Significance
Pancreatic ductal adenocarcinoma (PDAC) is projected to become the second deadliest cancer by 2030. Advances in therapeutic treatments are urgently required to fight against this fatal disease. Here, elucidation of the metabolic signature of PDAC has identified the low-density lipoprotein receptor (LDLR), which facilitates cholesterol uptake, as a promising therapeutic target. Blocking of LDLR reduces the proliferative and clonogenic potential of PDAC cells and decreases activation of the ERK1/2 survival pathway. Moreover, LDLR silencing sensitizes PDAC cells to chemotherapeutic drugs and potentiates the tumoral regression promoted by chemotherapy. Finally, Ldlr is highly expressed at all stages of human PDAC and expression is associated with an increased risk of PDAC recurrence.
Keywords: LDLR, cholesterol, pancreatic cancer, metabolism, gemcitabine
Abstract
The malignant progression of pancreatic ductal adenocarcinoma (PDAC) is accompanied by a profound desmoplasia, which forces proliferating tumor cells to metabolically adapt to this new microenvironment. We established the PDAC metabolic signature to highlight the main activated tumor metabolic pathways. Comparative transcriptomic analysis identified lipid-related metabolic pathways as being the most highly enriched in PDAC, compared with a normal pancreas. Our study revealed that lipoprotein metabolic processes, in particular cholesterol uptake, are drastically activated in the tumor. This process results in an increase in the amount of cholesterol and an overexpression of the low-density lipoprotein receptor (LDLR) in pancreatic tumor cells. These findings identify LDLR as a novel metabolic target to limit PDAC progression. Here, we demonstrate that shRNA silencing of LDLR, in pancreatic tumor cells, profoundly reduces uptake of cholesterol and alters its distribution, decreases tumor cell proliferation, and limits activation of ERK1/2 survival pathway. Moreover, blocking cholesterol uptake sensitizes cells to chemotherapeutic drugs and potentiates the effect of chemotherapy on PDAC regression. Clinically, high PDAC Ldlr expression is not restricted to a specific tumor stage but is correlated to a higher risk of disease recurrence. This study provides a precise overview of lipid metabolic pathways that are disturbed in PDAC. We also highlight the high dependence of pancreatic cancer cells upon cholesterol uptake, and identify LDLR as a promising metabolic target for combined therapy, to limit PDAC progression and disease patient relapse.
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest cancers, rated as the fourth leading cause of cancer-related death in the United States and Europe, with a 5-y survival rate of about 4% and a median survival of less than 6 mo (1). In the absence of early warning signs, only 15% of patients with localized PDAC can be cured by surgical resection. For the remaining patients diagnosed with late-stage pancreatic cancer with metastatic disease, the current chemotherapy with gemcitabine (GEM) is mainly palliative and remains the standard treatment despite limited benefits (5.6-mo survival) (2). Recent advances in treatment, such as combined regimens using fluorouracil, leucovorin, irinotecan, and oxaliplatin, or Nab-paclitaxel plus GEM, conferred a survival advantage compared with GEM alone (2).
The low response rate to chemotherapy is a result, in part, to the presence of a dense stroma, characterized by fibrillar networks around tumoral cells that compress vasculature and limit oxygen, nutrient, and drug delivery to the cells. A fundamental feature of tumoral cells is that they undergo metabolic reprogramming in response to these environmental constraints. Advances in tumor metabolism research reveal that PDAC cells primarily rely on glucose and glutamine catabolism to fulfill bioenergetic need and provide macromolecules required for growth and proliferation (3–5). However, metabolic reprogramming is a complex phenomenon that does not simply involve exacerbated glycolysis and glutaminolysis. Depending on intrinsic tumor properties (type, stage, genetic aberrations) and on constraints imposed by its microenvironment, the nature of nutrient up-taken by cancer cells and the metabolic routes used to sustain tumor growth vary greatly. Hence, establishing the metabolic signature of PDAC is fundamental for the understanding of mechanisms governing metabolic flexibility in this tumor, and for the identification of key metabolic actors/pathways that may constitute interesting therapeutic targets.
The metabolic fingerprint of advanced PDAC, defined in this study, demonstrates a strong enrichment of dysregulated transcripts involved in specific carbohydrate, amino acid, and lipid pathways. Lipid-enriched pathways were the most abundant in advanced tumors, and those related to lipoprotein catabolism and cholesterol synthesis were among the most activated in PDAC, compared with nonmalignant pancreas. These results emphasize that pancreatic cancer cells are highly dependent on cholesterol, a feature that may be exploited in PDAC therapy. Tumor cells have elevated cholesterol requirements that need to be finely regulated. These cells can increase their cholesterol content either through synthesis (i.e., mevalonate pathway), hydrolysis of cholesterol ester (CE) stores, or through receptor-mediated endocytosis of plasma cholesterol-rich low-density lipoproteins (LDL) via the LDL receptor (LDLR) (6). Cholesterol is most abundant in the plasma membrane, where it localizes to microdomain structures named lipid rafts, wherein reside key cell-signaling molecules associated with malignant progression (7). In cancer cells, lipid raft levels are increased and changes in their cholesterol content modulate growth-factor receptor signaling, such as the PI3K/Akt- and EGFR-dependent survival pathway (8). To prevent the toxic effects of free cholesterol (FC) loading of subcellular organelles, cells either esterify and retain excessive cholesterol into CE droplets or convert it into noncytotoxic oxysterols and steroid hormones (9). Therefore, the proportion of FC and CE fractions and their distribution within and among organelles and the plasma membrane need to be finely regulated at the transcriptional and posttranslational levels (10).
Evidence from preclinical studies shows that statins or zoledronic acid limit pancreatic tumor growth by inducing inhibition of cholesterol synthesis (11, 12), although clinically no significant benefits have been observed for advanced-PDAC patients (13). In this report, we propose a novel strategy based on the blockade of LDLR, the main selective routes of cholesterol-rich lipoprotein entrance into cancer cells. We first evaluate whether shRNA-silencing of LDLR suppresses the tumorigenic properties of pancreatic cancer cells and then elucidate the signaling pathways involved. Second, we examine whether a reduction in cholesterol uptake affects in vitro PDAC cell sensitivity to standard drugs, and the PDAC syngeneic graft regression in GEM-treated mice.
Results
Up-Regulation of Lipoprotein and Cholesterol Metabolic Pathways in PDAC.
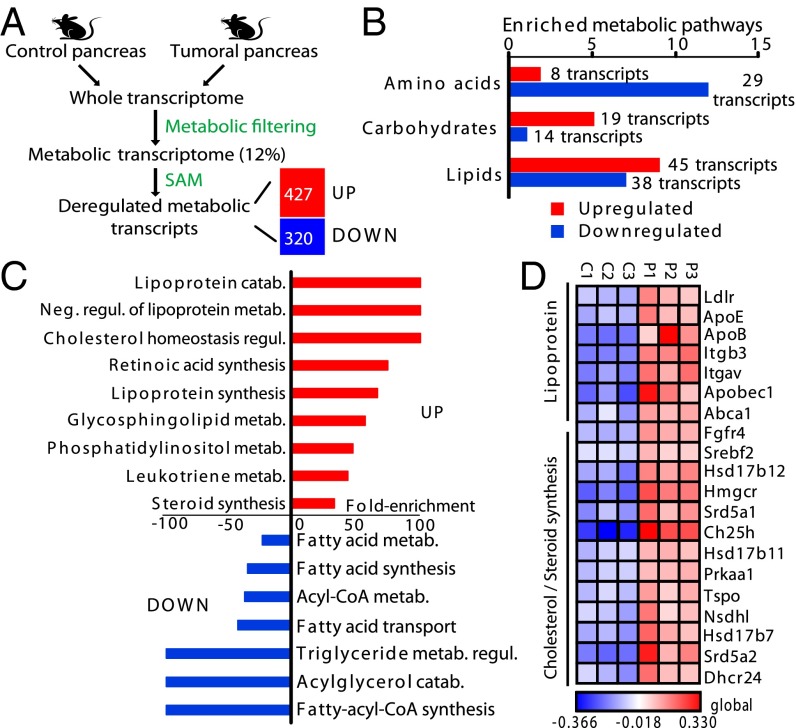
We used DNA microarray technology to identify transcripts involved in metabolic processes, which were differentially expressed between invasive PDAC and control pancreas. We used control mice (Ink4a/Arffl/fl; LSL-KrasG12D) and mice bearing spontaneous PDAC (Pdx1-Cre; Ink4a/Arffl/fl; LSL-KrasG12D) (14) with histological and clinical features similar to those reported in humans. We filtered the entire murine genome to select only metabolic transcripts, which constitute 12% of the mouse genome (i.e., 2,177 transcripts) and encode for known enzymes or transporters. Using the Significance Analysis of Microarrays method, we showed that 427 transcripts were significantly up-regulated and 320 were down-regulated in PDAC, compared with control pancreas (Fig. 1A and Dataset S1).
Fig. 1.
Metabolic transcript screening identifies lipoprotein and cholesterol/steroid pathways as key features of dysregulated metabolism in pancreatic tumors. (A) An outline of the transcriptomic approach used to highlight significant dysregulated metabolic transcripts in PDAC-bearing mice (n = 3) compared with control mice (n = 3). The number of significant up- and down-regulated transcripts in PDAC, compared with control pancreas, is indicated. (B) Number of significant enriched pathways in PDAC related to the indicated metabolism. The number of transcripts involved in each metabolism is provided. (C) Percentage of enrichment of overactivated or repressed lipid metabolic pathways in PDAC, compared with control pancreas. (D) A heat map illustrating overexpressed transcripts in PDAC, involved in lipoprotein or cholesterol/steroid metabolism. Values, based on microarray data obtained in A represent the log2-ratios of individual transcript expression values over the average expression profile. Each column is related to a single Affymetrix chip hybridized with individual cRNA from control pancreas (C1–C3) or PDAC (P1–P3). In A–D, up- and down-regulated transcripts or pathways are represented by red and blue, respectively. (B and C) P < 0.05.
Subsequent Bonferroni-corrected hypergeometric distribution analysis showed that the PDAC metabolic signature was highly enriched in up- and down-regulated pathways associated with lipid, amino acid, and carbohydrate metabolism (Fig. 1B and Dataset S2). Of these, the lipid class contained the greatest number of disrupted pathways in PDAC. In particular, pathways associated with lipoprotein catabolism and its negative regulation, and those involved in regulating cholesterol homeostasis, were the most highly enriched in PDAC (enrichment of 100%) (Fig. 1C and Dataset S3). Biosynthetic lipoprotein and retinoic acid pathways and glycosphingolipid metabolism were also overrepresented in PDAC (enrichment ranging from 57.1 to 75%). In contrast, enriched-fatty acid, acylglycerol, and triglyceride metabolic processes were highly depleted in PDAC compared with control pancreas (Fig. 1C and Dataset S3). Heat-map representations of the differential expression of lipoprotein and cholesterol-associated transcripts in PDAC and control pancreas revealed that LDLR, facilitating circulating lipoprotein uptake, the apolipoprotein B100 and E (ApoB, ApoE) that form lipoproteins, and the ATP binding cassette transporter A1 (ABCA1), mediating cholesterol efflux, were drastically up-regulated in the tumor (Fig. 1D). Moreover, up-regulation of transcripts involved in the cholesterol synthesis pathway and in the synthesis of its derivatives (oxysterols, steroid hormones) was also observed in PDAC compared with control pancreas (Fig. 1D). Finally, the master transcriptional activator of cholesterol synthesis and uptake, SREBF2 (10), was also overexpressed in PDAC (Fig. 1D). Therefore, the abundance and large diversity of transcripts encoding key enzymes, transporters, and apolipoproteins involved in cholesterol-related metabolic pathways, strongly indicate that pancreatic cancer cells have a high dependency on cholesterol.
Cholesterol Uptake Contributes to the Increase of Cholesterol Content in PDAC.
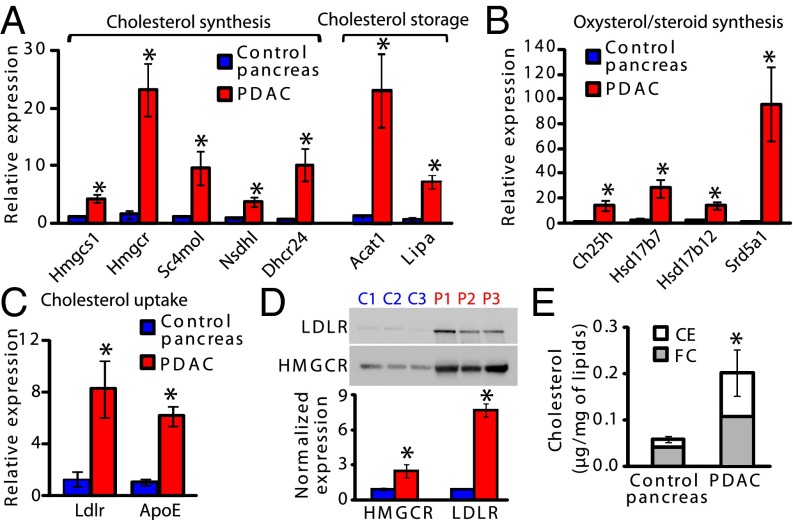
We next validated the overexpression of transcripts involved in cholesterol synthesis and storage (Fig. 2A), oxysterol and steroid synthesis (Fig. 2B), and cholesterol uptake (Fig. 2C) in additional mouse PDAC samples. The expression of Hmgcr, (3-Hydroxy-3-methylglutaryl CoA reductase), which encodes for the rate-limiting enzyme of cholesterol synthesis, was 23-times higher in PDAC than in control pancreas (Fig. 2A), although its protein level was not so abundant (Fig. 2D), probably because of an increase in its degradation rate (10). Other cholesterologenic transcripts and those involved in cholesterol storage, cholesterol acyltransferase 1 (Acat1) and Lipase A (Lipa), displayed high expression levels in the tumor compared with control pancreas (Fig. 2A). These data indicate that cholesterol synthesis and processes preventing cytotoxic FC loading, such as cholesterol esterification by ACAT1, or promoting LIPA-induced hydrolysis of CE, contained in droplets or lipoproteins, coexist in the tumor. Interestingly, in PDAC the most up-regulated transcript in the steroid hormone synthesis pathway encodes for SRD5A1, which promotes the conversion of testosterone into its active metabolite, the dihydrotestosterone (Fig. 2B). Finally, concerning lipoprotein-dependent cholesterol uptake, Ldlr and ApoE are 8.2- and 6.2-times higher in PDAC compared with control pancreas (Fig. 2C), suggesting that, along with cholesterol synthesis, cholesterol uptake is also strongly stimulated in the tumor. Importantly, whereas HMGCR levels are enhanced slightly in PDAC, the Ldlr increase is associated with a 7.7-fold increase in its protein level (Fig. 2D). This latter result demonstrates the pivotal role of the key cholesterol uptake facilitator, LDLR—and to a much lesser extent HMGCR—in cholesterol supply to PDAC cells. In the entire tumor, we effectively demonstrated a 3.5-fold increase in the total cholesterol (TC) content, which is furthermore composed of 50% stored CE (Fig. 2E). In PDAC, filipin-labeled FC is detected in epithelial cell membranes and the cytoplasm, whereas it is mainly present in islets of Langerhans in control pancreas (Fig. S1 A and B). These results demonstrate a high avidity of PDAC for cholesterol, which appears mostly satisfied by cholesterol uptake. Because targeting of cholesterol synthesis has proven ineffective for PDAC treatment, therefore blocking cholesterol uptake with LDLR silencing, to alter the content and distribution of cholesterol in the tumor, may help to define the impact of such a blockade on the tumorigenic properties of PDAC cells.
Fig. 2.
Up-regulation of cholesterol uptake correlates to cholesterol overload in PDAC. Expression of transcripts involved in the synthesis or storage of cholesterol (A), oxysterol/steroid synthesis (B), and cholesterol uptake (C) in control pancreas (n = 4) and PDAC (n = 4). Rplp0 mRNA levels were used for normalization. P value is relative to control pancreas mRNA levels. (D) LDLR and HMGCR proteins expression in control pancreas (C1–C3) and PDAC (P1–P3). Mean protein levels in each tissue (from n = 5 mice) are normalized to total loaded-protein (Amido black staining), and P value is expressed as in A. (E) TC, FC, and CE quantities in control pancreas (n = 5) and PDAC (n = 6) normalized to respective total lipid content. P value is expressed as in A. For all figures, data are mean ± SEM, *P < 0.05, Student's t test or Mann–Whitney U-test.
LDLR Is Expressed in the Epithelial Compartment of PDAC.
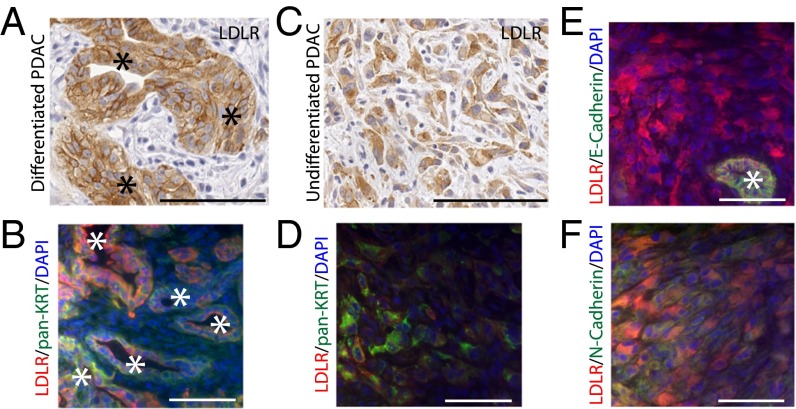
Using histological analysis, we determined the spatial LDLR expression in stromal and epithelial PDAC compartments, because no specific staining was observable in healthy pancreas (Fig. S1C). In tumors, LDLR was chiefly expressed in the epithelial cells and, more specifically, in well-differentiated cells organized into glands (Fig. 3A) and stained with the pan-cytokeratin (pan-KRT) epithelial marker (Fig. 3B). Moreover, LDLR was also present in undifferentiated cancer cells, which were disseminated into the stroma (Fig. 3C). Although these LDLR-undifferentiated cells were stained with the pan-KRT marker (Fig. 3D), they lost the E-cadherin epithelial marker, and acquired the N-cadherin mesenchymal marker (Fig. 3 E and F). These results illustrate that LDLR is present in the epithelial compartment of the tumor, both in differentiated and aggressive cells that exhibit an epithelial to mesenchymal transition phenotype.
Fig. 3.
Phenotypic characterization of LDLR-expressing cells in murine PDAC. LDLR staining in well-differentiated (A) and undifferentiated (C) epithelial pancreatic cancer cells. Magnification: 20×. Immunofluorescence costaining of LDLR with pan-cytokeratin (pan-KRT) epithelial marker in well-differentiated (B) and undifferentiated (D) PDAC. Immunofluorescence costaining of LDLR with (E) E-cadherin epithelial or (F) N-cadherin mesenchymal markers in undifferentiated PDAC. Magnification: 40×. (Scale bar, 100 µm.) In A, B, and E, tumoral glands are indicated with an asterisk (*).
LDLR Silencing, by Modifying Cholesterol Distribution, Inhibits ERK Survival Pathway and Reduces the Proliferative and Clonogenic Potential of Pancreatic Cancer Cells.
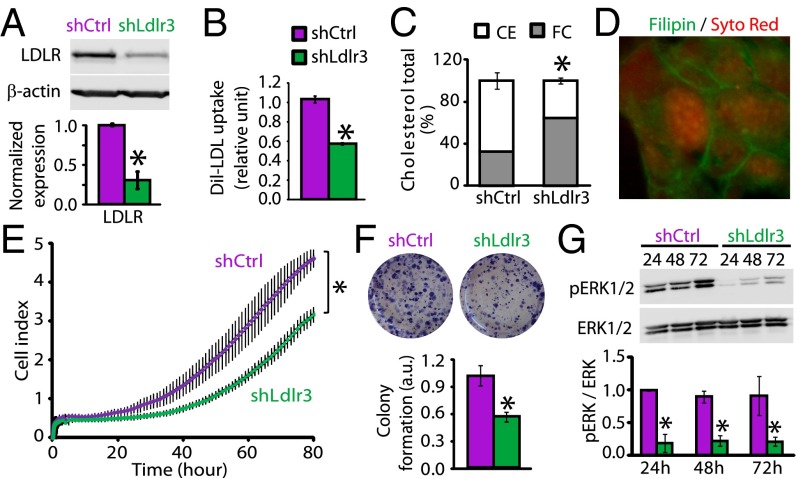
PK4A cells, established from Pdx1-Cre; LSL-KrasG12D; Ink4a/Arffl/fl tumors (15), were used to investigate the impact of LDLR inhibition on tumorigenic properties of PDAC cells. PK4A cells, expressing shRNA, which targeted different Ldlr sequences, were established and validated for LDLR knockdown (Fig. S2A). Successful LDLR knockdown was achieved with Ldlr3 shRNA (i.e., a 70% decrease in LDLR levels compared with control shRNA cells) (Fig. 4A), which significantly correlated to a 50% reduction in the uptake of labeled-LDL (1,1′-dioctadecyl-1-3,3,3,3-tetramethyl-indocarbocyanine perchlorate-LDL, Dil-LDL) (Fig. 4B). Interestingly, LDLR silencing did not disturb TC content but modified its distribution, because the CE content was reduced by 46%, whereas the FC fraction was 1.9-fold increased (Fig. 4C) and detected in the membrane and cytoplasm of PDAC cells (Fig. 4D). However, the increase in FC was not associated with an overactivation of cholesterol synthesis, because HMGCR protein levels were not altered by LDLR silencing (Fig. S2B), nor with an increase in the uptake of glucose, from which the cholesterol is synthesized (Fig. S2C). Moreover, the same quantities of glucose were shifted away from the tricarboxylic acid cycle, a prerequisite for cholesterol synthesis, toward lactate formation in the two shRNA PK4A cells (Fig. S2D). Thus, these data show that LDLR-depleted cells do not use compensatory mechanisms, as activation of cholesterol synthesis, to counteract the inefficient cholesterol uptake.
Fig. 4.
LDLR suppression prevents pERK1/2 activation and tumorigenic capacities of pancreatic cancer cells. (A) LDLR protein expression in nonmammalian control (shCtrl) and Ldlr3 shRNA (shLdlr3) pancreatic cancer cells (PK4A) normalized to respective β-actin levels. (B) Dil-LDL uptake in shCtrl and shLdlr3 PK4A cells normalized to cell number. (C) Quantities of CE and FC in shCtrl and shLdlr3 cells relative to TC. (D) Filipin-stained FC in shLdlr3 cells. Magnification: 60×. (E) Dynamic monitoring of shCtrl and shLdlr3 cell proliferation for 80 h. Cell index is expressed as mean ± SEM, *P < 0.05, ANOVA, n = 3. (F) Clonogenic assay and quantification of shCtrl and shLdlr3 colony-forming area. Values represent mean ± SE. (G) pERK1/2 protein expression, normalized to ERK1/2 protein levels, in shCtrl and shLdlr3 cells grown over 24, 48, and 72 h. In A–G, means represent at least three independent experiments, and P value is relative to control shRNA cell values.
To investigate the role of LDLR in supporting the growth and survival of PDAC cells, we performed real-time impedimetric cell proliferation monitoring along with colony-formation assays. We found that LDLR silencing, through shLdlr3, significantly decreased the cell proliferation rate, as well as the number of colonies formed, by 50% (Fig. 4 E and F). Similarly, shLdlr6, which inhibits the LDLR protein expression by 40%, reduced—albeit to a lesser extent than shLdlr3—the PK4A cell proliferation (Fig. S2E). Importantly, the morphology and size of PDAC cells were not impacted by LDLR silencing (Fig. S2 F and G). We then investigated which survival signaling pathways are disturbed in LDLR-depleted cells. By using a PathScan Intracellular Signaling array, we directly detected a reduction in expression of phosphorylated ERK1/2 (pERK1/2) and an increase of pGSK-3β in Ldlr3 shRNA PK4A cells compared with control cells, among other unchanged pro- and antiproliferative signaling pathways (Fig. S2H). The ERK1/2 survival pathway is particularly interesting because it is constitutively activated by the K-RasG12D oncogene in PDAC cells (16), and here we report that LDLR depletion drastically reduced pERK1/2 levels over time (24, 48, and 72 h) compared with control cells (Fig. 4G). Interestingly, we found that reduction in pERK1/2 did not result from a decreased activity of their upstream regulators, the phosphorylated MEK1/2 (Fig. S2I). Therefore, the ERK1/2 inactivation may result from an overactivation of ERK1/2-selective phosphatases. Thus, silencing LDLR may be an alternative strategy to prevent the constitutive K-RasG12D ERK activation.
LDLR Silencing Enhances Both the Cytotoxic Effects of Chemotherapy Drugs on PDAC Cells and GEM-Induced PDAC Regression.
GEM treatment showed limited benefits for PDAC patients; therefore, efforts are currently being made to increase their therapeutic efficacy by combination with other antitumoral agents. We investigated whether cholesterol distribution disorder, induced by inhibition of cholesterol uptake, altered the drug response of PDAC cells. We found that LDLR-depleted cells were more sensitive to GEM and SN38 (an active metabolite of irinotecan) than control cells (Fig. 5A and Fig. S3A). Importantly, the half-inhibitory concentration (IC50) of each of these drugs shifted, respectively, toward the inhibitory concentrations 1.9- and 3-times lower in LDLR-depleted cells compared with control cells (Fig. 5A and Fig. S3A). In contrast, LDLR depletion did not affect the oxaliplatin dose–response of PK4A cells (Fig. S3B). These results indicate that cholesterol-associated metabolic disruption strengthens the cytotoxic effects of several drugs on PDAC cells.
Fig. 5.
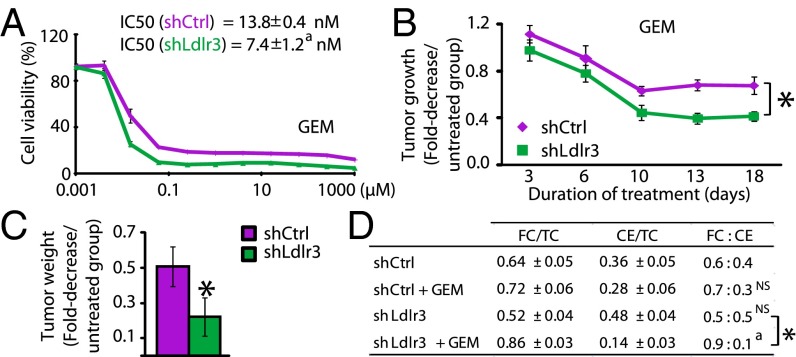
LDLR silencing, by modifying cholesterol distribution, increases sensitivity of pancreatic cancer cells to GEM and potentiates the GEM-induced PDAC regression. (A) Cytotoxicity dose–response curves for GEM performed on shCtrl and shLdlr3 PK4A cells, 48 h after starting treatment. The cell viability is expressed as a percentage of untreated cells. The IC50, determined for each cell line, is indicated. aP < 0.05 is relative to IC50 measured in shCtrl cells. Tumor regression (B) and end-point tumor weight (C) measured in GEM-treated mice implanted with shCtrl and shLdlr3 cells (n = 10 per group). Data are mean ± SEM and are expressed as the fold-decrease in tumor growth or weight measured in GEM-treated mice relative to untreated mice. P value is relative to growth curve obtained from GEM-treated mice implanted with shCtrl cells. (D) Proportion of FC and CE relative to TC in the four experimental groups (n = 5 in each group). FC:CE ratios are indicated. a, NS (Nonsignificant) are relative to FC:CE ratio determined in shCtrl untreated-mice. a,*P < 0.05. In A–D means represent at least two independent experiments.
Using pancreatic syngeneic-tumor graft mice, we then evaluated whether LDLR inhibition potentiates GEM-dependent tumor regression. One week after tumor establishment, half of the control and Ldlr3 shRNA PK4A implanted-mice were treated twice weekly with GEM. We noted that tumor growth was reduced by 50% in GEM-treated Ldlr3 shRNA mice compared with GEM-treated control shRNA mice (Fig. 5B), along with a 2.3-fold reduction in tumor weight in GEM-treated Ldlr3 shRNA mice compared with GEM-treated control mice (Fig. 5C). Importantly, LDLR-depleted tumors showed significantly higher apoptotic index than control shRNA-implanted tumors, as revealed by cleaved-caspase 3 staining (Fig. S3C), whereas no significant changes were detected for the Ki67 proliferative marker (Fig. S3D). Moreover, LDLR protein levels remained efficiently reduced in Ldlr3 shRNA-implanted tumors compared with control tumors, and HMGCR expression was not disturbed by LDLR silencing (Fig. S3E), as previously shown in vitro. Furthermore, LDLR silencing did not modify TC content compared with control tumors (Fig. S3F). Interestingly, when LDLR silencing was combined with GEM, the cholesterol distribution was chiefly altered (i.e., the FC:CE ratio) without any modification of TC content (Fig. 5D and Fig. S3F). Indeed, the FC was increased by 80% and CE stores were almost depleted in GEM-treated Ldlr3 shRNA tumors compared with untreated counterpart (FC:CE ratio of 0.5:0.5 in untreated vs. 0.9:0.1 in GEM-treated Ldlr3 shRNA tumors) (Fig. 5D). These data show that inhibition of LDLR combined with GEM treatment, by inducing cholesterol distribution damage, impedes tumor growth more efficiently than GEM alone.
Increased Ldlr Gene Expression Is an Indicator of Poor Prognosis in Human Pancreatic Cancer.
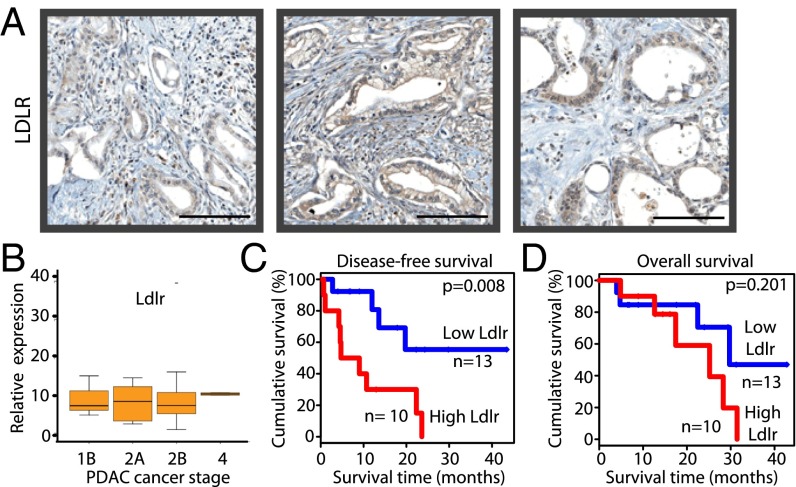
To investigate LDLR inhibition as a potential therapy for PDAC-resected patients, we used immunohistochemistry to clinically validate the LDLR expression in 10 human PDAC samples. All tumors expressed LDLR, predominantly in the membrane and cytoplasm compartments of epithelial cancer cells (Fig. 6A and Fig. S4A), as shown previously in mice (Fig. 3). The analysis of Ldlr transcript profiles in 23 other tumors showed that although expression varied between samples (Fig. S4B), this differential expression was not correlated with tumor stage (Fig. 6B). Then, two patient cohorts (low- and high-Ldlr expression groups) were defined on either side of a cut-off point determined by a maximally selected rank statistic test (Fig. S4 C and D). Next, the impact of such variation in Ldlr expression on disease-free and overall survival was evaluated by the Kaplan–Meier method. We found that high Ldlr expression was significantly associated with an increased risk of primary tumor and metastasis recurrence (showed by a reduction of disease-free survival, P = 0.008) (Fig. 6C), whereas the overall survival was not significantly impacted by Ldlr expression levels (P = 0.201) (Fig. 6D). Thus, in human PDAC, enhanced Ldlr expression—regardless of tumor stage—indicates a poor patient prognosis. Therefore, using a therapy designed to block the activity of this receptor, independently or in combination with chemotherapy, must be considered as a promising therapeutic strategy for these patients.
Fig. 6.
A high expression of Ldlr in human PDAC is associated with an increased risk of recurrence. (A) LDLR protein expression in three human PDAC collected during surgery. Magnification: 20×. (Scale bars, 100 µm.) (B) Ldlr mRNA levels in PDAC from different stages (n = 23). Kaplan–Meier disease-free survival (C) and overall survival (D) curves for PDAC patients, divided into high- and low-Ldlr expression groups based on the log-rank statistic test (n = 10 and n = 13, respectively).
Discussion
Here, we specifically determined the cholesterol-related metabolic signature of PDAC and the role of LDLR—the key mediator of cholesterol uptake—in pancreatic carcinogenesis. We found that LDLR-silenced pancreatic cancer cells have a reduced proliferation rate, accompanied by a profound inactivation of the ERK1/2 survival pathway, which highlights the direct antiproliferative effects of LDLR disruption on PDAC cells. Most notably, we outline the cumulative cytotoxic effects of cholesterol uptake inhibition and common chemotherapeutic drugs upon PDAC cells. In vivo, a combination of chemotherapy agents and LDLR targeting potentiates tumor regression by modifying cholesterol distribution in tumors. Importantly, we show that LDLR blockade may be considered as a promising therapy for PDAC patients diagnosed with localized, advanced, or metastatic PDAC because LDLR is highly expressed at any stage of the disease and high expression of its mRNA is directly correlated with an increased rate of disease relapse.
To identify metabolic pathways involved in pancreatic tumorigenesis, data from large-scale transcriptomic screenings of advanced pancreatic tumors and control pancreas were filtered to specifically highlight dysregulated metabolic genes in PDAC. This approach, combined with enrichment analysis, identified up-regulation of some well-known pathways related to carbohydrate (glucose, fructose, pentose) metabolism, and most notably, lipoprotein and cholesterol metabolic pathways. In contrast, despite an elevated number of dysregulated transcripts involved in amino acid metabolism, only two up-regulated pathways were enriched in PDAC, suggesting a secondary role of amino acid metabolism at this stage of the disease (Fig. 1B and Dataset S2). Then, in the cholesterol synthesis pathway, we found that most up-regulated transcripts are involved in the last steps of cholesterol synthesis, as well as in androgen and oxysterol productions, whereas none are implicated in the synthesis of isoprenoids [known as essential for trafficking and membrane anchoring of tumorigenic proteins (17)] (Fig. 2 A and B). It has been shown that de novo synthesized androgens strengthen the growth of androgen-dependent prostatic tumors (18), and oxysterols affect the balance between cell proliferation and apoptosis (19); therefore, it may be interesting to define their precise role in PDAC.
In the past decades, many studies aimed at limiting cholesterol content in cancer cells using statins to reduce cholesterol synthesis, although this strategy only had limited impact on cell fate specifically in epithelial tumors issued from exocrine pancreas, liver, and colon (12). Conversely, cholesterol uptake and lipoprotein metabolic pathways have received relatively little attention in the cancer research field; nevertheless, herein we showed that they undeniably contribute to PDAC progression. This report reveals that pancreatic cancer cells meet their excessive cholesterol demand by overactivating the LDLR-dependent uptake of cholesterol-rich lipoproteins, at the expense of the synthesis pathway. It also shows that the CE fraction, which is mainly yielded from LDL uptake, and stored in lipid droplets, was drastically increased in PDAC, compared with control pancreas (Fig. 2E). An increase in CE droplets, resulting from enhanced lipoprotein uptake, has already been described in prostate (20). Taking these data into account, and considering the fact that the targeting of synthetic cholesterol route did not show any decisive therapeutic benefits for pancreatic cancer patients (13), we decided to develop a novel cancer treatment approach consisting in the inhibition of the overactivated LDLR-dependent cholesterol uptake. We showed that LDLR silencing inhibited labeled-LDL uptake, and interestingly modified cholesterol repartition in PDAC cells, without altering total cholesterol content (Fig. 4 A–D). The CE fraction was decreased in favor of an increase in FC. In cancer cells, excessive FC is addressed to the plasma membrane, which elevates rafts/caveola content and stimulates raft-resident oncogenic signaling complexes (7, 8, 21). When FC exceeds the plasma membrane capacity, it is redistributed to intracellular organelles, the mitochondrial membrane or endoplasmic reticulum (ER), or to circulating plasma lipoproteins to avoid any cytotoxicity (22–24). In PDAC cells, increased FC following LDLR depletion impacts on their proliferative capacities (Fig. 4 E and F). Indeed, FC-loaded cells show a decrease in their proliferation rate that has been associated with compromised cell cycle progression. The extension of S-phase induced an impairment of cell entry in G2/M phase (Fig. S5A), as evidenced by the decrease in the G2/S ratio in LDLR-depleted cells, when compared with control cells (Fig. S5B). These results are in agreement with previous studies showing that cholesterol-lowering drugs inhibit myeloma cell and monocyte proliferation by induction of S-phase arrest (25, 26). Furthermore, using differential screening of signaling pathways, we showed that ERK1/2 activation was reduced in LDLR-depleted cells compared with control shRNA cells, thus explaining the slower growth rate of these cells (Fig. 4G). Interestingly, the inactivation of ERK1/2 by LDLR depletion does not rely on an inhibition of the Ras/MEK/ERK common pathway but may result from an overactivation of ERK1/2-selective phosphatases. We can already exclude the involvement of MKP-3 phosphatase because its expression is not altered by LDLR silencing (Fig. S5C). Other dual-specific pERK phosphatases, such as HePTP/PP2A complexes, could be responsible for the decrease in pERK1/2 (27). Interestingly, cholesterol, by binding to oxysterol-binding protein, drives the assembly of these phosphatases, which together have functionality that neither has alone (28). The control of HePTP/PP2A phosphatase activity by cholesterol may provide a molecular explanation for the dramatic dephosphorylation of pERK1/2 upon FC overload following LDLR depletion in pancreatic cancer cells. Interestingly, this critical cell-survival ERK pathway is aberrantly activated by the K-RasG12D oncogene mutation detected in 70–90% of PDAC patients, and its targeting has never been successful (16). Thus, our present findings strengthen the assumption that LDLR depletion, by inducing an excess of FC, impairs ERK activation, thus highlighting a novel strategy to block ERK dependent-proliferation in PDAC.
In this study, we found that pancreatic cancer cells are more sensitive to GEM than SN38, but they showed a very low response rate to oxaliplatin, with an IC50 1,000-times greater than that of GEM. This result is in accordance with clinical practice defining GEM as the more appropriate treatment for advanced PDAC patients. We then demonstrated that the targeting of cholesterol uptake, through LDLR silencing, potentiates the antitumor effect of GEM. Indeed, combination of GEM with LDLR depletion, leading to impaired cholesterol distribution, reduces the in vitro effective cytotoxic dose by two times, as well as the tumor growth. These results are in accordance with previous studies showing that cancer cells, which exhibit high LDLR-dependent uptake, and consequently CE accumulation, were resistant to chemotherapies (29). We also showed that, in syngeneic control shRNA tumors, the FC:CE ratio was completely inverted with respect to control shRNA PDAC cells in culture. FC fraction was higher in tumors than in cultured cells; this difference may be due to the low degree of tumor vascularization, limiting the supply of substrates for cholesterol synthesis to the tumor, and forcing PDAC cells to mobilize their CE stores to provide the cholesterol required for cell division. Importantly, in vivo, compared with LDLR depletion alone, a combination of GEM therapy and LDLR depletion disturbs the FC:CE ratio by depleting CE stores to excessively increase FC content. This imbalanced cholesterol distribution may weaken PDAC cells metabolically and therefore increase their vulnerability to cytotoxic drugs. In macrophages, FC-loading in the ER triggers cell death by inducing calcium store depletion and subsequent activation of the ER stress response (24, 30). Hence, it would be interesting to study the consequences of LDLR inactivation on ER stress response in PDAC cells. The increase in GEM cytotoxicity, when combined to LDLR silencing, may also result from excessive FC in the plasma membrane, which can facilitate transporter-dependent drug uptake and/or inhibit multidrug resistance-associated protein efflux. We can already exclude the notion of enhanced passive diffusion causing an increase in drug sensitivity in LDLR-depleted PDAC cells, because passive doxorubicin uptake is not modified by LDLR silencing (Fig. S6).
Finally, clinical data, revealing a higher risk of PDAC recurrence in patients with high Ldlr expression, illustrate that the more the tumor is metabolically active and eager for cholesterol, the more PDAC patient relapse is high. This finding supports the development of LDLR-inactivating agents as novel therapies that, combined with GEM, could be recommended to resected-PDAC patients with high Ldlr tumor levels. In these patients, this combined therapy may decrease tumor or metastasis recurrence risk and then improve their overall survival. Therefore, the development of novel preclinical strategies, abrogating LDL-cholesterol uptake through LDLR inactivating agents (blocking antibody, mimetic peptide, aptamers), appears as essential to identify the most relevant therapeutic approach for clinical trials. Collectively, our data highlight cholesterol uptake as a key metabolic pathway for the maintenance of tumoral cholesterol distribution, which is essential for PDAC progression and provides a novel metabolic therapeutic avenue for PDAC patients to delay or avoid PDAC or metastasis recurrence, regardless of disease stage.
Materials and Methods
All animal care and experimental procedures were performed in accordance with French Guidelines for animal handling and were approved by the local animal ethic committee (Marseille, C2EA-14).
Materials and methods are described in SI Materials and Methods. See Table S1 for primer sequences used to determine transcript expression profiles by real-time PCR.
Supplementary Material
Acknowledgments
We thank S. Granjeaud and M. Rubis for statistical and technical support, respectively, and staff at the cell culture, the Cibi integrative bioinformatic, the experimental histopathology platforms, as well as the mouse colony facility (U1068). This work was supported by grants from the National Institute of Cancer, Fondation de France (to F.G.); INSERM Plan Cancer 2009–2013 (to F.G., V.G., and J.R.); Cancéropôle PACA (to S.L.); Ministère de la Recherche (to O.O.); Site de Recherche Intégrée sur le Cancer (to C.L.); and European Research Council (to J.N.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE61412).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421601112/-/DCSupplemental.
References
- 1.Bosetti C, et al. Pancreatic cancer: Overview of descriptive epidemiology. Mol Carcinog. 2012;51(1):3–13. doi: 10.1002/mc.20785. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Guillaumond F, Iovanna JL, Vasseur S. Pancreatic tumor cell metabolism: Focus on glycolysis and its connected metabolic pathways. Arch Biochem Biophys. 2014;545:69–73. doi: 10.1016/j.abb.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149(3):656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9(2):125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- 7.Staubach S, Hanisch FG. Lipid rafts: Signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8(2):263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 8.Oh HY, et al. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate. 2007;67(10):1061–1069. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I. Consequences of cellular cholesterol accumulation: Basic concepts and physiological implications. J Clin Invest. 2002;110(7):905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Märten A, Lilienfeld-Toal Mv, Büchler MW, Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother. 2007;30(4):370–377. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- 12.Corcos L, Le Jossic-Corcos C. Statins: Perspectives in cancer therapeutics. Dig Liver Dis. 2013;45(10):795–802. doi: 10.1016/j.dld.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Hong JY, et al. Randomized double-blinded, placebo-controlled phase II trial of simvastatin and gemcitabine in advanced pancreatic cancer patients. Cancer Chemother Pharmacol. 2014;73(1):125–130. doi: 10.1007/s00280-013-2328-1. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillaumond F, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci USA. 2013;110(10):3919–3924. doi: 10.1073/pnas.1219555110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neuzillet C, Hammel P, Tijeras-Raballand A, Couvelard A, Raymond E. Targeting the Ras-ERK pathway in pancreatic adenocarcinoma. Cancer Metastasis Rev. 2013;32(1-2):147–162. doi: 10.1007/s10555-012-9396-2. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Ruiz C, Morales A, Fernandez-Checa JC. Statins and protein prenylation in cancer cell biology and therapy. Anticancer Agents Med Chem. 2012;12(4):303–315. doi: 10.2174/187152012800228715. [DOI] [PubMed] [Google Scholar]
- 18.Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012;12(6):751–759. doi: 10.1016/j.coph.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Weille J, Fabre C, Bakalara N. Oxysterols in cancer cell proliferation and death. Biochem Pharmacol. 2013;86(1):154–160. doi: 10.1016/j.bcp.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 20.Yue S, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19(3):393–406. doi: 10.1016/j.cmet.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li YC, Park MJ, Ye SK, Kim CW, Kim YN. Elevated levels of cholesterol-rich lipid rafts in cancer cells are correlated with apoptosis sensitivity induced by cholesterol-depleting agents. Am J Pathol. 2006;168(4):1107–1118, quiz 1404–1405. doi: 10.2353/ajpath.2006.050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steck TL, Lange Y. Cell cholesterol homeostasis: Mediation by active cholesterol. Trends Cell Biol. 2010;20(11):680–687. doi: 10.1016/j.tcb.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montero J, et al. Mitochondrial cholesterol contributes to chemotherapy resistance in hepatocellular carcinoma. Cancer Res. 2008;68(13):5246–5256. doi: 10.1158/0008-5472.CAN-07-6161. [DOI] [PubMed] [Google Scholar]
- 24.Zhang K, Kaufman RJ. Unfolding the toxicity of cholesterol. Nat Cell Biol. 2003;5(9):769–770. doi: 10.1038/ncb0903-769. [DOI] [PubMed] [Google Scholar]
- 25.Tu YS, et al. Involvement of Chk1-Cdc25A-cyclin A/CDK2 pathway in simvastatin induced S-phase cell cycle arrest and apoptosis in multiple myeloma cells. Eur J Pharmacol. 2011;670(2-3):356–364. doi: 10.1016/j.ejphar.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Fujino M, et al. Pitavastatin-induced downregulation of CCR2 and CCR5 in monocytes is associated with the arrest of cell-cycle in S phase. Atherosclerosis. 2006;187(2):301–308. doi: 10.1016/j.atherosclerosis.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Wang PY, Liu P, Weng J, Sontag E, Anderson RG. A cholesterol-regulated PP2A/HePTP complex with dual specificity ERK1/2 phosphatase activity. EMBO J. 2003;22(11):2658–2667. doi: 10.1093/emboj/cdg255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang PY, Weng J, Anderson RG. OSBP is a cholesterol-regulated scaffolding protein in control of ERK 1/2 activation. Science. 2005;307(5714):1472–1476. doi: 10.1126/science.1107710. [DOI] [PubMed] [Google Scholar]
- 29.Tatidis L, Masquelier M, Vitols S. Elevated uptake of low density lipoprotein by drug resistant human leukemic cell lines. Biochem Pharmacol. 2002;63(12):2169–2180. doi: 10.1016/s0006-2952(02)01018-3. [DOI] [PubMed] [Google Scholar]
- 30.Berisha SZ, Hsu J, Robinet P, Smith JD. Transcriptome analysis of genes regulated by cholesterol loading in two strains of mouse macrophages associates lysosome pathway and ER stress response with atherosclerosis susceptibility. PLoS ONE. 2013;8(5):e65003. doi: 10.1371/journal.pone.0065003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.