. After 2 days, we removed food for 4–6 h, then fed each bee a 10 µl inoculum containing 6000 Crithidia cells, which is similar to concentrations encountered in nature by bees [27–29]. We verified that bees consumed this inoculum, then provisioned them for 7 days with ad libitum pollen and a 30% sucrose solution either with or without one of the secondary metabolite compounds.
We purchased synthetic compounds (Indofine, Hillsborough, NJ, USA; Sigma-Aldrich, St. Louis, MO, USA) and prepared experimental secondary metabolite solutions at concentrations reported for floral nectars (table 1). For two compounds, we were only able to obtain data for concentrations in single-origin Apis mellifera honey, and chose secondary metabolite concentrations at approximately one-third that strength (table 1). Most compounds were tested in single, individual trials comparing the secondary metabolite treatment to a control. Amygdalin and anabasine were each tested in two separate trials to assess consistency across trials, and because results were similar, data were combined for analysis of each compound. Aucubin and catalpol were tested in the same trial with one control group.
After 7 days, we sacrificed bees and prepared intestines as described above (see Crithidia inoculum). We placed 10 µl aliquots of macerated intestine on a haemocytometer and counted amastigote and choanomastigote cell types in five subfields of the grid at 10× magnification with a dissecting microscope. We summed these counts and calculated Crithidia cells × ml−1, the variable we analyse below. For comparative purposes, we also calculated an estimate of total cells per bee. We mounted the right forewing of each bee on a microscope slide and measured radial cell length, an index of bee body size [30].
Statistical analyses. We conducted all analyses using JMP software [31], unless otherwise noted. Crithidia cell counts were log10-transformed to meet assumptions of parametric statistics. We fitted a separate analysis of covariance (ANCOVA) to compare each secondary metabolite treatment to its control, except that aucubin and catalpol, tested together, were analysed as separate treatments compared with a single control in the same ANCOVA model. For each analysis, we compared the Akaike information criterion (AIC) to select a best-fit model. Covariates retained varied among best-fit models, and included bee size estimates (weight and radial cell length), colony of origin for each bee, inoculation date, and, when multiple tests of the same compound were conducted, experimental trial number (see the electronic supplementary material, table S1). Because we made the a priori hypothesis that secondary metabolites would reduce Crithidia infection relative to controls, in our analysis of the aucubin and catalpol trial, we used linear contrasts to compare each compound to the control. To compare results across all compounds, we calculated Cohen's d effect sizes (as the difference between secondary metabolite and control treatment ANCOVA least square means divided by the root of the mean square error term from those models), with 95% confidence intervals (CI) and an overall effect size for all compounds [32,33].
Approximately 13% of bees had no detectable Crithidia infection after 7 days, suggesting they had cleared their infection. We excluded these replicates from the analysis described above, but used logistic regression to test whether diet treatment influenced the likelihood that bees would be Crithidia-free after 7 days.
We used logistic regression and Kaplan–Meier estimates to compare proportions of bees that died during experiments to assess mortality risk to bees of consuming secondary metabolites. We treated the data as right-censored, considering those bees that died as ‘failed’ and those that survived for 7 days as ‘censored’.
(iii). Experiment 2: costs and benefits of secondary metabolite consumption
In November to December 2013, we further investigated the effects of anabasine, the secondary metabolite that caused the greatest reduction in Crithidia cell number (see Results), on bee survival and reproduction in a factorial experiment crossing parasite status (infected with Crithidia versus control) with diet treatment (anabasine presence versus absence) in groups of worker-caste bees, hereafter referred to as microcolonies (electronic supplementary material, figure S1; [34]). Unmated workers lay haploid (male) eggs when isolated from the queen, and microcolonies are an effective means of estimating whole-colony reproduction as a function of diet [11,35].
We placed trios of newly emerged bees in 500 ml clear deli containers (RD 16C, Placon Products, Madison, WI, USA) with an absorbent paper insert and fed them ad libitum 30% sucrose solution and pollen for 2 days before treatments began. Bees came from nine source colonies, and each microcolony contained only sisters. Each of four treatment combinations had a sample size of 12–16 microcolonies, and source colony was randomly distributed across treatment groups. For the Crithidia treatment, bees were individually inoculated (see Crithidia inoculum), then returned to their microcolony, while bees in the control treatment were handled similarly but not inoculated. For the diet treatment, microcolonies were provisioned with 30% sucrose solution either with or without 20 ppm anabasine daily. This is a higher anabasine concentration than we used in the first experiment (table 1), but it falls within the natural range encountered in floral nectar [36] and was used in order to assess the highest potential costs and benefits to bees of consuming anabasine.
We provisioned microcolonies daily with 5 ml of their assigned nectar treatment in a 30 mm diameter Petri dish fitted with a dental cotton wick, and an approximately 400 mg ball of nectar-moistened pollen dipped in melted bees' wax. To measure food consumption we weighed each nectar and pollen ration before and 24 h after exposure to bees. To account for mass lost to evaporation, we also measured changes in mass of nectar solutions and pollen balls over 24 h in containers without bees (n = 29 for each treatment). We recorded daily observations of bee mortality and egg laying, and looked for stress indicators such as oophagy and larval ejection, but did not detect any. We removed replicates from the experiment after more than or equal to two bee deaths, and terminated all microcolonies 14 days after first egg laying (or 35 days after initiation if no reproduction had occurred). After two weeks of development, larvae have reached later instars but have not yet pupated, and we were thus able to study numbers and mass of offspring without introducing density-dependent effects of additional adult bees [35]. For each bee, we counted Crithidia cells and measured forewing radial cell length, and for each microcolony we counted and weighed all eggs and larvae. We include in our analyses a metric of within-microcolony size dimorphism [(largest bee radial cell/smallest bee radial cell)−1] [37], because size variation can affect microcolony food consumption and reproduction via social dominance behaviour [29].
Statistical analyses. We used linear models to analyse effects of parasite status and diet treatment on individual and microcolony response variables, including Crithidia cell counts, daily consumption of nectar and pollen, egg production and bee survival. Depending on the analysis, we also considered as fixed effects microcolony age, number of days since colony initiation (hereafter ‘day’), individual and mean radial cell length, within-microcolony size dimorphism, daily food consumption and interactions between effects. As random effects, we included colony of origin and microcolony. For each analysis, we report a best-fit model that had the lowest AIC score of those we considered.
We used a linear mixed model to compare log10-transformed Crithidia cells per bee between diet treatments. All bees in the uninfected treatment were verified as Crithidia-free at the end of the experiment, and excluded from this analysis. The best-fit model included diet treatment, day (of colony termination), size dimorphism and the diet × dimorphism interaction as fixed effects, and microcolony as a random effect. Because microcolony dimorphism had significant explanatory power in this analysis, we then used simple linear regression to ask whether the magnitude of size dimorphism was driven by the size of individual bees.
We used repeated-measures ANOVA to compare daily nectar and pollen consumption by microcolonies, specifying day as the repeated term. Nectar and pollen mass loss owing to evaporation did not differ between anabasine and control nectar treatments (nectar: F < 0.1, p > 0.7), but evaporation was significantly positively correlated with starting mass (nectar: r2 = 0.71, F1,81 = 193.91, p < 0.0001; pollen: r2 = 0.25, F1,81 = 27.37, p < 0.0001). We thus corrected daily nectar and pollen mass loss calculations with regression equations from these analyses. Best-fit models of nectar and pollen consumption each included as fixed effects, diet and Crithidia treatments and their interaction, as well as the number of bees alive in the microcolony each day, and as random effects, individual microcolony nested within parasite and diet treatments.
We used logistic regression to compare the probability of reproduction among microcolonies. For microcolonies that reproduced, we then used separate ANCOVAs to ask how parasite status and diet treatment affected days to first egg laying and total number and mass of eggs and larvae produced.
To compare bee survival among treatments, we fitted a mixed effects proportional hazards model with R statistical software using the package ‘coxme’ [38,39]. To select fixed and random effects, we compared log-likelihood scores between candidate and null models and report here the best-fit model where this comparison maximized AIC [38]. We treated survival data as right-censored, considering as ‘failed’ bees that died and as ‘censored’ those that survived. The best-fit model of bee survival data included as fixed effects, diet and parasite treatments, and as random effects, source colony and microcolony.
3. Results
(a). Experiment 1: effects of secondary metabolites on bee parasite infection
Crithidia populations grew rapidly in bees after inoculation, and individual bees in control treatments contained an average 9.36 × 105 cells after 7 days, more than 150 times that contained in the inoculum. Crithidia cell count was significantly reduced by four of the eight compounds we tested: anabasine (81% reduction), catalpol (61%), nicotine (62%) and thymol (67%; table 1; electronic supplementary material, table S1). Disease loads were not significantly reduced by amygdalin, aucubin, caffeine or gallic acid. Covariates, including colony of origin and body size measurements, explained some of the variation in Crithidia infection, but none had significant effects across all trials (electronic supplementary material, table S1). Across all eight compounds, meta-analysis revealed an overall strong, negative effect of secondary metabolites on Crithidia infection (Cohen's d = −1.01 ± 0.12 s.d.; figure 1). Effect sizes for all compounds were negative (range: −1.36 to −0.10; electronic supplementary material, table S1).
Figure 1.
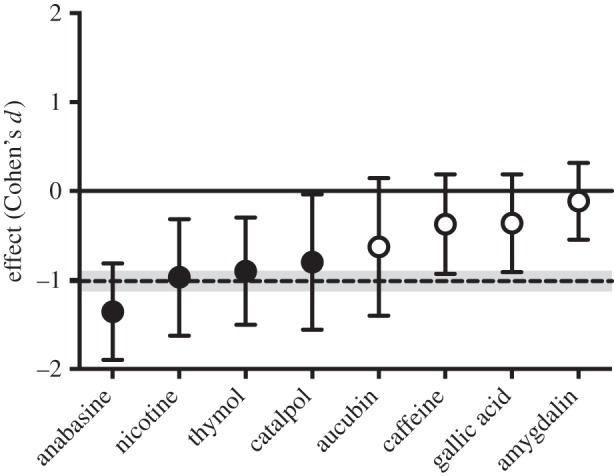
Experiment 1: nectar secondary metabolites reduced Crithidia bombi infections in the bumblebee Bombus impatiens, as demonstrated by Cohen's d effect sizes (±95% CI). Filled circles represent statistically significant differences in ANCOVAs comparing parasite load of bees consuming nectar secondary metabolites to that of bees consuming a control diet. The overall effect size for all compounds was −1.01 ± 0.12 s.d. (dashed line with shaded boundary).
Bees consuming anabasine were more likely to be Crithidia-free than their controls (12 of 27 bees receiving anabasine versus 6 of 39 controls; χ2 = 3.80, n = 84, p = 0.05), but other compounds had no effect on the probability of clearing infection (χ2 < 2.38, p > 0.12).
Nearly 23% of bees across all treatments died after inoculation but before the end of the experiment. We found no effect of individual secondary metabolites (χ2 ≤ 2.36, p ≥ 0.12) or all compounds considered together (χ2 = 3.02, n = 529, p = 0.08) on bee mortality. Similarly, there were no effects of individual compounds (log-rank tests: χ21 ≤ 2.36, p ≥ 0.12) or all compounds tested together (log-rank test: χ21 = 1.88, p = 0.17) on Kaplan–Meier survival functions.
(b). Experiment 2: costs and benefits of secondary metabolite consumption
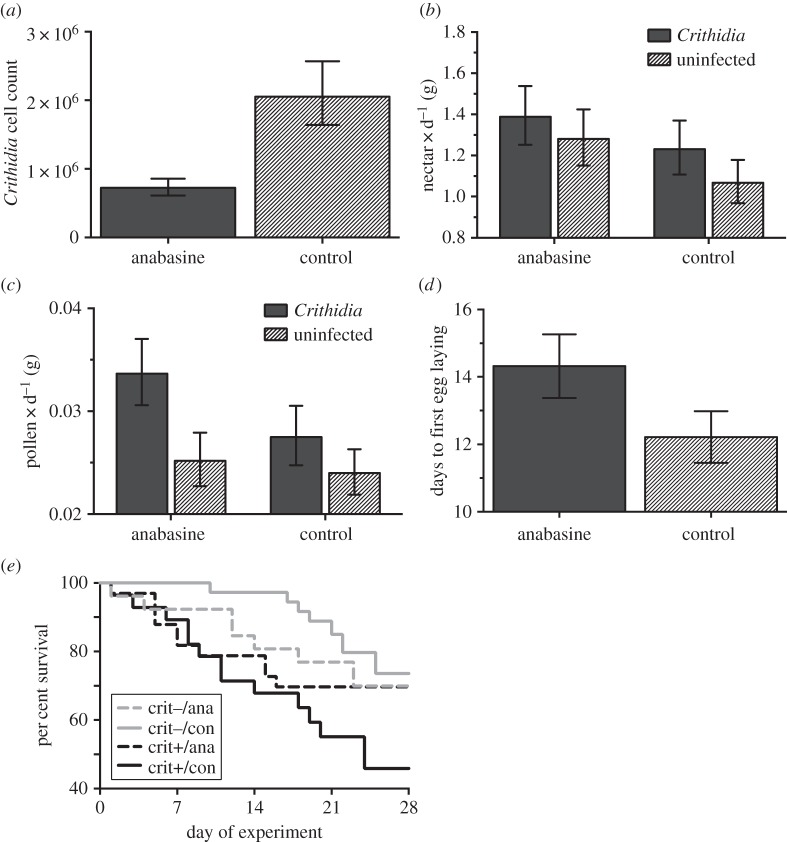
Infected bees from microcolonies provisioned with anabasine had 65% fewer Crithidia cells than controls (figure 2a), and infection was positively correlated with the number of days bees were infected (electronic supplementary material, tables S2–S3). Overall, Crithidia cell counts decreased with greater microcolony size dimorphism and there was a significant diet treatment × dimorphism interaction, in which disease load of bees consuming the control diet was strongly reduced when they inhabited microcolonies with greater size dimorphism, but bees receiving anabasine had similar disease loads regardless of size heterogeneity (electronic supplementary material, tables S2–S3). Microcolony dimorphism was not explained by individual bee size (linear regression: r2 = 0.006, F1,34 = 0.22, p = 0.64), suggesting that the explanatory power of this variable reflects importance of within-group size heterogeneity rather than individual bee size.
Figure 2.
Experiment 2: effects of diet treatment (anabasine versus control) on parasite load of Bombus impatiens workers (a). Effects of diet treatment and parasite treatment (presence/absence of Crithidia infection) on bumblebee microcolony consumption of nectar (b) and pollen (c); effect of diet treatment on number of days to egg laying (d); and effects of both treatments on worker mortality (e). Data are back-transformed means ± s.e. from ANCOVA (a) and repeated-measures ANOVA (b,c), means ± s.e. from ANCOVA (d), and survival curves from a proportional hazards model (e). ‘crit−’, uninfected; ‘crit+’, infected; ‘ana’, anabasine diet treatment; ‘con’, control diet.
Bees in the anabasine treatment consumed 16% more nectar than controls (figure 2b), but nectar anabasine had no effect on pollen consumption (figure 2c; electronic supplementary material, tables S2–S3). Crithidia infection increased pollen consumption by 24% (figure 2c) but did not affect nectar consumption (figure 2b). Nectar consumption was not affected by the diet × parasite treatment interaction, indicating that bees did not respond to Crithidia infection by increasing consumption of anabasine (figure 2b; electronic supplementary material, tables S2–S3). Pollen consumption was also not affected by this interaction. Daily microcolony nectar and pollen consumption were positively associated with number of surviving bees, and overall, nectar consumption increased over the course of the experiment while pollen consumption decreased. For consumption of both foods, the interactions between day and diet and parasite treatments, respectively, were not statistically significant (electronic supplementary material, tables S2–S3).
Anabasine affected some estimates of microcolony reproduction, but we found no trade-off for the costs and benefits of consuming anabasine dependent on parasite status. Approximately, 67% of microcolonies survived long enough to produce eggs (more than 10 days); of those, 89% did so, but neither diet nor parasite treatments nor their interaction affected likelihood of reproduction (χ2 < 1.41, n = 36, p > 0.27). Best-fit models in our analysis of reproductive responses included the effects of diet treatment, parasite treatment and their interaction, as well as colony of origin as a random effect. Microcolonies fed with anabasine began laying eggs 14% later than controls (mean ± 1 s.e. difference of 2.0 ± 1.9 days), but there was no effect of parasite status or the diet × parasite interaction (figure 2d; electronic supplementary material, tables S2–S3). Neither diet treatment nor parasite status affected other estimates of reproductive success, including total number and mass of eggs and larvae produced (in all cases, F < 2.40, p > 0.16).
Infection with Crithidia significantly reduced bee survival, with infected bees living 4.5 ± 1.4 s.e. fewer days than uninfected bees. However, anabasine consumption had no effect on bee survival (figure 2e; electronic supplementary material, tables S4–S6).
4. Discussion
The role of plant secondary metabolites in mediating interactions between herbivores and their natural enemies and parasites has long been recognized [40], but how such compounds influence other key interactions, such as those of mutualist pollinators and their parasites, has remained comparatively unexplored. By contrast, it is well known that parasites of pollinators can exert negative fitness effects on their hosts [16], including by reducing bee survival, as we demonstrate in this study. Stimulated by insights from research on tritrophic dynamics of herbivory, we tested the hypothesis that nectar secondary metabolites mediate interactions among pollinators and their parasites. We demonstrate that alkaloids (anabasine and nicotine), a terpenoid (thymol) and an iridoid glycoside (catalpol) naturally present in nectar can reduce infection levels of a common bumblebee parasite by as much as 81% (figure 1). We also found that compared with controls, bees consuming anabasine were more likely to be completely parasite-free after a week. These results are consistent with those of one other published study showing that the alkaloid gelsemine, found in Gelsemium sempervirens nectar, lowered Crithidia infection in B. impatiens workers by 65% [13]. Although not all secondary metabolites significantly reduced Crithidia infection, the direction of each effect was negative, and the overall effect across all compounds was strongly negative (figure 1).
At least three non-exclusive mechanisms could explain the effects of plant secondary metabolites on bee–parasite interactions. These mechanisms are drawn from the well-studied chemical ecology of plant–herbivore–parasite interactions [1] as well as research aimed at fighting Chagas and other trypanosome diseases of humans [4,5]. First, secondary metabolites may be directly toxic to trypanosomatids such as Crithidia. Exposure to numerous alkaloid, terpenoid and phenolic compounds is lethal to Trypanosoma species, probably via interruption of protein synthesis, DNA intercalation, interaction with neurotransmitters or induction of programmed cell death [41,42]. Second, secondary metabolites could affect bee immune function, including by upregulating bee immune response [43], stimulating bees' endosymbiotic gut bacteria [44], causing physical or chemical changes to gut lining [45], or simply by inducing diuresis [17]. This last possibility deserves further study, as we observed an increase in nectar consumption among microcolony bees that consumed anabasine (figure 2b). Third, secondary metabolites could alter metacyclogenesis, a critical process by which trypanosomes transition between amastigote and choanomastigote life stages [45]. Indeed, some efforts to combat Chagas Disease focus on the chemical interruption of metacyclogenesis [46]. Additional research is needed to identify the mechanisms responsible for the reduction of Crithidia infection by secondary metabolite ingestion by bees.
Given mounting concerns over global declines in natural and managed bees and the role of parasites in such declines, the chemistry of bee diets could play an important role in mitigating bee-disease dynamics, assuming anti-parasite benefits outweigh any costs of consumption. While we identified an anti-parasite benefit to consuming the alkaloid anabasine, we found little trade-off in terms of reproduction or survival, contrary to our original prediction. The only cost of anabasine consumption we detected was a 2-day delay in onset of egg laying, with no effect on the eventual number or mass of eggs and larvae. These results are in contrast to studies showing lethal and sublethal effects of anabasine on other insects [47]. Moreover, although bumblebees are known to be deterred from feeding by some secondary metabolites in nectar and pollen [48,49], bees in this experiment increased rather than decreased consumption of nectar containing a high concentration (20 ppm) of anabasine. This is striking, given previous work demonstrating that honeybees are deterred from consuming nectar containing more than or equal to 5 ppm anabasine [36]. In our investigation of costs and benefits, we uncovered the interesting result that bee-disease load was lower in microcolonies with greater size dimorphism, but only for bees consuming the control rather than anabasine diet. While we do not know the mechanism underlying this pattern, we note that one previous study highlighted the influence of a size-linked trait, colony dominance hierarchy, on outcomes of secondary metabolite consumption by bees [29].
We found that Crithidia infection reduced bee survival, as has also been shown in Bombus terrestris [16], suggesting common negative effects of Crithidia infection across Bombus species. Survival of bees infected with Crithidia that consumed the control diet was 46%, whereas infected bees consuming anabasine had 70% survival (figure 2e); despite the substantial disparity between these proportions, the difference was not statistically significant. However, because Crithidia has its biggest fitness impacts on food-stressed bees [50], the consequences of anabasine consumption may be underestimated by laboratory studies with ad libitum food. Future research should investigate sources of the high variation in survival and potential relationship to diet in more natural settings. One hypothesis is that the variation in the magnitude of infection due to anabasine consumption affected bee survival probability. Understanding how the magnitude of infection within bees as well as within colonies relates to individual bee and colony survival and reproduction will generate predictive insight into the degree to which secondary metabolites must reduce infection level to influence bee-disease dynamics.
Although we did not observe a significant fitness benefit of consuming anabasine when bees were parasitized, the consumption of secondary metabolites in nectar could still have important prophylactic effects on bee colony health and transmission dynamics. Crithidia spreads via infected faeces in the nest and at flowers, and gut cell counts, such as those we assessed in our experiments, are tightly correlated with faecal cell counts [27]. Thus, by reducing infection intensity, nectar secondary compounds could play important roles in reducing Crithidia transmission among nest-mates within colonies as well as among bees foraging in the wild. Understanding how floral traits such as nectar secondary chemistry mediate vertical and horizontal disease transmission represents an unexplored frontier in need of future research [51].
Four caveats are important to consider in the interpretation of our results. First, we did not account for the presence of horizontally transmitted endosymbiotic gut bacteria, which contribute to the immune response of bumblebees to Crithidia [44]. However, commercial bumblebee colonies probably retain their naturally occurring gut fauna, and newly eclosed experimental bees may have acquired these bacteria from contaminated pupa cases. Future work should investigate the effect of secondary metabolites on bee disease in the context of symbiont communities. Second, our experiments examined effects of single compounds at single concentrations, but concentrations in nectar are variable within and between plant populations [52], bees also consume compounds in pollen [10,11], and bumblebees may consume multiple secondary metabolites as larvae and adults [53]. Thus, the concentrations used in this study probably underestimate bees' actual exposure to plant chemicals. Moreover, there could be non-additive effects between suites of compounds present in single floral nectars, such as aucubin and catalpol (in Chelone glabra nectar; table 1), or gathered by bees from multiple sources. Third, our study focused only on the effects of secondary metabolites post-infection in adult workers, but secondary metabolites could also affect Crithidia viability in floral nectar prior to ingestion and transmission to new hosts [26]. If these compounds are deleterious to Crithidia within the gut of its host, they may also render nectar in flowers inhospitable to the parasites, although for one alkaloid, this is not the case [13]. Secondary metabolites could also confer parasite resistance to larval and adult bees when they are consumed before infection, perhaps by priming bees' immune response to disease [43]. Fourth, our microcolony experiment examined the effects of anabasine and Crithidia only during egg and larval development, but reproductive costs and benefits to bees could accrue after that time. For example, anabasine consumption could affect the proportion of offspring that successfully eclose as adults or horizontal transmission of Crithidia to adult offspring. These important questions were beyond the scope of this study, but should be addressed in future research.
As autotrophs, plants are key components of terrestrial ecosystems, and plant secondary metabolite chemistry plays a significant role in mediating interactions with herbivores [1]. Here we demonstrate that some compounds with well-described anti-herbivore effects can reduce parasite loads within pollinators. We found little evidence for direct negative effects of secondary metabolites on the consumers themselves, and for anabasine, bees were stimulated to consume rather than avoid the compound in nectar. Future work should examine how secondary metabolites such as anabasine can function as deterrents to insect herbivores in leaves while also increasing consumption by pollinating mutualists. One possibility suggested by our results is that bees could self-medicate by consuming plant secondary metabolites when they are infected with parasites [3,54].
Supplementary Material
Acknowledgements
We thank A. Carper Z. Gezon R. Schaeffer C. Urbanowicz and anonymous reviewers for comments on the manuscript, and Biobest Canada for donating bees. We thank A. Hogeboom Z. Getman-Pickering and M. Hammond for laboratory assistance.
Disclaimer
Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation.
Data accessibility
Data from this study have been made publically available at the Dryad Digital Repository (doi:http://dx.doi.org/10.5061/dryad.m2n6f).
Authors' contributions
L.L.R., R.E.I., L.S.A., A.S.L. and J.S.M. contributed to study design. L.L.R., R.E.I., L.S.A., A.S.L., K.H.R., W.E.A. and J.A. conducted the research. L.L.R. conducted statistical analysis and wrote the first draft of the manuscript, and all authors contributed significantly to revisions.
Funding statement
This research was supported by the National Science Foundation (DEB-0841862, DEB-0743535/0742923 and DEB-1258096/1256817).
Conflict of interests
The authors declare no competing interests.
References
- 1.Bennett RN, Wallsgrove RM. 1994. Tansley review no. 72. Secondary metabolites in plant defence mechanisms. New Phytol. 127, 617–633. ( 10.1111/j.1469-8137.1994.tb02968.x) [DOI] [PubMed] [Google Scholar]
- 2.Singer MS, Farkas TE, Skorik CM, Mooney KA. 2012. Tritrophic interactions at a community level: effects of host plant species quality on bird predation of caterpillars. Am. Nat. 179, 363–374. ( 10.1086/664080) [DOI] [PubMed] [Google Scholar]
- 3.De Roode JC, Lefèvre T, Hunter MD. 2013. Self-medication in animals. Science 340, 150–151. ( 10.1126/science.1235824) [DOI] [PubMed] [Google Scholar]
- 4.Schmidt TJ, et al. 2012. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases: part II. Curr. Med. Chem. 19, 2176–2228. ( 10.2174/092986712800229023) [DOI] [PubMed] [Google Scholar]
- 5.Schmidt TJ, et al. 2012. The potential of secondary metabolites from plants as drugs or leads against protozoan neglected diseases: part I. Curr. Med. Chem. 19, 2128–2175. [DOI] [PubMed] [Google Scholar]
- 6.Adler LS. 2000. The ecological significance of toxic nectar. Oikos 91, 409–420. ( 10.1034/j.1600-0706.2000.910301.x) [DOI] [Google Scholar]
- 7.Baker HG. 1977. Non-sugar chemical constituents of nectar. Apidologie 8, 349–356. ( 10.1051/apido:19770405) [DOI] [Google Scholar]
- 8.Knight TM, Chase JM, Hillebrand H, Holt RD. 2006. Predation on mutualists can reduce the strength of trophic cascades. Ecol. Lett. 9, 1173–1178. ( 10.1111/j.1461-0248.2006.00967.x) [DOI] [PubMed] [Google Scholar]
- 9.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detzel A, Wink M. 1993. Attraction, deterrence or intoxication of bees (Apis mellifera) by plant allelochemicals. Chemoecology 4, 8–18. ( 10.1007/BF01245891) [DOI] [Google Scholar]
- 11.Arnold SEJ, Idrovo MEP, Arias LJL, Belmain SR, Stevenson PC. 2014. Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. J. Chem. Ecol. 40, 878–881. ( 10.1007/s10886-014-0467-4) [DOI] [PubMed] [Google Scholar]
- 12.Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC. 2013. Caffeine in floral nectar enhances a pollinator's memory of reward. Science 339, 1202–1204. ( 10.1126/science.1228806) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manson J, Otterstatter M, Thomson J. 2010. Consumption of a nectar alkaloid reduces pathogen load in bumble bees. Oecologia 162, 81–89. ( 10.1007/s00442-009-1431-9) [DOI] [PubMed] [Google Scholar]
- 14.Simone-Finstrom MD, Spivak M. 2012. Increased resin collection after parasite challenge: a case of self-medication in honey bees? PLoS ONE 7, 1–7. ( 10.1371/journal.pone.0034601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams PH, Thorp RW, Richardson LL, Colla SR. 2014. Bumble bees of North America: an identification guide. Princeton, NJ: Princeton University Press. [Google Scholar]
- 16.Schmid-Hempel P. 1998. Parasites in social insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Tadmor-Melamed H, Markman S, Arieli A, Distl M, Wink M, Izhaki I. 2004. Limited ability of Palestine sunbirds Nectarinia oseato to cope with pyridine alkaloids in nectar of tree tobacco Nicotiana glauca. Funct. Ecol. 18, 844–850. ( 10.1111/j.0269-8463.2004.00929.x) [DOI] [Google Scholar]
- 18.Kretschmar JA, Baumann TW. 1999. Caffeine in citrus flowers. Phytochemistry 52, 19–23. ( 10.1016/S0031-9422(99)00119-3) [DOI] [Google Scholar]
- 19.Su R, Dong Y, Dong K, He SY. 2012. The toxic honey plant Camellia oleifera. J. Apic. Res. 51, 277–279. ( 10.3896/IBRA.1.51.3.09) [DOI] [Google Scholar]
- 20.London-Shafir I, Shafir S, Eisikowitch D. 2003. Amygdalin in almond nectar and pollen: facts and possible roles. Plant Syst. Evol. 238, 87–95. ( 10.1007/s00606-003-0272-y) [DOI] [Google Scholar]
- 21.Socha R, Juszczak L, Pietrzyk S, Gałkowska D, Fortuna T, Witczak T. 2011. Phenolic profile and antioxidant properties of Polish honeys. Int. J. Food Sci. Technol. 46, 528–534. ( 10.1111/j.1365-2621.2010.02517.x) [DOI] [Google Scholar]
- 22.Guyot C, Bouseta A, Scheirman V, Collin S. 1998. Floral origin markers of chestnut and lime tree honeys. J. Agric. Food Chem. 46, 625–633. ( 10.1021/jf970510l) [DOI] [PubMed] [Google Scholar]
- 23.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 24.Gillespie S. 2010. Factors affecting parasite prevalence among wild bumblebees. Ecol. Entomol. 35, 737–747. ( 10.1111/j.1365-2311.2010.01234.x) [DOI] [Google Scholar]
- 25.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisarovsky G, Schmid-Hempel P. 2014. Combining laboratory and field approaches to investigate the importance of flower nectar in the horizontal transmission of a bumblebee parasite. Entomol. Exp. Appl. 152, 209–215. ( 10.1111/eea.12218) [DOI] [Google Scholar]
- 27.Otterstatter MC, Thomson JD. 2006. Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133, 749–761. ( 10.1017/S003118200600120X) [DOI] [PubMed] [Google Scholar]
- 28.Schmid-Hempel P, Schmid-Hempel R. 1993. Transmission of a pathogen in Bombus terrestris, with a note on division of labour in social insects. Behav. Ecol. Sociobiol. 33, 319–327. ( 10.2307/4600887) [DOI] [Google Scholar]
- 29.Manson JS, Thomson JD. 2009. Post-ingestive effects of nectar alkaloids depend on dominance status of bumblebees. Ecol. Entomol. 34, 421–426. ( 10.1111/j.1365-2311.2009.01100.x) [DOI] [Google Scholar]
- 30.Harder LD. 1982. Measurement and estimation of functional proboscis length in bumblebees (Hymenoptera: Apidae). Can. J. Zool. 60, 1073–1079. ( 10.1139/z82-148) [DOI] [Google Scholar]
- 31.SAS Institute, Inc. 2014. JMP. Cary, NC: SAS Institute, Inc. [Google Scholar]
- 32.Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. Orlando, FL: Academic Press New York. [Google Scholar]
- 33.Algina J, Keselman HJ, Penfield RD. 2006. Confidence interval coverage for Cohen's effect size statistic. Educ. Psychol. Meas. 66, 945–960. ( 10.1177/0013164406288161) [DOI] [Google Scholar]
- 34.Cnaani J, Schmid-Hempel R, Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Sociaux 49, 164–170. ( 10.1007/s00040-002-8297-8) [DOI] [Google Scholar]
- 35.Gradish AE, Scott-Dupree CD, McFarlane AD, Frewin AJ. 2013. Too much work, not enough tarsi: group size influences Bombus impatiens (Hymenoptera: Apidae) worker reproduction with implications for sublethal pesticide toxicity assessments. J. Econ. Entomol. 106, 552–557. ( 10.1603/EC12154) [DOI] [PubMed] [Google Scholar]
- 36.Singaravelan N, Ne'eman G, Inbar M, Izhaki I. 2005. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J. Chem. Ecol. 31, 2791–2804. ( 10.1007/s10886-005-8394-z) [DOI] [PubMed] [Google Scholar]
- 37.Lovich J, Gibbons J. 1992. A review of techniques for quantifying sexual size dimorphism. Growth Dev. Aging 56, 269–281. [PubMed] [Google Scholar]
- 38.Therneau T. 2012. coxme: mixed effects Cox models. R package version 2.2–3 See www.cran.-project.org/package=coxme.
- 39.R Core Team 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 40.Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 11, 41–65. ( 10.1146/annurev.es.11.110180.000353) [DOI] [Google Scholar]
- 41.Merschjohann K, Sporer F, Steverding D, Wink M. 2001. In vitro effect of alkaloids on bloodstream forms of Trypanosoma brucei and T. congolense. Planta Med. 67, 623–627. ( 10.1055/s-2001-17351) [DOI] [PubMed] [Google Scholar]
- 42.Rosenkranz V, Wink M. 2008. Alkaloids induce programmed cell death in bloodstream forms of trypanosomes (Trypanosoma b. brucei). Molecules 13, 2462–2473. ( 10.3390/molecules13102462) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmid-Hempel P. 2005. Natural insect host–parasite systems show immune priming and specificity: puzzles to be solved. BioEssays 27, 1026–1034. ( 10.1002/bies.20282) [DOI] [PubMed] [Google Scholar]
- 44.Koch H, Schmid-Hempel P. 2011. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl Acad. Sci. USA 108, 19 288–19 292. ( 10.1073/pnas.1110474108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kollien A, Schaub G. 2000. The development of Trypanosoma cruzi in Triatominae. Parasitol. Today 16, 381–387. ( 10.1016/S0169-4758(00)01724-5) [DOI] [PubMed] [Google Scholar]
- 46.Cardoso J, Soares MJ. 2010. In vitro effects of citral on Trypanosoma cruzi metacyclogenesis. Mem. Inst. Oswaldo Cruz 105, 1026–1032. ( 10.1590/S0074-02762010000800012) [DOI] [PubMed] [Google Scholar]
- 47.González-Coloma A, et al. 2004. Structural diversity and defensive properties of norditerpenoid alkaloids. J. Chem. Ecol. 30, 1393–1408. ( 10.1023/B:JOEC.0000037747.74665.0a) [DOI] [PubMed] [Google Scholar]
- 48.Manson JS, Cook D, Gardner DR, Irwin RE. 2013. Dose-dependent effects of nectar alkaloids in a montane plant–pollinator community. J. Ecol. 101, 1604–1612. ( 10.1111/1365-2745.12144) [DOI] [Google Scholar]
- 49.Irwin RE, Cook D, Richardson LL, Manson JS, Gardner DR. 2014. Secondary compounds in floral rewards of toxic rangeland plants: impacts on pollinators. J. Agric. Food Chem. 62, 7335–7344. ( 10.1021/jf500521w) [DOI] [PubMed] [Google Scholar]
- 50.Brunner FS, Schmid-Hempel P, Barribeau SM. 2014. Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris. Proc. R. Soc. B 281, 20140128 ( 10.1098/rspb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McArt SH, Koch H, Irwin RE, Adler LS. 2014. Arranging the bouquet of disease: floral traits and the transmission of plant and animal pathogens. Ecol. Lett. 17, 624–636. ( 10.1111/ele.12257) [DOI] [PubMed] [Google Scholar]
- 52.Lanza J, Smith GC, Sack S, Cash A. 1995. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 102, 113–119. ( 10.1007/BF00333318) [DOI] [PubMed] [Google Scholar]
- 53.Gosselin M, Michez D, Vanderplanck M, Roelants D, Glauser G, Rasmont P. 2013. Does Aconitum septentrionale chemically protect floral rewards to the advantage of specialist bumblebees? Impact of toxic rewards on visitors. Ecol. Entomol. 38, 400–407. ( 10.1111/een.12032) [DOI] [Google Scholar]
- 54.Gherman BI, Denner A, Bobiş O, Dezmirean DS, Mărghitaş LA, Schlüns H, Moritz RFA, Erler S. 2014. Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 68, 1777–1784. ( 10.1007/s00265-014-1786-8) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study have been made publically available at the Dryad Digital Repository (doi:http://dx.doi.org/10.5061/dryad.m2n6f).