Abstract
Extended periods of darkness have long been used to study how the mammalian visual system develops in the absence of any instruction from vision. Because of the relative ease of implementation of darkness as a means to eliminate visually driven neural activity, it has usually been imposed earlier in life and for much longer periods than was the case for other manipulations of the early visual input used for study of their influences on visual system development. Recently, it was shown that following a very brief (10 days) period of darkness imposed at five weeks of age, kittens emerged blind. Although vision as assessed by measurements of visual acuity eventually recovered, the time course was very slow as it took seven weeks for visual acuity to attain normal levels. Here, we document the critical period of this remarkable vulnerability to the effects of short periods of darkness by imposing 10 days of darkness on nine normal kittens at progressively later ages. Results indicate that the period of susceptibility to darkness extends only to about 10 weeks of age, which is substantially shorter than the critical period for the effects of monocular deprivation in the primary visual cortex, which extends beyond six months of age.
Keywords: visual development, deprivation, visual loss, visual cortex, critical period, acuity
1. Introduction
Since the initial experiments of Hubel & Wiesel [1], exploration of the role of visual experience in the development of the visual pathways has been informed by myriad studies of the consequences of various forms of unusual early visual input that have been summarized in several long reviews [2–6]. For laboratory animals, where strict control of the nature and timing of the visual input is possible, development that occurs in complete darkness defines what can be achieved by self-organization and programmes of gene activation in the absence of visually driven neural activity. In past studies [3] conducted mainly on cats, darkness has typically been imposed from near birth for periods lasting several weeks or many months. While the immediate effects of extended dark rearing on the vision of cats are very profound, such that they appear blind, signs of vision gradually emerge followed by a slow improvement of visual acuity that can eventually reach normal levels so long as the deprivation ends before four months of age [7,8]. Surprisingly, in the course of a study [9] of monocularly deprived kittens, it appeared that similar severe effects on vision can follow very short (10 days) periods of darkness even when imposed at five weeks of age. The period of vulnerability to the devastating visual consequences of short periods of darkness appeared to be short as the vision of the non-deprived eye was unaffected in all three monocularly deprived animals placed in darkness when 15 weeks old and another at 10 weeks. The dramatic effect of short periods of darkness for the vision of the non-deprived eye of monocularly deprived kittens predicts a similar outcome for the vision of normal kittens following imposition of brief episodes of darkness. This prediction was confirmed on a single normal animal as part of our original investigation of monocularly deprived kittens [9].
We report here the results of a systematic documentation of the age-related decline in susceptibility to visual loss after short (10 days) periods of complete darkness in eight additional otherwise normally reared kittens. With delay in the age at which darkness occurred from five weeks of age, there was a decline in the severity of the immediate effects on vision, but the speed of recovery nevertheless remained slow. Darkness imposed at 70 days of age or later had no effect on visual acuity. The critical period for the effects of darkness on vision is thus very short in relation to the well-established period of vulnerability of the primary visual cortex to monocular deprivation [10–13].
2. Material and methods
(a). Animals and rearing
All 16 animals of non-specific breed were reared from birth in a closed cat breeding colony at Dalhousie University. Seven of the kittens (C150, C151, C152, C153, C155, C156 and C157) participated in an earlier study [9] and had experienced a 7-day period of monocular deprivation beginning at postnatal day 30 (P30). The other nine kittens from two litters (C174–C178; C313–316) were reared 2 years apart and did not experience any selective visual deprivation prior to the single period of dark rearing that was imposed on all of them. The kittens were housed in colony rooms illuminated on a 14 : 10 light–dark cycle. The normal kittens were placed for 10 days in a darkroom beginning at either P37 (n = 1), P47 (n = 2), P57 (n = 2), P66 (n = 3) or P77 (n = 1). One of the kittens (C176) that was placed in the darkroom at P47 developed uveitis, so that the recovery of vision could be followed for only five weeks.
(b). Darkroom facility
For the 10-day period spent in total darkness, kittens were placed with their mother in a large cage (1.7 × 0.7 × 0.9 m) within a darkroom that formed part of a facility designed to exclude all light. A detailed description of the facility and its operation is provided elsewhere [14].
(c). Behavioural testing
Measurements of the visual acuity for square-wave gratings were made by use of a jumping stand [15], and followed procedures identical to those described in recent papers [9,14] from this laboratory. The spatial frequency of the gratings was measured in cycles per degree (cpd). The key features of the procedure were the use of a two-alternative forced choice discrimination task between a vertical (positive) and horizontal (negative) grating, and the use of a descending method of limits in which the step sizes were very small and equated logarithmically (12 steps per octave as compared with three steps in a conventional human eye chart). The large (18.7 × 18.7 cm) square-wave gratings had a Michelson contrast of 1.0 and a luminance of 55 cd m−2, and were surrounded by a grey uniform border 3 cm wide. Training on the discrimination began as early as the fifth postnatal week so as to ensure that measurements of acuity could be made immediately after the 10 days in the darkroom. During the training period and in the ensuing weeks, the height of the jumping platform was raised gradually and commensurate with the kitten's ability to jump until it was able to jump from the maximum height (72 cm) that was employed. The minimum number of trials increased with spatial frequency from one at the beginning (a 32 mm period grating) to three when about an octave from the threshold and five at the four highest spatial frequencies. Correct responses were reinforced with food and petting, and incorrect responses resulted in denial of the reward and immediate repetition of the trial. After an error, the animal was required to make five consecutively correct responses or a minimum of seven correct responses out of a maximum of 10 trials provided at any spatial frequency. Most animals performed flawlessly until one or two gratings from threshold when their performance fell abruptly to chance and was accompanied by balking, meowing and long latencies. Thresholds (the visual acuity) were defined as the highest spatial frequency for which performance was at 70% correct or better. The thresholds could be titrated quite precisely, because performance dropped from flawless to chance within a couple of one-twelfth octave steps.
With the exception of animals from the prior investigation [9], all acuity measurements were made binocularly. The first measurements of vision after the kittens were removed from the darkroom were made after they began to actively crawl around and explore their new environment, which usually occurred after 20–30 min in the light. Kittens placed in darkness at P37 all appeared blind when they emerged from the darkroom and were placed with other kittens in a colony room. In contrast to their prior behaviour and those of other kittens, they froze in position when placed on the floor and remained immobile unless surprised by a sudden sound or in response to physical contact with another kitten. Formal tests of visual–motor functions were all negative. Thus, they did not exhibit visual placing responses with their front paws when lowered slowly to the edge of a horizontal surface such as a tabletop and only did so when the paws made tactile contact. Likewise, they made no following gaze movements in response to slow movements of objects or even laser spots; however, by contrast, they made accurate following head movements in response to the sound of an object moved on the floor, but would stop as soon as it was lifted above the floor and made no sound even though the object was moved as before. In addition, these kittens did not respond by a head turn or withdrawal upon the sudden visual presentation of a large object to one side or its gradual approach towards them. Some animals began to move very slowly after 15 min in the light, but would walk into objects such as chair legs or even other kittens. The apparent blindness was confirmed formally on the jumping stand by their inability to choose between an easy step onto a grating as opposed to an adjacent 40 cm drop as illustrated in figure 1, which shows a photograph of C157 as it attempted to find the grating. The ability to make this easy discrimination represented the first stage of recovery and could possibly be performed on the basis of brightness (luminance) differences.
Figure 1.

A photograph of a kitten as it attempts to locate and step onto a grating adjacent to a ‘cliff’ for a food reward not long after it had emerged from a 10-day period of total darkness. The grating could only be located by use of tactile information.
3. Results
It would seem unlikely that the apparent blindness exhibited by the three monocularly deprived kittens from the previous study [9] when they emerged from the darkroom was linked to their prior selective deprivation. Rather, the result predicts the previously unsuspected finding that normal kittens placed in darkness for just 10 days at P37 would emerge blind. That this prediction was borne out is readily apparent from the data displayed in figure 2, which shows a comparison of the immediate effects on binocular visual acuity and the subsequent recovery for a normal animal (C175) with the data from three animals (C150, C153, C156) from the prior study [9] that were all placed in darkness at the same age. For each animal, the improvement in acuity over time was fitted to a linear function using the least-squares method, and the goodness of fit was assessed with R2. The recovery of acuity in all three animals was very regular, and linear fits provided a satisfactory description with R2-values ranging from 0.83 to 0.99. Like the other animals, the normal kitten (C175) appeared blind when first tested an hour after emerging from the darkroom, but the next day behaved quite differently as it ran around with other kittens and showed obvious evidence of vision. Formal tests of visual placing were positive and on the jumping stand it readily showed that it could pass the open door discrimination as well as discriminate a vertical from a horizontal grating, thereby permitting measurement of its visual acuity, which was quite poor (only 0.34 cpd). As with the other animals, the subsequent recovery of visual acuity was linear and lasted about seven weeks. The close similarity of the recovery observed in the normal animal to that of animals from the earlier study [9] is evident from the similarity of the slope of the line fitted to the former (0.14 cpd per day) to those fitted to the latter (C150: 0.15; C153: 0.10; C156: 0.11 cpd per day).
Figure 2.
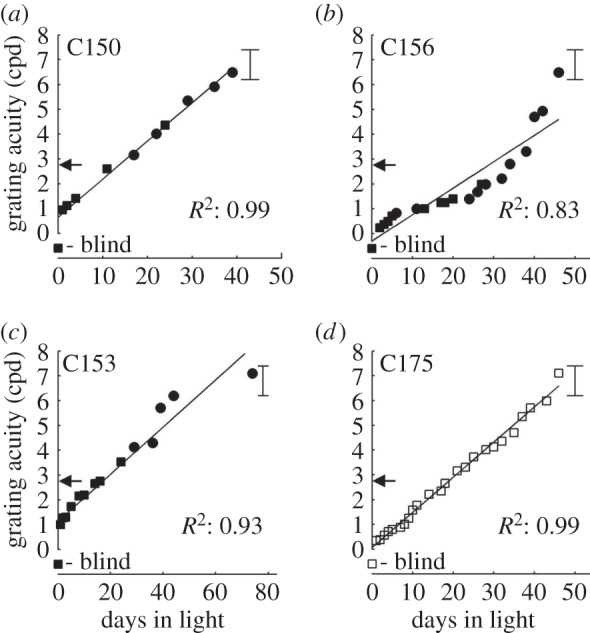
The recovery of vision of four kittens after a 10-day period of darkness imposed at 37 days of age. Black symbols depict the data from three monocularly deprived kittens (C150, C153 and C156) from a prior study that had received a one week period of monocular deprivation from P30 to P37. White symbols show the data from a normal kitten (C175) placed in darkness at the same age. Squares show the results of binocular measurements of acuity and circle symbols depict the results of monocular measurements made with the non-deprived eye. The brackets to the right in each graph show the normal range of values for the visual acuity of kittens at about 85 days of age. The poor vision of the animals when they emerged from darkness is highlighted by the horizontal arrows, which depict the average acuity of normal animals 10 days earlier when they were placed in darkness. Linear functions have been fitted to the recovery data for each kitten and the individual coefficients of determination (R2) are shown adjacent to the data.
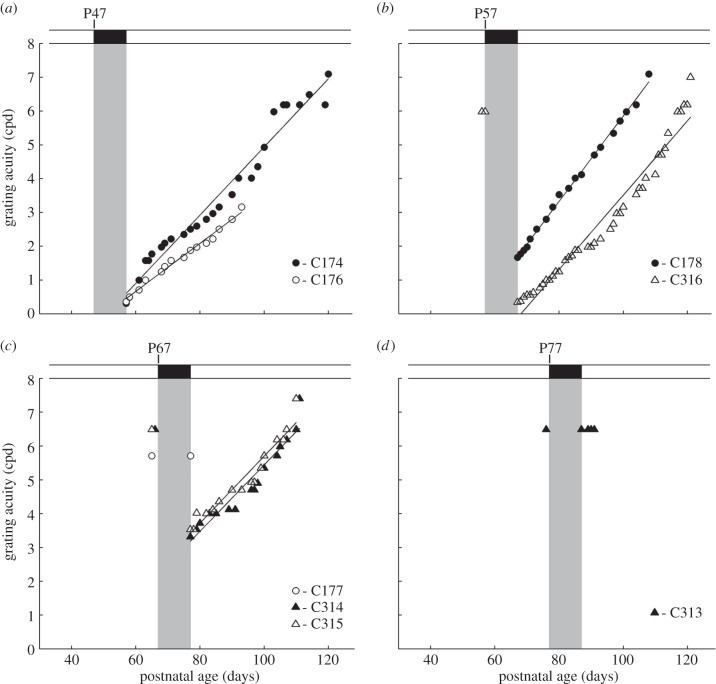
To determine the period of vulnerability to short periods of darkness, eight additional normal kittens were placed in the darkroom for 10 days at progressively later ages in 10-day increments at P47 (n = 2), P57 (n = 2), P67 (n = 3) and P77. The immediate effects on vision and the time course of recovery in these additional rearing conditions are displayed in figure 3. Circles and triangles are used to depict the results for individual kittens from, respectively, litters 1 and 2 in order to highlight a consistent difference between the two. In contrast to the kitten (C175) placed in darkness at P37 (figure 2) that appeared blind immediately afterwards, the kittens that were deprived later possessed measurable form vision when they emerged from the darkroom. The initial visual acuity on emergence from the darkroom was progressively higher as the period of darkness was delayed. The decline of the effects of darkness on acuity with age was fast such that the vision of one of the three kittens (C177) that was placed in darkness at P66 as well as the kitten (C313) so deprived at P77 was unchanged. The reduced sensitivity to darkness with age was also evident in measurements from the two remaining kittens placed in darkness at P67 (C314, C315), both of which exhibited a much smaller decrease in visual acuity compared with all animals placed in darkness at P47 or P57. Although the immediate effects of darkness were consistently greater on members of the second litter than the first, in all other respects the results were very similar, as reflected by the nearly identical slopes (C174: 0.10; C176: 0.07; C178: 0.13; C316: 0.11; C314: 0.10; C315: 0.10) of the linear fits to the individual data. In fact, a correlation analysis between all non-zero slopes and the age that animals emerged from darkness revealed no significant relationship (R2 = 0.11, p < 0.36). The diminishing influence of darkness applied at later ages on visual acuity is highlighted in figure 4, which displays a plot of the initial acuity observed on emergence from darkness as a function of the age at which it was imposed. A linear regression analysis (excluding that for the animal placed in darkness at P37 that emerged blind) indicated a significant relationship between the age darkness was imposed and grating acuity after darkness (R2: 0.82; p < 0.0019).
Figure 3.
The recovery of binocular visual acuity of eight normal kittens placed in darkness for 10 days that began at the ages shown. Grey regions depict the period of darkness. One of the two kittens that experienced darkness from P47 (C176; white circle symbols) developed uveitis, so that measurements of the recovery of vision had to be curtailed after five weeks. Solid lines indicate linear functions that have been fitted to the individual data for each kitten.
Figure 4.
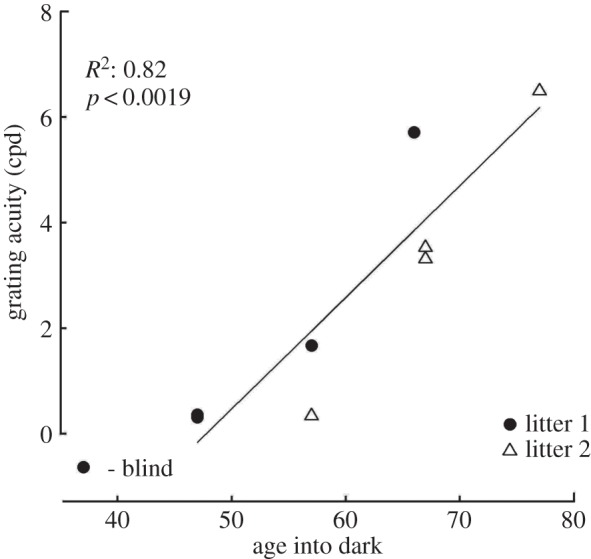
The initial visual acuity of nine normal kittens following 10 days of darkness that began at the ages plotted on the abscissa. Black and white symbols depict, respectively, data from the first and second litter. The data for the two animals placed in darkness at P47 were nearly identical. A linear regression has been fitted to the data with the coefficient of determination (R2) correlation shown at the top left.
The lack of any impairment of the vision of the animal placed in darkness at P77 indicates that the period of vulnerability to darkness is very short. To better illustrate this point, the immediate effects of darkness expressed as an index have been plotted in figure 5 as a function of the age at which darkness was imposed. The data for the two normal litters are once again depicted by circles and triangles, and results are also shown (squares) for the monocularly deprived animals placed in darkness from the earlier study [9]. The deprivation effect is expressed as the difference between the acuities measured immediately prior to (B) and after (A) the period of darkness divided by the former [(B − A)/B] and adopts a value of 1.0 when the animal appears blind after darkness and 0 if vision is unimpaired. The least-squares method was used to fit the data to a modified Hill function [16] of the form
where D(t) is the deprivation metric at time t, h is the Hill coefficient that determines the slope of the curve, and t50 is the age at which the curve is at half-height. R2 was used to assess the goodness of fit. Pooled data that included normal kittens and monocularly deprived animals were fitted well by this sigmoid function (solid curve). The age at which immersion in darkness for 10 days resulted in a deprivation effect of 0.5 (t50th) was 61 days. The difference between the results from the two normal litters that was evident in figure 3 was further captured by the two additional Hill functions fitted to the data from each litter. The separate fits to the data from the two litters were remarkably precise (both had R2-values of 0.99), but also quite different in terms of their t50-values, which were 55 and 67 days, respectively, for litters 1 and 2. For the t50 parameter, the 95% confidence intervals for litters 1 and 2 do not overlap with each other, but both overlap with the pooled fit. The 95% confidence intervals for the Hill coefficients overlap for all three fits. The rapid decline in the deprivation effect with age is evident not just in the low t50-value of the combined data (61 days), but also by the lack of loss of acuity in all five animals immersed in darkness beyond 70 days of age, suggesting that the upper bound of the period of vulnerability to darkness of spatial vision as assessed by visual acuity extends only to 10 weeks of age.
Figure 5.
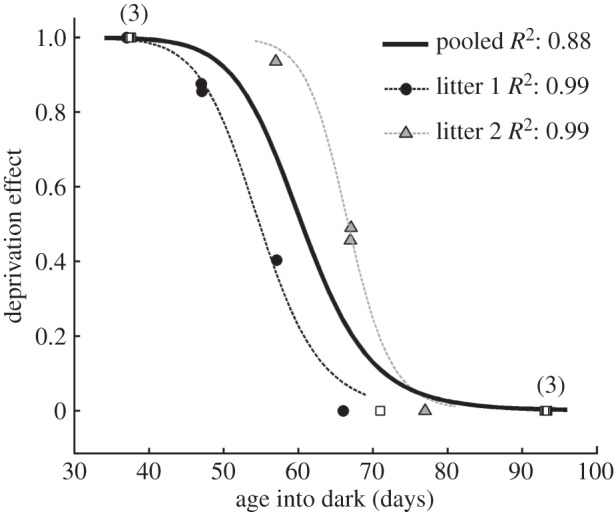
The profile of the critical period for the effects of exposure to darkness for 10 days beginning at the ages plotted on the abscissa. The effect of darkness on visual acuity is expressed as an index [(B – A)/B], where B and A represent the acuity respectively before (B) and after (A), the period of darkness. White square symbols show data from the seven monocularly deprived kittens placed in the dark from an earlier study [9], whereas black and grey symbols depict the data from normal animals placed in the dark. The numbers in brackets adjacent to the symbols indicate the number of animals for which the deprivation metric was identical. Individual data for the kittens from the two normal litters are depicted separately (litter 1, black circles; litter 2, grey triangles). The combined data for all animals have been fit by a Hill function and is indicated by the solid line. Dashed lines indicate separate Hill functions fitted to the data from each normal litter (litter 1, black; litter 2, grey).
4. Discussion
The severity and longevity of the effects of the 10-day period of darkness on vision were very surprising as they came after an extended prior period of normal visual exposure. Most prior studies [2] of the consequences of darkness on development of vision and of the visual pathways employed darkness for longer periods starting from birth. Remarkably, darkness has not been viewed in the same way as other experiential manipulations (most notably monocular deprivation) that produce fast effects on the developing visual system and on vision. One notable exception was a study [17] of the consequences of a short, 3–6-day period of darkness, imposed at four weeks of age on the responsiveness of single neurons in the kitten visual cortex. Several aspects of cortical responsiveness were diminished, including a broadening of orientation tuning, a reduction in the peak response frequency for optimal stimuli and increased response variability. Nevertheless, these changes at the level of striate cortex would not predict the dire behavioural consequences reported here. However, the generally high level of responsiveness observed in the visual cortex after darkness rules out the possibility that the blindness results from a loss of retinal function owing to darkness. Although the effects of darkness on retinal function have not been studied in cats, a study on rats [18] revealed long-lasting changes of retinal ganglion cell organization in animals reared for very long periods in darkness from birth to four months of age. On the other hand, there is a growing realization [19,20] that various forms of selected visual deprivation exert substantial effects beyond the striate cortex, and so it is possible that the behavioural consequences of the short periods of darkness are rooted in these widespread events in other interconnected cortical areas.
The period of vulnerability of vision to darkness is short in comparison with the well-known critical period for ocular dominance shifts in the cat visual cortex, which extends well beyond 16 weeks of age in response to 10-day periods of monocular deprivation [11] and to between six and eight months following longer periods of deprivation [12,13]. On the other hand, the critical period for the effects of darkness on vision is longer than those relating to the cortical processing of motion as reflected by the ability to modify the directional tuning of cells in striate cortex [21], or the perception of global motion [22], both of which extend only to six weeks of age. It is most unlikely that the deficits of spatial vision reported here were influenced in any meaningful way by modification of cortical mechanisms of global motion perception. It is not known whether the latter are influenced by short (10-day) periods of darkness, and even so, in only one of the rearing conditions employed here where darkness was imposed at P37 did it occur within the short critical period for the perception of global motion. Moreover, the visual stimuli employed for measurement of grating acuity were static and so did not require the ability to detect motion.
An interesting and unexpected finding from this study of the period of vulnerability to darkness was the consistent difference between results from the two litters of kittens that were examined. The separate Hill functions fitted to the results from the two litters (figure 5) revealed both the consistent nature of the results from each litter but also the clear differences between the two, as reflected by the t50-values that were separated by 12 days. There were no obvious procedural differences that could account for this variation. Even though they were born 2 years apart, from the same stud but different unrelated queens, they were reared and tested by the same people, and by use of the same stimuli and procedures. Both litters were of similar size and healthy. The consistency of the data within each litter combined with the systematic difference between the two suggests that genetic variation plays a role in determining when the vulnerability to darkness ends.
A remarkable feature of the visual loss that followed imposition of darkness was the slow speed of the subsequent recovery, which took seven weeks. Furthermore, the data of figure 3 suggest that the speed of recovery remained just as slow even as darkness was imposed at progressively later ages. The slow rate of the recovery throws into relief the asymmetry between the ages (less than P70 days) at which darkness can induce visual loss as opposed to the upper age limit during which gradual recovery from the darkness-induced loss occurs, which extends at least to P120 days (figure 3). It has been suggested ([6], ch. 9) that asymmetries between the plasticity that underlie induction and recovery may represent a general feature of critical periods in the visual system, and that the two forms of plasticity may derive from different molecular events.
Another asymmetry that occurs in response to 10 days of darkness was evident in the data from monocularly deprived animals in an earlier study [9]. When darkness was imposed early and immediately after the period of monocular deprivation, the recovery of vision in both the deprived and the non-deprived eye was very slow, as confirmed by the data from the normal animal (C175) of the present study, which was placed in darkness at the same age. In marked contrast, the recovery of the acuity of the deprived eye of animals placed in darkness when 15 weeks old was very fast, such that the vision of the deprived eye improved rapidly in 5–7 days to normal levels. This finding highlights two striking asymmetric responses to darkness in terms of the ages at which they occur and the speed of the subsequent visual recovery. When imposed within a critical period that ends at about 10 weeks of age, darkness exerts severe effects on vision, from which recovery is very slow in both normal and monocularly deprived kittens. By contrast, darkness can produce very rapid (5–7 days) improvement in the vision of the deprived eye of monocularly deprived kittens at least until 15 weeks of age, during which time the vision of the fellow eye remains unchanged. The upper limit of the latter critical period has not yet been defined, but our preliminary data suggests that it extends to at least six months of age.
Extended periods of darkness have long been known to have consequences for vision and for the functional properties of neurons in the visual cortex. In addition, it has been well documented that long periods of darkness extend the critical period for ocular dominance plasticity for many months, and possibly indefinitely [23–25]. Our experiments show that very brief periods of darkness can have at least two major consequences for vision. The first is the dramatic loss of vision when imposed within a short critical period, whereas the second is a rapid improvement in the vision of the deprived eye of monocularly deprived kittens promoted by darkness at much later ages [9]. The second period, which is evident to at least 15 weeks, represents a time when darkness can promote fast recovery of visual acuity in an amblyopic eye, without any impairment of the acuity of the fellow (normal) eye. At present, there is no obvious explanation for the almost 10-fold difference in the speed of recovery of vision following an early episode of darkness as opposed to the darkness-induced fast recovery reported earlier in the vision of the amblyopic eye of monocularly deprived kittens at much later ages. An intriguing possibility is that the fast and seemingly complete recovery from monocular deprivation may be instructed by the fellow eye that is unaffected by darkness beyond about P70. Although the molecular events that underlie the dramatic consequences for vision of short periods of darkness are as yet unknown, it is likely that significant differences exist between those involved in the disruption of vision in normal animals prior to P70 and those linked to the recovery of vision observed for several more months in monocularly deprived animals. With respect to a possible asymmetry in the molecular events that underlie these two consequences of darkness, it is of interest that several molecular changes that have been linked to the physiological changes produced by monocular deprivation in the visual cortex have been shown to play little or no part in the recovery from such deprivation [26–28], whereas others have been linked to recovery from deprivation but not to the initial disruption [29].
The severity of the consequences of brief periods of darkness when imposed early in life on kittens implies that the use of darkness as a possible therapy for amblyopia in humans [9,14,30] cannot be contemplated at any age with impunity. At the very least, the intervention should be delayed to an age where adverse effects, albeit temporary, on the vision of the non-amblyopic eye can be avoided. Identification of the critical age in humans beyond which darkness can be contemplated as a therapy will require information from many sources, including documentation of key psychophysical and molecular milestones during development in both cats and humans.
Ethics statement
The breeding, special rearing and experimental procedures were conducted in conformance with protocols approved by the Dalhousie University Committee on Laboratory Animals. Procedures were in compliance with guidelines dictated by the Canadian Council on Animal Care.
Data accessibility
The data for the various figures have been deposited in Dryad (doi:10.5061/dryad.cj64p).
Funding statement
This research was supported by a grant (no. 102653) to K.R.D., N.A.C. and D.E.M. from the Canadian Institute of Health Research and by individual Discovery grants from the Natural Sciences and Engineering Research Council (K.R.D. 298167, N.A.C. 355847 and D.E.M. 7660).
References
- 1.Hubel DH, Wiesel TN. 1963. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J. Neurophysiol. 26, 1003–1017. [DOI] [PubMed] [Google Scholar]
- 2.Movshon JA, Van Sluyters RC. 1981. Visual neuronal development. Annu. Rev. Psychol. 32, 477–522. ( 10.1146/annurev.ps.32.020181.002401) [DOI] [PubMed] [Google Scholar]
- 3.Mitchell DE, Timney B. 1984. Postnatal development of function in the mammalian visual system. In Handbook of physiology section I: the nervous system, vol. 3 part i sensory processes (ed. Darian-Smith I.), pp. 507–555. Bethesda, MD: American Physiological Society. [Google Scholar]
- 4.Movshon JA, Kiorpes L. 1990. The role of experience in visual development. In Development of sensory systems in mammals (ed. Coleman JR.), pp. 155–202. New York, NY: Wiley. [Google Scholar]
- 5.Rauschecker JP. 1991. Mechanisms of visual plasticity: Hebb synapses, NMDA receptors and beyond. Physiol. Rev. 71, 587–615. [DOI] [PubMed] [Google Scholar]
- 6.Daw NW. 2006. Visual development, 2nd edn New York, NY: Springer. [Google Scholar]
- 7.Timney B, Mitchell DE, Giffin F. 1978. The development of vision in cats after extended periods of dark-rearing. Exp. Brain Res. 31, 547–560. ( 10.1007/BF00239811) [DOI] [PubMed] [Google Scholar]
- 8.Blake R, Di Gianfilippo A. 1980. Spatial vision in cats with selective neural deficits. J. Neurophysiol. 43, 1197–1205. [DOI] [PubMed] [Google Scholar]
- 9.Duffy KR, Mitchell DE. 2013. Darkness alters maturation of visual cortex and promotes fast recovery from prior monocular deprivation. Curr. Biol. 23, 382–386. ( 10.1016/j.cub.2013.01.017) [DOI] [PubMed] [Google Scholar]
- 10.Hubel DH, Wiesel TN. 1970. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. (Lond.) 206, 419–436. ( 10.1113/jphysiol.1970.sp009022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olson CR, Freeman RD. 1980. Profile of the sensitive period for monocular deprivation in kittens. Exp. Brain Res. 39, 17–21. ( 10.1007/BF00237065) [DOI] [PubMed] [Google Scholar]
- 12.Jones KR, Spear PD, Tong L. 1984. Critical periods for effects of monocular deprivation: differences between striate and extrastriate cortex. J. Neurosci. 4, 2543–2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daw NW, Fox KD, Sato H, Czepita D. 1992. Critical period for monocular deprivation in the cat visual cortex. J. Neurophysiol. 67, 197–202. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell DE. 2013. A shot in the dark: the use of darkness to investigate visual development and as a therapy for amblyopia. Clin. Exp. Optom. 96, 363–372. ( 10.1111/cxo.12084) [DOI] [PubMed] [Google Scholar]
- 15.Mitchell DE, Giffin F, Timney B. 1977. A behavioural technique for the rapid assessment of the visual capabilities of kittens. Perception 6, 181–193. ( 10.1068/p060181) [DOI] [PubMed] [Google Scholar]
- 16.Atkins GL. 1973. A simple digital-computer program for estimating the parameter of the Hill Equation. Eur. J. Biochem. 33, 175–180. ( 10.1111/j.1432-1033.1973.tb02667.x) [DOI] [PubMed] [Google Scholar]
- 17.Freeman RD, Mallach R, Hartley S. 1981. Responsivity of normal kitten striate cortex deteriorates after brief binocular deprivation. J. Neurophysiol. 45, 1074–1084. [DOI] [PubMed] [Google Scholar]
- 18.Di Marco S, Nguyen VA, Bisti S, Protti DA. 2009. Permanent functional reorganization of retinal circuits induced by early long-term visual deprivation. J. Neurosci. 29, 13 691–13 701. ( 10.1523/jneurosci.3854-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell DE, Lomber SG. 2013. An examination of linking hypotheses drawn from the perceptual consequences of experimentally-induced changes in neural circuitry. Vis. Neurosci. 30, 271–276. ( 10.1017/S095252381300028X) [DOI] [PubMed] [Google Scholar]
- 20.Kiorpes L, McKee SP. 1999. Neural mechanisms underlying amblyopia. Curr. Opin. Neurobiol. 9, 480–486. ( 10.1016/S0959-4388(99)80072-5) [DOI] [PubMed] [Google Scholar]
- 21.Daw NW, Wyatt HJ. 1976. Kittens reared in a unidirectional environment: evidence for a critical period. J. Physiol. (Lond.) 257, 155–170. ( 10.1113/jphysiol.1976.sp011361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell DE, Kennie J, Kung D. 2009. Development of global motion perception requires early postnatal exposure to patterned light. Curr. Biol. 19, 645–649. ( 10.1016/j.cub.2009.02.038) [DOI] [PubMed] [Google Scholar]
- 23.Cynader MS, Mitchell DE. 1980. Prolonged sensitivity to monocular deprivation in dark-reared cats. J. Neurophysiol. 43, 1026–1040. [DOI] [PubMed] [Google Scholar]
- 24.Mower GD, Caplan CJ, Christen WG, Duffy FH. 1985. Dark rearing prolongs physiological but not anatomical plasticity of the cat visual cortex. J. Comp. Neurol. 235, 448–466. ( 10.1002/cne.902350404) [DOI] [PubMed] [Google Scholar]
- 25.Cynader M. 1983. Prolonged sensitivity to monocular deprivation in dark-reared cats: effects of age and visual exposure. Dev. Brain Res. 8, 155–164. ( 10.1016/0165-3806(83)90002-0) [DOI] [PubMed] [Google Scholar]
- 26.Taha S, Stryker MP. 2002. Rapid ocular dominance plasticity requires cortical but not geniculate protein synthesis. Neuron 34, 425–436. ( 10.1016/S0896-6273(02)00673-6) [DOI] [PubMed] [Google Scholar]
- 27.Liao DS, Mower AF, Neve RL, Sato-Bigbee C, Ramoa AS. 2002. Different mechanisms for loss and recovery of binocularity in the visual cortex. J. Neurosci. 22, 9015–9023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krahe TE, Medina AE, de Bittencourt-Navarrete RE, Colello RJ, Ramoa A. 2005. Protein synthesis- independent plasticity mediates rapid and precise recovery of deprived eye responses. Neuron 48, 329–343. ( 10.1016/j.neuron.2005.09.016) [DOI] [PubMed] [Google Scholar]
- 29.Müller CM, Griesinger CB. 1998. Tissue plasminogen activator mediates reverse occlusion plasticity in visual cortex. Nat. Neurosci. 1, 47–53. ( 10.1038/248) [DOI] [PubMed] [Google Scholar]
- 30.He HY, Ray B, Dennis K, Quinlan EM. 2007. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat. Neurosci. 10, 1134–1138. ( 10.1038/nn1965) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the various figures have been deposited in Dryad (doi:10.5061/dryad.cj64p).