Abstract
Intervertebral disc (IVD) degeneration is one of the major causes of low back pain, a problem with a heavy economic burden, which has been increasing in prevalence as populations age. Deeper knowledge of the complex spatial and temporal orchestration of cellular interactions and extracellular matrix remodelling is critical to improve current IVD therapies, which have so far proved unsatisfactory. Inflammation has been correlated with degenerative disc disease but its role in discogenic pain and hernia regression remains controversial. The inflammatory response may be involved in the onset of disease, but it is also crucial in maintaining tissue homeostasis. Furthermore, if properly balanced it may contribute to tissue repair/regeneration as has already been demonstrated in other tissues. In this review, we focus on how inflammation has been associated with IVD degeneration by describing observational and in vitro studies as well as in vivo animal models. Finally, we provide an overview of IVD regenerative therapies that target key inflammatory players.
Keywords: intervertebral disc, degenerative disc disease, inflammation, regeneration, intervertebral disc homeostasis
1. Introduction
Between 70 and 85% of all people have low back pain (LBP) at some time in their life. LBP can limit the activity in people younger than 45, causing a tremendous socio-economic impact [1]. The aetiology of LBP is unclear but in 40% of cases it is related to intervertebral disc (IVD) degeneration [2]. In 90% of sciatica cases, it is also associated with a herniated IVD that compresses a nerve root causing pain [3]. Novel strategies such as gene therapy, growth factor injection, cell-based therapies and tissue engineering approaches are being developed towards impairing degeneration or promoting regeneration of the IVD [4]. However, to achieve full IVD regeneration it is also necessary to recover the biomechanical properties of a native IVD and restore the biological behaviour of resident cells, including production of healthy extracellular matrix (ECM), while ensuring reduction of IVD-associated pain.
Traditionally, inflammation has mostly been seen as detrimental and correlated with disease progression, but it remains unclear whether it is a cause or consequence of IVD degeneration and herniation. A balanced inflammatory response may be required for restoring IVD function as recently suggested for other tissues [5,6]. In this review, we will discuss the inflammatory reaction in IVD, both in homeostasis and IVD degeneration, and comprehensively cover the strategies applied to inflammatory cells and factors targeted towards IVD regeneration.
2. The origin of inflammation in intervertebral disc
Inflammation has mostly been regarded as a response to infection or tissue injury, but the scientific community has been increasingly researching its physiological role in maintaining tissue homeostasis [7]. In general, the mechanisms of inflammation are dependent on the inducing agent and context, with the inflammatory response in the context of infection having been investigated the most. In the infection instigated inflammatory response, plasma and leucocytes are recruited to the site of infection and soluble mediators that lead to recruitment and activation of other cell types are secreted. A complex cascade of events is triggered that eventually leads to clearing infection from the tissue and resolution of inflammation. In response to tissue injury, there also exists a vascular response and an orchestrated recruitment and activation of various cell types. However, the IVD is an avascular tissue and therefore it is unsurprising that the inflammatory response is different in this context.
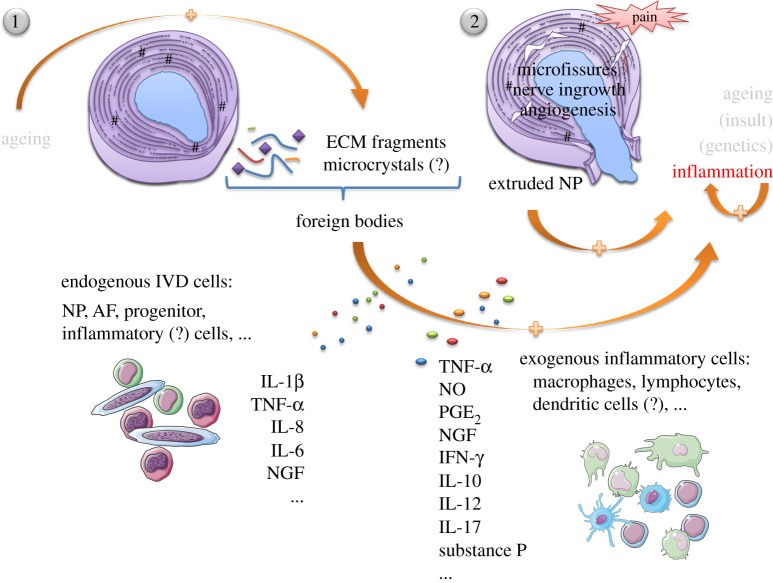
The process of IVD degeneration implies a cascade of structurally disrupting events, normally starting with declining nutrition of cells within the central IVD, followed by accumulation of cell waste products and degraded matrix molecules. This creates an increasingly acidic environment which further compromises cell viability [8]. Various causes have been hypothesized to play a role in the pathogenesis of degenerative disc disease (DDD), such as endplate calcification, leading to an impairment of normal nutrition routes, excessive mechanical loading, genetic pre-disposition, unhealthy habits, ageing and spine infection [9–16]. Regardless of the cause, inflammation is an omnipresent player, and its association to LBP is clear [17]. Yet, it remains uncertain what may trigger the recruitment of immune cells to IVD and the associated inflammatory response (figure 1).
Figure 1.
Inflammation in the IVD. It is unclear whether inflammation is the cause or consequence of disc degeneration and herniation, and what may trigger activation and recruitment of different immune cells. The normal ageing process allied to some genetic pre-disposition causes the IVD to degenerate giving rise to profound changes in the ECM—loss of proteoglycan content, dehydration, malnutrition, decrease of native cell population, matrix breakdown and calcifications. In this scenario, the natural response to mechanical loading is compromised and the IVD becomes prone to microfissures and consequent ingrowth of blood and nerve vessels. (1) Disc herniation may also occur when the AF is no longer able to sustain the NP. ECM fragments and microcrystals may internally elicit an inflammatory response, stimulating endogenous IVD cells to produce pro-inflammatory mediators, that will further feed the cascade of tissue degeneration—IL-1β, IL-8, IL-6. (2) NP is recognized as non-self by the immune system. Hence, its exposure (both in microfissures and herniation) may propagate an immunologic response, with recruitment of macrophages, lymphocytes and other possible inflammatory cells, in order to eliminate the foreign body. Discogenic pain has been many times attributed to TNF-α, PGE2, NO and IFN-γ secretion by macrophages, concomitantly with NGF and substance P production, accompanying the processes of nerve ingrowth and angiogenesis inwards the degenerated IVD. Activated B and T lymphocytes are also recruited to the site, contributing to the positive pro-inflammatory feedback loop established. It is not well understood how endogenous IVD cells interact with exogenous inflammatory cells and whether they positively contribute to tissue resorption and regeneration or not. Spontaneous disc regression is currently believed to be a consequence of macrophage activity.
One hypothesis for the cause of the IVD inflammatory response relies on endogenous factors, such as crystals and ECM breakdown products, which could induce the inflammatory response [7]. Crystal deposits of calcium pyrophosphate dihydrate (CPPD), cuboid microstructures (characterized as magnesium whitlockite) and hydroxyapatite (HA) have been observed in degenerated IVD specimens [15,18–20]. In articular cartilage, regions with crystals showed altered amounts of collagen, calcium-binding proteins, decorin and large proteoglycan content, as well as abnormal pericellular matrix deposition [21,22]. Phagocytosis of crystals present in joints and pericellular tissues can trigger activation of the NOD-like receptor family pyrin domain containing 3 (NALP3) inflammasome. This cytoplasmic multimolecular protein complex regulates activity of caspase-1 and maturation and release of interleukin (IL)-1β [7,23], the latter being commonly found in degenerated IVD [24].
ECM breakdown products generated during tissue dysfunction or damage may also promote an inflammatory response as has been shown in various models [25,26]. The IVD is mostly composed of ECM molecules, including collagens, proteoglycans and other matrix proteins (see table 1 for more information), which are continuously synthesized and degraded by local existing proteases to maintain homeostasis (table 2) [66,74–76]. However, when an imbalance occurs, degradation products might trigger inflammation. For instance, fibronectin fragments alleviate metalloproteinase (MMP) inhibition and so promote monocyte migration in vitro [77]. This certainly facilitates their recruitment into the inflamed region. Fragments of laminin, collagen type XIV and fibrin can also modulate inflammatory cell infiltration and proliferation in other systems [26]. In cartilage explant cultures, fibromodulin fragments are produced following IL-1 stimulation [78]. In different in vitro settings, fragments originated from elastin, laminins, collagen (type I and IV), fibronectin, ectactin/nidogen, thrombospondin and hyaluronan also induce protease and cytokine production, independent of their chemotactic activity [79]. Some of the aforementioned studies were performed in highly vascularized model systems (i.e. cardiovascular, lung or renal tissues). While these systems are very different from healthy adult IVDs, which are largely avascular, their findings might still be of relevance when studying phenomena associated with disc herniation or sequestration, in which blood vessels are much more abundant [80].
Table 1.
Main extracellular matrix components of a young and healthy IVD.
| name | distribution/localization | putative/possible function |
|---|---|---|
| collagens | ||
| fibril-forming collagens | ||
| type I | AF and NP | confers tensile stiffness allowing torsion and flexion [27–30] |
| type II | AF and NP | confines PG within the matrix to retain more water to allow larger deformations and withstand greater compressive loads [31,32] |
| type III | NP and outer AF | organizes pericellular environment; allows extensibility of tissue [33–35] |
| type V | AF and NP (increased in AF cells when compared to NP cells) | regulates fibril diameter (smaller if this collagen is more abundant) influencing mechanical properties [35,36] |
| type XI [37] | all over, mostly NP | regulates fibril diameter (smaller if this collagen is more abundant) influencing mechanical properties [35,38] |
| beaded-filament forming collagens | ||
| type VI | all over, mostly NP | helps cell fixation to the matrix and facilitates collagen bundles' sliding and lubrication [39,40] |
| FACIT collagens | ||
| type IX | NP | maintains matrix integrity [41,42] |
| type XII | AF | might regulate fibrillogenesis [29,43] |
| type XIV | AF | might regulate fibrillogenesis [29,43] |
| proteoglycans | ||
| aggregating PGs | ||
| aggrecan | AF and NP | maintains IVD's osmotic pressure; may act as an anti-angiogenic factor due to its inhibition of endothelial cell migration [40,44,45] |
| versican | all over, mostly AF | favours the attachment of adjacent lamellae, contributes to resistance to compressive forces and facilitates cell migration, since it is an anti-adhesive molecule [35,40] |
| non-aggregating PGs | ||
| small leucine-rich proteoglycans (SLRPs) | ||
| decorin | outer AF and fibrillar NP | regulates collagen fibril diameter and spacing, maintaining uniform patterning; GFs' reservoir (TGF-β), modulating ECM metabolism [46–48] |
| biglycan | outer AF and NP (fibrillar and pericellular region) | GFs' reservoir (TGF-β), modulating ECM metabolism [46,47] |
| asporin | outer and inner AF, rarely NP | GFs' reservoir (TGF-β), modulating ECM metabolism; may play a major role in modulating chondrocyte matrix homeostasis [49,50] |
| fibromodulin | AF and NP | regulates collagen fibril diameter and spacing, maintaining uniform patterning; GFs' reservoir (TGF-β), modulating ECM metabolism [44,47] |
| lumican | AF and NP | regulates collagen fibril diameter and spacing, maintaining uniform patterning [44,51] |
| prolargin (encoded by PRELP*) | all over, mostly AF | anchors basement membranes to the underlying connective tissue [44,52] |
| chondroadherin | AF and NP | binds integrin ad collagen; regulates cell metabolism and ECM structure, promoting matrix homeostasis [44,53,54] |
| osteoglycin/mimecan | AF and NP | unknown [44] |
| other matrix proteins | ||
| other PGs | ||
| perlecan | AF and NP | has a role in cell proliferation and differentiation by acting as co-receptor for FGFs; matrix organization and stabilization; role in FGF signalling [40,55] |
| fibronectin | all over the disc | preserves structural integrity of the ECM; involved in cell adhesion through interaction with integrins [56–58] |
| elastin | all over the disc | preserves structural integrity of the ECM; helps to regain disc height and shape after deformation [59–61] |
| COMP | all over, mostly AF | preserves structural integrity of the ECM; binds other matrix proteins and catalyses polymerization of type II collagen fibrils; prevents vascularization of cartilage [62,63] |
| thrombospondin | AF | preserves structural integrity of the ECM; mediates cell adhesion, matrix–matrix interactions, cell migration and proliferation in other tissues; might prevent vascularization of the tissue; activates TGF-β complex [64,65] |
Table 2.
Main IVD proteinases.
| name | distribution/localization | putative/possible function |
|---|---|---|
| aggrecanases | ||
| ADAMTS1, 4, 5, 9 and 15 |
ADAMTS1: NP and AF ADAMTS4: low levels NP and AF ADAMTS5: low levels NP and AF ADAMTS9: NP and AF ADAMTS15: low levels NP and AF |
degrades aggrecan [66–69], as well as versican, biglycan, fibromodulin, COMP, TSP1, TSP2, nidogen, among other substrates [70] |
| collagenases | ||
| MMP1, 8 and 13 |
MMP1: low levels, mostly inner AF and NP MMP8: low levels MMP13: low levels, mostly NP |
cleaves fibrillar collagen [66–68] |
| gelatinases | ||
| MMP2 and 9 |
MMP2: low levels, mostly inner AF and NP MMP9: low levels AF and NP |
degrades denatured collagen and basement membrane collagen [68] |
| stromelysin | ||
| MMP3 and 10 |
MMP3: low levels, mostly in the adult NP MMP10: only checked in the NP |
digests non-collagenous matrix proteins and denatured collagen [66,68,71] |
| matrilysin | ||
| MMP7 | NP and inner AF | degrades aggrecan and collagen type II [72] |
| other MMPs | ||
| MMP19 | AF and NP | cleaves aggrecan, COMP, types I and IV collagen, and fibronectin and acts on tenascin; can interfere with stabilization of capillary-like structures, possibly playing a role in the avascular status of the disc; regulates IGF-mediated proliferation in other tissues by proteolysis of IGFBP3 [73] |
It appears that fragment release initiates and propagates the inflammatory response locally. Many of these fragments (e.g. originated from biglycan, fibronectin, hyaluronan) signal through toll-like receptor-2 (TLR2) and/or TLR4 in other model systems [81,82]. TLR4, in particular, is a well-known pattern recognition receptor involved in innate immune responses that has been implicated in inflammatory degeneration [83]. In human IVD cells, hyaluronic acid fragments (fHA) lead to increased mRNA expression levels of inflammatory and catabolic genes IL-1β, IL-6, IL-8, cyclooxygenase (COX)-2, metaloprotease-1 and -13, and IL-6 [84]. However, while IL-6 production is dependent on TLR2 it is independent of TLR4. It should be noted that low and high molecular weight molecules can have different effects, even through the same pathways. For example, high molecular weight hyaluronan protects epithelial cells against pro-apoptotic stimuli through NF-κB activation, in a TLR dependent way. Low molecular weight degradation products can induce inflammation, promoting macrophage mediated production of IL-1β and tumour necrosis factor alpha (TNF-α), through activation of the NF-κB/IκBα complex [82]. It is difficult to assess the overall role of ECM proteins within an immune setting because of their dual roles and because many proteases and a variety of fragments are released simultaneously. This difficulty is exacerbated by the scarcity of in vivo data, owing to limitations in the techniques used to detect fragments and immune cells, which are present at low concentrations and are short lived [85].
Numerous studies suggest that the IVD might endogenously include inflammatory-like cells [86,87]. In particular, it has been shown in vitro that a population of IVD cells can phagocytize beads and apoptotic bodies [86]. In turn, human surgical non-herniated nucleus pulposus (NP) samples presented a high number of resident CD68+ cells [87]. Furthermore, a recent robust analysis of cytokine/chemokine expression profile of human NP cells has presented clear evidence that NP cells, or at least some of them, are producers of specific inflammation-associated molecules, even in basal conditions (non-degenerated NP) [88]. In addition, infiltrated leukocytes (CD11b-positive cells) were found even in prolapsed IVDs, where NP is supposedly intact and isolated from any vascular source of immune cells [88]. The question of whether these cells could be resident macrophages or macrophage-like cells remains. Although pleiotropic, cytokines and chemokines have three modes of action: (i) stimulating the production of other inflammatory mediators and MMPs, (ii) enhancing matrix degradation, and (iii) recruiting inflammatory cells and activating phagocytosis [89–94]. Together, these effects can contribute to disease progression in the IVD.
It should be stressed that extreme mechanical loading has also been shown to alter ECM properties (through proteinase activation) and promote inflammation, contributing to IVD degeneration [95]. Apart from in vitro studies, organ cultures of bovine caudal IVDs have shown that compression induces apoptosis, produces inflammatory mediators and alters matrix integrity, leading to development of the disease [96].
In a more advanced degenerative stage, the well contained and apparently ‘sealed’ NP (immune privileged) becomes exposed to immune cells, which, responding to an inflammatory stimulus, may arise from newly formed blood vessels that invade pathological clefts and tears found in the annulus fibrosus (AF). Nociceptive nerve fibre ingrowth also accompanies angiogenesis and is believed to be the origin of discogenic pain that actively contributes to LBP [97–100]. Indeed, while the probable sites for focal damage and inflammation are vertebral endplates and AF (the only sites where the IVD is vascularized) [101], the NP is capable of attracting leukocytes and increasing vascular permeability when implanted subcutaneously [100]. An increase in expression of some cytokines and MMPs in herniated IVD may occur when molecules seen as ‘non-self’ by immune cells become exposed. This may also be linked to the phenomenon of spontaneous regression or disappearance of extruded IVD fragments, which has been attributed to matrix degradation and phagocytosis by recruited/infiltrated macrophages [102–106]. In cases with transligamentous extrusion, which can occur when the NP is potentially more exposed to immune cells, regression occurs [107]. Furthermore, the survival rate of subcutaneously transplanted rat NP cells is higher in immunocompromized NOD mice, and both rat macrophages and NK cells lyse autologous NP cells in vitro, indicating that immune cell populations respond to NP tissues [108].
In the next sections, we will review observational, in vitro and in vivo studies of the inflammatory milieu in IVD.
3. Inflammatory key players in intervertebral disc
3.1. Observational studies
A range of cytokines have been found in human IVDs in varying amounts, depending on whether the IVD is healthy, degenerated or herniated. Table 3 groups by methodology (observational, in vitro or pathway analysis) some of the most important studies that have clarified which inflammatory factors are expressed during homeostasis and with degeneration. Importantly, the identity of the cells producing these mediators (i.e. NP cells, AF cells, native IVD cells only, native cells plus infiltrating inflammatory cells) is highlighted.
Table 3.
Inflammatory mediators found in the human IVD.
| mediator | condition | producing cells | references |
|---|---|---|---|
| inflammatory factors that are expressed during homeostasis | |||
| observational studies | |||
| TNF-α | post-mortem and non-degenerate samples | IVD cells | [24,71,109] |
| IL-1β | IVD cells | [71,109–112] | |
| IL-1α, IL-1Ra, IL-1RI, and ICE | NP and AF cells | [112] | |
| IL-6, IL-8, RANTES | AF and NP cells | [110] | |
| NGF | NP and AF cells in monolayer and alginate bead culture | [71,113] | |
| NGF receptor (trkA) | NP and AF cells in monolayer and alginate bead culture | [113] | |
| substance P | [71] | ||
| PLA2 | NP and AF cells | [114] | |
| CCL3 and CCL4 | IVD cells | [115] | |
| NOTCH | IVD cells | [109] | |
| MMPs | IVD cells | [71,92] | |
| inflammatory factors that are expressed with degeneration | |||
| observational studies | |||
| TNF-α | herniations (including subligamentous and transligamentous), protrusion, extrusion, sequestration, spondylosis, scoliosis, degenerated or discogenic pain | IVD cells and infiltrating cells | [24,71,116–121] |
| IL-1β | [71,111,117,118,120,121] | ||
| IL-1α | [117–119] | ||
| IL1-Ra, NO | [118] | ||
| IL-6 | [17,117,118,122] | ||
| IL-8 | [17,119] | ||
| IL-12, IL-17, IFN-γ | [122] | ||
| IL-20 (and its receptors) | [90] | ||
| IL-10, TGF-β, RANTES | [119] | ||
| IL-16, CCL2, CCL7, CXCL8 | [88] | ||
| substance P | [71,118] | ||
| PGE2 | [17,118] | ||
| COX-2 | [121] | ||
| PLA2 | [114,121] | ||
| NGF | [71] | ||
| VEGF, bFGF | [116] | ||
| GM-CSF | [117] | ||
| CD20, CD45RO, CD68 | [107] | ||
| MMPs | [71,107,116,118] | ||
| in vitro studies | |||
| TNF-α | degenerate, sciatica, discogenic pain, extrusion, sequestration | IVD cells and infiltrating cells treated with different inflammatory stimulus (including IL-1β, TNF-α, substance P, IL-17, IL-20, IFN-γ, LPS) in monolayer or three-dimensional cell culture, or co-cultured with macrophage-like cells, or exposed to high mechanical strain | [95,121,123–125] |
| IL-1β | discogenic pain and post-mortem | [110,112,124–126] | |
| IL-1α, IL-1Ra, IL-1RI, and ICE | degenerate and post-mortem | [112] | |
| IL-6 | discogenic pain, scoliosis, sciatica, extrusion, sequestration, degenerate, myelopathy or radiculopathy and post-mortem | [89,90,95,110,124,126–129] | |
| IL-8 | discogenic pain, scoliosis, sciatica, extrusion, sequestration, degenerate, myelopathy or radiculopathy and post-mortem | [90,95,110,124,126–128] | |
| IL-17A | protrusion, extrusion and scoliosis | [123] | |
| IL-15, IFN-γ, CXCL9, TLR-2, TLR-4, MCP-3 | post-mortem | [95] | |
| RANTES | discogenic pain and post-mortem | [110] | |
| MCP-1 | discogenic pain, scoliosis, sciatica, extrusion, sequestration and post-mortem | [90,95,127] | |
| TFGF-β1 | discogenic pain, scoliosis, sciatica and post-mortem | [95,127] | |
| substance P | myelopathy or radiculopathy | [126] | |
| bFGF | scoliosis, sciatica and discogenic pain | [127] | |
| PGE2 | degenerate, scoliosis, sciatica and discogenic pain | [89,121,128–130] | |
| COX-2 | extruded and sequestrated IVD tissue | [121] | |
| NGF | post-mortem | [95,113] | |
| NGF receptor (trkA) | post-mortem | [113] | |
| PGF2α | degenerate | [128] | |
| NO | degenerate, scoliosis | [89,124,129,130] | |
| ICAM-1 (CD54) | degenerate and scoliosis | [89] | |
| MMPs | degenerate, non-degenerate, extrusion and sequestration | [90,92,112,130] | |
| NOTCH | protrusion | [109] | |
| pathway analysis | |||
| NF-κB, MAPK and C/EBPβ→ CCL3 | discectomy | IVD cells and infiltrating cells | [115] |
| NF-κB→ADAMTS-4 and -5 | DDD and myelopathy | NP and infiltrating cells treated with IL-1β and TNF-α | [131] |
| NF-κB→Sox9 and collagen type II | spine trauma | IVD cells treated with IL-1 | [132] |
| NF-κB and MAPK→NOTCH pathway | protrusion | NP cells | [109] |
3.1.1. Post-mortem samples
Separating NP from AF tissue upon discectomy is a very challenging task, particularly when dealing with degenerated human IVD tissue. In cases of disc herniation (e.g. extruded, sequestered), the IVD is invaded by other cell types, confounding analysis of molecules released by regions of the IVD. Samples taken post-mortem, which are not contaminated by infiltrating inflammatory cells (or at least not to the same extent as herniated discs), are therefore superior when investigating IVD homeostasis. For instance, TNF-α was substantially expressed in autopsy material in fetal/infantile and older adult NP, whereas it was sparsely expressed in adolescent and young adult NP. It was not found in the AF of young adults (below 25 years), but significantly increased in older individuals [24]. Also, calcium-dependent phospholipase A2 (PLA2), a regulator of prostaglandin E2 (PGE2) production, has been found in both cadaveric and surgical samples, and IVDs of middle-aged cases had higher PLA2 activity than those of younger and older subjects, indicating an important physiological role in maintaining homeostasis [114]. Like TNF-α or PLA2, many other inflammatory key players have been localized in non-degenerated human IVD tissue (table 3). Importantly, IVD native enzyme activity has also been studied. It was shown in the intact IVD that IL-1 is a key cytokine mediating IVD matrix degradation, by measuring enzyme activity (in situ zymography (ISZ)) against gelatin, collagen II and casein matrices [92]. Also, MMP-10 expression (at mRNA and protein levels) was increased in the symptomatic degenerate IVD, when compared to non-symptomatic one—possibly contributing to matrix degradation and initiation of nociception [71]. An additional perspective is given by studies that identified factors not naturally produced by native IVD cells: immunoreactivity for IL-4, IL-6, IL-12 and interferon (IFN)-γ was modest in surgical IVD tissue, being higher in herniated IVD samples and virtually non-existent in the control samples taken from post-mortem non-degenerated IVDs [122]. The majority of these reports used post-mortem IVD samples as healthy controls, exposing the role of IVD native cells in IVD homeostasis. This knowledge is of potential interest for the development of endogenous therapeutic routes to restore homeostasis in DDD.
3.1.2. Degenerated samples
Regarding human degenerated IVD samples, early studies detected the presence of IL-1, intracellular adhesion molecule-1 (ICAM-1), lymphocyte function-associated antigen (LFA) and fibroblast growth factor (FGF) [133]. Immunoreactivity for some cytokines (IL-4, IL-6, IL-12, IFN-γ, TNF-α) seemed modest but evident in herniated and degenerated discs, with substantial macrophage infiltration [116,122]. Also, pathologic discs highly expressed IL-17, suggesting the involvement of Th17 lymphocytes in disc herniation [123]. Others have shown a higher expression of TNF-α, IL-1β, IL-6, IL-8, IL-20, PGE2 and nitric oxide (NO) in herniated discs [17,90,117]. Some inflammatory key players have also been associated with pain in human IVD: RANTES and IL-1β expression was significantly higher in painful versus painless discs, contrarily to IL-6 and IL-8 [110]. A strong difference was observed in the levels of nerve growth factor (NGF), neurofilament-68, growth-associated protein (GAP)-43, and substance P in invading nerve fibers, in and around the outer layer of uncontained herniated versus spondylotic IVDs [116]. Another study that evaluated 91 cytokine- and chemokine-associated genes in human NP cells showed that NP cells are a source of IL-16, CCL2, CCL7 and CXCL8 [88]. Some of the pro-inflammatory cytokines usually present at increased levels in human degenerated discs, such as IL-1β and TNF-α, may mediate catabolic effects, decreasing proteoglycan production and enhancing MMP expression [71,111,134,135].
3.2. In vitro studies
Different in vitro studies have focused on studying the sources and role of some inflammatory mediators associated with herniated and degenerated IVD. An increase in IL-6, IL-8 and PGE2 was observed in control and degenerated human IVD tissues upon lipopolysaccharide (LPS) stimulation [127]. Furthermore, it was shown that substance P, expressed by IVD cells, upregulates IL-1β, IL-6 and IL-8 in both NP and AF, and RANTES and TNF-α in AF only [126]. Also, NP cells were shown to express the CCL3 ligand (also known as macrophage inflammatory protein (MIP)-1α), which is well known for its chemotactic and pro-inflammatory effects, through activation of the MAPK, NF-κB and C/EBP signalling pathways after treatment with IL-1β or TNF-α [115]. These studies suggest a contribution of native IVD cells to the inflammatory milieu. However, others have defended the hypothesis that immune cells, such as macrophages, neutrophils and T cells, can be recruited to degenerated IVD [135]. This hypothesis is supported by evidence that Th17 cells expressing CCR6 are recruited to degenerated IVD by CCL20 secretion from NP [123], and that macrophages can migrate after stimulation with conditioned medium from rat NP cells treated with IL-1β or TNF-α [115].
TNF-α, which is one of the most studied pro-inflammatory molecules, is known to promote aggrecan degradation, disc catabolism and expression of pro-inflammatory cytokines and NGF, without any recovery [136]. TNF-α is an adipokine that has been associated with higher numbers of bovine IVD senescent cells and is therefore implicated with the inability of degenerated IVD to repopulate by itself [137]. Curiously, although many studies have focused on the role of TNF-α in IVD degeneration [136], Hoyland and co-authors suggest instead that IL-1β is the key regulator of matrix degradation in degenerated IVD: IL-1 has greater expression in the IVDs clinically associated with chronic LPB and treatments against IL-1β were shown to inhibit matrix degradation [92]. IL-1 is upregulated in degenerated human discs, inducing MMP7, MMP13 and ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs)—suggesting a deregulation of the normal IVD homoeostasis [138]. TNF-α blockers had no effect on matrix-degrading activity, suggesting that its upregulation in DDD is not associated with matrix degradation [92] but instead with neighbouring nerve root irritation, which is confirmed by other studies [134,139]. Hence, TNF-α might be contributing to discogenic pain in cases where nerve ingrowth into IVD degenerative fissures occurs [140,141].
3.3. In vivo studies
Although some IVD degeneration related inflammatory mediators identified in vitro have not yet been studied in vivo, recent evidence has shed light on the role of several molecules. The majority of studies have been conducted in rat, rabbit and porcine animal models.
In a rat animal model of IVD herniation, NP exposure led to increased IL-6, TNF-α and IFN-γ levels. Other cytokines (IL-1β, IL-10, IL-1α and IL-2), already increased by the surgical procedure, were not altered [142]. In another study, TNF-α was identified in rat herniated IVDs and associated with radicular pain [139]. Also, a rat model of caudal annular incision demonstrated a transient peak in IL-1β 4 days following injury. This model was characterized by NP size decrease, annular collagen layer disorganization, and cellular metaplasia of annular fibroblasts to chondrocyte-like cells. However, no significant changes in TNF-α or IL-6 were seen [143]. In a lumbar rabbit annular incision model, no alteration in IL-1α or TNF genes was observed in whole IVDs at either one or three weeks after injury [144]. In the same model, IL-1β, transforming growth factor (TGF)-β1 and iNOS (inducible nitric oxide synthase) gene expression increased after three weeks, but decreased between weeks 6 and 12, having a second peak at 24 weeks—possibly showing a long-term pro-inflammatory action [145]. In a rabbit model of IVD herniation, the presence of TNF-α, IL-1β and MCP-1 (which has been demonstrated to be a potent macrophage chemoattractant [146]) was also analysed: TNF-α and IL-1β were detected after day 1 (via immunohistochemistry) followed by MCP-1 3 days post-injury. Infiltrating cells, mainly macrophages, were also observed after day 3 [147]. Interestingly, in a lumbar porcine model of annular incision, a significant increase in IL-8 accompanied by a decrease in IL-1 was observed in IVDs subjected to discectomy at 12 weeks post-injury, while no difference was observed in disc morphology, proteoglycan content, or in levels of IL-6 and TNF-α expression between untreated and injured IVDs. Whereas both IL-1 and IL-8 have pro-inflammatory properties, the authors propose that such discectomy procedure may be capable of initiating a repair response in the IVD, given that expression of IL-8 (an anabolic agent) is increased when the catabolic IL-1 decreases [148].
The TLR4-ligand LPS triggered inflammation when injected in a rat caudal IVD [149]. LPS injection in a rat IVD led to an increase in the levels of IL-1β, TNF-α, HMGB1 (high-mobility group box 1) and MIF (macrophage migration inhibitory factor), which correlated with morphological changes in tissue organization, namely interruption of NP/AF border, contraction of NP shape and decrease of IVD height [149]. Recently, different components of the tissue renin-angiotensin system (tRAS) (angiotensin converting enzyme, Ang II, Ang II receptor type 1, Ang II receptor type 2 and cathepsin D), that contribute to inflammation in many organs, have been found for the first time in the normal rat IVD, at both mRNA and protein levels [150]. However, the association between tRAS and IVD degeneration and its relationship to IVD inflammation has yet to be elucidated.
Most of these in vivo studies mainly identify and quantify inflammatory mediators in IVD, but fail to produce a mechanistic explanation of their role in either IVD degeneration or regeneration. One of the reasons for this failure may be the difficulty of unravelling the complex inflammatory mechanisms in injury models of IVD pathology. In this context, animal models of spontaneous IVD degeneration such as the sand rat [151] and both younger chondrodystrophic (with cervical or thoracolumbar IVD disease) and older non-chondrodystrophic (caudal cervical or lumbosacral IVD disease) dogs [152,153] could bring new insights to the clinic. However, as far as we are aware, the inflammatory response has not been addressed in these models. Importantly, differences between species could also bring some intricacy to this issue. For instance, notochordal cells (NC) seem to disappear in the adult human IVD, while in many other species they are retained throughout adulthood [154]. As more models become available, it is central to translate information between species and to interpret the models appropriately to understand in greater depth the process of inflammation.
4. Strategies to target and modulate inflammation towards intervertebral disc regeneration
Promoting IVD regeneration relies on restoring naive IVD properties by: (i) recovering IVD biomechanics, (ii) re-establishing cell biological activity, including production of healthy ECM, and (iii) reducing IVD-associated pain. Biological approaches focusing on IVD regeneration or IVD-associated pain relief begun in the early 1990s, and have since increased in number and diversity as reviewed elsewhere [4].
A well-balanced approach supporting tissue regeneration and control of inflammatory response could be successful in reducing IVD-associated pain. Although the molecular mechanisms behind IVD pathology and inflammatory response remain to be elucidated in detail, some inflammatory-related molecules are key targets of novel therapies in DDD [155,156]. In this section, an overview of the strategies targeting inflammatory mediators towards IVD regeneration will be given.
4.1. Injection of molecules
The more direct approaches to control inflammation in IVD are to inject regulating agents close to the IVD. Example agents are TNF-α blockers, such as infliximab, adalimumab, etanercept [136,157–160], or IL-1 inhibitors, such as IL-1 receptor antagonist (IL-1Ra) [161]. Other TNF-α blockers include a monoclonal antibody tested in herniated IVD patients, who showed less leg and back pain after antibody administration [162], and a p38-TNF-α inhibitor, which was tested in the spine to address neuroinflammation but not specifically for IVD [163]. Other strategies involve injecting corticosteroids into the IVD [164], or the anti-cholesterolemic drug simvastatin [165], which appeared to retard IVD degeneration in animal models.
The therapeutic potential of IL-1Ra for sustained attenuation of IL-1β has also been explored using poly(lactic-co-glycolic acid) (PLGA) microspheres as a delivery system [166]. IL-1Ra-PLGA microspheres inhibited NO production in NP cell cultures and partially restored the levels of iNOS, ADAMTS-4, MMP-13, IL-1β, IL-6 and TLR-4, which were increased in the presence in IL-1β [166].
Another candidate to control inflammation in IVDs is COX-2, which regulates PGE2 synthesis in inflammatory conditions. Epidural injection of COX-2 inhibitors was shown to reduce pain in a rat model of IVD herniation [167]. Another approach uses platelet-rich plasma (PRP) as a therapy for degenerated IVD [168]—PRP was able to rescue chondrocyte degeneration induced by IL-1β and TNF-α [169].
Other approaches to reduce IVD-associated pain have been suggested. Resveratrol, a naturally occurring polyphenol present in red wine, was able to reduce IL-6, IL-8, MMP1, MMP3 and MMP13 expression when injected into the IVD [170]. Rhein (4,5-dihydroxyanthraquinone-2-carboxylic acid), an anthraquinone molecule derived from the rhizome of Rheum palmatum that exhibits anti-inflammatory activity and is used in the treatment of osteoarthritis and pain relief, was hypothesized to be a therapeutic agent for IVD through the regulation of IL-1 activity [171]. Fullerol, a derivative from fullerene and known anti-oxidant, retards cellular apoptosis and suppresses dorsal root ganglion (DRG) and neuron TNF-α-induced inflammatory responses, which when incorporated into nanoparticles is relevant for LBP treatment [172].
A very recent and promising approach to target inflammation in IVD is the inhibitor of IκB kinase-β (IKKβ), involved in NF-κB activation. The intradiscal injection of IKKβ reduced the levels of TNF-α, IL-1β and IL-6 of an injured IVD while suppressing high levels of neuropeptides within DRG neurons [173].
Despite good results in other therapeutic areas, injected molecules might be inefficient in DDD due to the short half-life of proteins in solution and the limited effect of a single protein in a complex process such as IVD degeneration [174]. Also, the risk of inducing IVD degeneration by puncturing of the IVD should be considered [175], although more recent studies describe alternative routes for molecule delivery [176]. Furthermore, given the predominantly avascular nature of IVD, systemic delivery of soluble molecules is unlikely to be effective in this situation. This view is supported by the report that the concentration of antibiotics in IVDs was undetectable in the NP of patients with IVD infection under systemic administration of antibiotics [177]. In addition, it has been shown that the rate of diffusion of antibiotics into the IVD is reduced by endplate calcification, increase in IVD size and solute molecular weight [178,179]. Moreover, although some nutrients' diffusion to the NP may occur via the endplates, the short half-life of pharmaceutical drugs or proteins can result in limited therapeutic doses that reach the NP [166].
4.2. Gene therapy
Gene therapy promises more prolonged effects in DDD, by introducing the possibility of locally modulating the expression of a specific gene and the consequent production of its protein [174]. As early as 1997, a study suggested genetic modifications as a therapy for DDD [180]. At that point, a retrovirus vector was proposed to transduce bovine chondrocytic endplate cells with IL-1Ra [180]. Cell transfection resulted in IL-1Ra production in 48 h, and injection of transfected cells in degenerated NP explants considerably reduced expression of several enzymes (such as MMP3) for two weeks after injection. This strategy aims at decreasing IL-1 mediated matrix degradation and stopping DDD progression [181]. In vivo, TGF-β1 transfection of rabbit IVD cells also enhanced proteoglycan synthesis for six weeks [182]. In agreement with this result, cells from human degenerated IVD transfected with TGF-β1 increased both proteoglycan and collagen production [183,184].
Gene therapy in a clinical setting may be limited by the safety of the gene transfer vector. Aspects such as exposure to high doses, long-term use, misplaced injections and the possibility of oncogenesis are key concerns when treating a chronic disease like DDD [185]. Progress in the development of more reliable viral vector constructs and in a better control of transgene expression would improve the safety of these therapies. Also, elucidation of molecular mechanisms behind the degenerative process and characterization of cell populations in IVD, as well as their role in ECM production, could bring new advancements to this field [174].
4.3. Cell-based therapies
Several cell-based therapies to stimulate IVD regeneration have been proposed in recent years: haematopoietic stem cells (HSC) [186], fetal spine cells [187], immortalized NP-cell lines [188], autologous IVD chondrocytes [189], embryonic stem cells (SC) [190], induced pluripotent SC [191], olfactory SC [192] and MSCs (derived either from bone marrow [193] or from umbilical cord blood [194]) have all been suggested to have potential for IVD repair/regeneration. NP progenitor cells were isolated from the NP (with approximately 1% frequency) and differentiated into chondrogenic and neurogenic lineages, suggesting potential for IVD regeneration [195]. Besides IVD regeneration, progenitor cells might play a protective role in regulating inflammation in IVD: rabbit NC were shown to reduce the levels of pro-inflammatory cytokines, IL-6 and IL-8, as well as iNOS, in in vitro co-cultures of AF cells with macrophages [196].
MSCs are one of the most attractive candidate cell types for IVD regeneration, partly because they could be autologous transplants. In a canine model, MSCs were able to increase collagen type II expression while decreasing cell apoptosis in IVD [197]. In rabbits, MSCs were able to remain in the IVD up to 24 weeks [198]. However, the number of transplanted MSCs is crucial; in the canine model 106 MSCs per IVD was ideal, since 105 MSCs resulted in decreased cell viability while 107 MSCs induced cell apoptosis [197]. Besides MSC multi-differentiation capacity, an associated immuno-modulatory effect has been suggested [199]. MSC role in inflammation is based on their active role as cytokine-release factories that interact directly with injured cells [200]. In this novel scenario, MSCs were shown to secrete IL-1Ra in a mouse model of lung injury [201] or produce a potent anti-inflammatory protein (TNF-α stimulated gene/protein 6, TSG-6) in a mouse infarct model [202]. Interestingly, TSG-6 was also identified as a key player in a rat model of corneal injury after MSC systemic administration [203]. After implantation of MSCs into beagle nucleotomized IVDs, the expression of Fas ligand (FasL) (a protein found in other immune privileged sites) was restored. It was suggested that MSCs either differentiated into cells expressing FasL, or stimulated the few remaining NP cells to produce this molecule—thus contributing to the recovery of immune privilege in degenerated IVDs [204]. Although the beneficial effects of these cells have been demonstrated in several models, the mechanisms behind MSC-based therapies are not clear.
In vitro studies showed that MSCs repress IgG production of peripheral blood lymphocytes co-cultured with IVD fragments from the same donors [205]. MSC influence in IVD inflammatory response has not been fully dissected until now due to its multi-factorial complexity and time dependence [205]. Human MSCs were able to downregulate gene expression of pro-inflammatory cytokines (IL-3, IL-6, IL-11, IL-15, TNF-α) and MMPs when in co-culture with rat NP cells [198].
In humans, two clinical trials took advantage of autologous MSCs, albeit with controversial results. MSCs were either directly injected in NP [206] or implanted in the IVD after seeding in collagen sponges [207]. In the first case, when MSCs were directly injected in patients diagnosed with DDD, but with preserved external AF and persistent LBP, the lumbar pain was strongly reduced after three months. However, no improvement on IVD height was detected by imaging [206]. Injection of MSCs in degenerated IVDs seems to promote an analgesic effect, due to trophic effects, which can occur quicker than detecting possible regenerative effects [206]. Given this, the authors suggest that the MSCs exhibited immuno-modulatory properties. The second case reports the implantation of MSCs after seeding in collagen sponges [207]. Two years post-surgery, the published results reported relief or disappearance of LBP and improvement of the vacuum phenomenon (gas in the intervertebral space, associated with intervertebral regressive degeneration). However, besides the small number of patients used (two), this study also lacks experimental details such as the controls used and effective number of cells transplanted [207]. More recent studies propose IVD injection of umbilical cord-derived MSCs as a promising therapy to overcome chronic discogenic LBP [208]. In this study, pain and lumbar function were recovered after cell transplantation and preserved over a 2-year follow-up period; however, only two patients were studied. Another recent study injected bone marrow concentrate cells into 26 patients [209]. In the 1-year follow-up study, the majority of the patients showed improvement of pain score and reduced impairment, with only some of them presenting IVD rehydration. The authors emphasize the use of critical unmanipulated cell doses. Usually, MSC-based therapies involve cell expansion in vitro to obtain sufficient cell numbers, but this in vitro manipulation risks modifying their receptor expression and can introduce contaminants.
One interesting feature of MSCs is their capacity to migrate into injured tissues and participate in the regenerative process, interacting with the surrounding environment through secretion of numerous molecules such as growth factors, cytokines and chemokines [210]. Nevertheless, contrary to leukocyte migration and haematopoietic SC homing, the mechanisms that regulate MSC migration to injured sites are not well characterized [211]. In vitro, in a pro-inflammatory environment stimulated by TNF-α, MSCs migrate towards SDF-1, RANTES and MDC gradients, amongst others [212]. Furthermore, MMPs and their inhibitors have also been shown to enhance MSC migratory capacity [213]. In a recent study, MSCs were recruited in vitro by conditioned medium from IVDs cultured under degenerative-simulated conditions [214]. CCL5/RANTES has been identified as a key chemoattractant released by degenerative IVD in organ culture [215]. Moreover, CXCL12/SDF-1 delivery in IVD organ cultures promotes MSC recruitment towards NP, especially if MSCs were harvested from young donors [216]. This does not exclude the possibility that other cytokines involved in IVD degeneration pathogenesis, namely TNF-α and IL-1β, might play a role in regulation of MSC recruitment to the IVD [181]. A hypothetical migration route of high-proliferative cells lateral to the epiphyseal plate and the outer border region of the IVD was recently described [217]—if this route is confirmed, new strategies envisaging IVD regeneration may be attempted.
5. Future perspectives
Recent findings from in vitro studies, animal models and clinical trials have started to unveil the role of inflammation in IVD degeneration. However, no evidence for a beneficial role of inflammation in maintaining homeostasis has been presented, owing to the difficulty in studying IVD tissue homeostasis. In other tissues, such as bone [218,219] or cardiovascular tissue [220], the control of inflammation has already proven to be critical in shifting the degeneration/regeneration balance towards regeneration. In particular, our group has focused on modulating inflammation in bone [5,221–224]. Hence, we believe that novel therapies for DDD should aim at restoring the homeostatic inflammatory conditions in the disc, rather than totally inhibiting inflammation, thus enabling endogenous repair mechanisms to operate.
Our group has recently shown that incorporating fibrinogen, a well-known inflammatory protein, into a biomaterial leads to increased bone formation [5]. In vitro studies have shown that fibrinogen-modified biomaterial stimulates NK cell-mediated MSC recruitment without affecting the MSC differentiation marker alkaline phosphatase [222]. Moreover, a broad analysis of macrophage-secreted factors showed that fibrinogen modified macrophage response, leading to a downregulation of the expression of inflammatory cytokines and a stimulation in the production of growth factor [224]. Factors such as MIP-1δ, platelet-derived growth factor-BB, bone morphogenetic protein (BMP)-5 and BMP-7 were significantly promoted by fibrinogen [224], which may impact tissue regeneration.
Recent advances in development biology also highlight the crucial role of immune cells. An efficient nuclear reprogramming to obtain induced pluripotent stem (iPS) cells was shown to require activation of an innate response [225] and was achieved via activation of TLR3 in the work of Yamanaka and colleagues [226]. The importance of this finding in physiological situations remains unclear, but it is becoming increasingly evident that activation of the immune response, particularly the innate response, may contribute to regulation of stem cell behaviour [225,227]. Moreover, it has been known for a long time that the post-inflammatory wound repair process recapitulates basic phenomena that occur during embryogenesis [228]. Therefore, future studies should focus on trying to understand what happens early in development, to discover more cues on how to modulate inflammation in a disease situation.
Although diverse studies have presented data on inflammatory key players in IVD, the inherent variability and contradictions arising from the different in vitro culture conditions and animal models used in these studies may be hampering translation of the research to a clinical setting. The standardization of methods, the correlation of results with different IVD clinical problems, the use of alternative ex vivo models (based on organotypic cultures or bioreactors) and the use of more physiologically accurate in vivo models of IVD degeneration could bring further advances to the IVD research field.
For IVD regeneration therapies to succeed, it will be important to address IVD degeneration together with inflammation. Until now, most studies have focused on only one of these two aspects. An integrated strategy, which addresses both the synergistic interplay that exists between the multiple factors associated with IVD degeneration and balances the inflammatory response, could be a step closer to the success of IVD regenerative strategies and bring relief for those suffering from LBP.
Glossary
- ADAMTS
A disintegrin and metalloproteinase with thrombospondin motifs
- AF
Annulus fibrosus
- BMP
Bone morphogenic protein
- C/EBPβ
CCAAT/enhancer-binding protein beta
- CCL
Chemokine ligand
- CEP
Cartilaginous endplates
- COX
Cyclooxygenase
- CPPD
Calcium pyrophosphate dihydrate
- CXCL9
Monokine induced by gamma interferon
- DDD
Degenerative disc disease
- DRG
Dorsal root ganglion
- ECM
Extracellular matrix
- FasL
Fas ligand
- FGF
Fibroblast growth factor
- fHA
Hyaluronic acid fragments
- GAG
Glycosaminoglycan
- GAP
Growth-associated protein
- GDF-1
Growth and differentiation factor-1
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- HA
Hyaluronic acid
- HMGB1
High-mobility group protein box 1
- HSC
Haematopoietic stem cells
- ICAM-1
Intracellular adhesion molecule-1
- ICE
IL-1β-converting enzyme
- IFN-γ
Interferon-γ
- IKKβ
3-Phosphoinositide-dependent protein kinase-1-mediated IκB kinase β
- IL
Interleukin
- IL-1Ra
Interleukin-1 receptor antagonist
- iNOS
Inducible nitric oxide synthase
- iPS
Induced pluripotent stem cells
- IVD
Intervertebral disc
- LBP
Low back pain
- LFA
Lymphocyte function-associated antigen
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinases
- MCP
Monocyte chemoattractant protein
- MDC
Macrophage-derived chemokine
- MIF
Macrophage migration inhibitory factor
- MIP
Macrophage inflammatory protein
- MMP
Metalloproteinase
- mRNA
Messenger ribonucleic acid
- MSC
Mesenchymal stromal cells
- NALP3
NACHT, LRR and PYD domains-containing protein 3
- NC
Notochordal cells
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NGF
Nerve growth factor
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- NO
Nitric oxide
- NP
Nucleus pulposus
- PGE2
Prostaglandin E2
- PGF2α
Prostaglandin F2 alpha
- PLA2
Calcium-dependent phospholipase A2
- PLGA
Poly(lactic-co-glycolic acid)
- PRP
Platelet-rich plasma
- RANTES
Regulated on activation, normal T-cell expressed and secreted
- Rhein
4,5-Dihydroxyanthraquinone-2-carboxylic acid
- SC
Stem cells
- Sox-9
Transcription factor Sox-9
- TGF
Transforming growth factor
- TGS-6
TNF-α stimulated gene/protein 6
- TIMP
Tissue inhibitor of metalloproteinase
- TLR
Toll-like receptor
- TNF-α
Tumour necrosis factor alpha
- TrkA
High affinity NGF receptor
- tRAS
Tissue renin-angiotensin system
References
- 1.Andersson GB. 1999. Epidemiological features of chronic low-back pain. Lancet 354, 581–585. ( 10.1016/S0140-6736(99)01312-4) [DOI] [PubMed] [Google Scholar]
- 2.Schwarzer AC, Aprill CN, Derby R, Fortin J, Kine G, Bogduk N. 1995. The prevalence and clinical features of internal disc disruption in patients with chronic low back pain. Spine (Phila Pa 1976) 20, 1878–1883. ( 10.1097/00007632-199509000-00007) [DOI] [PubMed] [Google Scholar]
- 3.Koes BW, Van Tulder MW, Peul WC. 2007. Diagnosis and treatment of sciatica. Br. Med. J. 334, 1313–1317. ( 10.1136/bmj.39223.428495.BE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hughes SP, Freemont AJ, Hukins DW, Mcgregor AH, Roberts S. 2012. The pathogenesis of degeneration of the intervertebral disc and emerging therapies in the management of back pain. J. Bone Joint Surg. Br. 94, 1298–1304. ( 10.1302/0301-620X.94B10.28986) [DOI] [PubMed] [Google Scholar]
- 5.Santos SG, et al. 2013. Adsorbed fibrinogen leads to improved bone regeneration and correlates with differences in the systemic immune response. Acta Biomater. 9, 7209–7217. ( 10.1016/j.actbio.2013.04.008) [DOI] [PubMed] [Google Scholar]
- 6.Sun Z, Zhang M, Zhao XH, Zhao ZH, GAO Y, Samartziz D, Wang HQ, Luo ZJ. 2013. Immune cascades in human intervertebral disc: the pros and cons. Int.J. Clin. Exp. Pathol. 6, 1009–1014. [PMC free article] [PubMed] [Google Scholar]
- 7.Medzhitov R. 2008. Origin and physiological roles of inflammation. Nature 454, 428–435. ( 10.1038/nature07201) [DOI] [PubMed] [Google Scholar]
- 8.Urban JP, Smith S, Fairbank JC. 2004. Nutrition of the intervertebral disc. Spine (Phila Pa 1976) 29, 2700–2709. ( 10.1097/01.brs.0000146499.97948.52) [DOI] [PubMed] [Google Scholar]
- 9.Moore RJ. 2006. The vertebral endplate: disc degeneration, disc regeneration. Eur. Spine J. 15(Suppl. 3), S333–S337. ( 10.1007/s00586-006-0170-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2005. 2004 Young Investigator Award winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine (Phila Pa 1976) 30, 167–173. ( 10.1097/01.brs.0000150833.93248.09) [DOI] [PubMed] [Google Scholar]
- 11.Adams MA, Roughley PJ. 2006. What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31, 2151–2161. ( 10.1097/01.brs.0000231761.73859.2c) [DOI] [PubMed] [Google Scholar]
- 12.Battie MC, Videman T, Kaprio J, Gibbons LE, Gill K, Manninen H, Saarela J, Peltonen L. 2009. The Twin Spine Study: contributions to a changing view of disc degeneration. Spine J. 9, 47–59. ( 10.1016/j.spinee.2008.11.011) [DOI] [PubMed] [Google Scholar]
- 13.Cook CE, Taylor J, Wright A, Milosavljevic S, Goode A, Whitford M. 2013. Risk factors for first time incidence sciatica: a systematic review. Physiother. Res. Int. 19, 65–78. ( 10.1002/pri.1572) [DOI] [PubMed] [Google Scholar]
- 14.Cheung KM, Karppinen J, Chan D, Ho DWH, Song Y-Q, Sham P, Cheah KSE, Leong JCY, Luk KDK. 2009. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand forty-three individuals. Spine (Phila Pa 1976) 34, 934–940. ( 10.1097/BRS.0b013e3181a01b3f) [DOI] [PubMed] [Google Scholar]
- 15.Hristova GI, Jarzem P, Ouellet JA, Roughley PJ, Epure LM, Antoniou J, Mwale F. 2011. Calcification in human intervertebral disc degeneration and scoliosis. J. Orthop. Res. 29, 1888–1895. ( 10.1002/jor.21456) [DOI] [PubMed] [Google Scholar]
- 16.Desanto J, Ross JS. 2011. Spine infection/inflammation. Radiol. Clin. N. Am. 49, 105–127. ( 10.1016/j.rcl.2010.07.018) [DOI] [PubMed] [Google Scholar]
- 17.Burke JG, Watson RW, Mccormack D, Dowling FE, Walsh MG, Fitzpatrick JM. 2002. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J. Bone Joint Surg. Br. 84, 196–201. ( 10.1302/0301-620X.84B2.12511) [DOI] [PubMed] [Google Scholar]
- 18.Lee RS, Kayser MV, Ali SY. 2006. Calcium phosphate microcrystal deposition in the human intervertebral disc. J. Anat. 208, 13–19. ( 10.1111/j.1469-7580.2006.00504.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruber HE, Norton HJ, Sun YB, Hanley EN. 2007. Crystal deposits in the human intervertebral disc: implications. Spine J. 7, 444–450. ( 10.1016/j.spinee.2006.08.015) [DOI] [PubMed] [Google Scholar]
- 20.Feinberg J, Boachie-Adjei O, Bullough PG, Boskey AL. 1990. The distribution of calcific deposits in intervertebral discs of the lumbosacral spine. Clin. Orthop. Relat. Res. 254, 303–310. [PubMed] [Google Scholar]
- 21.Kalya S, Rosenthal AK. 2005. Extracellular matrix changes regulate calcium crystal formation in articular cartilage. Curr. Opin. Rheumatol. 17, 325–329. ( 10.1097/01.bor.0000160783.14798.10) [DOI] [PubMed] [Google Scholar]
- 22.Masuda I, Ishikawa K, Usuku G. 1991. A histologic and immunohistochemical study of calcium pyrophosphate dihydrate crystal deposition disease. Clin. Orthop. Relat. Res. 263, 272–287. [PubMed] [Google Scholar]
- 23.Stutz A, Golenbock DT, Latz E. 2009. Inflammasomes: too big to miss. J. Clin. Investig. 119, 3502–3511. ( 10.1172/JCI40599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiler C, Nerlich AG, Bachmeier BE, Boos N. 2005. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 30, 44–53; discussion 54 ( 10.1097/01.brs.0000174529.07959.c0) [DOI] [PubMed] [Google Scholar]
- 25.Noble PW. 2002. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 21, 25–29. ( 10.1016/S0945-053X(01)00184-6) [DOI] [PubMed] [Google Scholar]
- 26.Vaday GG, Lider O. 2000. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J. Leukoc. Biol. 67, 149–159. [DOI] [PubMed] [Google Scholar]
- 27.Eyre DR, Muir H. 1977. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim. Biophys. Acta 492, 29–42. ( 10.1016/0005-2795(77)90211-2) [DOI] [PubMed] [Google Scholar]
- 28.Eyre DR, Dickson IR, Van Ness K. 1988. Collagen cross-linking in human bone and articular cartilage. Age-related changes in the content of mature hydroxypyridinium residues. Biochem. J. 252, 495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivan SS, Hayes AJ, Wachtel E, Caterson B, Merkher Y, Maroudas A, Brown S, Roberts S. 2014. Biochemical composition and turnover of the extracellular matrix of the normal and degenerate intervertebral disc. Eur. Spine J. 23(Suppl. 3), S344–S353. ( 10.1007/s00586-013-2767-8) [DOI] [PubMed] [Google Scholar]
- 30.Eyre DR, Muir H. 1976. Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J. 157, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinckmann J, Notbohm H, Müller PK. 2005. Collagen: primer in structure, processing and assembly, vol. 247, p. 252 Berlin, Germany: Springer. [Google Scholar]
- 32.Ippolito E, Ponseti IV. 1981. Juvenile kyphosis: histological and histochemical studies. J. Bone Joint Surg. Am. 63, 175–182. [PubMed] [Google Scholar]
- 33.Gruber HE, Ingram JA, Hanley EN., Jr 2007. Morphologic complexity of the pericellular matrix in the annulus of the human intervertebral disc. Biotech. Histochem. 82, 217–225. ( 10.1080/10520290701713999) [DOI] [PubMed] [Google Scholar]
- 34.Roberts S, Menage J, Duance V, Wotton SF. 1991. Type III collagen in the intervertebral disc. Histochem. J. 23, 503–508. ( 10.1007/BF01041176) [DOI] [PubMed] [Google Scholar]
- 35.Culav EM, Clark CH, Merrilees MJ. 1999. Connective tissues: matrix composition and its relevance to physical therapy. Phys. Ther. 79, 308–319. [PubMed] [Google Scholar]
- 36.Clouet J, et al. 2009. Identification of phenotypic discriminating markers for intervertebral disc cells and articular chondrocytes. Rheumatology 48, 1447–1450. ( 10.1093/rheumatology/kep262) [DOI] [PubMed] [Google Scholar]
- 37.Tow BP, Hsu WK, Wang JC. 2007. Disc regeneration: a glimpse of the future. Clin. Neurosurg. 54, 122–128. [PubMed] [Google Scholar]
- 38.Mio F, et al. 2007. A functional polymorphism in COL11A1, which encodes the alpha 1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am. J. Hum. Genet. 81, 1271–1277. ( 10.1086/522377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts S, Ayad S, Menage PJ. 1991. Immunolocalisation of type VI collagen in the intervertebral disc. Ann. Rheum. Dis. 50, 787–791. ( 10.1136/ard.50.11.787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melrose J, Smith SM, Appleyard RC, Little CB. 2008. Aggrecan, versican and type VI collagen are components of annular translamellar crossbridges in the intervertebral disc. Eur. Spine J. 17, 314–324. ( 10.1007/s00586-007-0538-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyre DR, Wu JJ, Fernandes RJ, Pietka TA, Weis MA. 2002. Recent developments in cartilage research: matrix biology of the collagen II/IX/XI heterofibril network. Biochem. Soc. Trans. 30, 893–899. ( 10.1042/BST0300893) [DOI] [PubMed] [Google Scholar]
- 42.Brinckmann J, et al. 2005. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol. 24, 459–468. ( 10.1016/j.matbio.2005.07.002) [DOI] [PubMed] [Google Scholar]
- 43.Eyre DR, Matsui Y, Wu JJ. 2002. Collagen polymorphisms of the intervertebral disc. Biochem. Soc. Trans. 30, 844–848. ( 10.1042/BST0300844) [DOI] [PubMed] [Google Scholar]
- 44.Onnerfjord P, Khabut A, Reinholt FP, Svensson O, Heinegard D. 2012. Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J. Biol. Chem. 287, 18 913–18 924. ( 10.1074/jbc.M111.298968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson WE, Caterson B, Eisenstein SM, Roberts S. 2005. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine (Phila Pa 1976) 30, 1139–1147. ( 10.1097/01.brs.0000162624.95262.73) [DOI] [PubMed] [Google Scholar]
- 46.Gotz W, Barnert S, Bertagnoli R, Miosge N, Kresse H, Herken R. 1997. Immunohistochemical localization of the small proteoglycans decorin and biglycan in human intervertebral discs. Cell Tissue Res. 289, 185–190. ( 10.1007/s004410050864) [DOI] [PubMed] [Google Scholar]
- 47.Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. 1994. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem. J. 302, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reed CC, Iozzo RV. 2002. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 19, 249–255. ( 10.1023/A:1025383913444) [DOI] [PubMed] [Google Scholar]
- 49.Gruber HE, Ingram JA, Hoelscher GL, Zinchenko N, Hanley EN, Jr, Sun Y. 2009. Asporin, a susceptibility gene in osteoarthritis, is expressed at higher levels in the more degenerate human intervertebral disc. Arthritis Res. Ther. 11, R47 ( 10.1186/ar2660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kizawa H, et al. 2005. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat. Genet. 37, 138–144. ( 10.1038/ng1496) [DOI] [PubMed] [Google Scholar]
- 51.Chakravarti S. 2002. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj. J. 19, 287–293. ( 10.1023/A:1025348417078) [DOI] [PubMed] [Google Scholar]
- 52.Bengtsson E, Morgelin M, Sasaki T, Timpl R, Heinegard D, Aspberg A. 2002. The leucine-rich repeat protein PRELP binds perlecan and collagens and may function as a basement membrane anchor. J. Biol. Chem. 277, 15 061–15 068. ( 10.1074/jbc.M108285200) [DOI] [PubMed] [Google Scholar]
- 53.Haglund L, Ouellet J, Roughley P. 2009. Variation in chondroadherin abundance and fragmentation in the human scoliotic disc. Spine (Phila Pa 1976) 34, 1513–1518. ( 10.1097/BRS.0b013e3181a8d001) [DOI] [PubMed] [Google Scholar]
- 54.Akhatib B, Onnerfjord P, Gawri R, Ouellet J, Jarzem P, Heinegard D, Mort J, Roughley P, Haglund L. 2013. Chondroadherin fragmentation mediated by the protease HTRA1 distinguishes human intervertebral disc degeneration from normal aging. J. Biol. Chem. 288, 19 280–19 287. ( 10.1074/jbc.M112.443010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melrose J, Smith S, Ghosh P, Whitelock J. 2003. Perlecan, the multidomain heparan sulfate proteoglycan of basement membranes, is also a prominent component of the cartilaginous primordia in the developing human fetal spine. J. Histochem. Cytochem. 51, 1331–1341. ( 10.1177/002215540305101010) [DOI] [PubMed] [Google Scholar]
- 56.Oegema TR, Jr, Johnson SL, Aguiar DJ, Ogilvie JW. 2000. Fibronectin and its fragments increase with degeneration in the human intervertebral disc. Spine 25, 2742–2747. ( 10.1097/00007632-200011010-00005) [DOI] [PubMed] [Google Scholar]
- 57.Kadler KE, Hill A, Canty-Laird EG. 2008. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol. 20, 495–501. ( 10.1016/j.ceb.2008.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leiss M, Beckmann K, Giros A, Costell M, Fassler R. 2008. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr. Opin. Cell Biol. 20, 502–507. ( 10.1016/j.ceb.2008.06.001) [DOI] [PubMed] [Google Scholar]
- 59.Yu J, Fairbank JC, Roberts S, Urban JP. 2005. The elastic fiber network of the anulus fibrosus of the normal and scoliotic human intervertebral disc. Spine (Phila Pa 1976) 30, 1815–1820. ( 10.1097/01.brs.0000173899.97415.5b) [DOI] [PubMed] [Google Scholar]
- 60.Yu J, Winlove PC, Roberts S, Urban JP. 2002. Elastic fibre organization in the intervertebral discs of the bovine tail. J. Anat. 201, 465–475. ( 10.1046/j.1469-7580.2002.00111.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson EF, Chetty K, Moore IM, Stewart A, Jones W. 1982. The distribution and arrangement of elastic fibres in the intervertebral disc of the adult human. J. Anat. 135, 301–309. [PMC free article] [PubMed] [Google Scholar]
- 62.Ishii Y, Thomas AO, Guo XE, Hung CT, Chen FH. 2006. Localization and distribution of cartilage oligomeric matrix protein in the rat intervertebral disc. Spine (Phila Pa 1976) 31, 1539–1546. ( 10.1097/01.brs.0000221994.61882.4a) [DOI] [PubMed] [Google Scholar]
- 63.Rutges J, Creemers LB, Dhert W, Milz S, Sakai D, Mochida J, Alini M, Grad S. 2010. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage 18, 416–423. ( 10.1016/j.joca.2009.09.009) [DOI] [PubMed] [Google Scholar]
- 64.Gruber HE, Bornstein P, Sage EH, Ingram JA, Zinchenko N, Norton HJ, Hanley EN. 2008. Disruption of the thrombospondin-2 gene alters the lamellar morphology but does not permit vascularization of the adult mouse lumbar disc. Arthritis Res. Ther. 10, R96 ( 10.1186/ar2483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dicesare PE, Morgelin M, Mann K, Paulsson M. 1994. Cartilage oligomeric matrix protein and thrombospondin 1. Purification from articular cartilage, electron microscopic structure, and chondrocyte binding. Eur. J. Biochem. 223, 927–937. ( 10.1111/j.1432-1033.1994.tb19070.x) [DOI] [PubMed] [Google Scholar]
- 66.Le Maitre CL, Freemont AJ, Hoyland JA. 2004. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J. Pathol. 204, 47–54. ( 10.1002/path.1608) [DOI] [PubMed] [Google Scholar]
- 67.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. 2013. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 13, 331–341. ( 10.1016/j.spinee.2012.02.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur. Spine J. 11, 308–320. ( 10.1007/s00586-002-0472-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pockert AJ, Richardson SM, Le Maitre CL, Lyon M, Deakin JA, Buttle DJ, Freemont AJ, Hoyland JA. 2009. Modified expression of the ADAMTS enzymes and tissue inhibitor of metalloproteinases 3 during human intervertebral disc degeneration. Arthritis Rheum. 60, 482–491. ( 10.1002/art.24291) [DOI] [PubMed] [Google Scholar]
- 70.Nagase H. 2012. The ADAMTS family of metalloproteinases. In Extracellular matrix: pathobiology and signalling (ed. Karamanos N.). Berlin, Germany: De Gruyter. [Google Scholar]
- 71.Richardson SM, Doyle P, Minogue BM, Gnanalingham K, Hoyland JA. 2009. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res. Ther. 11, R126 ( 10.1186/ar2793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Le Maitre CL, Freemont AJ, Hoyland JA. 2006. Human disc degeneration is associated with increased MMP 7 expression. Biotech. Histochem. 81, 125–131. ( 10.1080/10520290601005298) [DOI] [PubMed] [Google Scholar]
- 73.Gruber HE, Ingram JA, Hanley EN., Jr 2005. Immunolocalization of MMP-19 in the human intervertebral disc: implications for disc aging and degeneration. Biotech. Histochem. 80, 157–162. ( 10.1080/10520290500387607) [DOI] [PubMed] [Google Scholar]
- 74.Feng H, Danfelter M, Stromqvist B, Heinegard D. 2006. Extracellular matrix in disc degeneration. J. Bone Joint Surg. Am. 88(Suppl. 2), 25–29. ( 10.2106/JBJS.E.01341) [DOI] [PubMed] [Google Scholar]
- 75.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. 2000. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976) 25, 3005–3013. ( 10.1097/00007632-200012010-00007) [DOI] [PubMed] [Google Scholar]
- 76.Roughley PJ. 2004. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine (Phila Pa 1976) 29, 2691–2699. ( 10.1097/01.brs.0000146101.53784.b1) [DOI] [PubMed] [Google Scholar]
- 77.Marom B, Rahat MA, Lahat N, Weiss-Cerem L, Kinarty A, Bitterman H. 2007. Native and fragmented fibronectin oppositely modulate monocyte secretion of MMP-9. J. Leukoc. Biol. 81, 1466–1476. ( 10.1189/jlb.0506328) [DOI] [PubMed] [Google Scholar]
- 78.Sztrolovics R, White RJ, Poole AR, Mort JS, Roughley PJ. 1999. Resistance of small leucine-rich repeat proteoglycans to proteolytic degradation during interleukin-1-stimulated cartilage catabolism. Biochem. J. 339, 571–577. ( 10.1042/0264-6021:3390571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arroyo AG, Iruela-Arispe ML. 2010. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc. Res. 86, 226–235. ( 10.1093/cvr/cvq049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Virri J, Sikk S, Gronblad M, Tolonen J, Seitsalo S, Kankare J, Karaharju EO. 1994. Concomitant immunocytochemical study of macrophage cells and blood vessels in disc herniation tissue. Eur. Spine J. 3, 336–341. ( 10.1007/BF02200147) [DOI] [PubMed] [Google Scholar]
- 81.Jiang D, Liang J, Noble PW. 2007. Hyaluronan in tissue injury and repair. Annu. Rev. Cell Dev. Biol. 23, 435–461. ( 10.1146/annurev.cellbio.23.090506.123337) [DOI] [PubMed] [Google Scholar]
- 82.Morwood SR, Nicholson LB. 2006. Modulation of the immune response by extracellular matrix proteins. Arch. Immunol. Ther. Exp. 54, 367–374. ( 10.1007/s00005-006-0043-x) [DOI] [PubMed] [Google Scholar]
- 83.Abdollahi-Roodsaz S, Joosten LA, Roelofs MF, Radstake TRDJ, Matera G, Popa C, van der Meer JWM, Netea MG, van den Berg WB. 2007. Inhibition of toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum. 56, 2957–2967. ( 10.1002/art.22848) [DOI] [PubMed] [Google Scholar]
- 84.Quero L, et al. 2013. Hyaluronic acid fragments enhance the inflammatory and catabolic response in human intervertebral disc cells through modulation of toll-like receptor 2 signaling pathways. Arthritis Res. Ther. 15, R94 ( 10.1186/ar4274) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorokin L. 2010. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 10, 712–723. ( 10.1038/nri2852) [DOI] [PubMed] [Google Scholar]
- 86.Jones P, Gardner L, Menage J, Williams GT, Roberts S. 2008. Intervertebral disc cells as competent phagocytes in vitro: implications for cell death in disc degeneration. Arthritis Res. Ther. 10, R86 ( 10.1186/ar2466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nerlich AG, Weiler C, Zipperer J, Narozny M, Boos N. 2002. Immunolocalization of phagocytic cells in normal and degenerated intervertebral discs. Spine 27, 2484–2490. ( 10.1097/00007632-200211150-00012) [DOI] [PubMed] [Google Scholar]
- 88.Phillips KL, Chiverton N, Michael AL, Cole AA, Breakwell LM, Haddock G, Bunning RAD, Cross AK, Le Maitre CL. 2013. The cytokine and chemokine expression profile of nucleus pulposus cells: implications for degeneration and regeneration of the intervertebral disc. Arthritis Res. Ther. 15, R213 ( 10.1186/ar4408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gabr MA, et al. 2011. Interleukin-17 synergizes with IFNgamma or TNFalpha to promote inflammatory mediator release and intercellular adhesion molecule-1 (ICAM-1) expression in human intervertebral disc cells. J. Orthop. Res. 29, 1–7. ( 10.1002/jor.21206) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Huang KY, Lin RM, Chen WY, Lee CL, Yan JJ, Chang MS. 2008. IL-20 may contribute to the pathogenesis of human intervertebral disc herniation. Spine (Phila Pa 1976) 33, 2034–2040. ( 10.1097/BRS.0b013e31817eb872) [DOI] [PubMed] [Google Scholar]
- 91.Smith LJ, Chiaro JA, Nerurkar NL, Cortes DH, Horava SD, Hebela NM, Mauck RL, Dodge GR, Elliott DM. 2011. Nucleus pulposus cells synthesize a functional extracellular matrix and respond to inflammatory cytokine challenge following long-term agarose culture. Eur. Cell Mater. 22, 291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoyland JA, Le Maitre C, Freemont AJ. 2008. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxf.) 47, 809–814. ( 10.1093/rheumatology/ken056) [DOI] [PubMed] [Google Scholar]
- 93.Seguin CA, Pilliar RM, Roughley PJ, Kandel RA. 2005. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine (Phila Pa 1976) 30, 1940–1948. ( 10.1097/01.brs.0000176188.40263.f9) [DOI] [PubMed] [Google Scholar]
- 94.Seguin CA, Pilliar RM, Madri JA, Kandel RA. 2008. TNF-alpha induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine (Phila Pa 1976) 33, 356–365. ( 10.1097/BRS.0b013e3181642a5e) [DOI] [PubMed] [Google Scholar]
- 95.Gawri R, Rosenzweig DH, Krock E, Ouellet JA, Stone LS, Quinn TM, Haglund L. 2014. High mechanical strain of primary intervertebral disc cells promotes secretion of inflammatory factors associated with disc degeneration and pain. Arthritis Res. Ther. 16, R21 ( 10.1186/ar4449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walter BA, Korecki CL, Purmessur D, Roughley PJ, Michalek AJ, Iatridis JC. 2011. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage 19, 1011–1018. ( 10.1016/j.joca.2011.04.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MTN, Ross ERS, O'Brien JPJ, Hoyland A. 2002. Nerve growth factor expression and innervation of the painful intervertebral disc. J. Pathol. 197, 286–292. ( 10.1002/path.1108) [DOI] [PubMed] [Google Scholar]
- 98.Melrose J, Roberts S, Smith S, Menage J, Ghosh P. 2002. Increased nerve and blood vessel ingrowth associated with proteoglycan depletion in an ovine anular lesion model of experimental disc degeneration. Spine (Phila Pa 1976) 27, 1278–1285. ( 10.1097/00007632-200206150-00007) [DOI] [PubMed] [Google Scholar]
- 99.Kawakami M, Weinstein JN, Chatani K, Spratt KF, Meller ST, Gebhart GF. 1994. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine (Phila Pa 1976) 19, 1795–1802. ( 10.1097/00007632-199408150-00002) [DOI] [PubMed] [Google Scholar]
- 100.Olmarker K, Blomquist J, Stromberg J, Nannmark U, Thomsen P, Rydevik B. 1995. Inflammatogenic properties of nucleus pulposus. Spine (Phila Pa 1976) 20, 665–669. ( 10.1097/00007632-199503150-00006) [DOI] [PubMed] [Google Scholar]
- 101.Lotz JC, Ulrich JA. 2006. Innervation, inflammation, and hypermobility may characterize pathologic disc degeneration: review of animal model data. J. Bone Joint Surg. Am. 88(Suppl. 2), 76–82. ( 10.2106/JBJS.E.01448) [DOI] [PubMed] [Google Scholar]
- 102.Kobayashi S, et al. 2009. Ultrastructural analysis on lumbar disc herniation using surgical specimens: role of neovascularization and macrophages in hernias. Spine (Phila Pa 1976) 34, 655–662. ( 10.1097/BRS.0b013e31819c9d5b) [DOI] [PubMed] [Google Scholar]
- 103.Ahn SH, Ahn MW, Byun WM. 2000. Effect of the transligamentous extension of lumbar disc herniations on their regression and the clinical outcome of sciatica. Spine (Phila Pa 1976) 25, 475–480. ( 10.1097/00007632-200002150-00014) [DOI] [PubMed] [Google Scholar]
- 104.Ikeda T, Nakamura T, Kikuchi T, Umeda S, Senda H, Takagi K. 1996. Pathomechanism of spontaneous regression of the herniated lumbar disc: histologic and immunohistochemical study. J. Spinal Disord. 9, 136–140. ( 10.1097/00002517-199604000-00009) [DOI] [PubMed] [Google Scholar]
- 105.Gezici AR, Ergun R. 2009. Spontaneous regression of a huge subligamentous extruded disc herniation: short report of an illustrative case. Acta Neurochir. (Wien) 151, 1299–1300. ( 10.1007/s00701-009-0370-x) [DOI] [PubMed] [Google Scholar]
- 106.Takada T, Nishida K, Maeno K, Kakutani K, Yurube T, Doita M, Kurosaka M. 2012. Intervertebral disc and macrophage interaction induces mechanical hyperalgesia and cytokine production in a herniated disc model in rats. Arthritis Rheum. 64, 2601–2610. ( 10.1002/art.34456) [DOI] [PubMed] [Google Scholar]
- 107.Matsui Y, Maeda M, Nakagami W, Iwata H. 1998. The involvement of matrix metalloproteinases and inflammation in lumbar disc herniation. Spine (Phila Pa 1976) 23, 863–868; discussion 868–869 ( 10.1097/00007632-199804150-00005) [DOI] [PubMed] [Google Scholar]
- 108.Murai K, Sakai D, Nakamura Y, Nakai T, Igarashi T, Seo N, Murakami T, Kobayashi E, Mochida J. 2010. Primary immune system responders to nucleus pulposus cells: evidence for immune response in disc herniation. Eur. Cell Mater. 19, 13–21. [DOI] [PubMed] [Google Scholar]
- 109.Wang H, et al. 2013. Inflammatory cytokines induce NOTCH signaling in nucleus pulposus cells: implications in intervertebral disc degeneration. J. Biol. Chem. 288, 16 761–16 774. ( 10.1074/jbc.M112.446633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kepler CK, Markova DZ, Dibra F, Yadla S, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. 2013. Expression and relationship of proinflammatory chemokine RANTES/CCL5 and cytokine IL-1beta in painful human intervertebral discs. Spine (Phila Pa 1976) 38, 873–880. ( 10.1097/BRS.0b013e318285ae08) [DOI] [PubMed] [Google Scholar]
- 111.Le Maitre C, Hoyland J, Freemont A. 2007. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res. Ther. 9, R77 ( 10.1186/ar2275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Le Maitre CL, Freemont AJ, Hoyland JA. 2005. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res. Ther. 7, R732–R745. ( 10.1186/ar1732) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abe Y, Akeda K, An HS, Aoki Y, Pichika R, Muehleman C, Kimura T, Masuda K. 2007. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine (Phila Pa 1976) 32, 635–642. ( 10.1097/01.brs.0000257556.90850.53) [DOI] [PubMed] [Google Scholar]
- 114.Miyahara K, Ishida T, Hukuda S, Horiike K, Okamoto M, Tojo H. 1996. Human group II phospholipase A2 in normal and diseased intervertebral discs. Biochim. Biophys. Acta 1316, 183–190. ( 10.1016/0925-4439(96)00023-3) [DOI] [PubMed] [Google Scholar]
- 115.Wang J, et al. 2013. Tumor necrosis factor alpha- and interleukin-1beta-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 65, 832–842. ( 10.1002/art.37819) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kokubo Y, et al. 2008. Herniated and spondylotic intervertebral discs of the human cervical spine: histological and immunohistological findings in 500 en bloc surgical samples. Laboratory investigation. J. Neurosurg. Spine 9, 285–295. ( 10.3171/SPI/2008/9/9/285) [DOI] [PubMed] [Google Scholar]
- 117.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. 1996. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine (Phila Pa 1976) 21, 218–224. ( 10.1097/00007632-199601150-00011) [DOI] [PubMed] [Google Scholar]
- 118.Kang JD, Georgescu HI, Mcintyre-Larkin L, Stefanovic-Racic M, Donaldson WF, III, Evans CH. 1996. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine (Phila Pa 1976) 21, 271–277. ( 10.1097/00007632-199602010-00003) [DOI] [PubMed] [Google Scholar]
- 119.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. 2002. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine (Phila Pa 1976) 27, 911–917. ( 10.1097/00007632-200205010-00005) [DOI] [PubMed] [Google Scholar]
- 120.Henriksson H, Thornemo M, Karlsson C, Hägg O, Junevik K, Lindahl A, Brisby H. 2009. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine 34, 2278–2287. ( 10.1097/BRS.0b013e3181a95ad2) [DOI] [PubMed] [Google Scholar]
- 121.Miyamoto H, Saura R, Harada T, Doita M, Mizuno K. 2000. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J. Med. Sci. 46, 13–28. [PubMed] [Google Scholar]
- 122.Shamji MF, et al. 2010. Proinflammatory cytokine expression profile in degenerated and herniated human intervertebral disc tissues. Arthritis Rheum. 62, 1974–1982. ( 10.1002/art.27444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang W, Nie L, Wang Y, Wang X, Zhao H, Dongol S, Maharjan S, Cheng L. 2013. CCL20 secretion from the nucleus pulposus improves the recruitment of CCR6-expressing Th17 cells to degenerated IVD tissues. PLoS ONE 8, e66286 ( 10.1371/journal.pone.0066286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. 2008. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976) 33, 2253–2259. ( 10.1097/BRS.0b013e318182c35f) [DOI] [PubMed] [Google Scholar]
- 125.Park JY, Kuh SU, Park HS, Kim KS. 2011. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. J. Spinal Disord. Tech. 24, 352–357. ( 10.1097/BSD.0b013e3181fee4df) [DOI] [PubMed] [Google Scholar]
- 126.Kepler CK, Markova DZ, Hilibrand AS, Vaccaro AR, Risbud MV, Albert TJ, Anderson DG. 2013. Substance P stimulates production of inflammatory cytokines in human disc cells. Spine (Phila Pa 1976) 38, E1291–E1299. ( 10.1097/BRS.0b013e3182a42bc2) [DOI] [PubMed] [Google Scholar]
- 127.Burke JG, Rw GW, Conhyea D, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. 2003. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine (Phila Pa 1976) 28, 2685–2693. ( 10.1097/01.BRS.0000103341.45133.F3) [DOI] [PubMed] [Google Scholar]
- 128.Kim JH, Studer RK, Vo NV, Sowa GA, Kang JD. 2009. p38 MAPK inhibition selectively mitigates inflammatory mediators and VEGF production in AF cells co-cultured with activated macrophage-like THP-1 cells. Osteoarthritis Cartilage 17, 1662–1669. ( 10.1016/j.joca.2009.06.004) [DOI] [PubMed] [Google Scholar]
- 129.Sinclair SM, Shamji MF, Chen J, Jing L, Richardson WJ, Brown CR, Fitch RD, Setton LA. 2011. Attenuation of inflammatory events in human intervertebral disc cells with a tumor necrosis factor antagonist. Spine (Phila Pa 1976) 36, 1190–1196. ( 10.1097/BRS.0b013e3181ebdb43) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Studer RK, Vo N, Sowa G, Ondeck C, Kang J. 2011. Human nucleus pulposus cells react to IL-6: independent actions and amplification of response to IL-1 and TNF-alpha. Spine (Phila Pa 1976) 36, 593–599. ( 10.1097/BRS.0b013e3181da38d5) [DOI] [PubMed] [Google Scholar]
- 131.Tian Y, Yuan W, Fujita N, Wang J, Wang H, Shapiro IM, Risbud MV. 2013. Inflammatory cytokines associated with degenerative disc disease control aggrecanase-1 (ADAMTS-4) expression in nucleus pulposus cells through MAPK and NF-kappaB. Am. J. Pathol. 182, 2310–2321. ( 10.1016/j.ajpath.2013.02.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yu ZG, Xu N, Wang WB, Pan SH, Li KS, Liu JK. 2009. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin. Med. J. (Engl.) 122, 2483–2488. [PubMed] [Google Scholar]
- 133.Doita M, Kanatani T, Harada T, Mizuno K. 1996. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine (Phila Pa 1976) 21, 235–241. ( 10.1097/00007632-199601150-00015) [DOI] [PubMed] [Google Scholar]
- 134.Bachmeier BE, Nerlich AG, Weiler C, Paesold G, Jochum M, Boos N. 2007. Analysis of tissue distribution of TNF-α, TNF-α-receptors, and the activating TNF-α-converting enzyme suggests activation of the TNF-α system in the aging intervertebral disc. Ann. NY Acad. Sci. 1096, 44–54. ( 10.1196/annals.1397.069) [DOI] [PubMed] [Google Scholar]
- 135.Risbud MV, Shapiro IM. 2014. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat. Rev. Rheumatol. 10, 44–56. ( 10.1038/nrrheum.2013.160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goupille P, Mulleman D, Paintaud G, Watier H, Valat JP. 2007. Can sciatica induced by disc herniation be treated with tumor necrosis factor alpha blockade? Arthritis Rheum. 56, 3887–3895. ( 10.1002/art.23051) [DOI] [PubMed] [Google Scholar]
- 137.Purmessur D, Walter BA, Roughley PJ, Laudier DM, Hecht AC, Iatridis J. 2013. A role for TNFalpha in intervertebral disc degeneration: a non-recoverable catabolic shift. Biochem. Biophys. Res. Commun. 433, 151–156. ( 10.1016/j.bbrc.2013.02.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. 2007. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 35, 652–655. ( 10.1042/BST0350652) [DOI] [PubMed] [Google Scholar]
- 139.Igarashi T, Kikuchi S, Shubayev V, Myers RR. 2000. Exogenous tumor necrosis factor-alpha mimics nucleus pulposus-induced neuropathology: molecular, histologic, and behavioral comparisons in rats. Spine 25, 2975–2980. ( 10.1097/00007632-200012010-00003) [DOI] [PubMed] [Google Scholar]
- 140.Kauppila LI. 1995. Ingrowth of blood vessels in disc degeneration. Angiographic and histological studies of cadaveric spines. J. Bone Joint Surg. Am. 77, 26–31. [DOI] [PubMed] [Google Scholar]
- 141.Hess A, et al. 2011. Blockade of TNF-alpha rapidly inhibits pain responses in the central nervous system. Proc. Natl Acad. Sci. USA 108, 3731–3736. ( 10.1073/pnas.1011774108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cuellar JM, Borges PM, Cuellar VG, Yoo A, Scuderi GJ, Yeomans DC. 2012. Cytokine expression in the epidural space: a model of non-compressive disc herniation-induced inflammation. Spine (Phila Pa 1976) 38, 17–23. ( 10.1097/BRS.0b013e3182604baa) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rousseau MA, Ulrich JA, Bass EC, Rodriguez AG, Liu JJ, Lotz JC. 2007. Stab incision for inducing intervertebral disc degeneration in the rat. Spine (Phila Pa 1976) 32, 17–24. ( 10.1097/01.brs.0000251013.07656.45) [DOI] [PubMed] [Google Scholar]
- 144.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. 2002. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine (Phila Pa 1976) 27, 1291–1296. ( 10.1097/00007632-200206150-00009) [DOI] [PubMed] [Google Scholar]
- 145.Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. 2005. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 30, 15–24. ( 10.1097/00007632-200511150-00023) [DOI] [PubMed] [Google Scholar]
- 146.Baggiolini M, Dewald B, Moser B. 1997. Human chemokines: an update. Annu. Rev. Immunol. 15, 675–705. ( 10.1146/annurev.immunol.15.1.675) [DOI] [PubMed] [Google Scholar]
- 147.Yoshida M, Nakamura T, Sei A, Kikuchi T, Takagi K, Matsukawa A. 2005. Intervertebral disc cells produce tumor necrosis factor alpha, interleukin-1beta, and monocyte chemoattractant protein-1 immediately after herniation: an experimental study using a new hernia model. Spine (Phila Pa 1976) 30, 55–61. ( 10.1097/01.brs.0000149194.17891.bf) [DOI] [PubMed] [Google Scholar]
- 148.O'Neill CW, Liu JJ, Leibenberg E, Hu SS, Deviren V, Tay BK-B, Chin CT, Lotz JC. 2004. Percutaneous plasma decompression alters cytokine expression in injured porcine intervertebral discs. Spine J. 4, 88–98. ( 10.1016/S1529-9430(03)00423-6) [DOI] [PubMed] [Google Scholar]
- 149.Rajan N, Bloom O, Maidhof R, Stetson N, Sherry B, Levine M, Chahine NO. 2012. Toll-like receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Phila Pa 1976) 38, 1343–1351. ( 10.1097/BRS.0b013e31826b71f4) [DOI] [PubMed] [Google Scholar]
- 150.Morimoto R, Akeda K, Iida R, Nishimura A, Tsujii M, Obata S, Kasai Y, Uchida A, Sudo A. 2012. Tissue renin-angiotensin system in the intervertebral disc. Spine (Phila Pa 1976) 38, E129–E136. ( 10.1097/BRS.0b013e31827b8c89) [DOI] [PubMed] [Google Scholar]
- 151.Gruber HE, Hanley EN., Jr 2002. Ultrastructure of the human intervertebral disc during aging and degeneration: comparison of surgical and control specimens. Spine (Phila Pa 1976) 27, 798–805. ( 10.1097/00007632-200204150-00004) [DOI] [PubMed] [Google Scholar]
- 152.Bach FC, Willems N, Penning LC, Ito K, Meij BP, Tryfonidou MA. 2014. Potential regenerative treatment strategies for intervertebral disc degeneration in dogs. BMC Vet. Res. 10, 3 ( 10.1186/1746-6148-10-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bergknut N, et al. 2013. Intervertebral disc disease in dogs—part 1: a new histological grading scheme for classification of intervertebral disc degeneration in dogs. Vet. J. 195, 156–163. ( 10.1016/j.tvjl.2012.05.027) [DOI] [PubMed] [Google Scholar]
- 154.Alini M, et al. 2008. Are animal models useful for studying human disc disorders/degeneration? Eur. Spine J. 17, 2–19. ( 10.1007/s00586-007-0414-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roberts S, Butler RC. 2005. Inflammatory mediators as potential therapeutic targets in the spine. Curr. Drug Targets 4, 257–266. ( 10.2174/1568010053586372). [DOI] [PubMed] [Google Scholar]
- 156.Wuertz K, Vo N, Kletsas D, Boos N. 2012. Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-kappaB and MAP kinases. Eur. Cell Mater. 23, 103–119; discussion 119–120. [DOI] [PubMed] [Google Scholar]
- 157.Genevay S, Viatte S, Finckh A, Zufferey P, Balague F, Gabay C. 2010. Adalimumab in severe and acute sciatica: a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 62, 2339–2346. ( 10.1002/art.27499) [DOI] [PubMed] [Google Scholar]
- 158.Genevay S, Stingelin S, Gabay C. 2004. Efficacy of etanercept in the treatment of acute, severe sciatica: a pilot study. Ann. Rheum. Dis. 63, 1120–1123. ( 10.1136/ard.2003.016451) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Olmarker K, Rydevik B. 2001. Selective inhibition of tumor necrosis factor-alpha prevents nucleus pulposus-induced thrombus formation, intraneural edema, and reduction of nerve conduction velocity: possible implications for future pharmacologic treatment strategies of sciatica. Spine (Phila Pa 1976) 26, 863–869. ( 10.1097/00007632-200104150-00007) [DOI] [PubMed] [Google Scholar]
- 160.Okoro T, Tafazal SI, Longworth S, Sell PJ. 2010. Tumor necrosis alpha-blocking agent (etanercept): a triple blind randomized controlled trial of its use in treatment of sciatica. J. Spinal Disord. Tech. 23, 74–77. ( 10.1097/BSD.0b013e31819afdc4) [DOI] [PubMed] [Google Scholar]
- 161.Muller-Ladner U, Roberts CR, Franklin BN, Gay RE, Robbins PD, Evans CH, Gay S. 1997. Human IL-1Ra gene transfer into human synovial fibroblasts is chondroprotective. J. Immunol. 158, 3492–3498. [PubMed] [Google Scholar]
- 162.Autio RA, Karppinen JNiinimäki J, Ojala R, Veeger N, Korhonen T, Hurri H, Tervonen O. 2006. The effect of infliximab, a monoclonal antibody against TNF-α, on disc herniation resorption: a randomized controlled study. Spine 31, 2641–2645. ( 10.1097/01.brs.0000244616.64962.9e) [DOI] [PubMed] [Google Scholar]
- 163.Tobinick E. 2006. Spinal delivery of p38: TNF-alpha inhibitors. PLoS Med. 3, e511 ( 10.1371/journal.pmed.0030511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ragab AA, Woodall JW, Jr, Tucci MA, Wingerter SA, Fosnaugh AW, Franklin LN, Benghuzzi HA. 2009. A preliminary report on the effects of sustained administration of corticosteroid on traumatized disc using the adult male rat model. J. Spinal Disord. Tech. 22, 473–478. ( 10.1097/BSD.0b013e31818d5e55) [DOI] [PubMed] [Google Scholar]
- 165.Zhang H, Wang L, Park JB, Park P, Yang VC, Hollister SJ, La Marca F, Lin C-Y. 2009. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis Res. Ther. 11, R172 ( 10.1186/ar2861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gorth DJ, Mauck RL, Chiaro JA, Mohanraj B, Hebela NM, Dodge GR, Elliott DM, Smith LJ. 2012. IL-1ra delivered from poly(lactic-co-glycolic acid) microspheres attenuates IL-1β-mediated degradation of nucleus pulposus in vitro. Arthritis Res. Ther. 14, R179 ( 10.1186/ar3932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Tamaki T. 2002. Epidural injection of cyclooxygenase-2 inhibitor attenuates pain-related behavior following application of nucleus pulposus to the nerve root in the rat. J. Orthop. Res. 20, 376–381. ( 10.1016/S0736-0266(01)00114-0) [DOI] [PubMed] [Google Scholar]
- 168.Nagae M, et al. 2007. Intervertebral disc regeneration using platelet-rich plasma and biodegradable gelatin hydrogel microspheres. Tissue Eng. 13, 147–158. ( 10.1089/ten.2006.0042) [DOI] [PubMed] [Google Scholar]
- 169.Wu C-C, Chen W-H, Zao B, Lai P-L, Lin T-C, Lo H-Y, Shieh Y-H, Wu C-H, Deng W-P. 2011. Regenerative potentials of platelet-rich plasma enhanced by collagen in retrieving pro-inflammatory cytokine-inhibited chondrogenesis. Biomaterials 32, 5847–5854. ( 10.1016/j.biomaterials.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 170.Wuertz K, Quero L, Sekiguchi M, Klawitter M, Nerlich A, Konno S, Kikuchi S, Boos N. 2011. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus-mediated pain in vitro and in vivo. Spine 36, 1373–1384. ( 10.1097/BRS.0b013e318221e655) [DOI] [PubMed] [Google Scholar]
- 171.Li H, Liang C, Chen Q, Yang Z. 2011. Rhein: a potential biological therapeutic drug for intervertebral disc degeneration. Med. Hypotheses 77, 1105–1107. ( 10.1016/j.mehy.2011.09.013) [DOI] [PubMed] [Google Scholar]
- 172.Liu Q, Jin L, Shen FH, Balian G, Li XJ. 2013. Fullerol nanoparticles suppress inflammatory response and adipogenesis of vertebral bone marrow stromal cells—a potential novel treatment for intervertebral disc degeneration. Spine J. 13, 1571–1580. ( 10.1016/j.spinee.2013.04.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kobori S, et al. 2014. Inhibiting IkappaB kinase-beta downregulates inflammatory cytokines in injured discs and neuropeptides in dorsal root ganglia innervating injured discs in rats. Spine (Phila Pa 1976) 39, 1171–1177. ( 10.1097/BRS.0000000000000374) [DOI] [PubMed] [Google Scholar]
- 174.Vadalà G, Sowa GA, Kang JD. 2007. Gene therapy for disc degeneration. Expert Opin. Biol. Ther. 7, 185–196. ( 10.1517/14712598.7.2.185) [DOI] [PubMed] [Google Scholar]
- 175.Carragee EJ, Don AS, Hurwitz EL, Cuellar JM, Carrino JA, Herzog R. 2009. 2009 ISSLS prize winner: does discography cause accelerated progression of degeneration changes in the lumbar disc: a ten-year matched cohort study. Spine (Phila Pa 1976) 34, 2338–2345. ( 10.1097/BRS.0b013e3181ab5432) [DOI] [PubMed] [Google Scholar]
- 176.Vadala G, Russo F, Pattappa G, Schiuma D, Peroglio M, Benneker LM, Grad S, Alini M, Denaro V. 2013. The transpedicular approach as an alternative route for intervertebral disc regeneration. Spine (Phila Pa 1976) 38, E319–E324. ( 10.1097/BRS.0b013e318285bc4a) [DOI] [PubMed] [Google Scholar]
- 177.Gibson MJ, Karpinski MR, Slack RC, Cowlishaw WA, Webb JK. 1987. The penetration of antibiotics into the normal intervertebral disc. J. Bone Joint Surg. Br. 69, 784–786. [DOI] [PubMed] [Google Scholar]
- 178.Motaghinasab S, Shirazi-Adl A, Urban JP, Parnianpour M. 2012. Computational pharmacokinetics of solute penetration into human intervertebral discs—effects of endplate permeability, solute molecular weight and disc size. J. Biomech. 45, 2195–2202. ( 10.1016/j.jbiomech.2012.06.033) [DOI] [PubMed] [Google Scholar]
- 179.Ferguson SJ, Ito K, Nolte LP. 2004. Fluid flow and convective transport of solutes within the intervertebral disc. J. Biomech. 37, 213–221. ( 10.1016/S0021-9290(03)00250-1) [DOI] [PubMed] [Google Scholar]
- 180.Wehling P, Schulitz KP, Robbins PD, Evans CH, Reinecke JA. 1997. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine 15, 1092–1097. ( 10.1097/00007632-199705150-00008) [DOI] [PubMed] [Google Scholar]
- 181.Le Maitre CL, Hoyland JA, Freemont AJ. 2007. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: an in situ zymographic and gene therapy study. Arthritis Res. Ther. 9, R83 ( 10.1186/ar2282) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nishida K, Kang JD, Gilbertson LG, Moon S-H, Suh J-K, Vogt MT, Robbins PD, Evans CH. 1999. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine (Phila Pa 1976) 24, 2419–2425. ( 10.1097/00007632-199912010-00002) [DOI] [PubMed] [Google Scholar]
- 183.Lee YJ, Kong MH, Song KY, Lee KH, Heo SH. 2008. The relation between Sox9, TGF-β1, and proteoglycan in human intervertebral disc cells. J. Kor. Neurosurg. Soc. 43, 149–154. ( 10.3340/jkns.2008.43.3.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tan Y, Hu Y, Tan J. 2003. Extracellular matrix synthesis and ultrastructural changes of degenerative disc cells transfected by Ad/CMV-hTGF-beta 1. Chin. Med. J. (Engl.) 116, 1399–1403. [PubMed] [Google Scholar]
- 185.Leckie S, et al. 2012. Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine 12, 7–20. ( 10.1016/j.spinee.2011.09.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Haufe SMW, Mork AR. 2006. Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 15, 136–137. ( 10.1089/scd.2006.15.136) [DOI] [PubMed] [Google Scholar]
- 187.Quintin A, Schizas C, Scaletta C, Jaccoud S, Gerber S, Osterheld MC, Juillerat L, Applegate LA, Pioletti DP. 2008. Isolation and in vitro chondrogenic potential of human foetal spine cells. J. Cell Mol. Med. 13, 2559–2569. ( 10.1111/j.1582-4934.2008.00630.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Iwashina T, Mochida J, Sakai D, Yamamoto Y, Miyazaki T, Ando K, Hotta T. 2006. Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine 31, 1177–1186. ( 10.1097/01.brs.0000217687.36874.c4) [DOI] [PubMed] [Google Scholar]
- 189.Hohaus C, Ganey TM, Minkus Y, Meisel HJ. 2008. Cell transplantation in lumbar spine disc degeneration disease. Eur. Spine J. 17(Suppl. 4), S492–S503. ( 10.1007/s00586-008-0750-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sheikh H, Zakharian K, De La Torre RP, Facek C, Vasquez A, Chaudhry GR, Svinarich D, Peter-Cruet MJ. 2009. In vivo intervertebral disc regeneration using stem cell-derived chondroprogenitors: laboratory investigation. J. Neurosurg. 10, 265–272. ( 10.3171/2008.12.SPINE0835) [DOI] [PubMed] [Google Scholar]
- 191.Chen J, Lee EJ, Jing L, Christoforou N, Leong KW, Setton LA. 2013. Differentiation of mouse induced pluripotent stem cells (iPSCs) into nucleus pulposus-like cells in vitro. PLoS ONE 8, e75548 ( 10.1371/journal.pone.0075548) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Murrell W, Sanford E, Anderberg L, Cavanagh B, Mackay-Sim A. 2009. Olfactory stem cells can be induced to express chondrogenic phenotype in a rat intervertebral disc injury model. Spine J. 9, 585–594. ( 10.1016/j.spinee.2009.02.011) [DOI] [PubMed] [Google Scholar]
- 193.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. 2005. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine 30, 2379–2387. ( 10.1097/01.brs.0000184365.28481.e3) [DOI] [PubMed] [Google Scholar]
- 194.Chon BH, Lee EJ, Jing L, Setton LA, Chen J. 2013. Human umbilical cord mesenchymal stromal cells exhibit immature nucleus pulposus cell phenotype in a laminin-rich pseudo-three-dimensional culture system. Stem Cell Res. Ther. 4, 120 ( 10.1186/scrt331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Erwin WM, Islam D, Eftekarpour E, Inman RD, Karim MZ, Fehlings MG. 2013. Intervertebral disc-derived stem cells: implications for regenerative medicine and neural repair. Spine 38, 211–216. ( 10.1097/BRS.0b013e318266a80d) [DOI] [PubMed] [Google Scholar]
- 196.Kim JH, Moon HJ, Lee JH, Kim JH, Kwon TH, Park YK. 2012. Rabbit notochordal cells modulate the expression of inflammatory mediators by human annulus fibrosus cells cocultured with activated macrophage-like THP-1 cells. Spine (Phila Pa 1976) 37, 1856–1864. ( 10.1097/BRS.0b013e3182579434) [DOI] [PubMed] [Google Scholar]
- 197.Serigano K, Sakai D, Hiyama A, Tamura F, Tanaka M, Mochida J. 2010. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J. Orthop. Res. 28, 1267–1275. ( 10.1002/jor.21147) [DOI] [PubMed] [Google Scholar]
- 198.Miyamoto T, Muneta T, Tabuchi T, Matsumoto K, Saito H, Tsuji K, Sekiya I. 2010. Intradiscal transplantation of synovial mesenchymal stem cells prevents intervertebral disc degeneration through suppression of matrix metalloproteinase-related genes in nucleus pulposus cells in rabbits. Arthritis Res. Ther. 12, R206 ( 10.1186/ar3182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yoo K, et al. 2009. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 259, 150–156. ( 10.1016/j.cellimm.2009.06.010) [DOI] [PubMed] [Google Scholar]
- 200.Prockop DJ, Oh JY. 2012. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol. Ther. 20, 14–20. ( 10.1038/mt.2011.211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, Phinney DG. 2007. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc. Natl Acad. Sci. USA 104, 11 002–11 007. ( 10.1073/pnas.0704421104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee RH, et al. 2009. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 5, 54–63. ( 10.1016/j.stem.2009.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Oh JY, Roddy GW, Choi H, Lee RH, Ylostalo JH, Rosa RH, Prockop DJ. 2010. Anti-inflammatory protein TSG-6 reduces inflammatory damage to the cornea following chemical and mechanical injury. Proc. Natl Acad. Sci. USA 107, 16 875–16 880. ( 10.1073/pnas.1012451107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Hiyama A, Mochida J, Iwashina T, Omi H, Watanabe T, Serigano K, Tamura F, Sakai D. 2008. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J. Orthop. Res. 26, 589–600. ( 10.1002/jor.20584) [DOI] [PubMed] [Google Scholar]
- 205.Bertolo A, Thiede T, Aebli N, Baur M, Ferguson S, Stoyanov J. 2011. Human mesenchymal stem cell co-culture modulates the immunological properties of human intervertebral disc tissue fragments in vitro. Eur. Spine J. 20, 592–603. ( 10.1007/s00586-010-1662-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Orozco L, Soler R, Morera C, Alberca M, Sanchez A, García-Sancho J. 2011. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation 92, 822–828. ( 10.1097/TP.0b013e3182298a15) [DOI] [PubMed] [Google Scholar]
- 207.Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y. 2010. Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine 35, E475–E480. ( 10.1097/BRS.0b013e3181cd2cf4) [DOI] [PubMed] [Google Scholar]
- 208.Pang X, Yang H, Peng B. 2014. Human umbilical cord mesenchymal stem cell transplantation for the treatment of chronic discogenic low back pain. Pain Phys. 17, E525–E530. [PubMed] [Google Scholar]
- 209.Pettine KA, Murphy MB, Suzuki RK, Sand TT. 2014. Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33, 146–156. ( 10.1002/stem.1845) [DOI] [PubMed] [Google Scholar]
- 210.Meirelles L, Fontes AM, Covas DT, Caplan AI. 2009. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytok. Growth Factor Rev. 20, 419–427. ( 10.1016/j.cytogfr.2009.10.002) [DOI] [PubMed] [Google Scholar]
- 211.Karp JM, Leng Teo GS. 2009. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell 4, 206–216. ( 10.1016/j.stem.2009.02.001) [DOI] [PubMed] [Google Scholar]
- 212.Ponte A, Marais E, Gallay N, Langonne A, Delorme B, Herault E, Charboard P, Domenech J. 2009. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells 25, 1737–1745. ( 10.1634/stemcells.2007-0054) [DOI] [PubMed] [Google Scholar]
- 213.Tondreau T, Meuleman N, Stamatopoulos B, De Bruyn C, Delforge A, Dejeneffe M, Martiat P, Bron D, Lagneaux L. 2009. In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy 11, 559–569. ( 10.1080/14653240903051541) [DOI] [PubMed] [Google Scholar]
- 214.Illien-Jünger SPG, Peroglio M, Benneker LM, Stoddart MJ, Sakai D, Mochida J, Grad S, Alini M. 2012. Homing of mesenchymal stem cells in induced degenerative intervertebral discs in a whole organ culture system. Spine 37, 1865–1873. ( 10.1097/BRS.0b013e3182544a8a) [DOI] [PubMed] [Google Scholar]
- 215.Pattappa G, Peroglio M, Sakai D, Mochida J, Benneker LM, Alini M, Grad S. 2014. CCL5/RANTES is a key chemoattractant released by degenerative intervertebral discs in organ culture. Eur. Cell Mater. 27, 124–136; discussion 136. [DOI] [PubMed] [Google Scholar]
- 216.Pereira CL, et al. 2014. The effect of hyaluronan-based delivery of stromal cell-derived factor-1 on the recruitment of MSCs in degenerating intervertebral discs. Biomaterials 35, 8144–8153. ( 10.1016/j.biomaterials.2014.06.017) [DOI] [PubMed] [Google Scholar]
- 217.Henriksson H, Svala E, Skioldebrand E, Lindahl AMD, Brisby H. 2012. Support of concept that migrating progenitor cells from stem cell niches contribute to normal regeneration of the adult mammal intervertebral disc. Spine 37, 722–732. ( 10.1097/BRS.0b013e318231c2f7) [DOI] [PubMed] [Google Scholar]
- 218.Claes L, Recknagel S, Ignatius A. 2012. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 8, 133–143. ( 10.1038/nrrheum.2012.1) [DOI] [PubMed] [Google Scholar]
- 219.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. 2011. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng. B 17, 393–402. ( 10.1089/ten.teb.2011.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Boccafoschi F, Mosca C, Cannas M. 2012. Cardiovascular biomaterials: when the inflammatory response helps to efficiently restore tissue functionality? J. Tissue Eng. Regen. Med. 8, 253–267. ( 10.1002/term.1526) [DOI] [PubMed] [Google Scholar]
- 221.Torres AL, Santos SG, Oliveira MI, Barbosa MA. 2013. Fibrinogen promotes resorption of chitosan by human osteoclasts. Acta Biomater. 9, 6553–6562. ( 10.1016/j.actbio.2013.01.015) [DOI] [PubMed] [Google Scholar]
- 222.Almeida CR, Vasconcelos DP, Goncalves RM, Barbosa MA. 2012. Enhanced mesenchymal stromal cell recruitment via natural killer cells by incorporation of inflammatory signals in biomaterials. J. R. Soc. Interface 9, 261–271. ( 10.1098/rsif.2011.0357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Oliveira MI, Santos SG, Oliveira MJ, Torres AL, Barbosa MA. 2012. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur. Cell Mater. 24, 136–152; discussion 152–153. [DOI] [PubMed] [Google Scholar]
- 224.Maciel J, Oliveira MI, Colton E, McNally AK, Oliveira C, Anderson JM, Barbosa MA. 2014. Adsorbed fibrinogen enhances production of bone- and angiogenic-related factors by monocytes/macrophages. Tissue Eng. A 20, 250–263. ( 10.1089/ten.tea.2012.0439) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lee J, Sayed N, Hunter A, Au KF,, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP. 2012. Activation of innate immunity is required for efficient nuclear reprogramming. Cell 151, 547–558. ( 10.1016/j.cell.2012.09.034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda M, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872. ( 10.1016/j.cell.2007.11.019) [DOI] [PubMed] [Google Scholar]
- 227.O'Neill LAJ. 2012. Transflammation: when innate immunity meets induced pluripotency. Cell 151, 471–473. ( 10.1016/j.cell.2012.10.006) [DOI] [PubMed] [Google Scholar]
- 228.Raghow R. 1994. The role of extracellular matrix in postinflammatory wound healing and fibrosis. FASEB J. 8, 823–831. [DOI] [PubMed] [Google Scholar]