Abstract
Many parasites modify their host behaviour to improve their own transmission and survival, but the proximate mechanisms remain poorly understood. An original model consists of the parasitoid Dinocampus coccinellae and its coccinellid host, Coleomegilla maculata; during the behaviour manipulation, the parasitoid is not in contact with its host anymore. We report herein the discovery and characterization of a new RNA virus of the parasitoid (D. coccinellae paralysis virus, DcPV). Using a combination of RT-qPCR and transmission electron microscopy, we demonstrate that DcPV is stored in the oviduct of parasitoid females, replicates in parasitoid larvae and is transmitted to the host during larval development. Next, DcPV replication in the host's nervous tissue induces a severe neuropathy and antiviral immune response that correlate with the paralytic symptoms characterizing the behaviour manipulation. Remarkably, virus clearance correlates with recovery of normal coccinellid behaviour. These results provide evidence that changes in ladybeetle behaviour most likely result from DcPV replication in the cerebral ganglia rather than by manipulation by the parasitoid. This offers stimulating prospects for research on parasitic manipulation by suggesting for the first time that behaviour manipulation could be symbiont-mediated.
Keywords: parasitoid wasp, virus, holobiont, symbiont, behavioural manipulation, host–parasite interaction
1. Introduction
Parasites have the capacity to alter the biology of their hosts in many ways to improve their own fitness [1,2], host behaviour manipulation being one of the most striking outcomes. Behaviour manipulations may favour completion of the parasite's life cycle by (i) rendering intermediate hosts more susceptible to predation by definitive hosts, (ii) inducing the parasitized host to move to habitats suitable for the parasite and/or its progeny, (iii) increasing the appetite of vectors in cases of vector-borne transmission, and (iv) providing protection to the developing parasite against biotic or abiotic factors, a condition called bodyguard manipulation [1,3].
Understanding how manipulation of host behaviour works remains a challenge. Until recently, the study of proximate mechanisms has mostly focused on neuromodulatory systems [4] and experimental evidence of parasite genes inducing a direct change in host behaviour is very limited [5,6]. Bodyguards have only been reported in hosts of parasitic wasps (parasitoids) that pupate outside of their hosts [7,8], and the mechanisms involved have never been explored. We investigated this question using the Dinocampus coccinellae—Coleomegilla maculata association as a model system [8].
The female D. coccinellae lays its eggs in the ladybeetle and the parasitoid larvae develop inside the body of the coccinellid host. After about 20 days, a single prepupa egresses and spins a cocoon between the ladybeetle's legs. At this time, the ladybeetle's behaviour is modified: it remains static and displays tremors. Throughout parasitoid pupation, the host remains alive and positioned on top of the parasitoid cocoon, serving as a bodyguard to protect the parasitoid cocoon from predation [8]. After a week, the adult parasitoid emerges from the cocoon. Some ladybeetles recover from the paralysis, resume feeding and can even reproduce [9,10].
Endoparasitoid larvae grow inside their hosts and rely on a variety of weapons, including polydnaviruses and venom proteins that are typically injected with parasitoid eggs and disturb the host's immune defence, or development [11,12]. Dinocampus coccinellae is a Braconidae from the Helconoid subfamily in which no polydnavirus had been found. However, we identified a virus, named D. coccinellae paralysis virus (DcPV), in the head of parasitized ladybeetles. Given (i) the delay between oviposition by D. coccinellae and the onset of bodyguard behaviour in C. maculata, (ii) that the parasitoid is no longer in physical contact with its host during the behaviour manipulation, and (iii) the frequent and diverse roles played by viruses in host–parasitoid relationships, we hypothesized that DcPV could be associated with D. coccinellae and infective for the nervous tissue of the coccinellid host, thus participating in the behaviour manipulation.
2. Material and methods
The detailed material and methods are available in the electronic supplementary material.
(a). Sampling
Adult C. maculata were exposed to female D. coccinellae from Quebec. Following parasitism, D. coccinellae larvae (L) and C. maculata heads (H) and abdomens (Ab) were sampled before parasitoid egression: 5 days after parasitism (Be D5), 13 days after parasitism (Be D13), 20 days after parasitism (Be D20), immediately after emergence (Ae) and following ladybeetle recovery from parasitism (R) (figure 1). The abdomens of resistant ladybeetles (Res) in which parasitoid eggs had been encapsulated were collected 25 days after parasitism. Note that the behaviour of these individuals was not affected. The heads and abdomens of unparasitized C. maculata (He) and adult D. coccinellae (Adult) were collected as controls.
Figure 1.
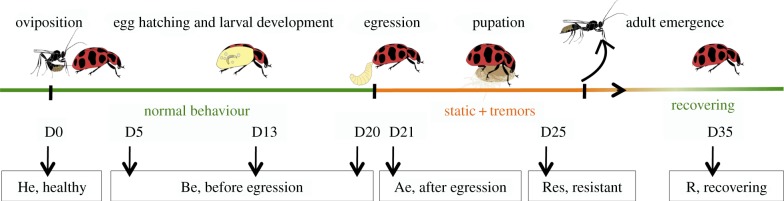
Life cycle of the parasitoid exploiting its host (drawing by Franz Vanoosthuyse). Boxes indicate when the samples were collected for analyses: healthy ladybeetle (He), before parasitoid larval egression (Be), after parasitoid larval egression (Ae), resistant ladybeetle (Res) and following host recovery (R).
In addition, larva of D. coccinellae from Poland, Japan and The Netherlands were collected separately.
(b). RNA sequencing
For conditions He, Be D20, Ae and R, a pool of RNA was generated for L, H and Ab and used to perform mRNA sequencing using an Illumina Genome Analyzer (electronic supplementary material, table S1). Data were used for de novo transcriptome assembly using Velvet and Oases (v. 0.2.05; http://www.ebi.ac.uk/~zerbino/oases/).
(c). Dinocampus coccinellae paralysis virus genome sequence
The complete DcPV genome initially assembled de novo from RNAseq data was re-sequenced using the Sanger method following a combination of PCR, RACE-PCR and cloning (electronic supplementary material, Note S2). Phylogenetic analysis was performed by using the Maximum-Likelihood Method based in MEGA5. Initial tree(s) were obtained automatically by applying BioNJ algorithms to a matrix of pairwise distances.
(d). Quantification of positive and negative strand Dinocampus coccinellae paralysis virus genomes
Strand-specific RT-qPCR assays were developed for quantification of positive-strand viral genomes and negative-strand replication intermediates with primers containing a 5′ tag sequence (electronic supplementary material, table S4 and figure S6) [13,14]. Absolute quantification was obtained using a standard curve of viral cDNA standards and data were natural-log-transformed before statistical analyses.
(e). Antiviral gene expression
RT-qPCR was used for quantification of C. maculata antiviral immune response genes. Relative gene expression was calculated using elongation factor 2 and carbonic anhydrase as reference genes and data were square-root-transformed (electronic supplementary material, table S5 and figure S8).
(f). Statistical analyses
The normal distribution of virus load and gene expression was confirmed with the Shapiro Wilk normality test for each dataset. Significant differences were revealed with a two-sided Student t-test with Welch correction and Bonferroni correction (electronic supplementary material, Note S3).
(g). Transmission electron microscopy
Samples were fixed in sodium cacodylate buffer and then in osmium tetroxide buffer. Once infiltrated in resin, 0.1 μm ultra-thin sections were stained with uranyl acetate and lead citrate and examined using a Zeiss EM 10 CR electron microscope.
3. Results and discussion
(a). The bodyguard behaviour: a neurological disorder
We initially used behavioural assays to analyse the symptoms that characterize the bodyguard behaviour (electronic supplementary material, Note S1). Parasitized ladybeetles are partially paralysed and exhibit tremors, gait disturbance and slow limited movements. These symptoms suggest a severe neurological disorder (electronic supplementary material, Note S1, figure S1 and Videos S1–S6).
(b). Comparative transcriptomic analysis identifies a RNA virus in parasitoids and parasitized ladybeetles
RNA sequencing data were used to generate de novo the transcriptomes of C. maculata, and D. coccinellae. Then, for each sample, reads were mapped against the reference transcriptomes and differentially expressed transcripts were identified (data not shown). Among the transcripts significantly upregulated in the head of parasitized ladybeetles, we identified numerous sequences highly similar to picorna-like virus polyproteins. These partial sequences were aligned and used to generate primers to sequence the complete virus genome (figure 2). Interestingly, DcPV was also present in parasitoid larvae.
Figure 2.
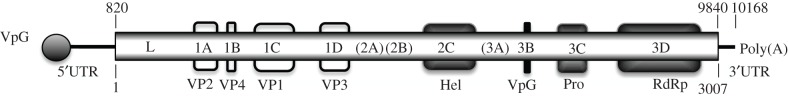
Schematic diagram of the predicted DcPV genome structure (electronic supplementary material, Note S2). Numbers on the top indicate nucleotide positions, numbers on the bottom indicate amino acid positions, and the long shaded box represents the single ORF. A leader sequence was found upstream of the viral capsid proteins (VP). Predicted proteins are indicated using the L434 nomenclature system. Boxes indicate the position of recognizable protein domains of structural proteins (VP 1 to 4; open boxes) and non-structural proteins (dark boxes).
(c). Molecular characterization of Dinocampus coccinellae paralysis virus
PCR, RACE-PCR, cloning and sequencing of the DcPV genome yielded a continuous sequence of 10 138 nucleotides, excluding the poly(A) tail (GenBank KF843822) (electronic supplementary material, Note S2 and table S2). The genome has one large open reading frame (ORF), from nucleotides 820 to 9840, that encodes a predicted polyprotein of 3007 residues (figure 2; electronic supplementary material, figure S2). The structural proteins are encoded by the N-terminal part of the precursor, and the non-structural helicase, protease and RNA-dependent RNA polymerase (RdRp) were encoded by the C-terminal part (electronic supplementary material, Note S2). The predicted proteins share functional motifs characteristic of picorna-like viruses and picornaviruses (electronic supplementary material, Note S2, and figures S3 and S4). The 5′ non-translated region was highly structured and included a cloverleaf-like structure and a 5′-UUUA-3′ loop similar to hairpin structures found in other picorna-like viruses (electronic supplementary material, Note S2 and figure S5). The phylogenetic analysis of conserved RdRp domains of viruses of Iflaviridae, Dicistroviridae and Picornaviridae further confirmed that DcPV is a member of the Iflaviridae family; it is closely related to VcPLV and NvitV-1, which have been found in the ichneumonid Venturia canescens [15] and the pteromalid Nasonia vitripennis [16], respectively (figure 3; electronic supplementary material, table S3). We found DcPV in all tested adult (four individual samples), larva (seven pooled samples) and pupa (six pooled samples) of D. coccinellae from Quebec. The worldwide distribution of the virus was confirmed as DcPV was detected in populations from Poland, Japan and The Netherlands (two pooled samples of larva each). DcPV is thus a new species of Iflavirus that belongs to a recently defined family of picorna-like single-strand positive RNA viruses that infect insects (http://www.picornavirales.org/iflaviridae/iflavirus_seq.htm and http://www.ictvonline.org/) [17].
Figure 3.
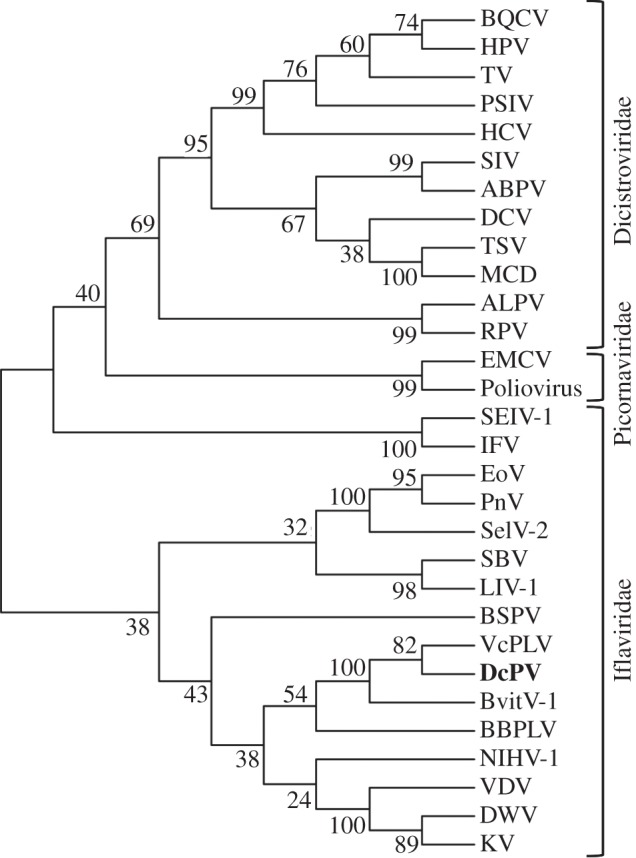
DcPV belongs to the Iflaviridae family. A phylogenetic tree was constructed from the alignment of 30 RdRp sequences (electronic supplementary material, table S3) by using the maximum-likelihood method (bootstrap on 1000 replicates). Branch lengths are proportional to the number of changes.
(d). Dinocampus coccinellae paralysis virus is associated to the parasitoid wasp Dinocampus coccinellae
In early development (e.g. eggs collected five days after oviposition, Be D5), DcPV genomes were below detectable level, suggesting that very few virus particles are present at this stage (figure 4). Thirteen days post-oviposition (Be D13), we collected either eggs or larva. Interestingly, virus was absent in eggs but abundant following egg hatching, resulting in a high variance at this time point. During larval development, virus load increased significantly (figure 4a,b, Be D20). In the adult stage, viral genome abundance was significantly higher than in larva, whereas the replication intermediates were significantly lower (figure 4). It resulted in a ratio of positive-strand to negative-strand genomes of about 100 : 1 in larva and 3000 : 1 in adult wasps, suggesting a high viral replication in larva and the storage of non-replicating viruses in adults. This is different from other described vertically transmitted endoparasitoid viruses that mainly replicate in the female oviducts and are produced in abundance to be injected within the host at oviposition [12]. As Iflaviruses are known to be released by virus-induced cell lysis or autophagy [18,19], this low replication in adult parasitoids could limit its pathogenicity towards D. coccinellae.
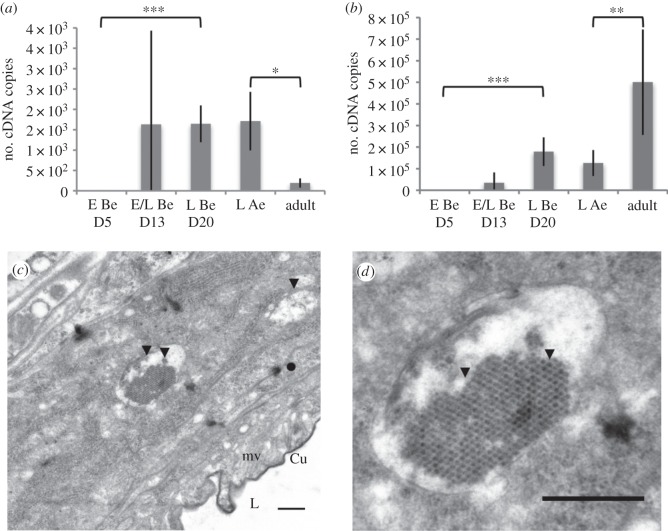
Figure 4.
Abundance and replication of DcPV in D. coccinellae. Quantity of negative- (a) and positive- (b) strand copies of DcPV in 500 μg of RNA from parasitoid eggs (E) and larvae (L) collected 5, 13 and 20 days following oviposition (E Be D5, E/L Be D13, L Be D20), immediately after larval egression from the host (L Ae) and in adult parasitoids. Results are mean ± s.e.m. of biological replicates. Asterisks (*, ** and ***) indicate results are significantly different for a two-sided Student's t-test (electronic supplementary material, Note S3), with q < 0.05, q < 0.01 and q < 0.001, respectively. (c) TEM image of the oviduct of D. coccinellae. Beneath the cuticular intima, a series of microvilli line the lumen. Viral particles are observed within unilamellar vesicles. (d) TEM image of a vesicle packed with viruses showing a typical crystal structure. Cu, cuticular intima lining the oviductal lumen; mv, microvilli; L, lumen; arrow heads, viral particles. Scale bars, 500 nm.
To assess if DcPV is transmitted to eggs, we studied the ultrastructure of the ovary and oviduct of parasitoid females by transmission electron microscopy (TEM). Virus particles of about 27 nm in diameter were found in large unilamellar vesicles of up to 2 µm in diameter in cells lining the oviductal lumen (figure 4c). Some vesicles were packed full of viruses, leading to their arrangement into a crystal-like structure characteristic of picorna-like viruses (figure 4d). However, no virus particle was observed within the oviduct lumen. This is the first observation of unilamellar vesicles within which viruses are packed in abundance. This new structure may have arisen from parasitoid and Iflavirus coevolution. Symbiosis has been shown to favour the formation of specialized and well-adapted structures including organs containing specialized cells hosting symbionts called bacteriocytes (inhabited by bacteria), mycetocytes (inhabited by yeast), virocytes (inhabited by viruses) or algocytes (inhabited by algae) [20]. These cells contain symbiosomes, i.e. intracellular vacuoles that contain one or more endocytobiotes [21]. Mechanistic studies would be necessary to demonstrate the adaptive value of the vesicles containing viruses found in the oviduct of D. coccinellae.
Overall, we provide numerous clues suggesting that DcPV is associated to D. coccinellae. Iflaviruses were first discovered in insects of economic importance (honeybee and silkworm); they are highly pathogenic and may cause colony collapse [17]. However, regarding DcPV, potential pathogenicity has not been observed in adult D. coccinellae, and similar observations were made for non-pathogenic VcPLV and NvitV-1 [12,15,16]. Nonetheless, the impact of DcPV, VcPLV and NvitV-1 on the parasitoid's physiology has not been investigated yet. Therefore, efforts should be made to generate non-infected parasitoid control lines to compare the life-history traits of control and natural individuals and decide on either parasitism (negative impact on parasitoid fitness), commensalism (no effect on parasitoid fitness) or mutualism (beneficial effects on parasitoid fitness). Alternatively, it would be possible to compare the fitness of individuals with different virus loads to assess how the virus impacts its host.
(e). Dinocampus coccinellae paralysis virus replicates in the cerebral ganglia of parasitized ladybeetles
To investigate if DcPV could be involved in the neurological disorder that characterizes manipulated coccinellid hosts, positive and negative strand genomes were quantified within the heads of parasitized ladybeetles—as this tissue was expected to be globally enriched in nervous cells—and data compared with that obtained in the abdomen. Before parasite egression (Be D20, figure 5), a significant increase in virus load in heads and abdomens indicated that the parasitoid larva, in which the virus is actively replicating at this time (see above; figure 4c), transmits DcPV to the ladybeetle. Note that in resistant ladybeetles where parasitoid eggs had been encapsulated (figure 5), no DcPV was detected, suggesting that DcPV do not replicate in the absence of a developing parasitoid larva. In addition, the quantity of viral genomes was significantly higher in abdomens than in heads (figure 5b, Be D20, p < 0.001), whereas in contrast the quantity of replication intermediates was not significantly different in heads and in abdomens. It resulted in a ratio of positive-strand to negative-strand genomes of 2500 : 1 in abdomens and 150 : 1 in heads, suggesting a higher replication of DcPV in heads, and a certain affinity of the virus for nervous tissues (neurotropism). Interestingly, neurotropism has been associated with paralytic symptoms of other picorna-like viruses such as Poliovirus [22,23], Aphid Lethal Paralysis Virus [24] and Chronic bee paralysis virus [25]. Remarkably, recovery of normal behaviour is associated with a significant reduction in virus load (figure 5).
Figure 5.
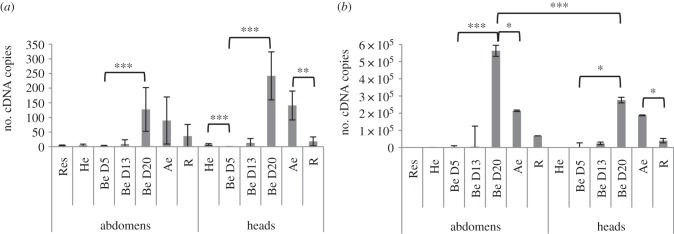
Abundance and replication of DcPV in healthy and parasitized C. maculata. Quantity of negative- (a) and positive- (b) strand copies of DcPV in 500 μg of RNA from abdomens and heads of ladybeetles collected healthy (He), 5, 13 and 20 days post-oviposition (Be D5, Be D13 and Be D20), immediately after larval egression (Ae), during recovery of a normal behaviour (R) and in resistant ladybeetles (Res). Results are mean ± s.e.m. of biological replicates. Asterisks (*, ** and ***) indicate that results are significantly different for a two-sided Student's t-test (electronic supplementary material, Note S3) with q < 0.05, q < 0.01 and q < 0.001, respectively.
To confirm DcPV neurotropism, we examined the ultrastructure of the nervous system over the course of parasitoid development using TEM. We initially studied the architecture and ultrastructure of cerebral ganglia of the healthy ladybeetle (electronic supplementary material, figure S7). It was very similar to Drosophila cerebral ganglia: a neural lamella surrounds the outer cortex of neural cell bodies and the central neuropile formed by neurites (electronic supplementary material, figure S7a) [26]. The neuropile is made up of a mass of nervous fibres crossing in all directions [26]. Glia between axons was observed as thin sections of cytoplasm. Glia cytoplasm protruded into the neuropile, where it could wrap around small axon bundles or individual axons (electronic supplementary material, figure S7b). In TEM, axons appear off-white and granular while glial cytoplasm is grey (electronic supplementary material, figure S7b). Lipid droplets were observed as well as trachea (electronic supplementary material, figure S7c).
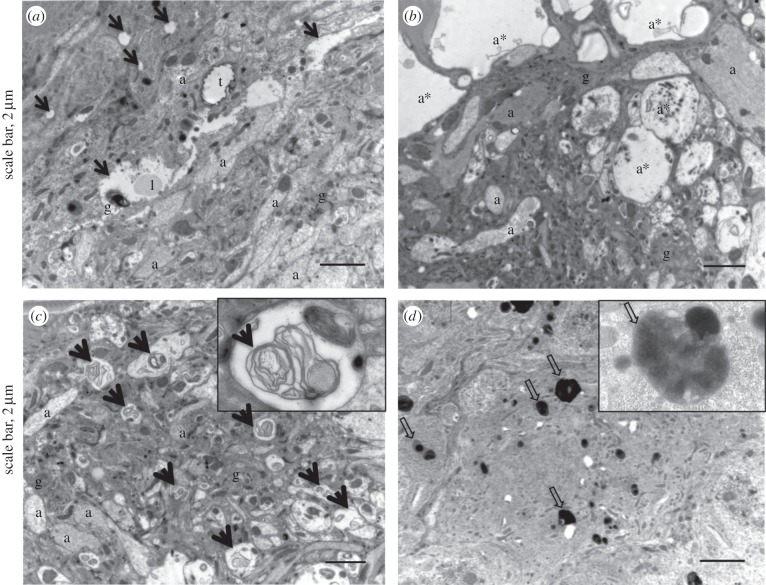
Before larva egression, the overall ultrastructure of the neuropile was unchanged (figure 6a). However, glial cells in the periphery of the neuropile were packed with virus particles of about 27 nm (figure 6). Visual observations suggest variations in the density of virus particles in the tissue, with a higher infection in the neuropile's periphery (not shown). No virus particle was found within axons. However, viruses were always associated with lipid droplets (figure 6b). Interestingly, all positive-strand viruses rely on lipids to replicate, mostly from the organelle membrane but sometimes from lipid droplets [27]. For example, lipid droplets play a key role in hepatitis C virus and rotavirus replication [28,29]. Thus, DcPV may use lipid droplets in a similar way to replicate in the ladybeetle nervous tissues. In addition, we observed the appearance of vacuoles within glial cells (figure 6a,c) and multilamellar structures surrounding the viruses (figure 6c).
Figure 6.
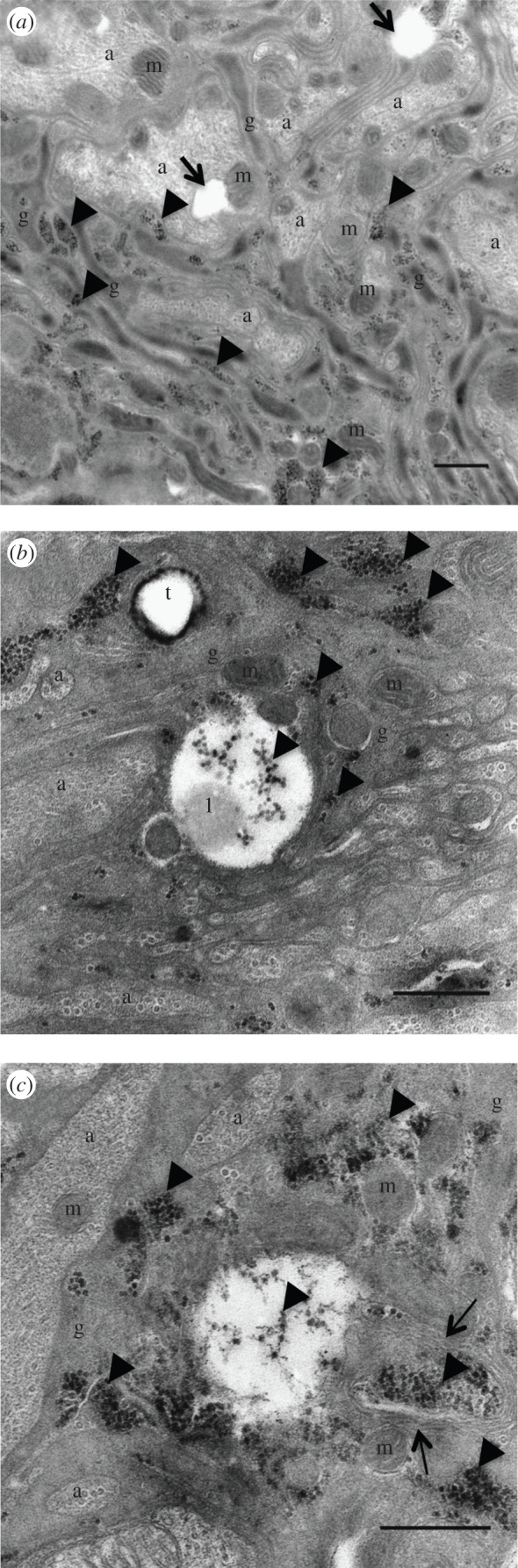
Ultrastructure of the neuropile of parasitized ladybeetles before larval egression. Virus particles were abundant in the cytoplasm of glial cells. (a,c) Numerous vacuoles formed within the neuropile. (b) Viruses were always abundant around lipid droplets and (c) in vacuoles that formed within glial cells. (c) Multilamellar structures were seen surrounding virus particles. a, axon; m, mitochondria; g, glia; t, trachea; l, lipid droplet; arrow heads, virus particles; black arrows, vacuoles; thin arrows, multilamellar structures. Scale bars, 500 nm.
Following larva egression, the predicted neuropathy was confirmed. Glial cells were highly vacuolated (figure 7), and axon swelling was sometimes observed (figure 7b). Multilamellar membranous structures accumulated in axons (figure 7c) and phagosomes were observed within the soma of neurons (figure 7d), confirming neuron degeneration. Autophagolysosomes observed after parasitoid egression could also be associated with antiviral immune defence [30–32]. Indeed, autophagy is a key feature of innate antiviral immunity, although mechanisms are poorly understood [30–32].
Figure 7.
Ultrastructure of the neuropile of ladybeetles after larval egression and onset of the bodyguard behaviour. (a) Samples were characterized by a marked vacuolization of glial cells and (b) axon swelling. (c) Fingerprint-like structures were numerous within axons. (d) Autophagolysosomes were found within the cell soma of neurons. a, axon; a*, axon swelling, l, lipid droplet; t, trachea; black arrows, vacuoles; thick arrows, fingerprint-like structures; large arrows, phagosomes.
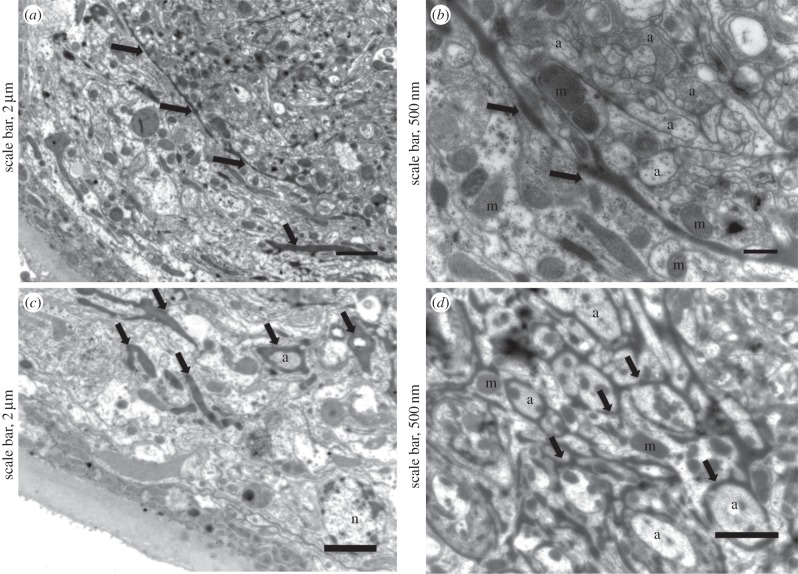
Finally, vacuolization was limited in ladybeetles that recovered from behaviour manipulation and survived parasitism, and we found few phagosomes and phagolysosomes. However, highly electron-dense glial cells expanded between the cortex and neuropile (figure 8a,b). These glial cells were observed surrounding axons and axon bundles (figure 8c,d). Images were characteristic of a strong glial regenerative response, i.e. the expansion of electron-dense glial cells around axons and axon bundles [33]. In Drosophila and cockroach, stabbing injury induces proliferation of glial cells and phagocytosis of cellular debris, which restore glial numbers, axonal enwrapment and normal nervous system function [33–37]; glia promote axonal regrowth and protect against axonal degeneration and neuronal death. Therefore, the spreading of glia in the neuropile of ladybeetles and engulfment of axons could explain how normal brain functions are restored and how the ladybeetle recovers from paralysis.
Figure 8.
Ultrastructure of the neuropile of parasitized ladybeetles that recovered from bodyguard manipulation. (a,b) Glia expanded between the cortex and neuropile, and (c,d) surrounded axons and axon bundles. a, axon; n, nucleus; m, mitochondria; thick arrows, expanding glial cells.
DcPV replication in glia may directly or indirectly alter the behaviour of the ladybeetle by glial cell lysis. Indeed, insect glia play a role in behavioural regulation via neurotransmitter clearance. Thus, glial cell lysis could ultimately affect vision, locomotion, sexual behaviour and host survival [38]. Also, neurons greatly depend on glia, so when glia integrity is threatened by DcPV replication, it would indirectly affect neuronal functioning. Alternatively, the virus may have been transmitted to neurons, which would explain the induction of autophagosis involved in virus clearing (xenophagosis) [30–32]. It has been demonstrated that xenophagic degradation allows virus clearance and limits virus replication [30,37,39]. Therefore, DcPV spread to neurons could result in a transient alteration of the central nervous system that is restored once the viruses have been eliminated. The impaired locomotion and overall lack of ladybeetle motricity are the most remarkable symptoms of the bodyguard behaviour. Based on our observations of the cerebral ganglia, we assume that the DcPV induced neuropathy spread to the entire nervous system, including the abdominal and thoracic ganglia. The latter is involved in the control of locomotion by innervating the legs and wings. Some aspects of the bodyguard behaviour, including the tremors and reduced reflex, may also be owing to other indirect side effects of the neuropathy, such as changes in ion concentration in haemolymph owing to malphighian tubule malfunction, endocrine disruption or an impairment of the processing of sensory information collected by antennae and eyes.
(f). The role of the antiviral immune response
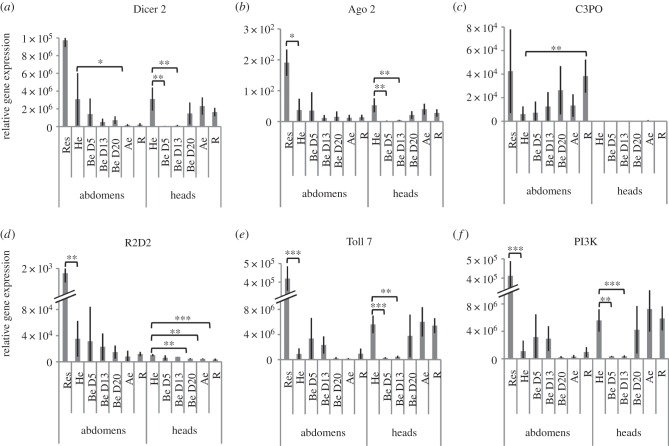
Presence of virus particles in the ladybeetle is expected to induce an antiviral response. We followed transcript levels of key genes involved in antiviral autophagy (Toll 7 and PI3K) and in antiviral RNA interference (Dicer 2, Ago 2, R2D2 and C3PO) throughout the infectious process (figure 9; electronic supplementary material, table S5 and figure S8) [32,39,40]. In abdomens, no significant variation of gene expression was observed except for a downregulation of Dicer 2 after egression and an upregulation of C3PO, a homologue of R2D2 [41], in recovering individuals. However, Ago 2, R2D2, Toll7 and PI3K were found significantly upregulated in ladybeetles resistant to parasitism (figure 9). Of significance, Dicer 2, Ago2, Toll 7 and PI3K expression was significantly downregulated in parasitized ladybeetle heads at D5 and D13 post-oviposition (figure 9). Their expression in heads was re-established from D20 onward, whereas R2D2 expression was significantly downregulated from D20 onward. Thus, the transient downregulation of multiple genes involved in the antiviral response in the ladybeetle nervous tissue could allow DcPV neurotropism in the first stages of the infectious process. Then, the re-establishment of an antiviral immune response in ladybeetle nervous tissue correlates with the appearance of phagolysosomes and with the onset of the bodyguard behaviour and result in the virus elimination. Thus, healthy ladybeetles are equipped to recognize and eliminate the virus. The antiviral immunosuppression (that may be induced by the developing D. coccinellae larva or by DcPV itself) could trigger the observed transient pathogenic infection and accumulation of DcPV in the nervous tissue. Then, elimination of the consequently high virus load could trigger the neuropathy responsible for the paralysis. A link between antiviral immune responses in nervous tissues and behavioural changes has previously been observed in other host–virus interactions. Immune response and associated widespread inflammatory response are known to be involved in behavioural changes induced by the Borna disease virus [42] or in patients with rabies [43]. Here, the antiviral immune response could result in the accumulation of phagosomes that induce paralytic symptoms characterizing the bodyguard behaviour.
Figure 9.
Gene expression profiles of selected genes involved in antiviral immune response as obtained by RT-qPCR analysis: gene expression profiles in abdomens and heads of ladybeetles collected healthy (He), 5, 13 and 20 days post-oviposition (Be D5, Be D13 and Be D20), immediately after larval egression (Ae), during recovery of a normal behaviour (R) and in resistant ladybeetles (Res). (a) Dicer 2; (b) Ago2, Argonaute 2; (c) C3PO; (d) R2D2 are involved in antiviral RNA interference. (e) Toll 7 and (f) PI3K, phosphatidylinositol 3 kinase are involved in antiviral autophagosis. Results are means ± s.d. of biological replicates for each experimental condition. Asterisks (*, ** and ***) indicate results are significantly different to He for a two-sided student's t-test (electronic supplementary material, Note S3) with q < 0.05, q < 0.01 and q < 0.001, respectively.
4. Concluding remarks
Until now, studies of the mechanisms responsible for host behaviour manipulation have mostly focused on neuromodulatory systems [1,4,44,45]. On rare occasions, a single parasite gene explains the extended phenotype [5]. Here, we revealed the involvement of a third protagonist, a symbiotic virus of the wasp—called DcPV—that is transmitted to the host during parasite larval development and is neurotropic. The presence of unilamellar vesicles containing large amounts of DcPV particles within the oviduct cells of D. coccinellae suggests that DcPV could be transmitted to the wasp eggs. Our results suggest that changes in ladybeetle behaviour most likely result from DcPV replication in the cerebral ganglia rather than by a direct manipulation by the parasitic wasp. We propose a theoretical scenario within which DcPV is employed as a biological weapon by D. coccinellae to manipulate the behaviour of C. maculata (life cycles in figure 10).
Figure 10.
Life cycles of the parasitoid D. coccinellae and its endosymbiotic virus (D. coccinellae paralysis virus, DcPV) together with responses to parasitism and infection of the ladybeetle host C. maculata (drawing by Franz Vanoosthuyse). The DcPV is stored in abundance in the oviduct of D. coccinellae female. Following oviposition and egg hatching, DcPV replicates in the parasitoid larva and is transmitted to C. maculata. The antiviral immune system of the ladybeetle is then suppressed, which allows DcPV to replicate in glial cells in the host's nervous system. The re-establishment of the antiviral immune response correlates with a severe neuropathy in the ladybeetle and the onset of the bodyguard behaviour. The synchronized egression of the larva allows it to take advantage of the paralyzed ladybeetle: it steals between the ladybeetle legs and spins its cocoon under its protection. The DcPV is being eliminated from the ladybeetle, which progressively recovers through nerve cell restoration. By then, the parasitoid resumes pupation and emerges with a new load of DcPV in its oviduct.
Further experiments are now necessary to characterize the nature of the symbiosis between D. coccinellae and DcPV. It remains unknown if symbiotic individuals display a better fitness than asymbiotic individuals and if the symbiosis is obligate for the host. In addition, and in order to validate the proposed scenario, the effect of DcPV and D. coccinellae on C. maculata physiology should be tested independently via a combination of RNA interference and injection of purified viruses. These complementary analyses will also provide information regarding the surprisingly precise simultaneous timing of larvae egression, re-establishment of antiviral immune response, accumulation of phagosomes and the induction of bodyguard behaviour by determining which mechanisms is responsible for the induction of the others.
The role of associated microorganisms, including eukaryotes, bacteria and viruses, in all components of organisms' biology has been recently revealed [46]. This study contributes to the realization that host–parasite interactions should be considered as holobiont–holobiont interactions [47] by revealing that parasite-associated microorganisms could participate in all components of host exploitation strategies, including behaviour manipulation.
Supplementary Material
Acknowledgements
We thank C. Cazevieille (CRIC, Montpellier, France) for help in TEM and L. Devine for English revision.
Data accessibility
DcPV genome sequence: EMBL GenBank accession KF843822. Raw fastq files: NCBI's sequence read archive reference PRJNA227418 for C. maculata samples and PRJNA227420 for D. coccinellae samples. de novo assembled transcriptomes: http://2ei.univ-perp.fr/telechargement/transcriptomes/ALL_Cocc_95.zip for parasitized C. maculata and http://2ei.univ-perp.fr/telechargement/transcriptomes/ALL_Larve_95.zip for D. coccinellae .
Funding statement
N.M.D. and F.M. were supported by the Agence Nationale de la Recherche (ANR) Blanc, SVSE7, project Bodyguard to F.T. and by FQRNT to J.B.
References
- 1.Hughes DP, Brodeur J, Thomas F. 2012. Host manipulation by parasites, p. 224 Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Moore J. 2002. Parasites and the behavior of animals, p. 315 Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–185. ( 10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- 4.Perrot-Minnot M-J, Cézilly F. 2013. Investigating candidate neuromodulatory systems underlying parasitic manipulation: concepts, limitations and prospects. J. Exp. Biol. 216, 134–141. ( 10.1242/jeb.074146) [DOI] [PubMed] [Google Scholar]
- 5.Hoover K, Grove M, Gardner M, Hughes DP, McNeil J, Slavicek J. 2011. A gene for an extended phenotype. Science 333, 1401 ( 10.1126/science.1209199) [DOI] [PubMed] [Google Scholar]
- 6.Kamita SG, Nagasaka K, Chua JW, Shimada T, Mita K, Kobayashi M, Maeda S, Hammock BD. 2005. A baculovirus-encoded protein tyrosine phosphatase gene induces enhanced locomotory activity in a lepidopteran host. Proc. Natl Acad. Sci. USA 102, 2584–2589. ( 10.1073/pnas.0409457102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maure F, Daoust SP, Brodeur J, Mitta G, Thomas F. 2013. Diversity and evolution of bodyguard manipulation. J. Exp. Biol. 216, 36–42. ( 10.1242/jeb.073130) [DOI] [PubMed] [Google Scholar]
- 8.Maure F, Brodeur J, Ponlet N, Doyon J, Firlej A, Elguero E, Thomas F. 2011. The cost of a bodyguard. Biol. Lett. 7, 843–846. ( 10.1098/rsbl.2011.0415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Triltsch H. 1996. On the parasitisation of the ladybird Coccinella septempunctata. J. Appl. Entomol. 120, 375–378. ( 10.1111/j.1439-0418.1996.tb01622.x) [DOI] [Google Scholar]
- 10.Maure F, Doyon J, Thomas F, Brodeur J. 2014. Host behavior manipulation as an evolutionary route toward attenuation of parasitoid virulence. J. Evol. Biol. 27, 2871–2875. ( 10.1111/jeb.12530) [DOI] [PubMed] [Google Scholar]
- 11.Whitfield JB, Asgari S. 2003. Virus or not? Phylogenetics of polydnaviruses and their wasp carriers. J. Insect Physiol. 49, 397–405. ( 10.1016/S0022-1910(03)00057-X) [DOI] [PubMed] [Google Scholar]
- 12.Beckage NE, Drezen J-M. 2012. Parasitoid viruses: symbionts and pathogens, p. 312 New York, NY: Academic Press. [Google Scholar]
- 13.Komurian-Pradel F, Perret M, Deiman B, Sodoyer M, Lotteau V, Paranhos-Baccalà G, André P. 2004. Strand specific quantitative real-time PCR to study replication of hepatitis C virus genome. J. Virol. Method 116, 103–106. ( 10.1016/j.jviromet.2003.10.004) [DOI] [PubMed] [Google Scholar]
- 14.Plaskon NE, Adelman ZN, Myles KM. 2009. Accurate strand-specific quantification of viral RNA. PLoS ONE 4, e7468 ( 10.1371/journal.pone.0007468) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reineke A, Asgari S. 2005. Presence of a novel small RNA-containing virus in a laboratory culture of the endoparasitic wasp Venturia canescens (Hymenoptera: Ichneumonidae). J. Insect Physiol. 51, 127–135. ( 10.1016/j.jinsphys.2004.05.005) [DOI] [PubMed] [Google Scholar]
- 16.Oliveira DCSG, Hunter WB, Ng J, Desjardins CA, Dang PM, Werren JH. 2010. Data mining cDNAs reveals three new single stranded RNA viruses in Nasonia (Hymenoptera: Pteromalidae). Insect Mol. Biol. 19, 99–107. ( 10.1111/j.1365-2583.2009.00934.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Oers MM. 2010. Genomics and biology of Iflaviruses. In Insect virology (eds Asgari S, Johnson K.), pp. 231–250. Norfolk, Virginia: Caister Academic Press. [Google Scholar]
- 18.Buck KW, Maramorosch K, Murphy FA, Shatkin AJ. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Vir. Res. 47, 159–251. ( 10.1016/S0065-3527(08)60736-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards AL, Jackson WT. 2013. Behind closed membranes: the secret lives of Picornaviruses? PLoS Pathog. 9, e1003262 ( 10.1371/journal.ppat.1003262) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seckbach J, Nardon P, Charles H. 2002. Morphological aspects of symbiosis. In Symbiosis, pp. 13–44. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 21.Ahn G, Choi E, Jeon K. 1990. A symbiosome-membrane-specific protein in symbiont-bearing Amoeba proteus as studied with a monoclonal antibody. Endocytobiosis Cell Res 7, 45–50. [Google Scholar]
- 22.Bodian D. 1949. Histopathologic basis of clinical findings in poliomyelitis. Am. J. Med. 6, 563–578. ( 10.1016/0002-9343(49)90130-8) [DOI] [PubMed] [Google Scholar]
- 23.Bodian D, Howe HA. 1941. Neurotropism and the genesis of cerebral lesions in poliomyelitis: an experimental study. Bull. Johns Hopkins Hosp. 68, 58–76. [Google Scholar]
- 24.Williamson C, Rybicki EP, Kasdorf GGF, Von Wechmar MB. 1988. Characterization of a new picorna-like virus isolated from aphids. J. Gen. Virol. 69, 787–795. ( 10.1099/0022-1317-69-4-787) [DOI] [Google Scholar]
- 25.Blanchard P, Ribière M, Celle O, Lallemand P, Schurr F, Olivier V, Iscache AL, Faucon JP. 2007. Evaluation of a real-time two-step RT-PCR assay for quantitation of Chronic bee paralysis virus (CBPV) genome in experimentally-infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods 141, 7–13. ( 10.1016/j.jviromet.2006.11.021) [DOI] [PubMed] [Google Scholar]
- 26.Cardona A, Saalfeld S, Preibisch S, Schmid B, Cheng A, Pulokas J, Tomancak P, Hartenstein V. 2010. An integrated micro- and macroarchitectural analysis of the Drosophila brain by computer-assisted serial section electron microscopy. PLoS Biol. 8, e1000502 ( 10.1371/journal.pbio.1000502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martín-Acebes MA, Vázquez-Calvo Á, Caridi F, Saiz J-C, Sobrino F. 2012. Lipid involvement in viral infections: present and future perspectives for the design of antiviral strategies. In Lipid metabolism, pp. 291–322. Rijeka, Croatia: InTech. [Google Scholar]
- 28.Cheung W, et al. 2010. Rotaviruses associate with cellular lipid droplet components to replicate in viroplasms, and compounds disrupting or blocking lipid droplets inhibit viroplasm formation and viral replication. J. Virol. 84, 6782–6798. ( 10.1128/JVI.01757-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogawa K, Hishiki T, Shimizu Y, Funami K, Sugiyama K, Miyanari Y, Shimotohno K. 2009. Hepatitis C virus utilizes lipid droplet for production of infectious virus. Proc. Jpn Acad. Ser. B Phys. Biol. Sci. 85, 217–228. ( 10.2183/pjab.85.217) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orvedahl A, Levine B. 2008. Autophagy and viral neurovirulence. Cell Microbiol. 10, 1747–1756. ( 10.1111/j.1462-5822.2008.01175.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richetta C, Faure M. 2013. Autophagy in antiviral innate immunity. Cell Microbiol. 15, 368–376. ( 10.1111/cmi.12043) [DOI] [PubMed] [Google Scholar]
- 32.Xu J, Cherry S. 2014. Viruses and antiviral immunity in Drosophila. Dev. Comp. Immunol. 42, 67–84. ( 10.1016/j.dci.2013.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato K, Forero MG, Fenton JC, Hidalgo A. 2011. The glial regenerative response to central nervous system injury is enabled by pros-notch and pros-NFκB feedback. PLoS Biol. 9, e1001133 ( 10.1371/journal.pbio.1001133) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato K, Awasaki T, Ito K. 2009. Neuronal programmed cell death induces glial cell division in the adult Drosophila brain. Development 136, 51–59. ( 10.1242/dev.023366) [DOI] [PubMed] [Google Scholar]
- 35.Smith P, Leech C, Treherne J. 1984. Glial repair in an insect central nervous system: effects of selective glial disruption. J. Neurosci. 4, 2698–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith PJ, Howes EA, Treherne JE. 1987. Mechanisms of glial regeneration in an insect central nervous system. J. Exp. Biol. 132, 59–78. [DOI] [PubMed] [Google Scholar]
- 37.Treherne J, Harrison J, Treherne J, Lane N. 1984. Glial repair in an insect central nervous system: effects of surgical lesioning. J. Neurosci. 4, 2689–2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edwards TN, Meinertzhagen IA. 2010. The functional organisation of glia in the adult brain of Drosophila and other insects. Prog. Neurobiol. 90, 471–497. ( 10.1016/j.pneurobio.2010.01.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding S-W, Voinnet O. 2007. Antiviral immunity directed by small RNAs. Cell 130, 413–426. ( 10.1016/j.cell.2007.07.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sabin LR, Zheng Q, Thekkat P, Yang J, Hannon GJ, Gregory BD, Tudor M, Cherry S. 2013. Dicer-2 processes diverse viral RNA species. PLoS ONE 8, e55458 ( 10.1371/journal.pone.0055458) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tomoyasu Y, Miller SC, Tomita S, Schoppmeier M, Grossmann D, Bucher G. 2008. Exploring systemic RNA interference in insects: a genome-wide survey for RNAi genes in Tribolium. Genome Biol. 9, R10 ( 10.1186/gb-2008-9-1-r10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carbone KM, Duchala CS, Griffin JW, Kincaid AL, Narayan O. 1987. Pathogenesis of Borna disease in rats: evidence that intra-axonal spread is the major route for virus dissemination and the determinant for disease incubation. J. Virol. 61, 3431–3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hemachudha T, Laothamatas J, Rupprecht CE. 2002. Human rabies: a disease of complex neuropathogenetic mechanisms and diagnostic challenges. Lancet Neurol. 1, 101–109. ( 10.1016/S1474-4422(02)00041-8) [DOI] [PubMed] [Google Scholar]
- 44.Adamo SA. 2013. Parasites: evolution's neurobiologists. J Exp Biol. 216, 3–10. ( 10.1242/jeb.073601) [DOI] [PubMed] [Google Scholar]
- 45.Van Houte S, Ros VID, van Oers MM. 2013. Walking with insects: molecular mechanisms behind parasitic manipulation of host behavior. Mol. Ecol. 22, 3458–3475. ( 10.1111/mec.12307) [DOI] [PubMed] [Google Scholar]
- 46.Brucker RM, Bordenstein SR. 2013. The capacious hologenome. Zoology 116, 260–261. ( 10.1016/j.zool.2013.08.003) [DOI] [PubMed] [Google Scholar]
- 47.Dheilly NM. 2014. Holobiont–Holobiont interactions: redefining host–parasite interactions. PLoS Pathog. 10, e1004093 ( 10.1371/journal.ppat.1004093) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
DcPV genome sequence: EMBL GenBank accession KF843822. Raw fastq files: NCBI's sequence read archive reference PRJNA227418 for C. maculata samples and PRJNA227420 for D. coccinellae samples. de novo assembled transcriptomes: http://2ei.univ-perp.fr/telechargement/transcriptomes/ALL_Cocc_95.zip for parasitized C. maculata and http://2ei.univ-perp.fr/telechargement/transcriptomes/ALL_Larve_95.zip for D. coccinellae .