Abstract
Successful establishment and range expansion of non-native species often require rapid accommodation of novel environments. Here, we use common-garden experiments to demonstrate parallel adaptive evolutionary response to a cool climate in populations of wall lizards (Podarcis muralis) introduced from southern Europe into England. Low soil temperatures in the introduced range delay hatching, which generates directional selection for a shorter incubation period. Non-native lizards from two separate lineages have responded to this selection by retaining their embryos for longer before oviposition—hence reducing the time needed to complete embryogenesis in the nest—and by an increased developmental rate at low temperatures. This divergence mirrors local adaptation across latitudes and altitudes within widely distributed species and suggests that evolutionary responses to climate can be very rapid. When extrapolated to soil temperatures encountered in nests within the introduced range, embryo retention and faster developmental rate result in one to several weeks earlier emergence compared with the ancestral state. We show that this difference translates into substantial survival benefits for offspring. This should promote short- and long-term persistence of non-native populations, and ultimately enable expansion into areas that would be unattainable with incubation duration representative of the native range.
Keywords: adaptive divergence, range expansion, incubation duration, wall lizard
1. Introduction
Non-native populations often encounter novel environments that impose strong directional selection. They therefore provide useful systems to study the process of adaptation [1–6]. However, evidence for adaptive divergence between native and non-native populations is currently limited, in particular for vertebrates [1]. Furthermore, the selective agents responsible are typically inferred from the pattern of phenotypic divergence, rather than being independently demonstrated, which makes it difficult to rule out non-adaptive processes [7]. The most convincing cases of adaptation in non-native species are therefore those where the causes of selection can be identified and the responses mirror locally adapted phenotypes within native ranges.
Recent studies of introduced insects and plants suggest that adaptive responses can facilitate spread into environments that were previously too stressful [8–10]. As climatic similarity is often the best predictor of establishment success for non-native species [11–13], climate is likely to exercise strong selection on many non-native populations. Such populations may therefore remain small and geographically isolated until they evolve tolerance to the new climatic regime, following which rapid range expansion is made possible. Understanding the mechanisms by which populations respond and adapt to novel climatic conditions is therefore crucial for predicting both the persistence and expansion of non-native species.
The common wall lizard, Podarcis muralis, is native to southern and western Europe but has been introduced multiple times to Germany, England and North America over the past 100 years [14–16]. These introduction sites represent different climatic conditions compared with the native range [14]. For example, air temperatures during the main activity season in populations in England are 5–10°C lower than their source regions in Tuscany and western France (electronic supplementary material, figure S1). Although behavioural thermoregulation enables non-native lizards to maintain annual activity patterns similar to those in their native range, these climatic differences put soil temperatures well below what females prefer for their nests (electronic supplementary material, table S1). Such cool conditions retard the rate of embryonic development [17], resulting in delayed hatching and even failure to complete embryogenesis before winter in cool summers [18]. Thus, we should predict strong selection for shorter incubation duration in non-native wall lizard populations, in particular at the cool temperatures rarely encountered in the native range but that are frequent in introduced populations in England.
Incubation duration can be reduced through several different mechanisms. First, embryos may be more advanced at oviposition. By capitalizing on the female's ability to maintain body temperatures substantially higher than those of nests, this reduces the overall time for completion of embryogenesis [19]. Egg retention is evolutionarily labile in lizards and has been shown to vary with climate within and between species [20–24]. Second, embryos can evolve a faster development rate. For example, in the Eastern fence lizard (Sceloporus undulatus), embryos from populations in cooler climates have a higher cardiac output and hence a more efficient delivery of nutrients and oxygen, which promotes faster embryogenesis [25]. Finally, embryos may hatch at an earlier developmental stage or at a smaller size and capitalize on residual yolk for completion of growth after emergence [26,27].
Here, we designed a set of common-garden experiments to test the hypothesis that non-native populations have adapted to the cool climate in their non-native range. First, we show that soil temperatures in the non-native range are physiologically stressful and impose significant selection for shorter incubation duration. Second, we test whether non-native populations have responded to this selection and establish the underlying mechanisms of adaptation. Finally, we use developmental reaction norms to infer the implications of shifts in incubation duration for the timing of hatching and recruitment under naturally fluctuating soil temperatures in the non-native range. Our results demonstrate prolonged embryo retention and faster embryonic growth at low temperatures in introduced wall lizards and suggest that these responses will have significant implications for the long-term persistence of this species in England.
2. Material and methods
The common wall lizard is a small (approx. 50–70 mm snout-to-vent length) diurnal lizard. It is native to southern and western Europe, but has established non-native populations in many places in Europe and in North America [14,15,28]. There are around 30 extant populations in southern England, the majority of which are highly geographically restricted with limited ecological impact [15].
(a). Female thermal preference and soil temperature
We first established preference for nest sites under unrestricted thermal conditions and in field enclosures. Unrestricted conditions were established by creating a thermal gradient in a large cage (1070 × 480 × 420 mm) of approximately 45°C to 20°C during the peak in the afternoon, falling to 15°C at night (electronic supplementary material, table S1). Five females were used in this experiment. Females were inspected in the morning and in the afternoon for signs of egg laying. Once they had laid, we recovered their clutch and replaced it with a thermal logger (Thermochron Ibutton, model number DS1921G) that logged the temperature for five consecutive days. A second dataset was generated from females housed in outdoor enclosures where suitable nesting sites with naturally variable thermal properties were available. Five female lizards were placed into the enclosure and allowed to lay eggs. The temperatures of nests were monitored using thermal loggers for 35 days following oviposition. The temperatures of these nest sites were compared to possible nest sites across the enclosure. Further details on these experiments are provided in the electronic supplementary material.
(b). Effects of hatching date on recruitment into adulthood
In 2012, we released 288 hatchlings from females of both French and Italian origin in a disused quarry close to the original non-native populations on the Dorset coast (50.59° N, −2.01° E). All eggs were incubated at a constant 24°C. This temperature is at the high end of the average expected temperature of nests in England but at the low end of temperatures in the native range (electronic supplementary material, table S1; [29,30]). Upon hatching, offspring were weighed to the nearest 0.01 g and their snout-to-vent length and total length were measured to the nearest 0.5 mm with a ruler. They were toe clipped for individual identification. Individuals were released in four batches, the timing of which represented a compromise between limiting the number of days offspring spent in captivity while maximizing the number of hatchlings released at a single field trip. The releases occurred on 15 July (offspring hatching from 9 July until 15 July), 24 July (16 July—24 July), 6 August (25 July—6 August) and 21 August (6 August until 14 August).
Lizards were recaptured in their second year (i.e. first year as adults) by repeated visits during the breeding season from late March until late June (n = 10). Upon recapture, individuals were measured for snout-to-vent length and mass (as above) before being temporarily marked with a marker pen before release to avoid unnecessary recapture. Overall, we recorded 41 individuals from the 288 released hatchlings, representing a recapture rate of 14% over the first two years of life. We used the recapture data to test how the timing of hatching affects recruitment into adulthood using models with a categorical variable with three levels representing release batch, lineage (Italian versus French), and their interaction as fixed effects, and included days in the laboratory and mass at hatching as covariates. Because the last release included only 27 offspring from seven clutches, we pooled the last two release batches to avoid having one level represented by very limited data. Furthermore, because our sample size is modest, we could not meaningfully take family effects into account (nine out of 65 families produced two recruits, the rest one or none). We therefore treated all offspring as independent for this analysis.
(c). Establishing differences in incubation duration between native and introduced populations
(i). Experiment at constant temperatures (20°C versus 24°C)
We caught 122 lizards by noosing at the early stages of gestation in early spring 2013 at three locations in Tuscany (Greve in Chianti (43.58° N, 11, 31° E), Prato (43.91° N, 11.10° E), Colle di Val d'Elsa (43.42° N, 11.11° E)), four in western France (Dinan (48.45° N, −2.07° E), Josselin (47.95° N, −2.54° E), Pontchateau (47.43° N, −2.09° E), Pouzagues (46.78° N, −0.44° E)) and four in southern UK (Italian origin: Ventnor Town (50.59° N, −1.21° E), Ventnor Botanical Garden (50.58° N, −1.22° E); French origin: Cheyne Weare (50.53° N, −2.43° E) and East Portland (50.54° N, −2.42° E)). The non-native populations represent at least two separate native sources for both the Italian and the French lineage that correspond well to the sampling locations of native animals [15]. Females had visible mating scars, large follicles or carried recently ovulated eggs (as determined by palpation), which ensured that they were fertile but would complete the large majority of gestation in the laboratory.
Females were housed individually in cages (590 × 390 × 415 mm) with sand as substrate, bricks as shelter, a water bowl, and held at a light cycle of 12 L : 12 D. They were given access to basking lights (60 W) for 8 h d−1 and a UV light (EXO-TERRA 10.0 UVB fluorescent tube) for 4 h d−1 and they were fed mealworms and crickets daily. Cages were inspected in the morning and in the late afternoon for signs of oviposition (which is easily assessed by visual inspection of females), such that eggs were recovered within 12 h of oviposition. Once laid, clutch size was assessed and the clutch and female were weighed to the nearest 0.01 g. All data in this paper are from the first clutch produced by females in that year (females can lay up to three clutches per year). The median lay date for native females was two weeks earlier than introduced females of both lineages (introduced French females: 30 May, native French females: 13 May, introduced Italian females: 29 May, native Italian females: 13 May).
For 65 clutches (each with more than four eggs), we selected one egg for dissection to assign the embryo to a developmental stage at oviposition according to the scheme for Lacertids [31]. For embryos that exhibited characters of two consecutive stages, we assigned an intermediate stage (e.g. 26.5). All staging was conducted by a single person (J.W.) who was unfamiliar with the coding system and hence embryos were scored blindly with respect to origin (native versus introduced) and lineage (French versus Italian). The remaining eggs were split into two groups and put into small plastic containers filled two-thirds with moist vermiculite (5 : 1 vermiculite : water volume ratio; changed every 20th day) and sealed with clingfilm. Half the clutch was incubated at constant 20°C and the other at constant 24°C in standard refrigerated incubators fitted with water baths to maintain humidity. During incubation, we measured the heart rate of all embryos within each of the 122 clutches as an estimate of cardiac output and hence developmental rate [32]. Following previous work [25,32], heart rate was measured using a digital egg monitor (Buddy, Avitronics, England) in a room at constant temperature set to either 20°C or 24°C (matching the incubation temperature of eggs). This was done twice during incubation, at 35 and 65 days following oviposition in the 24°C treatment and at 20 and 40 days in the 20°C treatment.
Eggs were inspected daily for signs of hatching (i.e. pipping) and the hatching date refers to the day of emergence. At hatching, the offspring snout-to-vent lengths and total lengths were measured to the nearest 0.5 mm with a ruler and mass was recorded to the nearest 0.01 g.
Because of the limited sample size per population (between five and 17 clutches), we pooled populations into the four main categories (Native Italian, Native French, Non-native Italian and Non-native French). We analysed differences in clutch size and the embryonic stage at oviposition between lineages (French versus Italian) and origin (native versus non-native). Because of the very large differences in incubation duration and heart rate in the 20°C and 24°C treatments (122.80 ± 0.60 days versus 56.15 ± 0.28 days, 47.45 ± 0.34 beats min−1 versus 76.23 ± 0.51 beats min−1), we analysed these variables separately for the two temperature treatments (heart rate was averaged across the two measurements for each clutch and temperature [25,32]). By contrast, hatchling size variables overlapped between incubation treatments and we therefore fitted a single model with lineage, origin and temperature to the mean hatching mass per clutch and treatment, including female identity as a random effect. All linear models were fitted in R using the car package to generate type III F-tests of fixed effects. When the interaction(s) was non-significant, results for main effects are presented from models excluding the interaction.
(ii). Experiment at shifting temperatures
To assess differences in incubation duration at temperatures too low for completion of embryogenesis, we designed a second experiment shifting eggs between temperatures. For this experiment, we only used animals of Italian origin, collecting gravid females in spring 2014 at three locations in Tuscany (Greve in Chianti (43.58° N, 11, 31° E), Peccioli (43.55° N, 10.72° E), Colle di Val'Elsa (43.42° N, 11.11° E)) and the same two focal non-native Italian populations as in the above experiment (Ventnor Town and Ventnor Botanical Garden). All animals were handled and treated as above. Clutches were split into four categories: (i) constant 28°C (n = 50), (ii) 15°C for 14 days followed by constant 28°C (n = 50), (iii) 18°C for 14 days followed by constant 28°C (n = 45) and (iv) 28°C for 14 days, followed by 18°C for 14 days, and finally 28°C until hatching (n = 40). The last treatment was included to address if there were any marked differences for embryos exposed to cool temperature in early-versus mid-development [33]. Eggs were inspected for hatching around the same time daily (in the late afternoon) and hatchlings were measured as described above.
We calculated for each clutch the differences in incubation duration between eggs at constant 28°C and eggs at the other treatments, and used these estimates as our response variable. A significant difference between native and non-native populations would be interpreted as faster (or slower) developmental rate at cool temperatures. Such effects may be more or less pronounced if embryos adjust their developmental rate to conditions experienced early in gestation [19,34]. Eggs incubated at 18°C for 14 days before being transferred to 28°C took on average half a day longer to hatch than eggs from the same clutch that were exposed to the 14 days 18°C treatment in mid-gestation (46.5 versus 45.9 days; paired t-test, t = 2.99, p = 0.01, d.f. = 28). However, because we were primarily interested in the overall effect between native and non-native populations, we use the average of the two treatments in our analyses and for fitting thermal reaction norms (see below), which maximizes sample sizes when embryos in one of the 18°C treatments failed to hatch.
To test for differences in preferred body temperature between native and introduced gravid females, in 2014, we recorded the body temperature of 72 individually housed, captive females from both native Italian and non-native UK populations (same sources as described above) eight times a day over a three-day period using an infrared thermometer. This has been demonstrated to be a reliable measure of internal body temperature in small lizards [35]. Measurements began one hour after basking lights came on and continued on the hour until basking lights turned off. At night, temperatures dropped to 15°C across all cages. Thus, there was limited potential for females to alter gestational temperature via shelter site choice. We analysed differences between native and introduced females using linear mixed models with body temperature as the response variable, origin (native or introduced) as the predictor variable and individual ID and observation day as random effects.
(d). Predicted hatching success in the UK
Temperature-dependent developmental rate at constant temperatures can be used to predict incubation duration at fluctuating temperatures [36–38]. To predict incubation duration of our non-native wall lizard populations, we used our data on developmental rate at constant temperatures at 20°C, 24°C and 28°C and the estimated developmental rate from the experiment switching eggs between 15°C or 18°C and 28°C (all for the Italian lineage; see the electronic supplementary material for further details). Data on developmental rate at higher temperatures were collected from the literature assuming that the developmental rate approaches a species-specific maximum at 35°C, data for which were provided by incubation experiments on a population from northern Spain (belonging to the same lineage as our French populations; [39,40]). Given that the difference between lineages and origins is small already at 24°C and that high temperatures are rarely encountered in the UK (see above), this should not bias our estimates. We fitted a four-parameter Weibull function and verified that it performed well for predicting incubation duration using experimental incubation under a daily fluctuating temperature regime similar to that of natural nests in England (see the electronic supplementary material for details).
We used soil data for locations south of the 53rd parallel north obtained from the British Meterological Office Integrated Data Archive System, Land and Marine Surface Stations Data for the period 2002–2013. We chose this period because of the availability of recent and yearly data across a range of consistent locations that are representative of annual variation, including relatively warm (e.g. 2006) and cool (e.g. 2011) summers, and because it covers the range of known wall lizard introductions in the UK. For each of the sites, soil temperatures are recorded every hour. We used the period from 15 May to 15 September in our analysis as mid-May is representative of the timing of oviposition for non-native lizards in the wild (the median lay date for females captured in the middle or end of gestation and brought to our laboratory for oviposition across years is 16 May; electronic supplementary material, table S2) and hatching past mid-September is unlikely because of the rapid decrease in daily soil temperature maximum (soil temperatures exceed 20°C—the minimum constant temperature for successful hatching in the laboratory—less than 0.3% of the time following 15 September across all years and sites in southern England). In addition, this four-month period is approximately equal to the predicted incubation duration for sites close to extant non-native populations and is in line with observations of newly hatched offspring in populations in England and at the northern native range limit ([41]; T.U. and G.M.W. 2008–2013, personal observation; T. Pashley 2000–2010, personal communication).
To generate expected hatching dates, we calculated the average proportion of time per day spent at different temperatures over this period and used this and the developmental rate data to generate predicted incubation durations for each location and year (see the electronic supplementary material for details).
3. Results
(a). Female thermal preference and soil temperature
Typical nesting sites in the introduced range are substantially cooler than is preferred by females for their nests under unrestricted thermal conditions (electronic supplementary material, table S1). We fitted thermal reaction norms for developmental rate of native Italian lizards to soil temperatures at depths representative of nesting sites in southern England (obtained from the UK Meteorological Office). This predicted incubation times well over three months even in relatively warm years, and failure to complete embryogenesis before winter in cooler years (electronic supplementary material, table S1 and figure S2).
(b). Effects of hatching date on recruitment into adulthood
There were strong effects of hatching date on juvenile recruitment into adulthood. Even under benign incubation temperatures that result in hatching in mid- to late summer, a two- to three-week difference in hatching translated into substantially reduced recruitment into the breeding population (logistic linear model: χ2 = 7.4, p = 0.02; table 1). Combined, these results suggest strong selection for earlier hatching in non-native wall lizard populations.
Table 1.
Recruitment of offspring into the breeding population as a function of offspring hatching date. Offspring were released in four batches corresponding to offspring hatch date. Because the last release included only 27 offspring from seven clutches, we pooled the last two release batches.
| release batch | hatch dates | proportion of offspring recruited (%) |
|---|---|---|
| 1 | 9 July to 15 July | 16.50 |
| 2 | 16 July to 24 July | 20.50 |
| 3 | 25 July to 21 Aug | 7.60 |
(c). Divergence in incubation duration between native and introduced populations
There was no difference in female snout-to-vent length between origins (native versus non-native; F1,118 = 0.01, p = 0.92) or lineages (French versus Italian; F1,118 = 1.63, p = 0.20). Females from non-native populations produced larger clutches than females from native populations, and Italian females produced larger clutches than French females (origin: F1,117 = 8.73, p = 0.004; lineage: F1,117 = 12.96, p < 0.001; snout-to-vent length: F1,117 = 63.4, p < 0.001). Eggs from French females were heavier than eggs from Italian females and tended to be smaller in non-native populations of both origins (origin: F1,118 = 5.37, p = 0.022; lineage: F1,118 = 3.31, p = 0.072). A total of 521 eggs were produced, of which 468 hatched. Embryonic mortality did not differ between origins (χ2 = 0.35, p = 0.55) or lineages (χ2 = 0.02, p = 0.90).
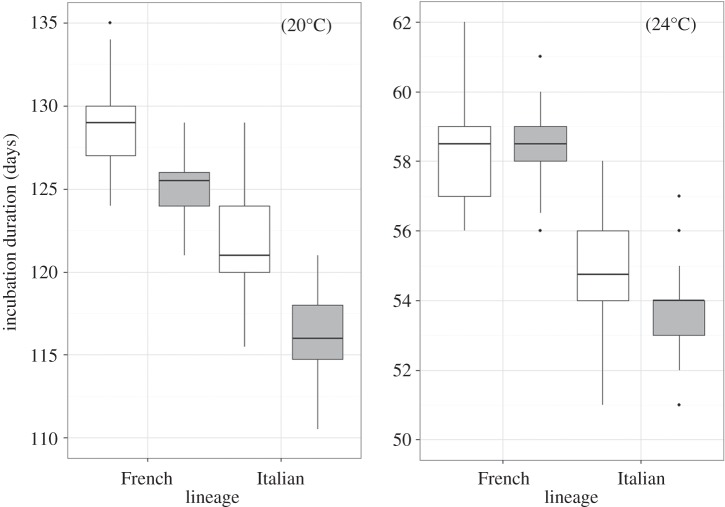
Incubation duration was strongly affected by incubation temperature, and it was significantly shorter in non-native populations of both Italian and French lineages at 20°C but not at 24°C (table 2 and figure 1). Embryos of the Italian lineage hatched sooner than embryos of the French lineage at both temperatures (table 2 and figure 1). Egg mass did not affect incubation duration at 20°C, but larger eggs had shorter incubation duration at 24°C (table 2). The shifting incubation temperature experiment confirmed shorter incubation duration for non-native animals at both 15°C and 18°C. Eggs from non-native females of Italian origin exhibited a 12.7 ± 0.33 day delay in incubation duration when held at 15°C for 14 days (compared to eggs held at a constant 28°C) compared with a delay of 13.6 ± 0.14 days for eggs from native females (F1,36 = 7.91, p = 0.007). The same pattern was observed at 18°C (non-native populations = 10.9 ± 0.21 day delay; native populations = 11.5 ± 0.18 day delay; F1,36 = 4.14, p = 0.049).
Table 2.
Output from linear models examining the effect of lineage and origin on the incubation duration of eggs kept at 20°C or 24°C. Statistically significant p-values are in bold. Main effects reported from model excluding interaction.
| 20°C |
24°C |
|||||
|---|---|---|---|---|---|---|
| factor | d.f. | F | p | d.f. | F | p |
| lineage | 1,96 | 151.77 | <0.001 | 1,99 | 197.23 | <0.001 |
| origin | 1,96 | 47.41 | <0.001 | 1,99 | 3.07 | 0.08 |
| egg mass | 1,96 | 0.83 | 0.36 | 1,99 | 4.27 | 0.04 |
| lineage × origin | 1,95 | 1.68 | 0.19 | 1,98 | 0.74 | 0.39 |
Figure 1.
Incubation duration of eggs kept at constant 20°C or 24°C from female wall lizards of French and Italian lineages sampled from native (white fill) and non-native (grey fill) populations.
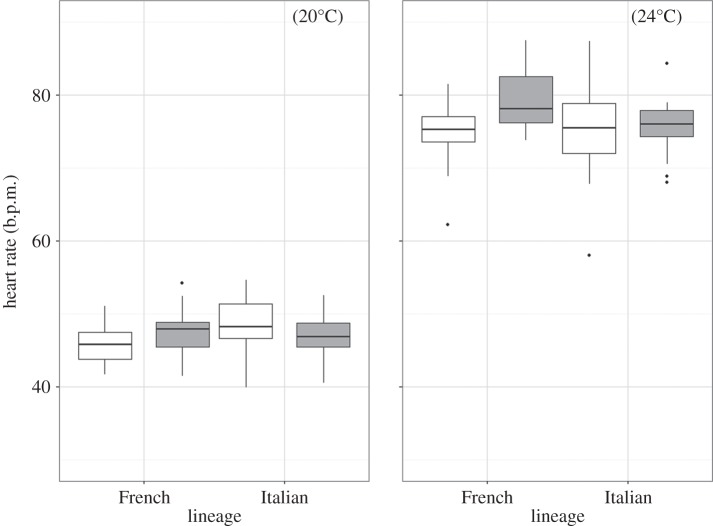
Embryos from non-native populations were significantly more advanced at oviposition compared with embryos from native populations, and this pattern was consistent for both lineages (F1,55 = 11.10, p = 0.002; figure 2). Differences in embryonic stage are unlikely to be explained by exposure to higher temperature before oviposition as there was no difference in gestational body temperature of non-native and native females (tested in the Italian lineage only: χ2 = 0.92, p = 0.34). Heart rate showed a more complex pattern, with a predicted higher heart rate in non-native populations of the French lineage compared with their native populations, but no difference in embryos from the Italian lineage. This lineage-by-origin interaction was significant at 20°C (F1,86 = 7.64, p = 0.007; figure 3) but failed to reach statistical significance in the 24°C treatment (F1,85 = 3.71, p = 0.057; figure 3). Refitting models of incubation duration with embryonic stage at oviposition as an additional predictor confirmed that it significantly contributed to the shorter developmental time at both 20°C (F1,49 = 14.23, p < 0.001) and 24°C (F1,52 = 16.72, p < 0.001). This was not the case for heart rate, which failed to significantly predict incubation duration at either temperature in refitted models (F1,81 = 1,38, p = 0.24 and F1,84 = 1.89, p = 0.17, respectively). In both cases, the difference between non-native and native populations at 20°C remained statistically significant (p < 0.001). Finally, Italian offspring were smaller than French offspring (F1,105.71 = 19.52, p = 0.001) and both lineages had smaller offspring at 20°C compared with 24°C (F1,65.96 = 135.32, p < 0.001), but offspring from non-native populations hatched at a similar size to offspring from native populations (F1,104.98 = 1.56, p = 0.21).
Figure 2.
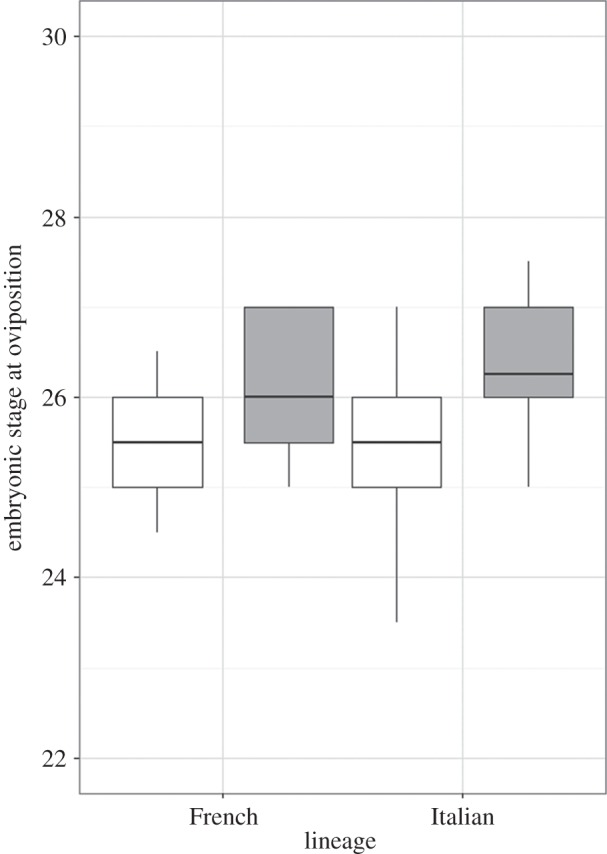
The embryonic stage of development at oviposition for eggs laid by French and Italian female wall lizards from both native (white fill) and non-native (grey fill) populations.
Figure 3.
The heart rate (b.p.m.) of developing embryos of eggs kept at a constant 20°C or 24°C from female wall lizards of French and Italian lineages sampled from native (white fill) and non-native (grey fill) populations.
(d). Consequences for the timing of hatching
To estimate the consequences of these responses for the timing of hatching, we modelled the predicted incubation duration of non-native and native lizards (of Italian origin) based on naturally fluctuating soil temperatures in sites representative of nesting locations across 34 sites in southern England. Predicted hatch dates for non-native eggs were one to three weeks earlier compared with the ancestral state, which greatly increased the chances of completing embryogenesis before autumn (figure 4).
Figure 4.
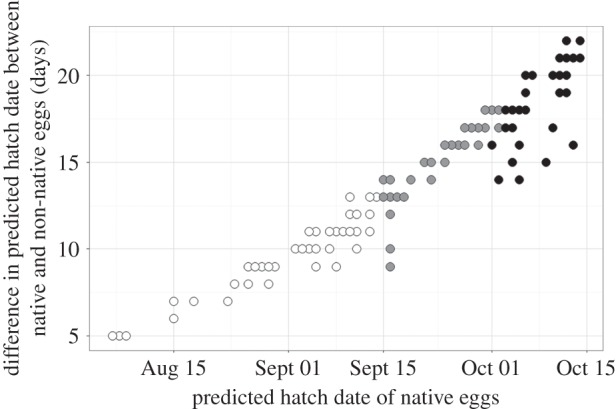
The predicted difference in the timing of hatching of eggs from native versus non-native wall lizard populations of the Italian lineage as a function of the estimated timing of hatching for native developmental rates. Estimates are based on soil temperatures across 34 sites in southern England over a 10-year period and a laying date of 15 May (data truncated at 15 Oct). Assuming hatching is unlikely past 15 Sept (see the electronic supplementary material), white dots represent soil temperatures that would allow successful hatching for both native and introduced lizards, grey dots represent soil temperatures that would allow successful hatching only for lizards from introduced populations and black dots represent soil temperatures that would not allow hatching for either native or introduced lizards.
4. Discussion
Our common-garden experiments demonstrate parallel reduction in incubation duration of embryos in wall lizard populations following independent introduction to cooler climate. This is consistent with adaptive evolutionary responses to the relatively cool nest temperatures in the introduced range, which necessitates sustained development at temperatures well below 24°C to complete embryogenesis (only 3% of soil temperature recordings at soil depths representative of nests in southern England are above 24°C for the relevant incubation period, i.e. from 15 May to 15 September). While embryo retention and faster developmental rate have evolved repeatedly in lizards in cool climates [21–24], our results demonstrate that such adaptations can arise very rapidly. Extant wall lizard populations in England were introduced less than 100 years ago, with the target populations tracing their origins back to between two and eight decades [15]. Thus, the results are consistent with recent evidence that geographical clines in introduced insects and plants can evolve within tens of generations (e.g. [42,43]), and with studies of vertebrates that have demonstrated adaptive divergence across a similar number of generations [2,3,5,44–47].
The reduction in incubation duration in non-native wall lizards appears to have multiple causes. Embryos in non-native populations are more advanced at the time of egg laying, which reduces the overall time to complete embryogenesis in the nest. We can rule out facultative egg retention as all females were housed under identical conditions and there was no difference in selected body temperature between native and introduced females (in addition, previous work has failed to experimentally demonstrate plasticity in egg retention in P. muralis [30], but see [24] for evidence from the skink Bassiana duperreyi). However, egg retention cannot fully account for the reduction in incubation duration in non-native populations, nor does it account for the difference between lineages, as both lineage and origin explained significant amounts of variation even when the differences in embryo stage were controlled for statistically. Embryos from non-native populations must therefore also develop faster at and below 20°C. Interestingly, there was no, or limited, increase in development rate at 24°C and 28°C. These responses thus demonstrate adaptive evolution of the slope and curvature of thermal reaction norms, which appears to be common for population divergence in plasticity [48].
What are the mechanisms underlying faster developmental rate in non-native populations? Eggs were somewhat smaller in non-native populations, but egg size cannot explain differences in incubation time between non-native and native populations (in fact, larger eggs hatched earlier at 24°C). Other maternal effects on yolk composition could be involved and the relative contribution to changes in yolk nutrients versus intrinsically upregulated metabolism in embryos warrants further study. The increased heart rate of embryos from non-native populations of the French lineage suggests that a faster development may partly be owing to increased cardiac output [32]. This mechanism has been shown to account for faster developmental rate at high latitudes in Sceloporus lizards [25]. However, heart rate itself was a poor predictor of incubation duration across our populations and lineages, suggesting that the divergence between non-native and native populations in the rate of development is not well explained by such simple estimates of nutrient and O2 delivery to developing tissues. The same applies to differences between lineages. Data from Spanish populations close to the presumed ice age refugia for the French lineage have even slower developmental rates at cool temperatures than our native French populations [39], suggesting that there may be a phylogenetic signal to developmental rate, which persists in non-native populations.
By predicting incubation duration of non-native and native lizards from soil temperatures across the introduced range, we show that the combined effects of egg retention and faster embryonic growth should lead to a one- to three-week earlier emergence compared with the ancestral state. Even two-weeks earlier hatching, which is a common prediction from the data, can make the difference between successful hatching and failing to hatch before the onset of autumn. Our recapture data show that this also constitutes a substantial (e.g. twofold) increase in survival after emergence (see also [30]). This increased survival of early hatched offspring could be the result of several non-mutually exclusive mechanisms, including increased opportunity for growth and production of fat bodies prior to hibernation ([49] see also [50]), positive effects of high embryonic temperature on physiological and morphological traits [51,52] or greater ability to capitalize on seasonally available food sources [53]. Earlier emergence and long-term persistence of non-native populations could be further enhanced if non-native lizards also initiated reproduction earlier than their native counterparts. However, the extent to which there may have been corresponding responses in female characters that promote egg laying early in spring in non-native populations is currently unknown.
The shorter incubation duration should increase short- and long-term persistence of non-native populations and may enable expansion into areas that would be unattainable with incubation durations representative of the native range. A time delay between introduction and range expansion appears to be a common pattern in biological invasions and recent studies of non-native insects and plants have demonstrated how adaptive divergence can facilitate spread into environments that were previously too stressful [8,9]. Wall lizards in England show limited natural dispersal [15] but the ability to recruit from nests with less benign thermal profiles may contribute to their expansion in several locations. Non-native populations with shorter incubation duration may also serve as sources for new introductions and hence make human-mediated range expansion more likely [54].
In summary, prolonged embryo retention and faster embryonic growth at low temperatures in non-native wall lizards suggest rapid adaptation following introduction to a cool climate. We show that these responses have significant effects on recruitment and hence are likely to contribute to the survival and eventual range expansion of the species in its introduced range.
Supplementary Material
Acknowledgements
We are grateful to Roberto Sacchi, Marco Zuffi and Fabien Aubret for logistical support, Hannah MacGregor, Lindall Kidd, Peter Ibbetson and Daniel Noble for field assistance, and the UK Meterological Office for access to soil temperature data. Two reviewers provided valuable comments on the paper.
Ethics statement
All work was approved by the University of Oxford's Local Ethical Review Process and the UK Home Office (PPL: 30/2560).
Data accessibility
All data associated with this MS will be made available on dryad (doi:10.5061/dryad.pd351).
Author contributions
T.U. and G.M.W. conceived of and managed the project, collected and analysed data, and wrote the paper. J.W., G.P., T.H., B.F., B.H. and S.M. collected data for different parts of the project, and N.J.B. assisted with analysis of climatic data. All authors commented on the paper.
Funding statement
We are grateful to the National Geographic Society, the British Ecological Society and the Royal Society of London for project funding (all to T.U.). G.M.W. was supported by an FP7 Marie Curie Fellowship, T.H. was supported by the European Science Foundation within the research networking ‘Thermal adaptation in ectotherms: linking life history, physiology, behaviour and genetics’ (EG/3312), B.H. was supported by an Australian Bicentennial Scholarship. T.U. is supported by the Royal Society and the Knut and Alice Wallenberg Foundations.
Conflict of interests
We have no competing interests.
References
- 1.Moran EV, Alexander JM. 2014. Evolutionary responses to global change: lessons from invasive species. Ecol. Lett. 17, 637–649. ( 10.1111/ele.12262) [DOI] [PubMed] [Google Scholar]
- 2.Reznick DA, Bryga H, Endler JA. 1990. Experimentally induced life-history evolution in a natural population. Nature 346, 357–359. ( 10.1038/346357a0) [DOI] [Google Scholar]
- 3.Losos JB, Schoener TW, Spiller DA. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432, 505–508. ( 10.1038/nature03039) [DOI] [PubMed] [Google Scholar]
- 4.Yeh PJ. 2004. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution 58, 166–174. ( 10.1111/j.0014-3820.2004.tb01583.x) [DOI] [PubMed] [Google Scholar]
- 5.Westley PAH, Ward EJ, Fleming IA. 2013. Fine-scale local adaptation in an invasive freshwater fish has evolved in contemporary time. Proc. R. Soc. B 280, 20122327 ( 10.1098/rspb.2012.2327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westley PAH. 2011. What invasive species reveal about the rate and form of contemporary phenotypic change in nature. Am. Nat. 177, 496–509. ( 10.1086/658902) [DOI] [PubMed] [Google Scholar]
- 7.Keller SR, Taylor DR. 2008. History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol. Lett. 11, 852–866. ( 10.1111/j.1461-0248.2008.01188.x) [DOI] [PubMed] [Google Scholar]
- 8.Colautti RI, Barrett SCH. 2013. Rapid adaptation to climate facilitates range expansion of an invasive plant. Science 342, 364–366. ( 10.1126/science.1242121) [DOI] [PubMed] [Google Scholar]
- 9.Kilkenny FF, Galloway LF. 2013. Adaptive divergence at the margin of an invaded range. Evolution 67, 722–731. ( 10.1111/j.1558-5646.2012.01829.x) [DOI] [PubMed] [Google Scholar]
- 10.Diaz F, Munoz-Valencia V, Juvinao-Quintero DL, Manzano-Martinez MR, Toro-Perea N, Cardenas-Henao H, Hoffmann AA. 2014. Evidence for adaptive divergence of thermal responses among Bemisia tabaci populations from tropical Colombia following a recent invasion. J. Evol. Biol. 27, 1160–1171. ( 10.1111/jeb.12387) [DOI] [PubMed] [Google Scholar]
- 11.Duncan RP, Bomford M, Forsyth DM, Conibear L. 2001. High predictability in introduction outcomes and the geographical range size of introduced Australian birds: a role for climate. J. Anim. Ecol. 70, 621–632. ( 10.1046/j.1365-2656.2001.00517.x) [DOI] [Google Scholar]
- 12.Hayes KR, Barry SC. 2008. Are there any consistent predictors of invasion success? Biol. Invasions 10, 483–506. ( 10.1007/s10530-007-9146-5) [DOI] [Google Scholar]
- 13.Rago A, While GM, Uller T. 2012. Introduction pathway and climate trump ecology and life history as predictors of establishment success in alien frogs and toads. Ecol. Evol. 2, 1437–1445. ( 10.1002/ece3.261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulte U, Hochkirch A, Loetters S, Roedder D, Schweiger S, Weimann T, Veith M. 2012. Cryptic niche conservatism among evolutionary lineages of an invasive lizard. Glob. Ecol. Biogeogr. 21, 198–211. ( 10.1111/j.1466-8238.2011.00665.x) [DOI] [Google Scholar]
- 15.Michaelides S, While GM, Bell C, Uller T. 2013. Human introductions create opportunities for intra-specific hybridization in an alien lizard. Biol. Invasions 15, 1101–1112. ( 10.1007/s10530-012-0353-3) [DOI] [Google Scholar]
- 16.Kwiat GA, Gist DH. 1987. Annual reproductive-cycle of an introduced population of European wall lizards (Podarcis muralis) in Ohio. J. Herpetol. 21, 205–209. ( 10.2307/1564484) [DOI] [Google Scholar]
- 17.Ji X, Brana F. 1999. The influence of thermal and hydric environments on embryonic use of energy and nutrients, and hatchling traits, in the wall lizards (Podarcis muralis). Comp. Biochem. Phys. A 124, 205–213. ( 10.1016/s1095-6433(99)00111-7) [DOI] [Google Scholar]
- 18.Strijbosch H, Bonnemayer JJAM, Dietvorst PJM. 1980. The northern most population of Podarcis muralis (Lacertilia, Lacertidae). Amphibia-Reptilia 1, 161–172. ( 10.1163/156853880X00150) [DOI] [Google Scholar]
- 19.Shine R. 2002. Reconstructing an adaptationist scenario: what selective forces favor the evolution of viviparity in montane reptiles? Am. Nat. 160, 582–593. ( 10.1086/342815) [DOI] [PubMed] [Google Scholar]
- 20.Shine R. 1983. Reptilian reproductive modes—the oviparity–viviparity continuum. Herpetologica 39, 1–8. [Google Scholar]
- 21.Angilletta MJ, Oufiero CE, Sears MW. 2005. Thermal adaption of maternal and embryonic phenotypes in a geographically widespread ectotherm. Int. Congr. Ser. 1275, 258–266. ( 10.1016/j.ics.2004.07.038) [DOI] [Google Scholar]
- 22.Oufiero CE, Angilletta MJ. 2006. Convergent evolution of embryonic growth and development in the eastern fence lizard (Sceloporus undulatus). Evolution 60, 1066–1075. ( 10.1111/j.0014-3820.2006.tb01183.x) [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Diaz T, Brana F. 2012. Altitudinal variation in egg retention and rates of embryonic development in oviparous Zootoca vivipara fits predictions from the cold-climate model on the evolution of viviparity. J. Evol. Biol. 25, 1877–1887. ( 10.1111/j.1420-9101.2012.02575.x) [DOI] [PubMed] [Google Scholar]
- 24.Telemeco RS, Radder RS, Baird TA, Shine R. 2010. Thermal effects on reptile reproduction: adaptation and phenotypic plasticity in a montane lizard. Biol. J. Linn. Soc. 100, 642–655. ( 10.1111/j.1095-8312.2010.01439.x) [DOI] [Google Scholar]
- 25.Du WG, Warner DA, Langkilde T, Robbins T, Shine R. 2010. The physiological basis of geographic variation in rates of embryonic development within a widespread lizard species. Am. Nat. 176, 522–528. ( 10.1086/656270) [DOI] [PubMed] [Google Scholar]
- 26.Doody JS. 2011. Environmentally cued hatching in reptiles. Integr. Comp. Biol. 51, 49–61. ( 10.1093/icb/icr043) [DOI] [PubMed] [Google Scholar]
- 27.Warkentin KM. 2011. Environmentally cued hatching across taxa: embryos respond to risk and opportunity. Integr. Comp. Biol. 51, 14–25. ( 10.1093/icb/icr017) [DOI] [PubMed] [Google Scholar]
- 28.Schulte U. 2008. Die Mauereidechse. Erfolgreich im Schlepptau des Menschen. Bielefeld, Germany: Laurenti. [Google Scholar]
- 29.Brana F, Ji X. 2000. Influence of incubation temperature on morphology, locomotor performance, and early growth of hatchling wall lizards (Podarcis muralis). J. Exp. Zool. 286, 422–433. () [DOI] [PubMed] [Google Scholar]
- 30.Le Henanff M, Meylan S, Lourdais O. 2013. The sooner the better: reproductive phenology drives ontogenetic trajectories in a temperate squamate (Podarcis muralis). Biol. J. Linn. Soc. 108, 384–395. ( 10.1111/j.1095-8312.2012.02005.x) [DOI] [Google Scholar]
- 31.Dufaure J, Hubert J. 1961. Table de developpement du lezard vivipare: Lacerta vivipara. Arch. Anat. Microsc. Morphol. Exp. 50, 309–328. [Google Scholar]
- 32.Du W-G, Radder RS, Sun B, Shine R. 2009. Determinants of incubation period: do reptilian embryos hatch after a fixed total number of heart beats? J. Exp. Biol. 212, 1302–1306. ( 10.1242/jeb.027425) [DOI] [PubMed] [Google Scholar]
- 33.Shine R. 2002. Eggs in autumn: responses to declining incubation temperatures by the eggs of montane lizards. Biol. J. Linn. Soc. 76, 71–77. ( 10.1111/j.1095-8312.2002.tb01715.x) [DOI] [Google Scholar]
- 34.Angilletta MJ, Lee V, Silva AC. 2006. Energetics of lizard embryos are not canalized by thermal acclimation. Physiol. Biochem. Zool. 79, 573–580. ( 10.1086/501062) [DOI] [PubMed] [Google Scholar]
- 35.Bucklin SE, Ferguson GW, Gehrmann WH, Pinder JE. 2010. Use of remote laser sensing equipment to measure surface temperature and to predict deep body temperatures of small lizards in the field. Herpetol. Rev. 41, 309–312. [Google Scholar]
- 36.Georges A, Beggs K, Young JE, Doody JS. 2005. Modelling development of reptile embryos under fluctuating temperature regimes. Physiol. Biochem. Zool. 78, 18–30. ( 10.1086/425200) [DOI] [PubMed] [Google Scholar]
- 37.Hagstrum DW, Milliken GA. 1991. Modelling differences in insect developmental times between constant and fluctuating temperatures. Ann. Entomol. Soc. Am. 84, 369–379. ( 10.1093/aesa/84.4.369) [DOI] [Google Scholar]
- 38.Birchard GF. 2005. Effects of incubation temperature. In Reptilian incubation environment, evolution and behaviour (ed. Deeming DC.), pp. 103–124. Nottingham, UK: Nottingham University Press. [Google Scholar]
- 39.Vandamme R, Bauwens D, Brana F, Verheyen RF. 1992. Incubation-temperature differentially affects hatching time, egg survival, and hatchling performance in the lizard Podarcis muralis. Herpetologica 48, 220–228. [Google Scholar]
- 40.Brana F, Ji X. 2007. The selective basis for increased egg retention: early incubation temperature determines hatchling phenotype in wall lizards (Podarcis muralis). Biol. J. Linn. Soc. 92, 441–447. ( 10.1111/j.1095-8312.2007.00871.x) [DOI] [Google Scholar]
- 41.Cornish N. 2011. Genetic diversity and conservation of wall lizards (Podarcis muralis) in Jersey, British Channel Islands. Brighton, UK: University of Sussex. [Google Scholar]
- 42.Huey R, Gilchrist G, Carlson M, Berrigan D, Serra L. 2000. Rapid evolution of a geographic cline in size in an introduced fly. Science 287, 308–309. ( 10.1126/science.287.5451.308) [DOI] [PubMed] [Google Scholar]
- 43.Dlugosch KM, Parker IM. 2008. Invading populations of an ornamental shrub show rapid life history evolution despite genetic bottlenecks. Ecol. Lett. 11, 701–709. ( 10.1111/j.1461-0248.2008.01181.x) [DOI] [PubMed] [Google Scholar]
- 44.Kolbe JJ, Van Middlesworth PS, Losin N, Dappen N, Losos JB. 2012. Climatic niche shift predicts thermal trait response in one but not both introductions of the Puerto Rican lizard Anolis cristatellus to Miami, Florida, USA. Ecol. Evol. 2, 1503–1516. ( 10.1002/ece3.263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Badyaev AV. 2010. The beak of the other finch: coevolution of genetic covariance structure and developmental modularity during adaptive evolution. Phil. Trans. R. Soc. B 365, 1111–1126. ( 10.1098/rstb.2009.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badyaev AV. 2009. Evolutionary significance of phenotypic accommodation in novel environments: an empirical test of the Baldwin effect. Phil. Trans. R. Soc. B 364, 1125–1141. ( 10.1098/rstb.2008.0285) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hendry AP, Hensleiogh JE, Reisenbichler RR. 1998. Incubation temperature, developmental biology, and the divergence of sockeye salmon (Oncorhynchus nerka) within Lake Washington. Can. J. Fish. Aquat. Sci. 55, 1387–1394. ( 10.1139/f98-020) [DOI] [Google Scholar]
- 48.Murren CJ, et al. 2014. Evolutionary change in continuous reaction norms. Am. Nat. 183, 453–467. ( 10.1086/675302) [DOI] [PubMed] [Google Scholar]
- 49.Warner DA, Shine R. 2007. Fitness of juvenile lizards depends on seasonal timing of hatching, not offspring body size. Oecologia 154, 65–73. ( 10.1007/s00442-007-0809-9|ISSN 0029-8549) [DOI] [PubMed] [Google Scholar]
- 50.Einum S, Fleming IA. 2000. Selection against late emergence and small offspring in Atlantic salmon (Salmo salar). Evolution 54, 628–639. ( 10.1111/j.0014-3820.2000.tb00064.x) [DOI] [PubMed] [Google Scholar]
- 51.Elphick M, Shine R. 1998. Longterm effects of incubation temperature on the morphology and locomotor performance of hatchling lizards. Biol. J. Linn. Soc. 63, 429–447. ( 10.1111/j.1095-8312.1998.tb01527.x) [DOI] [Google Scholar]
- 52.Shine R, Olsson M. 2003. When to be born? Prolonged pregnancy or incubation enhances locomotor performance in neonatal lizards. J. Evol. Biol. 16, 823–832. ( 10.1046/j.1420-9101.2003.00600.x) [DOI] [PubMed] [Google Scholar]
- 53.Crozier LG, Hendry AP, Lawson PW, Quinn TP, Mantua NJ, Battin J, Shaw RG, Huey RB. 2008. Potential responses to climate change in organisms with complex life histories: evolution and plasticity in Pacific salmon. Evol. Appl. 1, 252–270. ( 10.1111/j.1752-4571.2008.00033.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lombaert E, Guillemaud T, Cornuet J-M, Malausa T, Facon B, Estoup A. 2010. Bridgehead effect in the worldwide invasion of the biocontrol harlequin ladybird. PLoS ONE 5, e0009743 ( 10.1371/journal.pone.0009743) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this MS will be made available on dryad (doi:10.5061/dryad.pd351).