Abstract
Left–right asymmetries are common properties of nervous systems. Although lateralized sensory processing has been well studied, information is lacking about how asymmetries are represented at the level of neural coding. Using in vivo functional imaging, we identified a population-level left–right asymmetry in the honey bee's primary olfactory centre, the antennal lobe (AL). When both antennae were stimulated via a frontal odour source, the inter-odour distances between neural response patterns were higher in the right than in the left AL. Behavioural data correlated with the brain imaging results: bees with only their right antenna were better in discriminating a target odour in a cross-adaptation paradigm. We hypothesize that the differences in neural odour representations in the two brain sides serve to increase coding capacity by parallel processing.
Keywords: honey bee, brain asymmetry, odour coding, calcium imaging, odour discrimination, lateralization
1. Introduction
Most bilaterian animals have asymmetric brains. Brain asymmetry is thought to optimize neural circuitry, avoiding duplication of functions and promoting parallel processing [1,2]. Brain lateralization is evident both in cognitive functions and processing of sensory stimuli [1,3]. In humans, for example, there is an asymmetric activation of brain areas during auditory, visual and tactile processing [4–7]. Beside large-scale differences in the activation between the left and right brain hemispheres, there are also neuron-specific brain asymmetries. For example, in Caenorhabditis elegans a physiological left–right asymmetry in chemosensory neurons is required for normal chemotaxis [8], and in zebrafish (Danio rerio) a physiological and anatomical left–right asymmetry in habenula neurons is required for normal olfactory and visual processing [9,10]. However, to the best of our knowledge, left–right asymmetry in neural population coding in primary sensory brain areas has not yet been studied.
We addressed the issue of lateralized sensory coding using the olfactory system of the honey bee, Apis mellifera. In honey bees, there are population-level left–right asymmetries in odour-driven behaviour and sensory odour processing [11–15]. After associative odour learning, a right bias in short-term memory retention [12–14], and a left bias in long-term memory retention [12] have been shown. Moreover, there is a disproportionate distribution in the number of sensilla, the olfactory structures on the antenna, with the right antenna showing more sensilla in each segment [11,14]. To date however, evidence of lateralized morphology [15] or neural coding [16,17] in the honey bee brain is lacking.
Along the olfactory pathway, the AL is the first brain area for processing odour information. Different odourants activate different, but often overlapping, ensembles of olfactory receptor neurons [18]. From the antenna, olfactory receptor neurons project to subcompartments of the ipsilateral AL, called glomeruli. Thus, receptor neurons create a combinatorial odour code of activated glomeruli [19]. In the glomeruli, odour-evoked activity is transmitted to local interneurons and to projection neurons (PN), which connect higher brain areas [20]. There is good evidence that PN activity patterns indeed encode odour identity and underlie odour perception [21,22]. We performed in vivo imaging of PNs and found an asymmetry in odour information processing both at the level of neural representation and odour perception during a cross-adaptation paradigm.
2. Material and methods
All experiments were done with foragers of the honey bee, Apis mellifera. Calcium imaging data came from the same published and unpublished dataset from our previous study [23]. The recordings were performed on either the left or the right AL. In total, 66 bees were imaged, 33 left ALs and 33 right ALs. Details of the imaging method can be found elsewhere [23]. Briefly, PNs of both ALs were stained with the calcium-sensitive dye Fura-2 dextran (Invitrogen). The next day, bees were prepared for imaging and the AL that showed better staining and spontaneous activity was brought into focus.
PNs in the AL were imaged with a water immersion objective (20×, NA 0.95, Olympus) with a wide-field imaging system (Till Photonics) and a CCD camera (Imago QE, Till Photonics). 8 × 8 pixels of the camera were binned on-chip, resulting in a resolution of 172 × 130 pixels. Each acquisition lasted 29 s and consisted of 232 double frames, recorded with 340 and 380 nm excitation light, respectively, at a frame rate of 8 Hz. Excitation and emission light was separated by a 420 nm dichroic mirror and a 490–530 nm emission filter. Bees were stimulated with 1 : 100 dilutions of 1-hexanol, 2-octanol, 1-nonanol, a mixture of 1-hexanol and 2-octanol, and the solvent mineral oil as blank control (all from Sigma Aldrich). For all the experiments, odourants were prepared daily from stocks in mineral oil, which were renewed every four weeks. Odourant stimuli were delivered as 4 s pulses with a custom-built computer-controlled olfactometer [22]. Each channel of the olfactometer consisted of two syringes, an empty one for equalizing air flow and one containing the odourant. The olfactometer had 6 channels. The air stream through each channel was 300 ml min−1, each controlled by a flow meter (Analyt-MTC). Odourants were injected into a continuous carrier air stream (1200 ml min−1) in a glass tube (1 cm in diameter), which was directed to the bee positioned 1 cm in front of it. A solenoid three-way valve (Lee) controlled the odour pulses by diverting air from the empty syringe to the odourant syringe. The six channels added up to 1800 ml min−1, and total air stream was 3000 ml min−1. Continuous air exhaust behind the bee cleared residual odour. Odourants were presented in a pseudo-randomized order with an inter-trial interval of 2 min.
(a). Data analysis
Imaging data were analysed using custom-written programs in IDL (RSI). Movies were movement-corrected by aligning frames within and between measurements. Glomeruli were segmented with the help of an unsharp masked image of the raw fluorescence and a correlation image where the correlation of the signal traces between neighbouring pixels was calculated. For each animal, both identified and unidentified glomeruli were segmented (figure 1a). The number of segmented glomeruli differed across individuals (8–20 glomeruli per individual, both in the left and in the right ALs). The numbers of identified glomeruli were 12.9 ± 2.8 in the left AL and 13.8 ± 3.4 in the right AL. The ratio of the images acquired at 340 and 380 nm excitation wavelength was calculated, and the average background fluorescence (mean of 66 frames before stimulation) was subtracted from every frame, to get the fluorescence response signal ΔF340/380. No filtering was used for quantitative analysis.
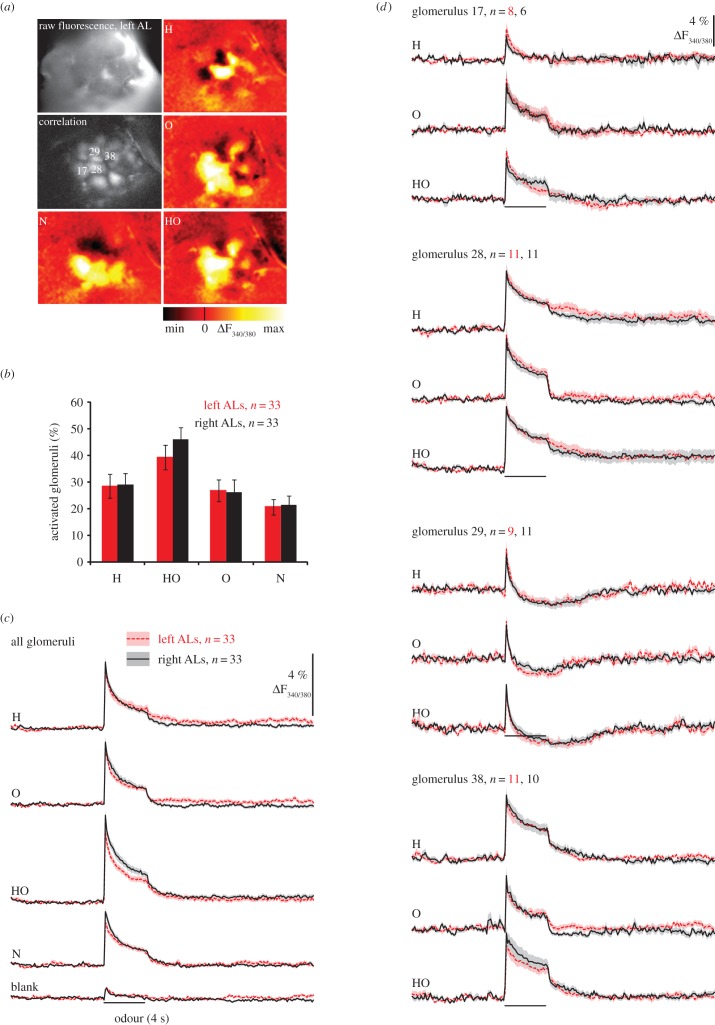
Figure 1.
PN response strength does not differ between sides. (a) Calcium imaging in PNs (raw fluorescence, left AL), correlation image of the same AL (correlation) with identified glomeruli whose odour responses is represented in (d)) and colour-coded images showing odour-evoked glomerular activity to 2-nonanol (N), 1-hexanol (H), 2-octanol (O) and a 1 : 1 mixture of 1-hexanol and 2-octanol (HO). (b) The number of odour-activated glomeruli is equal between sides. Percentage of activated glomeruli for different odour stimuli: each glomerulus was considered active when activity during the first second of odour response showed a signal greater than or equal to 6 × s.d. before stimulus onset. For each bee, the percentage of activated glomeruli was calculated as mean ± s.e.m. for left and right ALs (N = 33 per side). The percentage of activated glomeruli differed between odours (F3,192 = 23.436, p < 0.001) but not between sides (F1,64 = 0.143, p = 0.706) and there was no interaction between side and type of odourant (odour × side F3,192 = 0.706, p = 0.529). (c) Time course of global PN responses to odours along the whole recordings (29 s) (average over all glomeruli; mean ± s.e.m.); grey bars indicate the 4 s stimulus pulse. The RM ANOVA revealed an odour effect (F3,192 = 25.57, p < 0.001) but neither side effect (side F1,64 = 2.59; p = 0.112) nor interactions (odour × side F3,19 = 1.390; p = 0.25) were found. (d) Time course of PN responses to odours in identified glomeruli averaged across animals. (Online version in colour.)
Background activity was quantified by the standard deviation (s.d.) before stimulus onset (frames 1–70) averaged over all glomeruli. Left and right background activities were compared using an independent t-test (two tails).
The response strength was quantified as the mean signal during 1 s after stimulus onset (frames 72–79). To calculate the global response over the whole AL, for each bee, the response of each glomerulus was calculated and averaged over all glomeruli.
For the analysis in figure 1b, glomeruli with a response strength greater than or equal to 6 × s.d. before stimulus were considered as active. Numbers of activated glomeruli were compared at the level of the AL (averaged per bee) using a repeated measures analysis of variance (RM ANOVA) with odours as within-subject factor and side as a between-subjects factor.
In figure 2a,b, the distance between odour response patterns was quantified as Euclidean distances: for each AL, the odour response pattern of all glomeruli (response strength during frames 72–79) was represented as a vector in the glomerular response space and the Euclidean distance between the two vectors was calculated as follows:
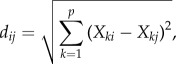 |
with i and j indicating two odours, p the number of glomeruli, and Xki the activity of glomerulus k when stimulated with odour i.
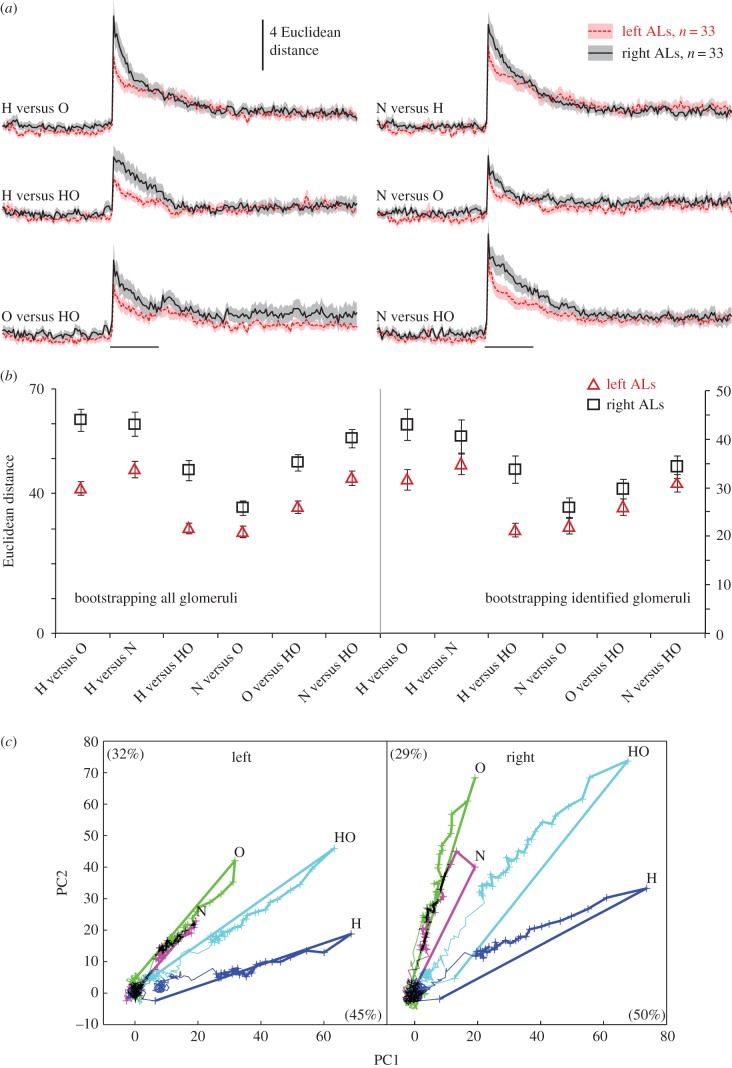
Figure 2.
Distances between odour response patterns are greater in the right AL. (a) Time course of Euclidean distances between odourant pairs across all glomeruli (average over all bees; mean ± s.e.m.) in left ALs (red online) and right ALs (black) ALs. The horizontal bars indicate the 4 s stimulus pulse. (b) Left graph: bootstrapping distributions of Euclidean distances (mean ± s.d.) in left and right ALs from 10 000 random samples with replacements within the pooled left and right AL data (balanced to 426 glomeruli within each group). Means of Euclidean distances are calculated over the first second of odour stimuli. Right graph: same as left graph but pooling the identified glomeruli only (158 glomeruli per side). (c) Trajectories of the odour responses in all glomeruli of left ALs (426 glomeruli) and right ALs (455 glomeruli) calculated using PCA. Bold lines indicate the 4 s odour stimulation. Crosses represent the time points of sampled frames during odour stimulation (125 ms inter-frame interval). Percentages of variability explained by PC1 and PC2 are given in brackets. H: 1-hexanol; O: 2-octanol; N: 2-nonanol; HO: 1 : 1 mixture of 1-hexanol and 2-octanol. (Online version in colour.)
Odour distances were analysed using RM ANOVA with distances as a within-subject factor and side as a between-subjects factor.
In order to test for the consistency of side differences, bootstrapping was performed on the Euclidean distances, resampling the total 66 bee datasets (left and right ALs together) randomly into two groups (33 ALs in each group), repeating that 1000 times. This provides two distributions of Euclidean distances, which were now compared to the original left and right data. For each resampled dataset, RM ANOVA allowed us to calculate a p-value for the between-subjects factor (i.e. the factor that represents side in the original dataset). The p-value of this permutation test was then calculated as the proportion of resampled datasets where the between-subjects factor was equal to or greater than the one observed in the original left and right data.
In a second approach, glomerular signals of all bees were pooled into two groups containing left and the right AL data, respectively. Group sizes were balanced to 426 glomeruli each. In a second bootstrapping analysis, glomeruli within the left and right groups were now randomly resampled with replacements, repeating this 10 000 times. This allowed us to characterize the distributions of the Euclidean distances in each of the two groups according to their mean and s.d. (figure 2b). One analysis was performed involving all glomeruli (balanced to 426 per side), a second with only the identified glomeruli (balanced to 158 per side). Results of the left and right groups of identified glomeruli were compared using RM ANOVA with distances as a within-subject factor and side as a between-subjects factor.
In figure 2c, a principal component analysis (PCA) was performed for left and right antennal lobes (ALs) separately (using the Matlab princomp function). For each odour, the PCA was applied to the multi-dimensional glomerular space (426 glomeruli for the left AL; 455 for the right AL) for the whole recording period (29 s).
Quantitative and statistical analyses were performed using Matlab (R2012b, Mathworks) and PASW Statistic v. 18 (SPSS).
The original data can be found in Data_PNs.txt in the electronic supplementary materials.
(b). Behavioural experiments
Honey bee foragers were collected from outdoor hives between March and June 2013 in Konstanz (Germany). They were individually mounted in plastic holders as described elsewhere [22], allowing for free movement of antennae and mouth parts. In the evening, bees were fed to satiation with 1 M sucrose solution, and experiments were performed the next day. Before odour conditioning, honey bees were randomly divided into two groups: in one group the right antenna was cut at the base of the pedicel, in the second group the left antenna was cut, 15–60 min before the experiment. Each bee was put in front of a custom-built olfactometer (see above) and an exhaust fan removed the odourant quickly after exposure.
Bees received three conditioning trials during which an odourant was presented for 4 s paired with 0.2 µl 1 M sucrose reward, which was presented for 3 s starting 2 s after odour onset. The odourants were 1-hexanol or 2-octanol diluted 1 : 100 v/v in mineral oil. The inter-trial intervals were 1 min in all behavioural experiments. In each conditioning session, eight bees with amputated left antenna and eight bees with amputated right antenna were conditioned in parallel.
In the cross-adaptation experiment (figure 3a), bees were trained to associate a target odourant that was superimposed on a different (figure 3a(ii), n = 262, see figure 3 for details on n) or the same background odourant (figure 3a(iii), n = 206). The background odourant started 20 s before the onset of the target odourant.
Figure 3.
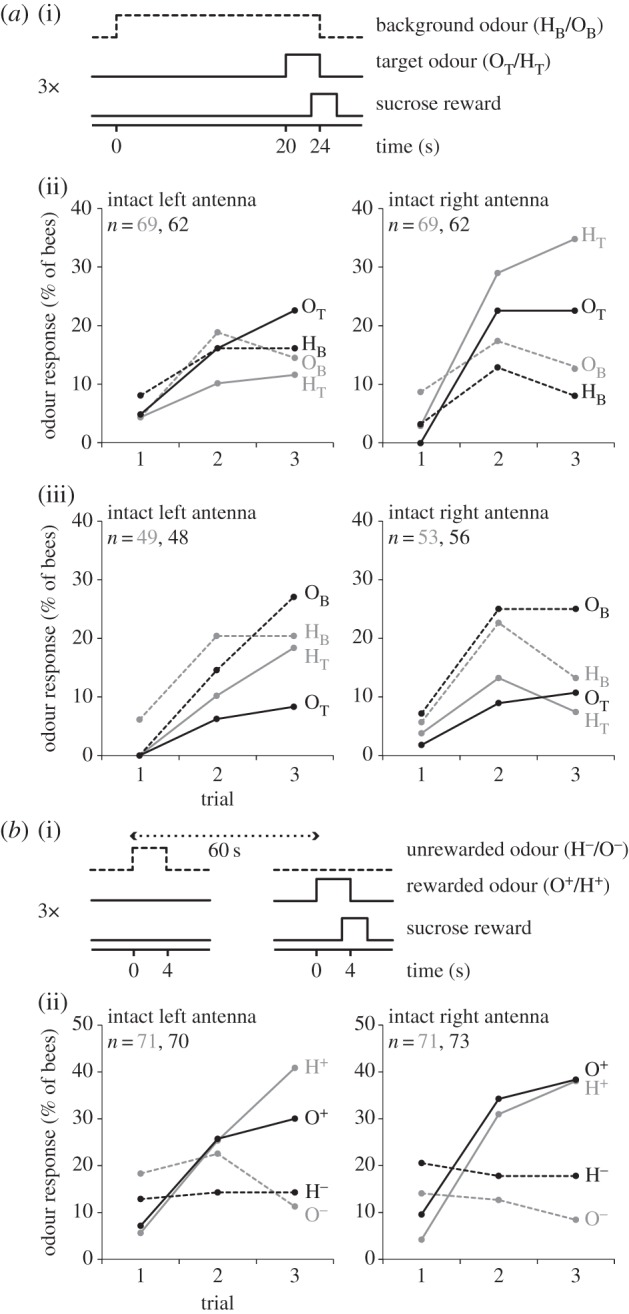
Bees with only the right antenna in use are better in discriminating a target from a background odourant in a cross-adaptation experiment. (a)(i) Timing of background odour, target odour and sucrose reward during the learning experiment. The background odour started 20 s before the onset of the target odour, and the sucrose reward started 3 s after the onset of the foreground odour. Bees received three conditioning trials. Inter-trial interval was 60 s. (a)(ii) Bees were trained to discriminate a target odour (solid lines) from a background odour (dashed lines). In one subgroup of bees (grey lines), the target odour was 1-hexanol (HT) and the background odour was 2-octanol, OB). In another subgroup of bees (black lines), the target odour was 2-octanol (OT) and the background odour was 1-hexanol (HB). Behavioural performance during conditioning was measured as proboscis extension response to the initial 3 s of the odourant stimuli. There were general effects of both trial (F2,516 = 28.113, p < 0.001) and stimulus (F1,258 = 4.16, p = 0.042) and effects of interaction of stimulus × trial (F2,516 = 7.11, p = 0.001) and stimulus × side (F2,516 = 8.36 p = 0.004). ANOVA revealed an odour × side interaction (F1,258 = 4.72, p = 0.03). There was an interaction effect between side × trial × stimulus (F2,516 = 4.84, p = 0.008) but this was not dependent on the odour used (odour × side × trial × stimulus F2,516 = 1.41, p = 0.247). (a)(iii) same as in (a)(ii) but the target odour was the same as the background odour. There were general effects of both trial (F2,404 = 24.94, p < 0.001) and stimulus (F1,202 = 21.30, p < 0.001) and effects of interaction of stimulus × trial (F2,516 = 7.11, p = 0.001). There was no interaction effect among side × trial × stimulus (F2,404 = 0.159, p = 0.853) nor among side × trial × stimulus × odour (F2,404 = 0.945, p = 0.389). (b)(i) Bees were differentially conditioned to discriminate between a rewarded odour (solid lines) and a non-rewarded odour (dashed lines). Bees received 3 × pseudo-randomized presentations of rewarded and non-rewarded odourants (inter-trial interval: 60 s). Odourants were delivered for 4 s, sucrose solution was presented for 3 s (1 s overlap with X+). (b)(ii) Behavioural responses over the three conditioning trials were calculated as in (a)(ii). In one subgroup of the bees (grey line), the rewarded odour was 1-hexanol (H+) and the non-rewarded odour was 2-octanol (O−). In another subgroup of bees (black line), the rewarded odour was 2-octanol (O+) and the non-rewarded odour was 1-hexanol (H−). A general effect of trial (F2,562 = 24.295 p < 0.001), stimulus (F1,281 = 30.492 p < 0.001) and trial × stimulus interaction effect was evident (F2,562 = 45.305 p < 0.001); no effects were found for trial × stimulus x side (F2,562 = 1.325, p = 0.267) nor for trial × stimulus × side × odour (F2,562 = 0.009, p = 0.991).
In the differential conditioning protocol (figure 3b, n = 285), a rewarded odour (X+) and a non-rewarded odour (Y−) were presented in a pseudo-randomized order (either X+, Y−, Y−, X+, Y−, X+ or Y−, X+, X+, Y−, X+, Y−). Behavioural data were analysed with RM ANOVA, with trials, odourants (1-hexanol versus 2-octanol) and stimuli (rewarded versus non-rewarded odourant) as a within-subject factor and side as a between-subjects factor.
The original data can be found in Data_Fig3Aii.txt, Data_Fig3Aiii.txt and Data_Fig3Bii.txt in the electronic supplementary materials.
3. Results
We used calcium imaging of PNs to test neural odour representations for differences between the left and right ALs of the honey bee. Antennae were frontally stimulated with the single odourants 1-hexanol (H), 2-octanol (O), 1-nonanol (N) and a 1 : 1 binary mixture (HO) (figure 1a).
(a). Background activity strength, odour response strength and number of activated glomeruli are equal between sides
We first compared the background activity, measured as standard deviation before stimulus onset, and found no difference in the fluorescence response (ΔF340/380) between the left and right ALs (left: 0.63 ± 0.05%, right: 0.66 ± 0.04% (mean ± s.e.m.)). Odours activated between 20 and 50% of the imaged glomeruli (figure 1a,b), and the percentage of activated glomeruli differed between odours but not between sides (figure 1b). We then compared the odour response strength (averaged signal during the first second of odour response) between the left and right AL and again, no difference was apparent (figure 1c,d).
(b). Distances between odour representations are higher in the right antennal lobe
We next asked whether there is a qualitative difference in odour representations between the left and right ALs and calculated the Euclidean distances between glomerular response patterns of different odours. The distances between odour-evoked glomerular response patterns correlate well with the perceived dissimilarity between them [21], showing that distances between odour-evoked glomerular response patterns contain behaviourally relevant odour information. Therefore, left–right differences in inter-odour distances would indicate differences in the discriminatory power of the odour code. Indeed, inter-odour Euclidean distances differed between sides and were higher in the right AL (RM ANOVA, p < 0.05; figure 2a). Permutation test of Euclidean distances in 1000 random subsets of data from pooled left and right datasets excluded a sampling artefact (p = 0.032), strengthening the robustness of the difference that we found between left and right ALs. To exclude the possibility that any difference in the imaged plane between the sides would have systematically affected the population of imaged glomeruli and thus the inter-odour distances, we calculated the Euclidean distances on the basis of identified glomeruli. We created subsets of identified glomeruli, whereby all of them were equal with respect to number and identity of glomeruli. We created 10 000 estimates of these subsets using the bootstrapping method and calculated their Euclidean distances. These Euclidean distances were then compared between the left and right sides using a general linear model that revealed a significant general difference that was consistent for all odour pairs (figure 2b, RM ANOVA, p < 0.001).
Finally, in order to better visualize the difference in odour distances over time in the two ALs, we performed a PCA and projected the odour-induced responses of the 426 left and 455 right glomeruli into a two-dimensional space (represented by the two first principal components, which capture 77% and 79% of the variability in the left and right ALs, respectively). Odour-evoked trajectories in PCA spaces were similar in the two sides but the relative distances between inter-odour patterns were larger across glomeruli in the right ALs (figure 2c).
(c). Perceptual odour distances depend on the antenna in use
In honey bees, the distance between PN activity patterns correlates with perceptual odour distances [21,22]. Accordingly, the higher neurophysiological odour distances in the right AL might support perceptual odour discrimination. To test this hypothesis, we used a cross-adaptation paradigm to measure the perceptual distance between odour representations. Cross-adaptation paradigms can be used as tool to assess perceptual odour distances [24–26]. In a cross-adaptation experiment, a target odourant is presented after pre-exposure to a background odour. The pre-exposure of the background odourant leads to adaptation, which can attenuate the perception of a target odourant. The attenuation of the target odour perception increases with increasing similarity between the target and the background odourant. We trained bees with a single antenna in use (the other one being amputated) to extend their proboscis when a target odourant was presented, which was preceded by a different, 20-s long background odourant (figure 3a(i)). We found that bees with only their right antenna in use performed better in this task than bees with only their left antenna in use (figure 3a(ii), RM ANOVA, p < 0.01). The right-antenna dominance on this task was not owing to a better discrimination for changes in odour concentrations: when both groups of bees were trained with the same odourants as background and target, they failed equally (figure 3a(iii)). These results suggest that smelling with the right antenna supports a higher perceptual distance between 1-hexanol and 2-octanol or better discriminatory odour learning. To distinguish between these alternative explanations, we tested bees' performance during discriminatory odour learning, and we trained bees in a differential conditioning paradigm to discriminate between a rewarded or a non-rewarded odourant with either their left or right antenna in use. Bees with either the right or the left antenna in use performed equally during differential conditioning (figure 3b). Thus, bees' better performance in segregating the target from the background odourant when using their right antenna (figure 3a(ii)) might reflect a higher perceptual discriminatory power rather than a better learning performance.
4. Discussion
We used in vivo calcium imaging and behavioural experiments to investigate population-level lateralization of odour processing in the honey bee brain. Using three alcohols and one binary mixture as odourants, we found that odour processing is asymmetric: the neural odour representations were more separated in the right AL, and bees with only their right antenna in use were better in segregating a target odour from a background odour in a cross-adaptation experiment. Similarly, in vertebrates the discriminatory power of sensory representations can be lateralized, as has been shown in the visual system of birds [27–31] both behaviourally and physiologically (fMRI during a discrimination task described in [31], electrophysiological recordings in the primary auditory area in [30] and in the auditory system of bats [32]; the latter was revealed by measuring inter-stimuli Euclidean distances).
The larger physiological inter-odour distances that we found could reflect a general higher discriminatory power of odour representations in the right brain side. Alternatively, inter-odour distances could differ in an odour-specific way, such that some pairs of odourants evoke more distinct activity patterns in the right side, while others evoke more distinct activity patterns in the left side. To distinguish between these alternative explanations, it is necessary to compare the physiological inter-odour distances for a larger number of odour pairs across different chemical classes.
The better odour–background segregation in bees with their right antenna in use could reflect a higher perceptual olfactory discriminatory power. Alternatively, right-antenna bees could learn faster in that odour–background segregation task, albeit equal performance during differential odour conditioning (figure 3b). To distinguish between these alternative conclusions and to better relate the physiological and perceptual inter-odour distances, the behavioural cross-adaptation experiment should be repeated with more odour pairs and more training trials.
The left–right asymmetry in neural inter-odour distances and behavioural performance in the cross-adaptation experiment that we report here add to previous studies that revealed anatomical and behavioural lateralization in bees [11–14]. After appetitive odour conditioning, bees show a right-antenna bias of short-term memory retention [12–14], and a left-antenna bias of long-term memory retention [12]. We did not find a significant difference in the discriminatory odour learning, suggesting that associative odour-reward learning is independent of the antenna in use (figure 3b). Rogers et al. [33] showed a right dominance for context-dependent behaviours, in particular during tasks possibly related to nest-mate recognition. It is possible that this behavioural specialization reflects a higher discriminatory power for colony odours with the right antenna, as we have found for alcohols.
What are the neural mechanisms underlying the side-specific differences in odour coding? Previous calcium imaging studies using bath staining techniques found symmetric odour response patterns in the honey bee ALs [16,17]. Bath staining is not selective for a particular neuron type, but the signal is probably dominated by activity in olfactory receptor neurons [34]. Therefore, the left–right difference we found might be owing to left–right difference in processing within the AL network rather than left–right difference in glomerular pattern of receptor neuron activity. The higher distances between odour-evoked glomerular response patterns in the right AL might reflect a left–right difference in the inter-glomerular network. In honey bees, glomeruli are interconnected by a dense network of extrinsic neurons [35–39] and local interneurons [19,40], many of which are inhibitory [41]. The strength of inter-glomerular inhibitory connections is variable within and across ALs [42]. Thus, it is possible that the inter-glomerular networks differ qualitatively between the left and right ALs owing to developmental or experience-dependent alterations. Such left–right differences in the inter-glomerular networks could increase inter-odour distances in the right side, while leaving the overall response strength equal between ALs.
What is the advantage of asymmetric odour coding? Being able to discriminate between similar odourants, concentrations and mixture compounds is equally important as being able to generalize across them. These opposing tasks require opposing coding strategies. To fulfill these opposing coding strategies, the olfactory system may use parallel processing, which is a common feature both in the insect AL [43–49] and in the mammalian olfactory bulb [50]. The left–right asymmetry in odour coding that we found here could reflect another type of parallel processing, which would allow the simultaneous use of different coding schemes and therefore increase the computational capacity of the olfactory system.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank C. Giovanni Galizia, Christoph Kleineidam and Jacob Stierle for fruitful discussions, Andrew J. Anderson, Nicholas Kirkerud and Martin Strauch for statistical advice, and Teresa Bluhmki, Mihaela Coric, Michael Lutz and Sina Rometsch for help with the behavioural experiments, and two anonymous reviewers for their valuable suggestions.
Funding statement
This study was supported by the Fondazione Cassa di Risparmio di Trento e Rovereto (to E.R., A.H., G.A., G.V.), Provincia Autonoma di Trento (Unità di Ricerca IBRAIM to A.H.) and Bundesministerium für Bildung und Forschung (01GQ0931 to P.S.).
References
- 1.Rogers LJ, Vallortigara G, Andrew RJ. 2013. Divided brains the biology and behaviour of brain asymmetries. New York, NY: Cambridge University Press. [Google Scholar]
- 2.Vallortigara G, Rogers LJ. 2005. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 28, 575–589; discussion 589–633 ( 10.1017/S0140525X05000105) [DOI] [PubMed] [Google Scholar]
- 3.Concha ML, Bianco IH, Wilson SW. 2012. Encoding asymmetry within neural circuits. Nat. Rev. Neurosci. 13, 832–843. ( 10.1038/nrn3371) [DOI] [PubMed] [Google Scholar]
- 4.Peelle JE. 2012. The hemispheric lateralization of speech processing depends on what ‘speech’ is: a hierarchical perspective. Front. Hum. Neurosci. 6, 309 ( 10.3389/fnhum.2012.00309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tervaniemi M, Hugdahl K. 2003. Lateralization of auditory-cortex functions. Brain Res. Rev. 43, 231–246. ( 10.1016/j.brainresrev.2003.08.004) [DOI] [PubMed] [Google Scholar]
- 6.Meng M, Cherian T, Singal G, Sinha P. 2012. Lateralization of face processing in the human brain. Proc. R. Soc. B 279, 2052–2061. ( 10.1098/rspb.2011.1784) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivry R, Robertson L. 1998. The two sides of perception. Cambridge, MA: The MIT Press. [Google Scholar]
- 8.Wes PD, Bargmann CI. 2001. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 410, 698–701. ( 10.1038/35070581) [DOI] [PubMed] [Google Scholar]
- 9.Jetti SK, Vendrell-Llopis N, Yaksi E. 2014. Spontaneous activity governs olfactory representations in spatially organized habenular microcircuits. Curr. Biol. 24, 434–439. ( 10.1016/j.cub.2014.01.015) [DOI] [PubMed] [Google Scholar]
- 10.Dreosti E, Vendrell Llopis N, Carl M, Yaksi E, Wilson SW. 2014. Left–right asymmetry is required for the habenulae to respond to both visual and olfactory stimuli. Curr. Biol. 24, 440–445. ( 10.1016/j.cub.2014.01.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letzkus P, Ribi WA, Wood JT, Zhu H, Zhang SW, Srinivasan MV. 2006. Lateralization of olfaction in the honey bee Apis mellifera. Curr. Biol. 16, 1471–1476. ( 10.1016/j.cub.2006.05.060) [DOI] [PubMed] [Google Scholar]
- 12.Rogers LJ, Vallortigara G. 2008. From antenna to antenna: lateral shift of olfactory memory recall by honey bees. PLoS ONE 3, e2340 ( 10.1371/journal.pone.0002340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anfora G, Frasnelli E, Maccagnani B, Rogers LJ, Vallortigara G. 2010. Behavioural and electrophysiological lateralization in a social (Apis mellifera) but not in a non-social (Osmia cornuta) species of bee. Behav. Brain Res. 206, 236–239. ( 10.1016/j.bbr.2009.09.023) [DOI] [PubMed] [Google Scholar]
- 14.Frasnelli E, Anfora G, Trona F, Tessarolo F, Vallortigara G. 2010. Morpho-functional asymmetry of the olfactory receptors of the honey bee (Apis mellifera). Behav. Brain Res. 209, 221–225. ( 10.1016/j.bbr.2010.01.046) [DOI] [PubMed] [Google Scholar]
- 15.Rigosi E, Frasnelli E, Vinegoni C, Antolini R, Anfora G, Vallortigara G, Haase A. 2011. Searching for anatomical correlates of olfactory lateralization in the honey bee antennal lobes: a morphological and behavioural study. Behav. Brain Res. 221, 290–294. ( 10.1016/j.bbr.2011.03.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galizia CG, Nägler K, Hölldobler B, Menzel R. 1998. Odour coding is bilaterally symmetrical in the antennal lobes of honey bees (Apis mellifera). Eur. J. Neurosci. 10, 2964–2974. ( 10.1111/j.1460-9568.1998.00303.x) [DOI] [PubMed] [Google Scholar]
- 17.Sandoz JC, Galizia CG, Menzel R. 2003. Side-specific olfactory conditioning leads to more specific odor representation between sides but not within sides in the honey bee antennal lobes. Neuroscience 120, 1137–1148. ( 10.1016/S0306-4522(03)00384-1) [DOI] [PubMed] [Google Scholar]
- 18.Galizia C, Menzel R. 2001. The role of glomeruli in the neural representation of odours: results from optical recording studies. J. Insect Physiol. 47, 115–130. ( 10.1016/S0022-1910(00)00106-2) [DOI] [PubMed] [Google Scholar]
- 19.Galizia CG, Szyszka P. 2008. Olfactory coding in the insect brain: molecular receptive ranges, spatial and temporal coding. Entomol. Exp. Appl. 128, 81–92. ( 10.1111/j.1570-7458.2007.00661.x) [DOI] [Google Scholar]
- 20.Sachse S, Galizia CG. 2002. Role of inhibition for temporal and spatial odor representation in olfactory output neurons: a calcium imaging study. J. Neurophysiol. 87, 1106–1117. ( 10.1152/jn.00325.2001) [DOI] [PubMed] [Google Scholar]
- 21.Guerrieri F, Schubert M, Sandoz JC, Giurfa M. 2005. Perceptual and neural olfactory similarity in honey bees. PLoS Biol. 3, e60 ( 10.1371/journal.pbio.0030060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szyszka P, Demmler C, Oemisch M, Sommer L, Biergans S, Birnbach B, Silbering AF, Galizia CG. 2011. Mind the gap: olfactory trace conditioning in honey bees. J. Neurosci. 31, 7229–7239. ( 10.1523/JNEUROSCI.6668-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rath L, Giovanni Galizia C, Szyszka P. 2011. Multiple memory traces after associative learning in the honey bee antennal lobe. Eur. J. Neurosci. 34, 352–360. ( 10.1111/j.1460-9568.2011.07753.x) [DOI] [PubMed] [Google Scholar]
- 24.Cleland TA, Morse A, Yue EL, Linster C. 2002. Behavioral models of odor similarity. Behav. Neurosci. 116, 222–231. ( 10.1037/0735-7044.116.2.222) [DOI] [PubMed] [Google Scholar]
- 25.Wilson DA. 2000. Odor specificity of habituation in the rat anterior Piriform cortex. J. Neurophysiol. 83, 139–145. [DOI] [PubMed] [Google Scholar]
- 26.Dalton P, Wysocki CJ. 1996. The nature and duration of adaptation following long-term odor exposure. Percept. Psychophys 58, 781–792. ( 10.3758/BF03213109) [DOI] [PubMed] [Google Scholar]
- 27.Güntürkün O, Diekamp B, Manns M, Nottelmann F, Prior H, Schwarz A, Skiba M. 2000. Asymmetry pays: visual lateralization improves discrimination success in pigeons. Curr. Biol. 10, 1079–1081. (doi:1016/S0960-9822(00)00671-0) [DOI] [PubMed] [Google Scholar]
- 28.Salva O, Regolin L, Mascalzoni E, Vallortigara G. 2012. Cerebral and behavioural asymmetries in animal social recognition. Comp. Cogn. Behav. Rev. 7, 110–138. ( 10.3819/ccbr.2012.70006) [DOI] [Google Scholar]
- 29.Vallortigara G, Cozzutti C, Tommasi L, Rogers LJ. 2001. How birds use their eyes: opposite left–right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr. Biol. 11, 29–33. ( 10.1016/S0960-9822(00)00027-0) [DOI] [PubMed] [Google Scholar]
- 30.George I, Vernier B, Richard J, Hausberger M, Cousillas H. 2004. Hemispheric specialization in the primary auditory area of awake and anesthetized starlings (Sturnus vulgaris). Behav. Neurosci. 118, 597–610. ( 10.1037/0735-7044.118.3.597) [DOI] [PubMed] [Google Scholar]
- 31.Poirier C, Boumans T, Verhoye M, Balthazart J, Van der Linden A. 2009. Own-song recognition in the songbird auditory pathway: selectivity and lateralization. J. Neurosci. 29, 2252–2258. ( 10.1523/JNEUROSCI.4650-08.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanwal JS. 2012. Right–left asymmetry in the cortical processing of sounds for social communication vs. navigation in mustached bats. Eur. J. Neurosci. 35, 257–270. ( 10.1111/j.1460-9568.2011.07951.x) [DOI] [PubMed] [Google Scholar]
- 33.Rogers LJ, Rigosi E, Frasnelli E, Vallortigara G. 2013. A right antenna for social behaviour in honey bees. Sci. Rep. 3, 2045 ( 10.1038/srep02045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galizia CG, Vetter R. 2004. Optical methods for analyzing odor-evoked activity in the insect brain. In Methods in insect sensory neuroscience (ed. Christensen TA.), pp. 349–392. Boca Raton, FL: CRC Press. [Google Scholar]
- 35.Arnold G, Masson C, Budharugsa S. 1985. Comparative study of the antennal lobes and their afferent pathway in the worker bee and the drone (Apis mellifera). Cell Tissue Res. 242, 593–605. ( 10.1007/BF00225425) [DOI] [Google Scholar]
- 36.Hammer M. 1993. An identified neuron mediates the unconditioned stimulus in associative olfactory learning in honey bees. Nature 366, 59–63. ( 10.1038/366059a0) [DOI] [PubMed] [Google Scholar]
- 37.Rybak J, Menzel R. 1993. Anatomy of the mushroom bodies in the honey bee brain: the neuronal connections of the alpha-lobe. J. Comp. Neurol. 334, 444–465. ( 10.1002/cne.903340309) [DOI] [PubMed] [Google Scholar]
- 38.Schröter U, Malun D, Menzel R. 2007. Innervation pattern of suboesophageal ventral unpaired median neurones in the honey bee brain. Cell Tissue Res. 327, 647–667. ( 10.1007/s00441-006-0197-1) [DOI] [PubMed] [Google Scholar]
- 39.Kirschner S, Kleineidam CJ, Rybak R, Gru B, Zube C. 2006. Dual olfactory pathway in the honey bee, Apis mellifera. J. Comp. Neurol. 952, 933–952. ( 10.1002/cne.21158) [DOI] [PubMed] [Google Scholar]
- 40.Kreissl S, Strasser C, Galizia CG. 2010. Allatostatin immunoreactivity in the honey bee brain. J. Comp. Neurol. 518, 1391–1417. ( 10.1002/cne.22343) [DOI] [PubMed] [Google Scholar]
- 41.Schäfer S, Bicker G. 1986. Distribution of GABA-like immunoreactivity in the brain of the honey bee. J. Comp. Neurol. 246, 287–300. ( 10.1002/cne.902460302) [DOI] [PubMed] [Google Scholar]
- 42.Girardin CC, Kreissl S, Galizia CG. 2013. Inhibitory connections in the honey bee antennal lobe are spatially patchy. J. Neurophysiol. 109, 332–343. ( 10.1152/jn.01085.2011) [DOI] [PubMed] [Google Scholar]
- 43.Müller D, Abel R, Brandt R, Zöckler M, Menzel R. 2002. Differential parallel processing of olfactory information in the honey bee, Apis mellifera L. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 188, 359–370. ( 10.1007/s00359-002-0310-1) [DOI] [PubMed] [Google Scholar]
- 44.Krofczik S, Menzel R, Nawrot MP. 2009. Rapid odor processing in the honey bee antennal lobe network. Front. Comput. Neurosci. 2, 9 ( 10.3389/neuro.10.009.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamagata N, Schmuker M, Szyszka P, Mizunami M, Menzel R. 2009. Differential odor processing in two olfactory pathways in the honey bee. Front. Syst. Neurosci. 3, 16 ( 10.3389/neuro.06.016.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carcaud J, Hill T, Giurfa M, Sandoz J-C. 2012. Differential coding by two olfactory subsystems in the honey bee brain. J. Neurophysiol. 108, 1106–1121. ( 10.1152/jn.01034.2011) [DOI] [PubMed] [Google Scholar]
- 47.Galizia CG, Franke T, Menzel R, Sandoz JC. 2012. Optical imaging of concealed brain activity using a gold mirror in honey bees. J. Insect Physiol. 58, 743–749. ( 10.1016/j.jinsphys.2012.02.010) [DOI] [PubMed] [Google Scholar]
- 48.Brill MF, Rosenbaum T, Reus I, Kleineidam CJ, Nawrot MP, Rossler W. 2013. Parallel processing via a dual olfactory pathway in the honey bee. J. Neurosci. 33, 2443–2456. ( 10.1523/JNEUROSCI.4268-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rössler W, Brill MF. 2013. Parallel processing in the honey bee olfactory pathway: structure, function, and evolution. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 199, 981–996. ( 10.1007/s00359-013-0821-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Igarashi KM, et al. 2012. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J. Neurosci. 32, 7970–7985. ( 10.1523/JNEUROSCI.0154-12.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.