Abstract
Background/Aim
The present study explores associations between hemoglobin (Hb) levels and patients with cardiac enlargement in end-stage kidney disease (ESKD) to help prevent cardiac remodeling during the predialysis phase of chronic kidney disease (CKD).
Methods
This cross-sectional study included 2,249 patients with ESKD (age, 67 ± 13 years; male, 67%; diabetic kidney disease, 41%) who started hemodialysis (HD) between January 2006 and October 2013 at eight participating hospitals. We examined associations between Hb levels immediately before the first HD session and cardiothoracic ratios (CTRs). Clinical factors associated with the CTR were also assessed.
Results
The mean Hb level was 8.7 ± 1.6 g/dl, and the mean and median CTRs were 55.0 and 54.7%, respectively. The correlation between the Hb level and the CTR was linear and negative (r = −0.129, p < 0.001). The mean CTR and the prevalence of patients with a CTR >50% obviously decreased with increasing Hb levels (both p < 0.001 for trend). Univariate logistic regression analysis revealed an approximately 20% reduction in the odds ratio for complicating CTRs >50% per 1 g/dl increase in Hb. Hb levels of <9 g/dl were significantly associated with CTRs >50%. Numerical and categorical Hb remained significantly associated with CTRs >50% after adjusting for confounding variables.
Conclusions
Lower Hb levels participate in progressive CTR enlargement in patients with ESKD, and maintaining Hb levels of >9 g/dl might help prevent cardiac remodeling during the predialysis phase of CKD.
Key Words: Cardiothoracic ratio, Volume overload, Erythropoiesis-stimulating agent, Anemia, Left ventricular systolic dysfunction
Introduction
Anemia plays a crucial role in the promotion of cardiac remodeling and thus in the onset of cardiac events in patients with chronic kidney disease (CKD) [1,2]. However, more patients in the advanced stages of CKD develop anemia despite treatment with erythropoiesis-stimulating agents (ESA) [3]. Indeed, data from our previous multicenter study of Japanese patients on dialysis revealed that the mean hemoglobin (Hb) level at the start of dialysis was 8.5 g/dl [4], which is lower than the target level recommended by current international guidelines [5,6,7]. The incidence of cardiac events such as congestive heart failure and myocardial ischemia increases close to the time of starting dialysis [8,9]. We speculated that progressive anemia plays some part in the occurrence of cardiac remodeling and cardiac events in the advanced stages of CKD.
Cardiac enlargement is associated with adverse outcomes in patients with heart disease [10,11]. The size of the heart can be estimated from chest X-rays as a proportion of the thoracic diameter, namely, the cardiothoracic ratio (CTR) [12]. Most chronic congestive heart failures due to acquired left ventricular (LV) systolic dysfunction are associated with chamber dilation [13]. The CTR negatively correlates with cardiac systolic dysfunction in patients with congestive heart failure [14]. A CTR >50% is associated with a poorer prognosis in community-dwelling elderly populations aged nearly 80 years [15] as well as in patients with congestive heart failure [16] and in those on dialysis [17,18]. Therefore, CTR would be an easier way to determine the status of cardiac remodeling. However, there is little evidence to evaluate the association of the Hb level with the CTR in the predialytic phase of advanced CKD patients.
We postulated that lower Hb values would complicate cardiac enlargement, which indicates cardiac remodeling in patients with end-stage kidney disease (ESKD). The present study investigates associations between Hb levels and CTRs at the start of maintenance dialysis in a large multicenter cohort of Japanese patients with ESKD. The results of this study could help physicians to manage renal anemia in order to prevent cardiac remodeling during the predialysis phase of CKD.
Patients and Methods
Study Design and Patients
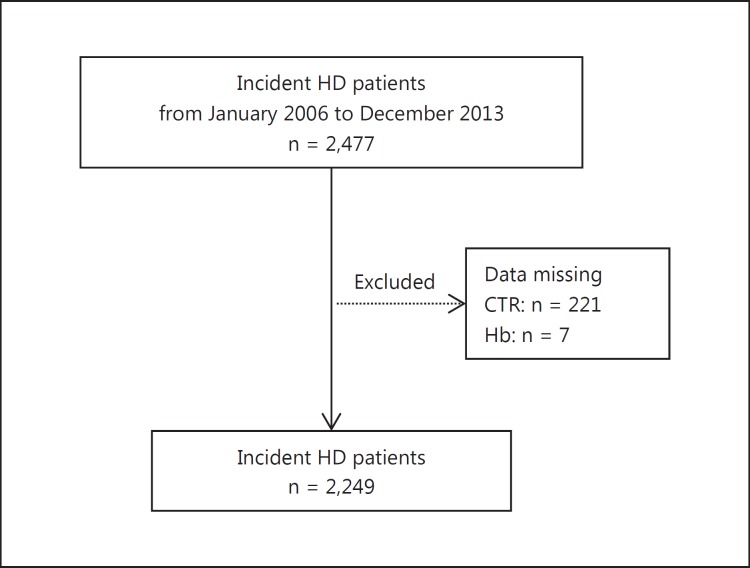
We conducted a cross-sectional study of information obtained from the database of the Japanese Study Group for Assessing Initiation of Renal Replacement Therapy (J-START), which includes the nephrology units at eight teaching hospitals in Japan. The objective of the J-START was to create a shared database of patients with ESKD at the time of starting renal replacement therapy for clinical research purposes. Between January 2006 and October 2013, 2,477 patients with ESKD started chronic dialysis at the eight hospitals. Clinical information about these patients was added to the J-START database. We excluded those with ESKD who had no Hb or CTR data. We therefore analyzed data from 2,249 patients with ESKD (fig. 1). Levels of Hb were measured immediately before the patients started their first hemodialysis (HD) session. Clinical factors associated with the CTR were also determined. The Ethics Committee for Clinical Research at the Toho University Ohashi Medical Center approved the study protocol (permission No. 13-52).
Fig. 1.
Schema of patient enrollment in the study.
Data Collection
Clinical information was collected from all patients at each nephrology unit immediately before their first HD session. Information about medication with ESA, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, calcium channel blockers, vitamin D receptor activator, and iron agents was also collected during the predialysis period. Blood pressure (BP) was recorded in the supine position, and blood samples were obtained immediately before the first HD session. Comorbid conditions at the start of dialysis were determined from medical records. The estimated glomerular filtration rate (eGFR) was calculated using the new Japanese equation [19]: eGFR (ml/min/1.73 m2) = 194 × creatinine-1.094 × age−0.287 (× 0.739 for women). The body mass index (BMI) at optimal weight was calculated as weight in kilograms divided by the square of height in meters.
Definition of Comorbid Disease
The presence or absence of cardiac, cerebral, and peripheral vascular disease was determined by medical record review. Cardiovascular diseases were defined as valvular heart disease, any cause of cardiomyopathy and myocarditis, pericarditis, endocarditis, and ischemic heart disease including myocardial infarction, acute coronary syndrome, and a history of coronary revascularization therapy in ESKD patients. Cerebrovascular diseases were defined as cerebral bleeding and infarction in ESKD patients. Peripheral vascular disease was defined as a history of amputation, gangrene, and clinically significant ischemic symptoms.
Reasons for Starting Dialysis
The J-START database includes information about the clinical reasons involved in deciding whether or not a patient should start a chronic dialysis therapy. ‘Volume overload’ means that patients with ESKD started dialysis due to excessive fluid retention such as lung congestion and/or massive edema. ‘Planned’ means that patients with ESKD and no critical sign of uremia started a schedule of dialysis based on discussions with their physicians.
Cardiothoracic Ratio
Standard chest radiography was performed on the day of the first HD session. The CTR was determined by dividing the maximal horizontal width of the heart by the horizontal inner width of the rib cage. To ensure accurate measurement, a vertical line was drawn on the radiograph through the midpoint of the spine from the sternum to the diaphragm. The maximum transverse diameter of the heart was determined by adding the widest distance of the right and left heart borders from and to the midline, respectively. This value was then divided by the maximum transverse diameter of the thorax. Patients with ESKD and cardiac enlargement were defined as having a CTR >50% in this study.
Statistical Analysis
Data are expressed as means ± SD or medians with interquartile ranges. Patient characteristics were compared across Hb levels using the χ2 test for categorical variables, analysis of variance for normally distributed variables, and the Kruskal-Wallis tests for nonnormally distributed variables. Trends that were in parallel with Hb concentrations were analyzed using the Jonckheere-Terpstra trend test as appropriate. Associations between various factors and the CTR or CTRs >50% were assessed using Pearson's regression analysis and logistic regression analysis. Dummy variables were used for gender (0 = female; 1 = male), primary renal diseases (0 = no; 1 = yes), and medication (0 = no; 1 = yes). Independent determinants of CTRs >50% were identified using multivariate regression analysis. A p value <0.05 was considered to indicate statistically significant differences in all tests. All data were statistically analyzed using SPSS for Windows version 20 (IBM, Armonk, N.Y., USA).
Results
Patient Characteristics
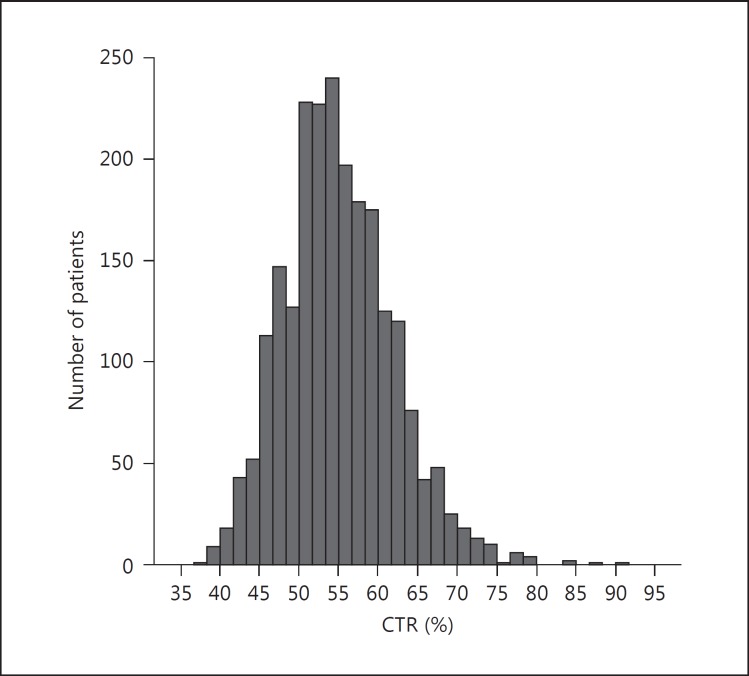
The mean age of the patient cohort was 67 ± 13 years. The ratios of males (67%) and of patients with diabetic kidney disease (41%) were similar to those reported for the entire Japanese population at the start of the dialysis [20]. The mean serum creatinine and eGFR at the start of the renal replacement therapy were 9.3 ± 3.5 mg/dl and 4.7 ± 2.6 ml/min/ 1.73 m2, respectively, being similar to the levels for the entire Japanese dialysis population [20]. Therefore, our cohort seemed representative of Japanese patients on dialysis in general. The mean Hb level was 8.7 ± 1.6 g/dl. The histogram in figure 2 shows that the distribution of the CTR was nearly normal. The mean and median CTRs were 55.0 and 54.7%, respectively, and 77% of our patients had an enlarged heart (CTR >50%). Table 1 shows other clinical characteristics of the patients.
Fig. 2.
Histogram of CTRs.
Table 1.
Characteristics of 2,249 patients with ESKD stratified according to Hb levels
| Total | Hb level |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| <7.0 g/dl | 7.0-7.9 g/dl | 8.0-8.9 g/dl | 9.0-9.9 g/dl | 10.0-10.9 g/dl | ≥11.0 g/dl | |||
| Patients, n | 2,249 | 299 | 417 | 568 | 501 | 312 | 152 | |
| Age, years | 67±13 | 66±14 | 68±13 | 67±13 | 68±14 | 69±13 | 67±14 | 0.059 |
| Male, % | 67 | 64 | 65 | 68 | 69 | 65 | 74 | 0.207 |
| BMI | 23.0±4.0 | 23.5±4.2 | 23.4±3.7 | 23.0±4.1 | 23.0±4.2 | 22.4±3.8 | 22.6±3.8 | 0.003 |
| Primary disease, % | 0.095 | |||||||
| Diabetic kidney disease | 41 | 36.1 | 42.2 | 44 | 42.5 | 38.1 | 36.2 | |
| Glomerulonephritis | 24.7 | 29.4 | 20.4 | 26.1 | 22.8 | 26 | 26.3 | |
| Renal sclerosis | 17.6 | 12.7 | 20.9 | 13.4 | 19 | 21.2 | 21.7 | |
| Polycystic kidney disease | 2.8 | 3.7 | 3.8 | 2.3 | 2.2 | 2.2 | 2.6 | |
| Obstructive kidney disease | 1 | 1.3 | 0.7 | 0.7 | 1.4 | 1 | 1.3 | |
| Others | 5.8 | 7.7 | 4.6 | 6.5 | 5.2 | 4.5 | 7.9 | |
| Unknown cause | 6.9 | 9 | 7.2 | 6.7 | 6.8 | 6.7 | 3.9 | |
| SBP, mm Hg | 152±25 | 150±23 | 152±26 | 151±26 | 152±24 | 151±27 | 152±27 | 0.907 |
| DBP, mm Hg | 77±15 | 77±15 | 77±15 | 76±15 | 77±15 | 78±16 | 78±16 | 0.234 |
| Cardiovascular disease, % | 21 | 12 | 19 | 26 | 22 | 22 | 16 | <0.001 |
| Cerebrovascular disease, % | 14 | 13 | 14 | 12 | 15 | 18 | 13 | 0.238 |
| Peripheral artery disease, % | 4 | 4 | 4 | 4 | 4 | 7 | 4 | 0.481 |
| Duration of nephrologist care, years | 3.1±6.9 | 2.3±3.4 | 3.4±8.3 | 3.1±5.6 | 3.4±8.9 | 3.4±7.1 | 3.0±3.5 | 0.250 |
| Cause of initiation, % | 37 | 41 | 42 | 38 | 35 | 32 | 27 | 0.003 |
| Volume overload | ||||||||
| Planned | 22 | 10 | 17 | 20 | 28 | 29 | 36 | <0.001 |
| Medication, | 69 | 60 | 71 | 73 | 71 | 68 | 68 | 0.006 |
| % ESA | ||||||||
| RAS-I | 58 | 53 | 58 | 59 | 61 | 59 | 56 | 0.335 |
| CCB | 71 | 63 | 69 | 74 | 75 | 72 | 57 | <0.001 |
| VDRA | 18 | 16 | 17 | 17 | 21 | 22 | 15 | 0.147 |
| Iron | 16 | 13 | 17 | 14 | 16 | 19 | 20 | 0.127 |
| Diuretics | 64 | 58 | 68 | 66 | 67 | 58 | 64 | 0.004 |
| Albumin, g/dl | 3.2±0.6 | 3.0±0.6 | 3.1±0.6 | 3.1±0.6 | 3.2±0.6 | 3.3±0.6 | 3.4±0.7 | <0.001 |
| Hb, g/dl | 8.7±1.6 | 6.2±0.7 | 7.5±0.3 | 8.5±0.3 | 9.4±0.3 | 10.4±0.3 | 11.9±0.9 | <0.001 |
| Fe, μg/dl | 58.4±38.0 | 55.3±42.5 | 57.1±46.8 | 56.1±34.0 | 60.2±32.7 | 67.6±41.3 | 60.7±31.8 | 0.017 |
| TSAT, % | 28.0±20.9 | 29.4±30.1 | 28.1±24.7 | 26.9±17.4 | 28.4±18.3 | 29.9±19.2 | 27.3±14.1 | 0.518 |
| Ferritin, ng/ml | 224±298 | 306±371 | 228±290 | 219±316 | 211±253 | 225±370 | 169±186 | <0.001 |
| Creatinine, mg/dl | 9.3±3.5 | 10.5±4.1 | 9.6±3.7 | 9.2±3.4 | 9.0±3.4 | 8.6±3.1 | 8.2±3.2 | <0.001 |
| eGFR, ml/min/1.73 m2 | 4.7±2.6 | 3.9±1.8 | 4.5±2.7 | 4.6±2.0 | 4.8±2.4 | 5.1±2.9 | 6.0±4.6 | <0.001 |
| Uric acid, mg/dl | 8.7±2.4 | 8.8±2.6 | 8.6±2.5 | 8.7±2.4 | 8.7±2.6 | 8.5±2.2 | 9.3±2.9 | 0.053 |
| Corrected calcium, mg/dl | 8.6±1.0 | 8.5±1.1 | 8.6±1.0 | 8.5±1.0 | 8.6±0.9 | 8.7±1.0 | 8.8±1.2 | 0.02 |
| Phosphate, mg/dl | 6.3±1.8 | 6.7±2.2 | 6.4±1.8 | 6.2±1.8 | 6.1±1.7 | 6.1±1.7 | 6.2±1.9 | <0.001 |
| Intact PTH, pg/ml | 322±261 | 339±273 | 333±335 | 314±239 | 305±187 | 348±296 | 293±211 | 0.152 |
| C-reactive protein, mg/dl | 2.0±4.2 | 2.9±4.9 | 2.1±3.8 | 1.9±3.9 | 1.9±4.6 | 1.6±3.6 | 1.8±4.7 | 0.005 |
| CTR, % | 55.0±7.0 | 56.4±7.0 | 55.9±6.8 | 55.2±6.9 | 54.0±6.8 | 54.3±73 | 54.0±7.0 | <0.001 |
| CTR >50%, % | 77 | 85 | 83 | 79 | 72 | 72 | 68 | <0.001 |
Figures are means±SD unless indicated otherwise. CCB = Calcium channel blockers; DBP = diastolic BP; intact PTH = intact parathyroid hormone; RAS-I = renin-angiotensin system inhibitor; SBP = systolic BP; TSAT = transferrin saturation; VDRA = vitamin D receptor activator.
Correlation between the Hb Level and CTR and Other Clinical Characteristics of Patients with ESKD
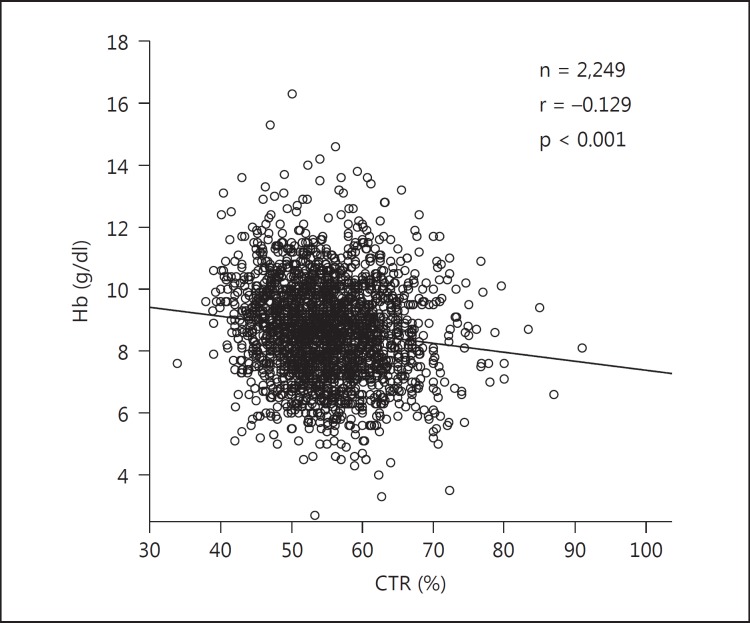
Figure 3 shows a weak, but significant linear and negative correlation between the Hb level and CTR (r = −0.129, p < 0.001) in the patients. Table 1 displays the clinical characteristics of the patients stratified according to their Hb level. The mean CTR and the rate of patients with a CTR >50% obviously decreased with increasing Hb levels (p < 0.001 for both trends). The trends for serum albumin and eGFR significantly increased, whereas that of volume overload significantly decreased with increasing Hb levels (p < 0.001 for all three trends). The presence of cardiovascular disease slightly increased in parallel with increasing Hb levels, but the significance was near borderline (p = 0.047 for trend).
Fig. 3.
Association between the Hb level and CTR in patients with ESKD at the start of dialysis. The linear correlation between Hb and CTR is significant and negative (r = −0.129, p < 0.001).
Odds for Complicating CTRs >50%
Factors associated with CTRs >50% were assessed using univariate logistic regression analysis. Table 2 shows a significant negative association between the Hb level and CTRs >50%. The odds ratio (OR) for complicating CTRs >50% was reduced by about 20% per 1 g/dl increase in Hb. Categorical assessment showed that lower Hb was associated with higher odds for complicating CTRs >50%, and an Hb level of <9 g/dl was significantly associated with CTRs >50%. Significant associations were similarly inverse between CTRs >50% and serum albumin as well as creatinine. The use of ESA, which is the most popular medicine for renal anemia, and the use of renin-angiotensin system inhibitor, which is one of the potential medicines for preventing cardiac remodeling, had a negative association with CTRs >50%. Cardiovascular disease and volume overload were closely associated with CTRs >50% as we expected.
Table 2.
Logistic regression analysis of factors associated with a CTR >50%
| OR | 95% CI | p | |
|---|---|---|---|
| Age (years) | 1.015 | 1.007 – 1.024 | 0.001 |
| Malea | 0.511 | 0.406 – 0.644 | <0.001 |
| BMI | 1.067 | 1.038 – 1.096 | <0.001 |
| Diabetic kidney diseaseb | 1.046 | 0.855 – 1.279 | 0.663 |
| SBP (mm Hg) | 1.004 | 1.000 – 1.008 | 0.067 |
| DBP (mm Hg) | 0.994 | 0.988 – 1.001 | 0.08 |
| Cardiovascular diseaseb | 1.506 | 1.157 – 1.959 | 0.002 |
| Cerebrovascular diseaseb | 1.084 | 0.813 – 1.447 | 0.582 |
| Peripheral artery diseaseb | 1.476 | 0.868 – 2.509 | 0.151 |
| Duration of nephrologist care (years) | 1.003 | 0.988 – 1.019 | 0.674 |
| Volume overloadc | 3.131 | 2.459 – 3.986 | <0.001 |
| Plannedd | 0.454 | 0.365 – 0.566 | <0.001 |
| ESAe (%) | 0.752 | 0.602 – 0.938 | 0.011 |
| RAS-Ie (%) | 0.812 | 0.663 – 0.994 | 0.044 |
| VDRAe (%) | 0.626 | 0.492 – 0.796 | <0.001 |
| Irone (%) | 1.021 | 0.779 – 1.339 | 0.878 |
| Diureticse (%) | 1.466 | 1.199 – 1.794 | <0.001 |
| Albumin (g/dl) | 0.667 | 0.564 – 0.789 | <0.001 |
| Hb (g/dl) | 0.815 | 0.765 – 0.869 | <0.001 |
| Fe µg/dl) | 0.993 | 0.991 – 0.996 | <0.001 |
| TSAT (%) | 0.994 | 0.989 – 0.998 | 0.008 |
| Ferritin (ng/ml) | 1.001 | 1.000 – 1.001 | 0.009 |
| Creatinine (mg/dl) | 0.933 | 0.908 – 0.958 | <0.001 |
| eGFR (ml/min/1.73 m2) | 1.077 | 1.028 – 1.128 | <0.001 |
| Corrected calcium (mg/dl) | 0.964 | 0.871 – 1.067 | 0.477 |
| Phosphate (mg/dl) | 0.971 | 0.920 – 1.025 | 0.284 |
| Intact PTH (pg/ml) | 1.000 | 1.000 – 1.000 | 0.708 |
| C-reactive protein (mg/dl) | 1.078 | 1.042 – 1.116 | <0.001 |
| Hb | |||
| <7.0 g/dl | 2.757 | 1.728 – 4.398 | <0.001 |
| 7.0 – 7.9 g/dl | 2.318 | 1.515 – 3.547 | <0.001 |
| 8.0 – 8.9 g/dl | 1.757 | 1.184 – 2.609 | 0.005 |
| 9.0 – 9.9 g/dl | 1.251 | 0.845 – 1.853 | 0.263 |
| 10.0 – 10.9 g/dl | 1.211 | 0.796 – 1.843 | 0.372 |
| ≥11.0 g/dl | 1.000 | ||
DBP = Diastolic BP; intact PTH = intact parathyroid hormone; RAS-I = renin-angiotensin system inhibitor; SBP = systolic BP; TSAT = transferrin saturation; VDRA = vitamin D receptor activator.
Male vs. female.
Patients with vs. without the disease.
Volume overload vs. no volume overload.
Planned vs. not planned.
Medication: user vs. nonuser.
Hb Is Independently Associated with CTRs >50%
Table 3 shows six models of multivariate logistic regression analysis. Numerical and categorical Hb values were significantly associated with CTRs >50% after adjusting for age, sex, BMI, diabetic kidney disease, and duration of nephrologist care in model 1. Similar to the results of the univariate analysis, an approximately 20% reduction in the OR per 1 g/dl increase in Hb and a higher OR for an Hb level of <9 g/dl remained statistically significant after adjustment. Hb levels were independently associated with CTRs >50% after adjusting for systolic BP, renin-angiotensin system inhibitor, calcium channel blockers, diuretics, previous cardiovascular disease, and volume overload in model 2, for serum data, albumin, eGFR, and C-reactive protein in model 3, for ESA, iron agent, Fe, transferrin saturation, and ferritin in model 4, for vitamin D receptor activator, serum calcium, phosphate, and intact parathyroid hormone in model 5, and for all factors included in models 1-5 in model 6.
Table 3.
Independent association between Hb and CTR >50%
| Model 1 |
Model 2 |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Hb | 0.816 | 0.764 – 0.873 | <0.001 | 0.832 | 0.779 – 0.888 | <0.001 |
| <7.0 g/dl | 2.795 | 1.713 – 4.559 | <0.001 | 2.581 | 1.600 – 4.164 | <0.001 |
| 7.0 – 7.9 g/dl | 2.157 | 1.380 – 3.371 | 0.001 | 2.057 | 1.329 – 3.185 | 0.001 |
| 8.0 – 8.9 g/dl | 1.710 | 1.129 – 2.591 | 0.011 | 1.583 | 1.053 – 2.380 | 0.027 |
| 9.0 – 9.9 g/dl | 1.210 | 0.800 – 1.831 | 0.366 | 1.142 | 0.762 – 1.714 | 0.52 |
| 10.0 – 10.9 g/dl | 1.118 | 0.718 – 1.743 | 0.621 | 1.177 | 0.763 – 1.815 | 0.462 |
| ≥11.0 g/dl | 1 | 1 | ||||
|
Model 3 |
Model 4 |
|||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Hb | 0.796 | 0.742 – 0.854 | <0.001 | 0.813 | 0.757 – 0.873 | <0.001 |
| <7.0 g/dl | 3.192 | 1.935 – 5.266 | <0.001 | 2.758 | 1.659 – 4.586 | <0.001 |
| 7.0 – 7.9 g/dl | 2.785 | 1.769 – 4.384 | <0.001 | 2.48 | 1.560 – 3.943 | <0.001 |
| 8.0 – 8.9 g/dl | 1.944 | 1.280 – 2.950 | 0.002 | 1.962 | 1.273 – 3.023 | 0.002 |
| 9.0 – 9.9 g/dl | 1.429 | 0.945 – 2.161 | 0.091 | 1.477 | 0.959 – 2.275 | 0.076 |
| 10.0 – 10.9 g/dl | 1.307 | 0.842 – 2.029 | 0.232 | 1.284 | 0.812 – 2.030 | 0.285 |
| ≥11.0 g/dl | 1 | 1 | ||||
|
Model 5 |
Model 6 |
|||||
| OR | 95% CI | p | OR | 95% CI | p | |
| Hb | 0.775 | 0.721 – 0.823 | <0.001 | 0.798 | 0.732 – 0.869 | <0.001 |
| <7.0 g/dl | 3.562 | 2.117 – 5.993 | <0.001 | 3.369 | 1.838 – 6.178 | <0.001 |
| 7.0 – 7.9 g/dl | 2.819 | 1.764 – 4.506 | <0.001 | 2.517 | 1.462 – 4.334 | 0.001 |
| 8.0 – 8.9 g/dl | 2.027 | 1.310 – 3.135 | 0.002 | 2.117 | 1.274 – 3.517 | 0.004 |
| 9.0 – 9.9 g/dl | 1.454 | 0.943 – 2.244 | 0.091 | 1.512 | 0.917 – 2.493 | 0.106 |
| 10.0 – 10.9 g/dl | 1.274 | 0.802 – 2.023 | 0.304 | 1.295 | 0.761 – 2.203 | 0.341 |
| ≥11.0 g/dl | 1 | 1 | ||||
Each model was analyzed by adjusting for the factors as follows:
Model 1: adjusting for age, sex, BMI, diabetic kidney disease, and duration of nephrologist care.
Model 2: adjusting for systolic BP, renin-angiotensin system inhibitor, calcium channel blockers, diuretics, previous cardiovascular disease, and volume overload.
Model 3: adjusting for albumin, eGFR, and C-reactive protein.
Model 4: adjusting for ESA, iron, Fe, transferrin saturation, and ferritin.
Model 5: adjusting for vitamin D receptor activator, calcium, phosphate, and intact parathyroid hormone. Model 6: adjusting for all factors included in models 1 – 5.
Discussion
Main Findings
The important findings of this multicenter study were as follows: the Hb level at the start of dialysis was closely associated with the CTR, which is a surrogate marker of cardiac enlargement; this association was independent of heart disease, volume overload, serum albumin level or renal function, all of which are potentially connected with an enlarged heart, and the association between an Hb level of <9.0 g/dl and CTRs >50% was closer. These findings suggest that renal anemia plays a role in promoting cardiac hypertrophy. Thus, maintaining Hb at a level of >9.0 g/dl during the predialysis phase of CKD could be a possibility to prevent CTRs >50%.
Assessment of the Impact of Hb on CTRs >50%
Hb plays an important role in delivering oxygen to all organs and peripheral tissues. The initial hemodynamic response to anemia is a decrease in vascular resistance [21], which causes a slight reduction in systemic BP. This response leads to a secondary compensation phenomenon to increase sympathetic activity [22,23], which induces peripheral vasoconstriction, resulting in a reduction in renal blood flow and glomerular filtration rate, as well as salt and water retention with subsequent expansion of the extracellular plasma volume that is associated with an increase in cardiac output [24]. A higher cardiac workload increases the LV mass and remodels it with more myocardial fibrosis deposition that consequently leads to cardiac events [25]. Therefore, chronic renal anemia is closely associated with the progression of cardiac remodeling.
Previous studies have associated Hb levels with the LV mass index [26] and found that correcting anemia improves the LV mass index in patients with CKD [27]. Considering the cardiac remodeling process due to anemia described above, increasing the extracellular plasma volume is one of the initial triggers of progression of cardiac remodeling. Therefore, eccentric hypertrophy, which is remodeling for adaptation to volume overload, occurs in the heart of patients with chronic anemia. The CTR is the simplest way to evaluate cardiac enlargement, which is one of the manifestations of eccentric cardiac hypertrophy. Thus, our main finding of a negative association between the CTR and the Hb level is relevant to these processes.
Evaluation for Cardiac Remodeling
An echocardiogram is the gold standard used to precisely analyze the degree of cardiac remodeling and to distinguish the type of cardiac remodeling, i.e. concentric or eccentric. Unfortunately, we do not have any echocardiogram data of our subjects. However, we feel that we have enough material to explore the association of the Hb level with the CTR. To our knowledge, there is no article on the influence of anemia on the CTR in nondialyzed ESKD patients. A recent report has shown that the Hb level is inversely associated with the CTR independent of confoundable variables in chronic peritoneal dialysis patients [18]. Our study has confirmed the same finding at the initiation of the dialysis, which may result from the management of anemia in the predialytic phase of the CKD course.
An echocardiogram is not used easily and routinely in the ‘real-world’ clinical setting in this field, especially in small clinics. A Japanese guideline for cardiovascular disease has recommended chest X-ray (CTR) as a first-line evaluation of cardiac disease in ESKD patients [28]. Therefore, evaluating the CTR as a marker of cardiac remodeling in ESKD patients is essential for first-line screening.
Hb Levels <9.0 g/dl and CTRs >50%
The OR for a complicating CTR >50% was higher in patients with lower Hb values. Foley et al. [2] showed that the LV volume index determined by echocardiography, a marker of cardiac enlargement, closely correlated with Hb levels in patients on HD. A fall in the Hb level of 1 g/dl was associated with an increase in the volume index of 8 ml/m2, which is supported by our finding that higher odds of a CTR >50% are associated with lower Hb levels. Akaishi et al. [29] evaluated the effects of anemia management with ESA in patients with CKD. They found that Hb levels after 32 weeks significantly correlated with changes in LV diastolic diameter; i.e. changes in LV diastolic diameter increased as Hb levels decreased. They also found a significant increase in LV diastolic diameter in CKD patients with an achieved Hb level of <10 g/dl after 32 weeks of ESA management. Our finding of a significant association between an Hb level of <9 g/dl and CTR >50% supports their results.
Implication of the CTR in Patients with ESKD
The mean and median CTRs in the present study were about 55%, which is similar to that found in a previous study of patients on HD [30]. On the other hand, about 75% of our patients had a CTR >50%, which was higher than the 42% [17], 59% [31] and 67% [30] previously described, although the prevalence of cardiovascular disease was about 20% in the present and previous studies. We used a database that was created from information obtained when patients with ESKD started renal replacement therapy. Indeed, about 40% of our patients had volume overload. This could explain why the prevalence of CTR >50% was higher in our patients with ESKD than in previous studies.
If a higher CTR indicates cardiac remodeling even in patients with ESKD, then the CTR could be a predictor of mortality in such patients. Recent studies have shown that the CTR has a considerable impact on predicting mortality in patients on HD [17,][31]. Therefore, these findings imply that an appropriate management of renal anemia would improve the prognosis of patients with ESKD on dialysis by preventing or retarding the progression of cardiac remodeling.
Hb Level at the Initiation of Dialysis
The Japanese guidelines for renal anemia in CKD recommend that ESA therapy in nondialysis CKD patients should target an Hb level of 11 g/dl or higher [7], which is a similar Hb level to those of Western countries [6,32]. About 60% of ESKD patients in our subject cohort showed an Hb level of <9.0 g/dl at the initiation of dialysis, which is far from the guideline target. However, this is not quite an unusual phenomenon in the real-world clinical setting. For instance, even among CKD patients who receive an ESA therapy, a higher percentage of anemia with Hb levels of <11 g/dl is observed in Japanese patients in the more advanced stages of CKD [3]. Also, recent data from a multicenter cohort study of 2,434 Japanese CKD patients revealed that the mean Hb level at the initiation of dialysis was 8.3 g/dl [33]. Moreover, in the United States, about 75% of ESKD patients showed an Hb concentration of <11 g/dl at the initiation of the dialysis [34]. These results may imply that an unachievable Hb target in the advanced stage of CKD and/or at the initiation of dialysis accelerates cardiac remodeling.
Hb levels in our study were measured by using the samples obtained immediately before the first dialysis session. Low responsiveness of ESA therapy is often seen just before the initiation of dialysis [33]. The accumulation of factors associated with the low responsiveness of ESA therapy, such as severe secondary hyperparathyroidism, inflammation, and cumulative uremic toxins among others, is considered to be the cause of it. Treatment with ESA might not sufficiently correct anemia in patients with severely reduced renal function. The relatively low maximum dose of erythropoietin approved in Japan (6,000 IU/week) may further limit the improvement in the Hb level.
Limitations
The cross-sectional design of the present study imposed the major limitation that a causal effect of Hb levels and CTRs >50% could not be confirmed. Our database did not provide any information about longitudinal Hb levels during the predialysis phase of CKD. Moreover, volume overload might decrease Hb levels via hemodilution. We minimized such bias by including previous cardiovascular disease and volume overload, which have a significant and positive impact on a complicating enlarged heart in multivariate analyses. The association of the Hb level with CTR >50% remained statistically significant after adjusting for these markers. Another limitation of the present study is that CTR does not always mean cardiac enlargement. For instance, significant pericardial effusion that occasionally arises in patients with ESKD at the start of dialysis causes a higher CTR. Therefore, we included factors associated with pericardial effusion, namely, volume overload, diabetes mellitus, serum albumin level, and the inflammation marker C-reactive protein, in a multivariate analysis. The association between the Hb level and CTR >50% remained statistically significant after adjusting for these markers. Although echocardiographic measurements could minimize such bias, we unfortunately did not have any echocardiographic data of our subjects. Therefore, we could not distinguish precisely between concentric and eccentric forms of LV hypertrophy, or other factors that caused a CTR >50%. Also, it is not clear whether or not the significant association of Hb levels with CTR remains after adjusting for echocardiographic parameters. Finally, we did not assess the intra- or interobserver variability for the measurement of CTR. It is undeniable that this could have biased our findings.
Conclusions
Hb levels were significantly and negatively associated with CTR, which is a surrogate marker of cardiac enlargement in patients with ESKD. The risk for complicating CTR >50% remarkably increased at an Hb level of <9 g/dl. Maintaining Hb levels of >9 g/dl might help prevent cardiac remodeling during the predialysis phase of CKD.
Disclosure Statement
None of the authors has any conflicts of interest to declare in connection with this paper.
Acknowledgements
The authors are grateful to Hiroyuki Sekihara, Moriaki Osaka, Hiroyuki Miyakawa, Hiromi Okamoto, Kazuhiko Kitano, and Ryoji Kijima for managing the J-START database.
References
- 1.Silva RP, Barbosa PH, Kimura OS, Sobrinho CR, Sousa Neto JD, Silva FA, Silva Junior GB, Mota RM, Daher EF. Prevalence of anemia and its association with cardio-renal syndrome. Int J Cardiol. 2007;120:232–236. doi: 10.1016/j.ijcard.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Foley RN, Parfrey PS, Morgan J, Barre PE, Campbell P, Cartier P, Coyle D, Fine A, Handa P, Kingma I, Lau CY, Levin A, Mendelssohn D, Muirhead N, Murphy B, Plante RK, Posen G, Wells GA. Effect of hemoglobin levels in hemodialysis patients with asymptomatic cardiomyopathy. Kidney Int. 2000;58:1325–1335. doi: 10.1046/j.1523-1755.2000.00289.x. [DOI] [PubMed] [Google Scholar]
- 3.Akizawa T, Makino H, Matsuo S, Watanabe T, Imai E, Nitta K, Ohashi Y, Hishida A. Management of anemia in chronic kidney disease patients: baseline findings from Chronic Kidney Disease Japan Cohort Study. Clin Exp Nephrol. 2011;15:248–257. doi: 10.1007/s10157-010-0396-7. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka Y, Joki N, Hase H, Iwasaki M, Ikeda M, Ando R, Shinoda T, Inaguma D, Sakaguchi T, Komatsu Y, Koiwa F, Yamaka T, Shigematsu T. Effect of erythropoietin-stimulating agent on uremic inflammation. J Inflamm (Lond) 2012;9:17. doi: 10.1186/1476-9255-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drueke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s) Kidney Int. 2012;82:952–960. doi: 10.1038/ki.2012.270. [DOI] [PubMed] [Google Scholar]
- 6.KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease 2007 update of hemoglobin target. Am J Kidney Dis. 2007;50:471–530. doi: 10.1053/j.ajkd.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Tsubakihara Y, Nishi S, Akiba T, Hirakata H, Iseki K, Kubota M, Kuriyama S, Komatsu Y, Suzuki M, Nakai S, Hattori M, Babazono T, Hiramatsu M, Yamamoto H, Bessho M, Akizawa T. 2008 Japanese Society for Dialysis Therapy: guidelines for renal anemia in chronic kidney disease. Ther Apher Dial. 2010;14:240–275. doi: 10.1111/j.1744-9987.2010.00836.x. [DOI] [PubMed] [Google Scholar]
- 8.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 9.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Johnson GR, Shabetai R, Loeb H, Tristani F, Rector T, Smith R, Fletcher R. Ejection fraction, peak exercise oxygen consumption, cardiothoracic ratio, ventricular arrhythmias, and plasma norepinephrine as determinants of prognosis in heart failure. The V-HeFT VA Cooperative Studies Group. Circulation. 1993;87:VI5–VI16. [PubMed] [Google Scholar]
- 11.Goor DA, Golan M, Bar-El Y, Modan M, Lusky A, Rozenman J, Mohr R. Synergism between infarct-borne left ventricular dysfunction and cardiomegaly in increasing the risk of coronary bypass surgery. J Thorac Cardiovasc Surg. 1992;104:983–989. [PubMed] [Google Scholar]
- 12.Hammermeister KE, Chikos PM, Fisher L, Dodge HT. Relationship of cardiothoracic ratio and plain film heart volume to late survival. Circulation. 1979;59:89–95. doi: 10.1161/01.cir.59.1.89. [DOI] [PubMed] [Google Scholar]
- 13.Parmley WW. Pathophysiology and current therapy of congestive heart failure. J Am Coll Cardiol. 1989;13:771–785. doi: 10.1016/0735-1097(89)90215-5. [DOI] [PubMed] [Google Scholar]
- 14.Philbin EF, Garg R, Danisa K, Denny M, Gosselin G, Hassapoyannes C, Horney A, Johnstone DE, Lang RM, Ramanathan K, Safford RE, Sarma RJ, Weiss R, Williford WO, Fleg JL. The relationship between cardiothoracic ratio and left ventricular ejection fraction in congestive heart failure. Digitalis Investigation Group. Arch Intern Med. 1998;158:501–506. doi: 10.1001/archinte.158.5.501. [DOI] [PubMed] [Google Scholar]
- 15.Frishman WH, Nadelmann J, Ooi WL, Greenberg S, Heiman M, Kahn S, Guzik H, Lazar EJ, Aronson M. Cardiomegaly on chest X-ray: prognostic implications from a ten-year cohort study of elderly subjects: a report from the Bronx Longitudinal Aging Study. Am Heart J. 1992;124:1026–1030. doi: 10.1016/0002-8703(92)90987-7. [DOI] [PubMed] [Google Scholar]
- 16.Spinar J, Vitovec J, Spac J, Blaha M, Spinarova L, Toman J. Non-invasive prognostic factors in chronic heart failure. One-year survival of 300 patients with a diagnosis of chronic heart failure due to ischemic heart disease or dilated cardiomyopathy. Int J Cardiol. 1996;56:283–288. doi: 10.1016/0167-5273(96)02740-4. [DOI] [PubMed] [Google Scholar]
- 17.Chen KH, Lin-Tan DT, Huang WH, Hung CC, Chang CT, Huang JY, Lin JL. Cardiothoracic ratio, malnutrition, inflammation, and two-year mortality in non-diabetic patients on maintenance hemodialysis. Kidney Blood Press Res. 2008;31:143–151. doi: 10.1159/000127388. [DOI] [PubMed] [Google Scholar]
- 18.Chen KH, Hung CC, Lin-Tan DT, Huang WH, Hsu CW, Weng SM, Lin JL. Cardiothoracic ratio association with mortality in patients on maintenance peritoneal dialysis. Ther Apher Dial. 2011;15:81–88. doi: 10.1111/j.1744-9987.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Nakai S, Iseki K, Itami N, Ogata S, Kazama JJ, Kimata N, Shigematsu T, Shinoda T, Shoji T, Suzuki K, Taniguchi M, Tsuchida K, Nakamoto H, Nishi H, Hashimoto S, Hasegawa T, Hanafusa N, Hamano T, Fujii N, Masakane I, Marubayashi S, Morita O, Yamagata K, Wakai K, Wada A, Watanabe Y, Tsubakihara Y. An overview of regular dialysis treatment in Japan (as of 31 December 2010) Ther Apher Dial. 2012;16:483–521. doi: 10.1111/j.1744-9987.2012.01143.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, Leung JM, Fisher DM, Murray WR, Toy P, Moore MA. Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA. 1998;279:217–221. doi: 10.1001/jama.279.3.217. [DOI] [PubMed] [Google Scholar]
- 22.Franchitto N, Despas F, Labrunee M, Vaccaro A, Lambert E, Lambert G, Galinier M, Senard JM, Pathak A. Cardiorenal anemia syndrome in chronic heart failure contributes to increased sympathetic nerve activity. Int J Cardiol. 2013;168:2352–2357. doi: 10.1016/j.ijcard.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Franchitto N, Despas F, Labrunee M, Roncalli J, Boveda S, Galinier M, Senard JM, Pathak A. Tonic chemoreflex activation contributes to increased sympathetic nerve activity in heart failure-related anemia. Hypertension. 2010;55:1012–1017. doi: 10.1161/HYPERTENSIONAHA.109.146779. [DOI] [PubMed] [Google Scholar]
- 24.Brannon ES, Merrill AJ, Warren JV, Stead EA. The cardiac output in patients with chronic anemia as measured by the technique of right atrial catheterization. J Clin Invest. 1945;24:332–336. doi: 10.1172/JCI101610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metivier F, Marchais SJ, Guerin AP, Pannier B, London GM. Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant. 2000;15((suppl 3)):14–18. doi: 10.1093/oxfordjournals.ndt.a027970. [DOI] [PubMed] [Google Scholar]
- 26.Levin A. Anemia and left ventricular hypertrophy in chronic kidney disease populations: a review of the current state of knowledge. Kidney Int Suppl. 2002;80:35–38. doi: 10.1046/j.1523-1755.61.s80.7.x. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi T, Suzuki A, Shoji T, Togawa M, Okada N, Tsubakihara Y, Imai E, Hori M. Cardiovascular effect of normalizing the hematocrit level during erythropoietin therapy in predialysis patients with chronic renal failure. Am J Kidney Dis. 2000;35:250–256. doi: 10.1016/s0272-6386(00)70334-9. [DOI] [PubMed] [Google Scholar]
- 28.Hirakata H, Nitta K, Inaba M, Shoji T, Fujii H, Kobayashi S, Tabei K, Joki N, Hase H, Nishimura M, Ozaki S, Ikari Y, Kumada Y, Tsuruya K, Fujimoto S, Inoue T, Yokoi H, Hirata S, Shimamoto K, Kugiyama K, Akiba T, Iseki K, Tsubakihara Y, Tomo T, Akizawa T. Japanese Society for Dialysis Therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial. 2012;16:387–435. doi: 10.1111/j.1744-9987.2012.01088.x. [DOI] [PubMed] [Google Scholar]
- 29.Akaishi M, Hiroe M, Hada Y, Suzuki M, Tsubakihara Y, Akizawa T. KRN321 Study Group. Effect of anemia correction to the modestly high hemoglobin level in patients with chronic kidney disease on left ventricular hypertrophy. J Cardiol. 2013;62:249–256. doi: 10.1016/j.jjcc.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Bohn E, Tangri N, Gali B, Henderson B, Sood MM, Komenda P, Rigatto C. Predicting risk of mortality in dialysis patients: a retrospective cohort study evaluating the prognostic value of a simple chest X-ray. BMC Nephrol. 2013;14:263. doi: 10.1186/1471-2369-14-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yen TH, Lin JL, Lin-Tan DT, Hsu KH. Cardiothoracic ratio, inflammation, malnutrition, and mortality in diabetes patients on maintenance hemodialysis. Am J Med Sci. 2009;337:421–428. doi: 10.1097/MAJ.0b013e31819bbec1. [DOI] [PubMed] [Google Scholar]
- 32.KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int. 2012;2(Suppl):279–279. [Google Scholar]
- 33.Akizawa T, Saito A, Gejyo F, Suzuki M, Nishizawa Y, Tomino Y, Tsubakihara Y, Akiba T, Hirakata H, Watanabe Y, Kawanishi H, Bessho M, Udagawa Y, Aoki K, Uemura Y, Ohashi Y. Co-JET Study Group. Impacts of recombinant human erythropoietin treatment during predialysis periods on the progression of chronic kidney disease in a large-scale cohort study (Co-JET study) Ther Apher Dial. 2014;18:140–148. doi: 10.1111/1744-9987.12066. [DOI] [PubMed] [Google Scholar]
- 34.Bradbury BD, Fissell RB, Albert JM, Anthony MS, Critchlow CW, Pisoni RL, Port FK, Gillespie BW. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Clin J Am Soc Nephrol. 2007;2:89–99. doi: 10.2215/CJN.01170905. [DOI] [PubMed] [Google Scholar]