Abstract
α-Enolase is a glycolytic enzyme and a surface receptor for plasminogen. α-Enolase-bound plasminogen promotes tumor cell invasion and cancer metastasis by activating plasmin and consequently degrading the extracellular matrix degradation. Therefore, α-enolase and plasminogen are novel targets for cancer therapy. We found that the amino acid sequence of a peptide purified from enzymatic hydrolysates of seahorse has striking similarities to that of α-enolase. In this study, we report that this peptide competes with cellular α-enolase for plasminogen binding and suppresses urokinase plasminogen activator (uPA)-mediated activation of plasminogen, which results in decreased invasive migration of HT1080 fibrosarcoma cells. In addition, the peptide treatment decreased the expression levels of uPA compared to that of untreated controls. These results provide new insight into the mechanism by which the seahorse-derived peptide suppresses invasive properties of human cancer cells. Our findings suggest that this peptide could emerge as a potential therapeutic agent for cancer. [BMB Reports 2014; 47(12): 691-696]
Keywords: α-enolase, Invasion, Plasminogen, Seahorse peptide
INTRODUCTION
α-Enolase is a metabolic enzyme involved in the synthesis of pyruvate. Recently, α-enolase has been reported to act as a plasminogen receptor (1-3). Binding of α-enolase with plasminogen leads to the activation of plasminogen to plasmin by the proteolytic action of either urokinase-type (uPA) or tissue-type (tPA) plasminogen activators (4). Plasmin is a serine protease with a broad spectrum of substrates that include fibrin, extracellular matrix components, and proteins involved in extracellular matrix degradation, such as matrix metalloproteinases (5). The α-enolase/plasminogen/plasmin system has been shown to play a role in a wide variety of physiological and pathophysiological processes involving extracellular matrix degradation, tissue remodeling, embryogenesis, wound healing, cell migration, invasion, inflammation, angiogenesis, and tumor cell metastasis (4). Overexpression and activation of α-enolase, plasminogen, and plasmin has been considered a diagnostic marker for many tumors (4). The plasminogen activators, which include the two isoforms, uPA and tPA, and the plasminogen activator inhibitors, PAI-1 and PAI-2, play a fundamental role in the degradation of intravascular clots and extracellular matrix proteolysis (6, 7). Several plasminogen inhibitors, which include α-2 antiplasmin and the protease nexin I, have been reported to control tumor metastasis facilitated by plasminogen and plasmin (8, 9). Antibodies directed against the plasminogen-binding site of α-enolase inhibit leukocyte-endothelial interaction and invasion of pathogens (4, 10). Thus, the regulatory mechanism of the α-enolase/plasminogen/plasmin system in cancer cell invasion and metastasis has drawn many researchers to target the pathway for identifying potential anti-cancer therapeutic agents.
A natural peptide isolated from the enzymatic hydrolysates of the seahorse Hippocampus kuda Bleeler was found to have anti-inflammatory effect (11). We found that this peptide has significant sequence similarity with the α-enolase of other species. Here, we examined the effects of this peptide on α-enolase and plasminogen functional regulation. The peptide was found to compete with α-enolase for plasminogen binding and suppress invasive migration of HT1080 fibrosarcoma cells by inhibiting the activation of plasminogen to plasmin.
RESULTS
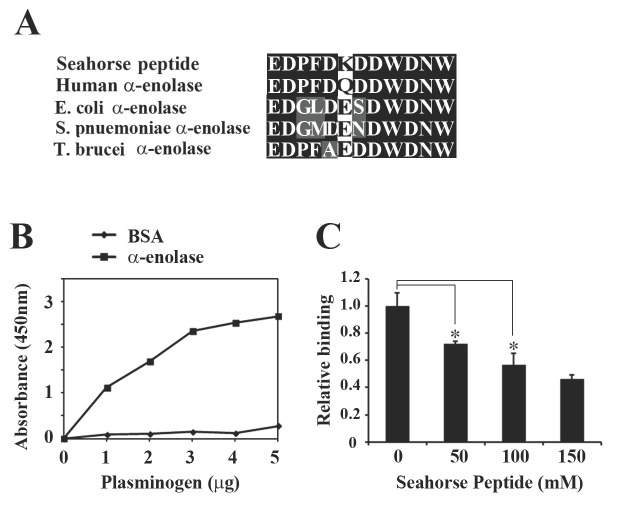
A seahorse peptide with amino acid sequence similarity to α-enolase decreases the interaction of α-enolase with plasminogen
Previously, we isolated a peptide from the enzymatic hydrolysates of seahorse and showed that it possesses anti-inflammatory activity (11). However, the cellular target molecule of this peptide has never been documented. When the peptide sequences were compared with known sequences in the translated GenBankTM database, over 90% of the peptide sequence corresponded with that of α-enolase from various sources. Further, 92% homology was found with the human α-enolase (Fig. 1A). Analysis of the peptide sequence revealed similarities in a region proximal to the plasminogen-binding site in human α-enolase (4, 5, 12). Therefore, in this study, we investigated whether the seahorse peptide influences the physiological role of endogenous α-enolase, such as binding and activation of plasminogen. First, to determine whether the peptide affects the interaction of α-enolase with plasminogen, we performed an ELISA assay using immobilized α-enolase and increasing concentrations of plasminogen. Fig. 1B and 1C shows a concentration-dependent binding of plasminogen to α-enolase coated wells. For controls, the wells were coated with only BSA, which had negligible, nonspecific binding of plasminogen. Significantly, the addition of seahorse peptide reduced the plasminogen binding to immobilized α-enolase. In these experiments, up to 52% inhibition was observed with 0.1 M peptide incubation, suggesting that the peptide is able to compete with α-enolase for plasminogen binding.
Fig. 1. The Seahorse-peptide with amino acid sequence similarity to α-enolase decreases the interaction of α-enolase and plasminogen. (A) A strong sequence similarity (92%) between human α-enolase (amino acids 293-304) and seahorse-derived peptide. All sequences were obtained from GenBank protein sequence data with accession numbers: Human (NP 001419), E. coli (AAC69289), S. pnuemoniae (AAC17130), and T. brucei (AAF73201). (B) Specific binding of plasminogen to α-enolase. Plasminogen binds to α-enolase immobilized on microtiter well plates in a concentration-dependent manner. ELISA was performed in a multiwall plate coated with α-enolase (1 μg/well) and increasing amounts of human plasminogen (1-5 μg). Control wells lacked α-enolase and were coated with only BSA (1 μg/well). The results represent the average from three independent experiments. (C) Inhibitory effect of seahorsepeptide on the interaction between α-enolase and plasminogen. Various concentration of seahorse peptide were added to wells containing 1 μg immobilized α-enolase, followed by the addition of 2 μg plasminogen, and ELISA was performed as described in Materials and Methods. The results represent the average from three independent experiments. *P < 0.05 compared with controls.
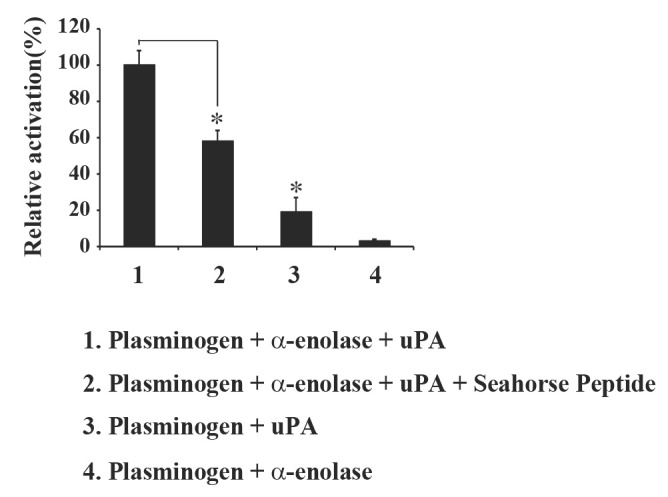
Peptide decreases the activation of plasminogen to plasmin
Next, we examined whether the proteolytic activation of α-enolase-bound plasminogen to plasmin is affected by peptide treatment. Plasminogen activation was performed by measuring the substrate-cleaving activity of generated plasmin. The reaction mixture containing plasminogen, α-enolase, and plasmin substrate Val-Leu-Lys-para-nitroanilide, were incubated in the presence or absence of uPA and peptide. Fig. 2 indicates that in the absence of uPA, plasminogen exhibited less proteolytic activity regardless of the presence of α-enolase, whereas the addition of uPA mediated the proteolytic activation of plasminogen to plasmin. Incubation with additional α-enolase in the presence of uPA led to significantly increased activation of plasminogen compared to those in reactions lacking α-enolase or uPA. The addition of 0.1 M peptide significantly inhibited uPA-mediated plasmin generation in the presence of α-enolase. These results strongly suggest that α-enolase plays a crucial role in facilitating uPA-mediated proteolytic activation of plasminogen while treatment with the peptide inhibits uPA-mediated activation of plasminogen by decreasing its interaction with α-enolase.
Fig. 2. Seahorse peptide decreases the activation of plasminogen into plasmin. The α-enolase (1 μg) was incubated with plasminogen (2 μg) in the presence or absence of seahorse peptide (0.1 M) and uPA (30 ng). Plasmin formation was measured as mentioned in Material and Methods. The results are expressed as the average of three different experiments. *P < 0.05 compared with 1.
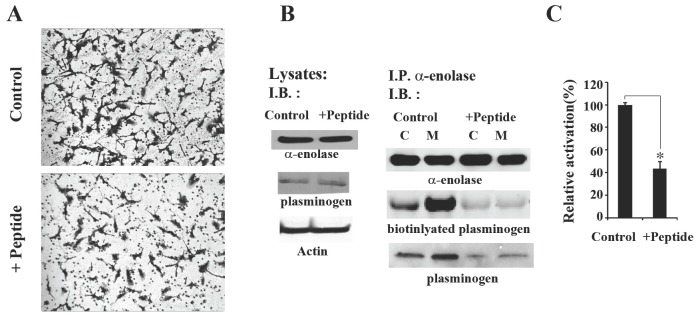
Seahorse peptide inhibits invasive migration of HT1080 cells by decreasing α-enolase-plasminogen interaction and uPA-dependent plasminogen activation
Interaction of α-enolase with plasminogen leads to proteolytic conversion of plasminogen to the active protease plasmin. Plasmin is the main enzyme responsible for matrix degradation, wound healing, cell migration and invasion, and tumor-cell metastasis. The major biological function of plasminogen activation is to degrade the extracellular matrix of basement membranes. Therefore, we examined whether peptide treatment affects invasive migration of tumor cells. As illustrated in Fig. 3A, treatment of HT1080 cells with 0.1 M peptide inhibited the invasive migration into Matrigel, with an inhibition ratio of approximately one half of the controls. To determine whether this inhibitory effect of the peptide on invasive migration is dependent on decreased association between endogenous α-enolase and plasminogen, we carried out immunoprecipitation of protein extracts obtained from HT1080 cells. 0.1 M peptide treatment did not affect the expression levels of α-enolase and plasminogen in HT1080 cells (Fig. 3B). Since many studies have reported that α-enolase binds plasminogen at the cell surface and the subsequent plasminogen activation on the cell surface is important for α-enolase and plasminogen-mediated physiological cell events such as invasive cell migration (4, 6, 14-16), we subfractionated HT1080 cell lysates into 100,000 g pellet (membrane) and supernatant (cytosol) fractions exactly as described previously (20). A total of 200 μg protein from each fraction was immunoprecipitated with an anti-α-enolase antibody, and immunoprecipitaes were separated by SDS-PAGE and transferred to PVDF. The protein levels in each immunoprecipitate were determined by immunoblotting with antibodies against α-enolase and plasminogen. Overlay assay with 0.1 μg biotinylated plasminogen was also performed to confirm to detectable binding between α-enolase and plasminogen. The results show that strong binding of plasminogen to α-enolase was observed more in the membrane fraction than in the cytosolic fraction of cells. An inhibitory effect of peptide treatment on this interaction was more detectable in the membrane sample. These results suggest that intracellular interaction of α-enolase and plasminogen primary occurred in membrane of HT1080 cells, which is more sensitive to peptide treatment (Fig. 3B). Additionally, plasminogen activation reaction with the membrane fraction of peptide-treated cells exhibited less proteolytic activity of plasminogen than that of the control cell membrane fraction (Fig. 3C). These results indicate that this peptide inhibits invasive migration of HT1080 cells by reducing α-enolase-plasminogen interaction in cell membrane and decreasing plasminogen activation. The peptide did not affect the viability of human fibrosarcoma HT1080 cells (Supplementary Fig. 1). When HT1080 cells were treated with 0.1 M peptide for 48 h, peptide treatment did not induce any significant inhibition of cell growth.
Fig. 3. Seahorse peptide inhibits invasive migration of HT1080 cells by decreasing interaction between α-enolase and plasminogen. (A) Matrigel invasion assays were performed with HT1080 cells incubated with 0.1 M peptide for 48 h. (B) Peptide treatment decreases the association of α-enolase and plasminogen. HT1080 cells were incubated with 0.1 M control peptide or seahorse peptide for 48 h and then the cell lysates were analyzed for α-enolase, plasminogen, and a-actin. Peptide treatment did not affect the expression levels of α-enolase and plasminogen in HT1080 cells. Additionally, the cell lysates were subfractionated into 100,000 g pellet (membrane) or supernatant (cytosol) fractions. Total 200 μg protein from each fraction was immunoprecipitated with an anti-α-enolase antibody, and immunoprecipitae were separated by SDS-PAGE and transferred to PVDF. The levels of protein in each immunoprecipitate were determined by immunoblotting with antibodies against α-enolase and plasminogen. Overlay assay with 0.1 μg biotinylated plasminogen was also performed to confirm to detectable binding between α-enolase and plasminogen. Specific protein interaction was shown as streptavidin signal. Results are representative of three separate experiments. (C) Peptide treatment attenuates activation of plasminogen in cell membrane. Cells incubated with 0.1 M control peptide or seahorse peptide were lysed, and then fractionated into membrane fraction. 20 μg of membrane fraction was added to assay for plasminogen activity. The results are expressed as the average of three different experiments. *P < 0.05 compared to controls peptide treatment.
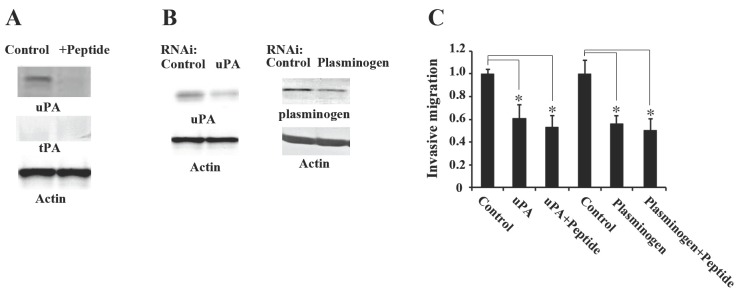
Since α-enolase-bound plasminogen activation is well correlated with function of plasminogen activators, uPA and tPA (13), we finally examined whether peptide treatment affected these two physiological plasminogen activators. HT1080 cells were treated with 0.1 M peptide for 48 h and the expression levels of uPA and tPA were examined. Peptide treatment decreased the expression levels of uPA compared to that of control, whereas tPA was expressed at very low levels in the total protein extract of HT1080 cells and its expression did not change after peptide treatment (Fig. 4A). Therefore, it is likely that uPA plays an important role in plasminogen activation in HT1080 cells. The role of uPA and plasminogen on invasive migration of HT1080 cells was determined by siRNA transfection of uPA and plasminogen. HT1080 cells were transfected with siRNA for uPA or plasminogen (Fig. 4B). These siRNA transfections attenuated the expression of uPA and plasminogen in HT1080 cells. We observed that the uPA siRNA as well as the plasminogen siRNA attenuated invasive migration of HT1080 cells (Fig. 4C), which was similar to that observed in the peptide-treated cells. Taken together, these results suggest that uPA-mediated plasminogen activation is important for invasive migration of HT1080 cells and seahorse-derived peptide inhibits the invasive migration of HT1080 cells by suppressing α-enolase-plasminogen interaction and uPA-dependent plasminogen activation.
Fig. 4. Seahorse peptide attenuates uPA and plasminogen-dependent invasive migration of HT1080 cells. (A) The 0.1 M peptide treatment decreased uPA expression. (B, C) HT1080 cells were transfected with uPA or plasminogen siRNA. After transfection with siRNA, the cells were incubated for 48 h in the absence or presence of 0.1 M peptide and then the cells were analyzed by western blot or harvested for invasive migration assays. All data are expressed as the average of three different experiments. *P < 0.05 compared to controls RNAi.
DISCUSSION
For many years, α-enolase was solely regarded as a soluble cytosolic glycolytic enzyme. However, recent studies have indicated that α-enolase is a receptor and activator of plasminogen. α-Enolase binds plasminogen at the surface of prokaryotic pathogens such as invasive yeasts and parasites, as well as that of mammalian cells including peripheral blood cells, endothelial cells, neuronal cells, and cancer cells (4, 6, 14-16). The cascade of protease activity initiated by the activation of α-enolase-dependent plasminogen on the cell surface has significant implications on various physiological and pathological events such as wound healing, tissue remodeling, embryogenesis, and transformed tumor-cell spread. In particular, overexpression of α-enolase in various cancerous cells is more likely to enhance the plasminogen activation and hence facilitate the spread of tumor cells. The major biological role by plasminogen activation is to degrade the extracellular matrix of basement membranes, which is why α-enolase and plasminogen have been considered as diagnostic markers for many tumors.
α-Enolase is expressed in high levels in HT1080 cells. The uPA-mediated plasminogen activation in the presence of α-enolase is essential for the invasive migration of HT1080 cells. Seahorse-derived peptide decreases uPA-mediated plasminogen activation by inhibiting the interaction between α-enolase and plasminogen, which results in the inhibition of invasive migration of HT1080 cells. The study with A549, adenocarinomic human alveolar basal epithelial cells also showed similar inhibitory effect of peptide on intracellular interaction of α-enolase and plasminogen and on invasive migration of cells (Supplementary Fig. 3). Therefore, it may be assumed that this peptide affects the activity of α-enolase and plasminogen in various physiological and pathological events as well as in tumor invasion and cancer metastasis. This peptide therefore has the potential to emerge as a novel agent for the treatment of tumor cells. In addition, the peptide treatment decreases expression level of uPA. Peptide treatment caused decrease of uPA in both protein and messenger RNA level (Supplementary Fig. 2). Cell treatment with MG132, a proteasome inhibitor, did not affect uPA expression in the presence or absence of peptide, suggesting that peptide inhibits uPA expression at the transcriptional level. Furthermore, peptide was found to reduce NF-kB p65 protein levels in both nuclear and cytosolic fraction (Supplementary Fig. 2). Therefore, NF-KB is likely to be an important transcription regulator regulating uPA expression in invasive HT1080 cells. This finding provides important information to explore the role of peptide suppressing tumor progression.
The biological functionality of plasminogen activation is dependent on its interaction with α-enolase. It has been reported that the C-terminal lysines of α-enolase are involved in its interaction with plasminogen (4). However, several α-enolases that lack C-terminal lysines also exhibit plasminogen binding. An internal sequence (248FYDKERKVY256) in the enolase of Streptococcus pneumoniae was first identified as an additional plasminogen-binding site (17). The crystal structure of this protein demonstrated that this internal motif is the primary site for plasminogen binding (18). Similar internal plasminogen-binding motifs were found not only in the enolases of other pathogenic organisms, but also in the central loop of human α-enolase (4, 12, 19). A common feature of the internal plasminogen-binding regions is that the corresponding residues contain highly positively and negatively charged residues exposed to the surface, a characteristic feature that has been suggested to be important for plasminogen recognition. Since the seahorse- derived peptide also has highly charged residues, these charged residues may interfere with the interaction between α-enolase and plasminogen. In fact, peptide effect on α-enolase-plasminogen interaction in vitro was sensitive to the addition of NaCl (data not shown), indicating the influence of the electrical properties of the charged residues in the inhibitory mechanism of plasminogen-enolase interaction by peptide treatment. In addition, we also suggest another possibility that the peptide influences catalytic conformational changes of α-enolase, in particular in cells. We investigated whether the peptide is able to directly bind to plasminogen since the peptide sequence is similar to α-enolase. Peptide did not directly bind to plasminogen in vitro (data not shown). Nevertheless, peptide treatment decreases plasminogen-enolase interaction both in vitro and in vivo and its inhibitory effect is likely to be greater in cells than in vitro (Fig. 1C and Fig. 3B). The amino acid sequence proximal to the internal plasminogen-binding motif is highly homologous to the seahorse peptide amino acid sequence. This region, which is similar to the seahorse peptide sequence, has been known to be responsible for the catalytic conformational change through its dimerization (18). Therefore, peptide treatment probably influences such catalytic conformational changes of the α-enolase, which consequently may affect the interaction of α-enolase with plasminogen. Further studies using site-directed mutagenesis of the peptide, α-enolase, and plasminogen are required to more clearly understand the inhibitory mechanism of the peptide in the interaction between α-enolase and plasminogen. These studies in turn would demonstrate the critical role of the seahorse peptide as a potential therapeutic agent for cancer.
MATERIALS AND METHODS
Materials and Methods, were provided in Supplementary data: http://www.bmbreports.org/jbmb_by_volume.html?vol=47.
Acknowledgments
This work was supported by the Pukyong National University Research Fund in 2011(PK-C-D-2011-0673).
References
- 1.Takei N., Kondo J., Nagaike K., Ohsawa K., Kato K., Kohsaka S. Neuronal survival factor from bovine brain is identical to neuron-specific enolase. J. Neurochem. (1991);57:1178–1184. doi: 10.1111/j.1471-4159.1991.tb08277.x. [DOI] [PubMed] [Google Scholar]
- 2.Fluit A. C., Wolfhagen M. J., Jansze M., Torensma R., Verhoef J. Toxin B of Clostridium difficile does not have enolase activity. FEBS Lett. (1993);316:103–105. doi: 10.1016/0014-5793(93)81745-L. [DOI] [PubMed] [Google Scholar]
- 3.al-Giery A. G., Brewer J. M. Characterization of the interaction of yeast enolase with polynucleotides. Biochim. Biophys. Acta. (1992);1159:134–140. doi: 10.1016/0167-4838(92)90017-8. [DOI] [PubMed] [Google Scholar]
- 4.Vanegas G., Quinones W., Carrasco-Lopez C., Concepcion J. L., Albericio F., Avilan L. Enolase as a plasminogen binding protein in Leishmania mexicana. Parasitol. Res. (2007);101:1511–1516. doi: 10.1007/s00436-007-0668-7. [DOI] [PubMed] [Google Scholar]
- 5.Mundodi V., Kucknoor A. S., Alderete J. F. Immunogenic and plasminogen-binding surface-associated alpha-enolase of Trichomonas vaginalis. Infect. Immun. (2008);76:523–531. doi: 10.1128/IAI.01352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plow E. F., Felez J., Miles L. A. Cellular regulation of fibrinolysis. Thromb. Haemost. (1991);66:32–36. [PubMed] [Google Scholar]
- 7.Plow E. F., Herren T., Redlitz A., Miles L. A., Hoover-Plow J. L. The cell biology of the plasminogen system. FASEB J. (1995);9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 8.Wiman B., Collen D. On the mechanism of the reaction between human alpha 2-antiplasmin and plasmin. J. Biol. Chem. (1979);254:9291–9297. [PubMed] [Google Scholar]
- 9.Lancefield R. C. Differentiation of group A streptococci with a common R antigen into three serological types, with special reference to the bactericidal test. J. Exp. Med. (1957);106:525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gentile F., Pizzimenti S., Arcaro A., Pettazzoni P., Minelli R., D'Angelo D., Mamone G., Ferranti P., Toaldo C., Cetrangolo G., Formisano S., Dianzani M. U., Uchida K., Dianzani C., Barrera G. Exposure of HL-60 human leukaemic cells to 4-hydroxynonenal promotes the formation of adduct(s) with alpha-enolase devoid of plasminogen binding activity. Biochem. J. (2009);422:285–294. doi: 10.1042/BJ20090564. [DOI] [PubMed] [Google Scholar]
- 11.Ryu B., Qian Z. J., Kim S. K. Purification of a peptide from seahorse, that inhibits TPA-induced MMP, iNOS and COX-2 expression through MAPK and NF-kappaB activation, and induces human osteoblastic and chondrocytic differentiation. Chem. Biol. Interact. (2010);184:413–422. doi: 10.1016/j.cbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Quinones W., Pena P., Domingo-Sananes M., Caceres A., Michels P. A., Avilan L., Concepcion J. L. Leishmania mexicana: molecular cloning and characterization of enolase. Exp. Parasitol. (2007);116:241–251. doi: 10.1016/j.exppara.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell. Mol. Life Sci. (2001);58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle M. D., Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb. Haemost. (1997);77:1–10. [PubMed] [Google Scholar]
- 15.Coleman J. L., Benach J. L. Use of the plasminogen activation system by microorganisms. J. Lab. Clin. Med. (1999);134:567–576. doi: 10.1016/S0022-2143(99)90095-1. [DOI] [PubMed] [Google Scholar]
- 16.Lahteenmaki K., Edelman S., Korhonen T. K. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends. Microbiol. (2005);13:79–85. doi: 10.1016/j.tim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann S., Wild D., Diekmann O., Frank R., Bracht D., Chhatwal G. S., Hammerschmidt S. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol. Microbiol. (2003);49:411–423. doi: 10.1046/j.1365-2958.2003.03557.x. [DOI] [PubMed] [Google Scholar]
- 18.Ehinger S., Schubert W. D., Bergmann S., Hammerschmidt S., Heinz D. W. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: crystal structure and evaluation of plasmin(ogen)-binding sites. J. Mol. Biol. (2004);343:997–1005. doi: 10.1016/j.jmb.2004.08.088. [DOI] [PubMed] [Google Scholar]
- 19.Felez J., Chanquia C. J., Levin E. G., Miles L. A., Plow E. F. Binding of tissue plasminogen activator to human monocytes and monocytoid cells. Blood. (1991);78:2318–2327. [PubMed] [Google Scholar]
- 20.Del Pozo V., Rojo M., Rubio M. N., Cortegano I., Cardaba B., Gallardo S., Ortega M., Civantos E., Lopez E., Martin-Mosquero C., Peces-Barba G., Palomino P., Gonzalez-Mangado N., Lahoz C. Gene therapy with galectin-3 inhibits bronchial obstruction and inflammation in antigen-challenged rats through inerleukin-5 gene downregulation. Am. J. Respir. Crit. Care Med. (2002);166:732–737. doi: 10.1164/rccm.2111031. [DOI] [PubMed] [Google Scholar]