Significance
Healthy systems are characterized by scale invariance across multiple timescales. We investigated whether loss of scale invariance that occurs with aging and disease can be counteracted by exercise, in four age groups of mice. Surprisingly, we observed that lack of exercise was detrimental not only in old but also in young mice, raising the possibility of an unforeseen role of behavioral activity for health in aged and young subjects alike. Moreover, we show that scale invariance could be restored by high levels of exercise, even in old animals. The World Health Organization has pinpointed lack of exercise and a sedentary lifestyle as a major risk factor for various diseases. Our measures may guide health programs.
Keywords: circadian rhythms, wheel running, behavioral activity, multiple timescales, aging
Abstract
In healthy humans and other animals, behavioral activity exhibits scale invariance over multiple timescales from minutes to 24 h, whereas in aging or diseased conditions, scale invariance is usually reduced significantly. Accordingly, scale invariance can be a potential marker for health. Given compelling indications that exercise is beneficial for mental and physical health, we tested to what extent a lack of exercise affects scale invariance in young and aged animals. We studied six or more mice in each of four age groups (0.5, 1, 1.5, and 2 y) and observed an age-related deterioration of scale invariance in activity fluctuations. We found that limiting the amount of exercise, by removing the running wheels, leads to loss of scale-invariant properties in all age groups. Remarkably, in both young and old animals a lack of exercise reduced the scale invariance in activity fluctuations to the same level. We next showed that scale invariance can be restored by returning the running wheels. Exercise during the active period also improved scale invariance during the resting period, suggesting that activity during the active phase may also be beneficial for the resting phase. Finally, our data showed that exercise had a stronger influence on scale invariance than the effect of age. The data suggest that exercise is beneficial as revealed by scale-invariant parameters and that, even in young animals, a lack of exercise leads to strong deterioration in these parameters.
Many physiologic variables, such as heart rate, respiration, and locomotor activity, display scale-invariant or “fractal” properties, characterized by temporal structures that are similar across different timescales (1–6). Fractal patterns in physiological systems are intrinsic characteristics that are independent of external stimuli (7–9). The presence of fractal patterns is associated with health advantages, such as system integrity, as well as adaptability, and physiologic systems lose their scale invariance under diseased or aging conditions. The scale invariance in, for example, heart rate is reduced in congestive heart failure patients, and moreover, changes in fractal patterns have been demonstrated to be better predictors of morbidity and mortality than classical biomarkers (2, 3, 10).
Detrended fluctuation analysis (DFA) is a widely used method to quantify correlations in nonstationary physiological time series, such as heart rate and gait (1–3, 11, 12). The DFA provides information on the amplitude of the fluctuations at different timescales. The correlation in the fluctuations is described by the scaling exponent α. A value of α close to 1.0 indicates a delicate balance between uncorrelated randomness (α = 0.5) and regularity (α = 1.5), and is observed in healthy physiological systems (1). Under pathological conditions such as Huntington’s disease and Alzheimer’s disease, the physiological fluctuations are measurably different from 1 and are either too random or too regular (11, 12).
Locomotor activity in both humans and rodents displays scale-invariant fluctuations for timescales of seconds up to ∼24 h (8, 12, 13). The mechanism for generating scale-invariant activity patterns is not understood. Recent studies showed that scale-invariant activity patterns are disrupted with aging and in patients suffering from dementia (12). The degree of the disruption is strongly correlated with the circadian neurotransmitter content in the suprachiasmatic nucleus (SCN) (14), the master clock in mammals that regulates circadian rhythms in physiology and behavior (15).
In the past few years, it has become increasingly clear that exercise is beneficial for the circadian system (16–18). Furthermore, exercise improves the immune system, helps to prevent obesity, cardiovascular disease, and type 2 diabetes mellitus, and improves memory (19–23). The World Health Organization has pinpointed a lack of exercise as a major risk factor for diseases, including metabolic, cardiovascular, and immune diseases (24, 25). The aim of the present study is to determine whether a lack of exercise affects age-related deterioration in scale invariance of locomotor activity and whether, in turn, enhanced activity levels can lead to improvement. We found that, unexpectedly, a lack of exercise leads to loss of scale invariance not only in aged but also in young animals. Following a period of inactivity, exercise resulted in complete restoration of scale invariance in young animals and substantial improvement in old animals.
Results
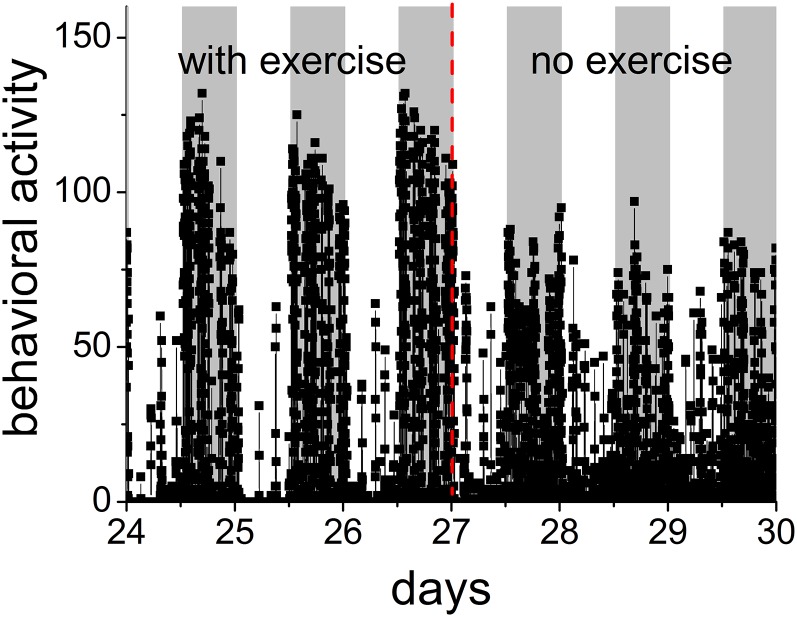
The activity patterns at baseline were determined by making and analyzing passive infrared (PIR) recordings for at least 1 mo in the presence of a running wheel. We then examined how the activity patterns changed after the running wheels were removed. Immediately after removal of the wheels in mice of 0.5 y, the amount of behavioral activity was reduced during the active period (nighttime for mice) (P = 0.001, paired t test), whereas during the rest period (daytime) the amount of behavioral activity increased (P = 0.04, paired t test, based on detrended time series), resulting in a blunted day–night rhythm amplitude (Fig. 1). The DFA method was applied to examine the activity patterns of the four groups with the age of 0.5 y (n = 9), 1 y (n = 6), 1.5 y (n = 12), and 2 y (n = 6). The mice had access to running wheels from the age of 3 mo onward.
Fig. 1.
Time series of behavioral activity in one representative animal in the presence and absence of a running wheel shows 24-h rhythm in activity levels. The PIR scores the animal’s movement in the cage per 1 min, and higher levels of movement lead to a higher score for behavioral activity levels. The animals are kept in alternating 12-h intervals of light and dark. Gray background indicates lights off. In the absence of the running wheel, the pattern in behavioral activity was altered compared with the pattern in the presence of a running wheel: the amount of activity was reduced during the night (active period) and was increased during the day (rest period).
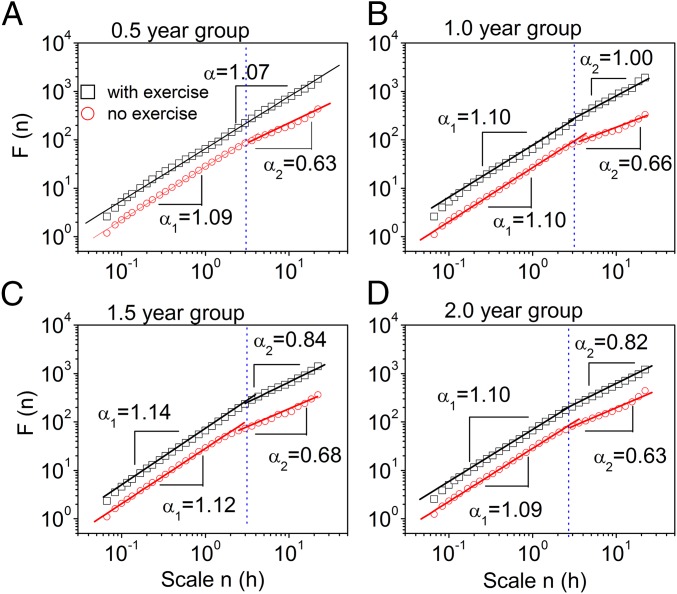
In the presence of running wheels, 0.5-y-old mice exhibited scale-invariant behavior in activity fluctuations over multiple timescales ranging from minutes to 24 h (Fig. 2A). The scaling exponent α = 1.07, close to 1, indicates strong scale-invariant correlations in activity fluctuations (Table 1). In the three other groups, we observed different correlations over two regions of timescales with a crossover at ∼3 h (Fig. 2 B–D). The correlations at timescales below the crossover (region I) characterized by a scaling exponent α1 remained the same (P > 0.35, Tukey’s test, ANOVA; Table S1) in all age groups, whereas the correlations at timescales above the crossover (region II) characterized by a scaling exponent α2 differ among the groups. In the mice of 1 y, α2 at timescales above the crossover (region II) was 1.00 and was not significantly different from the value in young mice (P = 0.13, unpaired t test). The oldest two groups of age 1.5 and 2 y showed significant alterations in activity patterns. Specifically, the two older groups showed weaker correlations at timescales above the crossover (region II) than the younger groups (P < 0.05, Tukey’s test, ANOVA; Table 1). The significant difference in the group of 1.5 and 2 y compared with the younger groups suggests that a key process of aging occurs between 1 and 1.5 y of age.
Fig. 2.
Removal of the running wheel as well as aging weakened the scale-invariant correlation of behavioral activity fluctuations. The scale-invariant correlation of behavioral activity was assessed in the presence or absence of a running wheel in four age groups: (A) 0.5 y, (B) 1 y, (C) 1.5 y, and (D) 2 y. The recorded PIR data of the last 9 d in the presence of running wheels and the first 9 d in the absence of running wheels were chosen for analysis. The data were averaged from at least six mice in each group.
Table 1.
α2 at timescales from 3 to 24 h in region II
| Condition | 0.5 y, m ± SE | 1 y, m ± SE | 1.5 y, m ± SE | 2 y, m ± SE |
| With wheels | 1.07 ± 0.007 | 1.00 ± 0.03 | 0.84 ± 0.04 | 0.82 ± 0.08 |
| Without wheels | 0.63 ± 0.03 | 0.66 ± 0.03 | 0.68 ± 0.02 | 0.63 ± 0.05 |
| P value* | 0.001 | 0.001 | 0.003 | 0.02 |
The presence of a wheel was followed by the absence of a wheel, and the last 9 d with a wheel are compared with the first 9 d without a wheel. m, mean; SE, standard error.
Paired t test.
In the absence of running wheels, the scale-invariant behavior of activity fluctuations was disturbed in 0.5-y-old mice, resulting in different correlations over two timescale regions with a crossover at ∼3 h (Fig. 2A). In region I, activity fluctuations possessed a strong correlation as characterized by a scaling exponent α1 = 1.09. In region II, the correlation was reduced as characterized by a scaling exponent α2 = 0.63, which was significantly smaller than α1 (P = 0.001, paired t test, Table 1). Notably, in the absence of a running wheel, α2 was close to 0.5, which corresponds to uncorrelated “white” noise behavior patterns. Thus, removal of the running wheels resulted in deterioration of the scale-invariant correlation in activity fluctuations in young mice. Also, in the age groups of 1, 1.5, and 2 y, scale invariance of activity fluctuations in region II broke down after removing the running wheels (Fig. 2 B–D and Table 1). Interestingly, α2 reached the same level in all groups in the absence of exercise (P > 0.23, Tukey’s test, ANOVA). The change in α2 was already observable during the first 3 d without exercise (Table S2). In contrast, the scaling exponent α1 in region I remained the same independent of the presence of running wheels (Table S1).
Following removal of the running wheels, in all age groups, the scaling exponents α2 in region II (3–12 h) in the resting period deteriorated significantly (P < 0.01, paired t test). Thus, the scale-invariant correlation in activity fluctuations during daytime (resting period) is influenced by the running wheel activity during nighttime (active period; Table S3).
The Scale-Invariant Correlation in Activity Fluctuations Is Restored After Returning the Running Wheels.
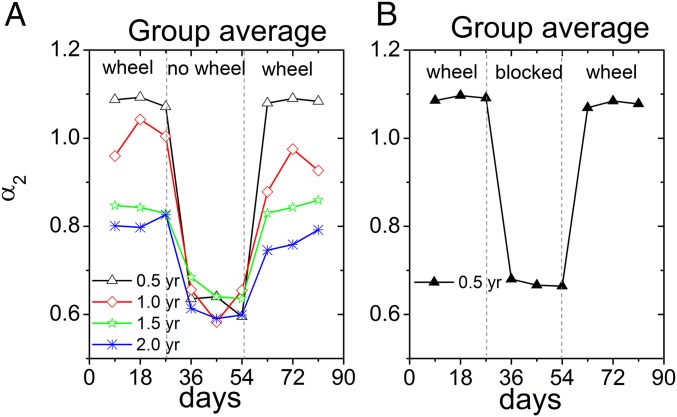
Having established that removal of the running wheels led to deterioration in scale-invariant activity regulation, we next tested whether returning the running wheels could restore the scale-invariant correlation. For all age groups, Fig. 3A shows that the scaling exponents in region II increased (P < 0.04, paired t test) after returning the running wheels (0.5 y: α2 = 1.08 ± 0.02; 1 y: α2 = 0.88 ± 0.04; 1.5 y: α2 = 0.83 ± 0.06; 2 y: α2 = 0.74 ± 0.09; mean ± SE). These values are not significantly different from the values obtained before removal of the wheels (P > 0.18, paired t test), suggesting a full restoration of correlations at large timescales (3–24 h). The restoration of α2 was already visible over the first 3-d period when the wheel was returned (Table S4). Returning the running wheels did not significantly affect the scaling exponents α1 in region I (P > 0.3, paired t test), which is not surprising because removal of the wheels had not affected scale invariance in this timescale domain either.
Fig. 3.
(A) Voluntary exercise restored the scale-invariant correlation of behavioral activity fluctuations. The scaling exponent α2 at timescales from ∼3 to 24 h obtained from the DFA versus experimental time in days. The scale-invariant correlation of behavioral activity was measured in the presence or absence of a running wheel in four age groups: 0.5 y, 1 y, 1.5 y, and 2 y. There were three successive stages in our recording: in the presence of the running wheels (day ≤27), after removal of the running wheels (day ≥28 and day ≤54) and after returning the running wheels (day ≥55). The deterioration in α2 was apparent following removal of the running wheel in all age groups. In addition, directly following returning of the running wheel, α2 improved in all age groups. Each data point is averaged from at least six mice in the same group. (B) Blocking the running wheel weakened the scale-invariant correlation of behavioral activity fluctuations similar as removing the wheel. The scale-invariant correlation of behavioral activity in response to blocking of the wheel was assessed in 0.5-y-old mice. The data were averaged from nine mice.
In a separate group of 0.5-y-old mice (n = 9), the running wheels were blocked rather than being removed from the home cage (Fig. 3B). Blocking the running wheels resulted in a similar deterioration in scale invariance in activity fluctuations in region II as removing the running wheel (blocking: α2 = 0.68 ± 0.03, P = 0.19, unpaired t test). Similarly, unblocking the running wheels had a similar effect as returning the running wheels (unblocking: α2 = 1.06 ± 0.02, P = 0.81, unpaired t test). This suggests that it is exercise rather than cage enrichment that accounts for the observed effects.
The Scale-Invariant Correlation in Activity Fluctuations Is Determined Both by the Amount of Exercise and by Aging.
In the presence of the running wheels, the scale-invariant correlation in activity fluctuations decreases with aging. However, it is well known that the amount of wheel rotations per day also decreases with aging of the animal (26, 27). To determine the relative contribution of aging versus the number of wheel rotations to the scale-invariant behavior in activity fluctuations, we used a mixed-effect model, including age and amount of wheel running per 9 d as covariates. We found that the value of adjusted R2 is 0.63, and the standardized multiple regression coefficients for the amount of wheel running was 0.53 (P = 0.001) and for the age was −0.37 (P = 0.01). These findings suggest that aging has an independent effect on the scaling exponent α2, but that voluntary exercise has a stronger influence on α2 at timescales from 3 to 24 h.
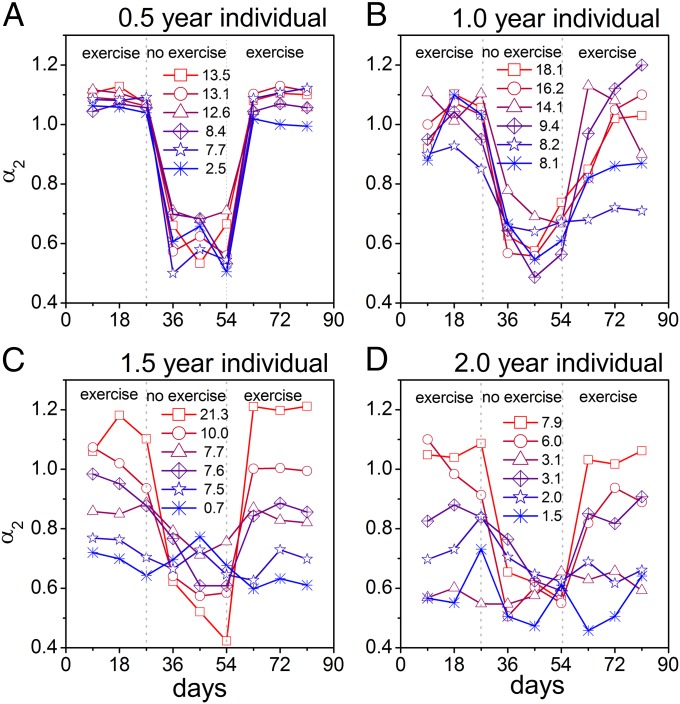
Within the oldest groups (1.5 and 2 y), there were larger differences between individual mice (represented by larger SE) in the scaling exponent α2 than in the younger groups (0.5- and 1-y groups) (Table 1). This larger SE suggests that α2 is not so well predicted by age alone. Therefore, we analyzed α2 as a function of the amount of activity of individual animals in all age groups (Fig. 4). In each age group, α2 is larger in the more active animals, which becomes apparent especially in the two older age groups.
Fig. 4.
The scale-invariant correlation from ∼3 to 24 h in activity fluctuations in individual mice demonstrates the dependence of scale invariance on activity levels. Shown are separate curves for individual mice of (A) 0.5 y, (B) 1 y, (C) 1.5 y, and (D) 2 y. The mice expressed different numbers of running wheel revolutions, indicated in the middle of each panel. Values are the mean from before and after wheel removal, and expressed in kilometers per day. There was a small SE of the scaling exponent α2 in the group of 0.5 and 1 y. There was a large SE in the group of 1.5 and 2 y. The activity levels of the aged animals were highly related to α2. For visibility and comparison, six mice are shown for each age group. See Fig. S1 for all animals.
Summary and Discussion
This study addressed the question of whether a lack of exercise affects scale invariance in locomotor activity and, if so, whether scale invariance can be restored. We showed that in young-adult mice that had access to running wheels the scale-invariant correlation was high (α ∼ 1) and persisted over a wide range of timescales (minutes to 24 h). Aging resulted in reduced scale-invariant correlation in activity fluctuations. In the absence of running wheels, scale invariance was attenuated at timescales from ∼3 to 24 h, whereas returning running wheels restored the deteriorated correlation in activity fluctuations. The data indicate that voluntary exercise restores the scale-invariant correlation in activity fluctuations, resulting in a healthy behavioral activity profile. This is the first study (to our knowledge) showing that restoration of scale invariance is possible. Analysis using a mixed-effect model showed that spontaneous activity levels are a better and independent predictor for changes in scale-free behavior than age.
Removal of the running wheel resulted in loss of scale invariance in activity fluctuations of mice, leading to a crossover at ∼3 h with reduced correlations at larger timescales (>3 h) and persisting correlations at the smaller timescales. A similar crossover has been observed in humans and rats. Aging humans and humans suffering from, e.g., dementia and/or Alzheimer’s disease display different fractal patterns in activity fluctuations in two scale regions, resulting in a crossover at 1.5–2 h (12, 28). In rats, lesioning the SCN significantly reduced the scale-invariant correlation at larger timescales (between 4 and 24 h), whereas the patterns at smaller timescales (<4 h) were only minimally affected, resulting in a crossover at ∼4 h (7). The presence of two scale regions (one at smaller timescales and the other at larger timescales) in the scale invariance suggests the involvement of different neuronal networks in the regulation of activity fluctuations at the smaller and larger timescales.
The loss of scale invariance that we observed in aged mice is consistent with previous studies in humans (12), whereas the effect of exercise on scale invariance of locomotor activity has not been studied before either in animals or in humans. When we determined the activity fluctuations exclusively during the resting period of the animal (i.e., the daytime), we observed differences, depending on the presence/absence of running wheels. We found that the correlation in activity fluctuations at timescales from ∼3 to 12 h during the resting period was reduced in all age groups when no running wheel was present. Interestingly, this was the case, whereas the total amount of locomotor activity during daytime was larger in the absence of a running wheel. This suggests that (i) the correlation in activity fluctuations is not dependent only on the total amount of activity, but rather reflects a complementary property of the temporal activity profile; and (ii) exercise during the active period is beneficial for scale-invariant control during the resting period.
The SCN is one of several neuronal nodes of the brain network controlling behavioral activity (8, 18). The deterioration in circadian activity rhythms as a result of aging and night shift work has been linked to deficits in functioning of the SCN (27, 29). Furthermore, it has been shown that proper SCN functioning is crucial for maintaining scale invariance in activity and heart rate fluctuations over multiple timescales (8, 9, 30). Simulated shift work as well as SCN lesions in rats reduces the scale-invariant correlation of the locomotor patterns (7, 29). Moreover, scale invariance is lost in the behavioral activity fluctuations of aged humans and in Alzheimer’s disease patients, and this has been attributed to dysfunction of the SCN (12, 28). Together, these results indicate that proper functioning of the SCN is crucial for scale-invariant patterns of activity (14). In the presence of running wheels, the scale-invariant correlation in activity fluctuations was improved in aging mice (1.5- and 2-y group). This result is consistent with the previous finding that voluntary exercise can strengthen the circadian system (17, 31–34), suggesting that the enhancement of scale-invariant correlation in activity fluctuations in the presence of running wheels may be the result of a restoration in circadian regulation.
Our mixed-effect model analysis showed that both the amount of exercise and age play a role in the correlation of activity fluctuation at timescales from ∼3 to 24 h (represented by the scaling exponent α2), and that the amount of exercise influences α2 more than age does. In the older mice, the difference in α2 among animals is pronounced with the scaling exponent α2 close to 1 in active mice and close to 0.5 in sedentary mice.
When the scaling exponents α2 and α1 are similar, as occurs in active mice, the behavioral activity fluctuations exhibit scale invariance across timescales from minutes to 24 h (28). As demonstrated by numerous studies, scale-invariant correlations with α ∼ 1 indicate the most complex dynamic patterns in physiological fluctuations that are associated with healthy physiology (1). Thus, understanding the causal relation between the amount of exercise and scaling exponent α of activity fluctuations is of measurable relevance to health. In this study, we show that, immediately following removal of the running wheels, the correlation in activity fluctuations decreased in the first 9-d epoch, whereas returning the running wheels immediately restored the scale invariance. To better understand the time course of these effects, we repeatedly analyzed changes in α2 over the first 3 d after removal of the wheel, and over the first 3 d after returning the wheel. Again, we found that the effects were immediately measurable.
It is possible that the effect of exercise on scale-invariant properties in activity patterns is not limited to mice. Verifying whether the same effect exists in humans may be important with the relevance to public health. For example, the sedentary lifestyle of young individuals in modern society is linked to various health problems. Finding objective measures to guide proper exercise is still a challenge. Scale-invariant activity patterns may serve as one of such measures that will help people to maintain health.
Materials and Methods
Animals.
All animal experiments were performed in accordance with the regulations of Dutch law on animal welfare, and the institutional ethics committee for animal procedures from the Leiden University Medical Center (Leiden, The Netherlands) approved the protocol. Male C57BL/6JOlaHSD mice were housed individually in a controlled environment (21 °C, 40–50% humidity) on a 12-h/12-h light/dark cycle. Food and tap water were available ad libitum during the entire experiment. All of the mice had access to running wheels (diameter: 26 cm) for voluntary exercise, from the age of 3 mo. The amount of exercise was defined as the number of wheel revolutions. To investigate the influence of a lack of activity, the wheels were removed or blocked for 27 d, while recordings of PIR were continued. To investigate possible restoration of scale-free behavior, we additionally analyzed the first 27 d after the wheel was reintroduced in the cage or unblocked. Six of the 0.5-, 1.0-, and 1.5-y-old mice were the same mice measured 6 mo apart; all other mice were separate groups measured only once.
Data Acquisition.
Behavioral activity of the mice was recorded using PIR motion detection sensors (Hygrosens Instruments) that were mounted underneath the lid of the cage and connected to a ClockLab data collection system (Actimetrics Software) that recorded the amount of sensor activation in 1-min bins.
Assessment of Scale Invariance Using DFA.
The DFA method was used to determine the scale-invariant correlation of activity fluctuations and was calculated as follows: (i) The integrated time series of N data points was divided into m nonoverlapping “windows.” The number of data points n = N/m was the same in each window, where n represents the timescale. (ii) In each window, a second-order polynomial function was used as a “local trend” to fit the n data points. (iii) In each window, the n data points were subtracted from the “local trends” to obtain the residuals. (iv) For each window, we calculated the root-mean-square of the residuals. For the entire integrated time series, the fluctuation amplitude F(n) was defined as the average value of the root-mean-square from each of the m windows. (v) We changed the number m (timescale n) and repeated i–iv (note that 4 ≤ n ≤ N/9). (vi) We plotted the amplitude fluctuation F(n) as a function of the timescale n in double-logarithmic coordination.
Throughout the present study, the “integrated time series” was regarded as one set of continuous PIR recoding data of 9 d (n = 1,440*9; 1,440 data points per d) or 3 d (n = 1,440*3; 1,440 data points per d). The fluctuation function was calculated from these N data points and can be written as a power-law form: F(n) = n−α, if activity fluctuations are scale-invariant feature. The scaling exponent α quantifies the scale-invariant correlation of the fluctuations as follows: (i) α = 0.5 represents there is no correlation in activity fluctuations, which corresponds to white noise; (ii) α < 0.5 indicates there are negative correlations, i.e., large recording data values are more likely to be followed by small recording data values and vice versa; (iii) α > 0.5 means there are positive correlations, i.e., large recording data values have more probability of being followed by large recording data values and vice versa.
Statistical Analysis.
Effects of voluntary exercise and aging on the scaling exponents.
We used Student paired t test to examine the influence of exercise on the scaling exponents within the same mouse. To assess the difference of the scaling exponents in four age groups, we performed Tukey’s test, ANOVA. The difference between two sets of data are considered to be significant when P < 0.05.
Mixed-effect model.
We performed the multiple linear regression analysis (Origin 7.0; Origin Lab) to calculate the dependence of the scaling exponent α2 on the amount of voluntary exercise and/or the age. For this analysis, each variable is standardized to ignore the variable’s units in the following steps: (i) each variable subtracts its mean and the new values are obtained; (ii) the standardized variable is achieved by the quotient of the new values divided by the SD of the variable.
Supplementary Material
Acknowledgments
We thank H. Post-van Engeldorp Gastelaars for excellent technical support. This research was supported by the European Commission Grant EUCLOCK (018741) (to J.H.M.), by the Netherlands Organization for Scientific Research TOPGO Grant 818.02.016 (to J.H.M.), and by Dutch Diabetes Research Foundation Grant 2013.81.1663 (to C.P.C.). K.H. is supported by NIH Grants R00-HL102241 and P01AG009975. F.A.J.L.S. was supported in part by NIH Grants R01 HL094806 and R01 HL118601. H.E.S. thanks the National Science Foundation Cyber-Enabled Discover and Innovation program for support under Award 1125290, and NIH for support under Award 5R01AG021133.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1424706112/-/DCSupplemental.
References
- 1.Goldberger AL, et al. Fractal dynamics in physiology: Alterations with disease and aging. Proc Natl Acad Sci USA. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng CK, et al. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28(Suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 3.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5(1):82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 4.Proekt A, Banavar JR, Maritan A, Pfaff DW. Scale invariance in the dynamics of spontaneous behavior. Proc Natl Acad Sci USA. 2012;109(26):10564–10569. doi: 10.1073/pnas.1206894109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barabási AL. The origin of bursts and heavy tails in human dynamics. Nature. 2005;435(7039):207–211. doi: 10.1038/nature03459. [DOI] [PubMed] [Google Scholar]
- 6.Peng CK, et al. Quantifying fractal dynamics of human respiration: Age and gender effects. Ann Biomed Eng. 2002;30(5):683–692. doi: 10.1114/1.1481053. [DOI] [PubMed] [Google Scholar]
- 7.Hu K, Scheer FA, Ivanov PCh, Buijs RM, Shea SA. The suprachiasmatic nucleus functions beyond circadian rhythm generation. Neuroscience. 2007;149(3):508–517. doi: 10.1016/j.neuroscience.2007.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu K, et al. Fractal patterns of neural activity exist within the suprachiasmatic nucleus and require extrinsic network interactions. PLoS One. 2012;7(11):e48927. doi: 10.1371/journal.pone.0048927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu K, Scheer FA, Buijs RM, Shea SA. The endogenous circadian pacemaker imparts a scale-invariant pattern of heart rate fluctuations across time scales spanning minutes to 24 hours. J Biol Rhythms. 2008;23(3):265–273. doi: 10.1177/0748730408316166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin LY, et al. Detrended fluctuation analysis predicts successful defibrillation for out-of-hospital ventricular fibrillation cardiac arrest. Resuscitation. 2010;81(3):297–301. doi: 10.1016/j.resuscitation.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hausdorff JM, et al. Altered fractal dynamics of gait: Reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol (1985) 1997;82(1):262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Van Someren EJ, Shea SA, Scheer FA. Reduction of scale invariance of activity fluctuations with aging and Alzheimer’s disease: Involvement of the circadian pacemaker. Proc Natl Acad Sci USA. 2009;106(8):2490–2494. doi: 10.1073/pnas.0806087106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macintosh AJ, Alados CL, Huffman MA. Fractal analysis of behaviour in a wild primate: Behavioural complexity in health and disease. J R Soc Interface. 2011;8(63):1497–1509. doi: 10.1098/rsif.2011.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pittman-Polletta BR, Scheer FA, Butler MP, Shea SA, Hu K. The role of the circadian system in fractal neurophysiological control. Biol Rev Camb Philos Soc. 2013;88(4):873–894. doi: 10.1111/brv.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vansteensel MJ, Michel S, Meijer JH. Organization of cell and tissue circadian pacemakers: A comparison among species. Brain Res Brain Res Rev. 2008;58(1):18–47. doi: 10.1016/j.brainresrev.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Harrington ME. Exercise strengthens circadian clocks. J Physiol. 2012;590(Pt 23):5929. doi: 10.1113/jphysiol.2012.245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder AM, et al. Voluntary scheduled exercise alters diurnal rhythms of behaviour, physiology and gene expression in wild-type and vasoactive intestinal peptide-deficient mice. J Physiol. 2012;590(Pt 23):6213–6226. doi: 10.1113/jphysiol.2012.233676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oosterhout F, et al. Amplitude of the SCN clock enhanced by the behavioral activity rhythm. PLoS One. 2012;7(6):e39693. doi: 10.1371/journal.pone.0039693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruunsgaard H, Pedersen BK. Special feature for the Olympics: Effects of exercise on the immune system: Effects of exercise on the immune system in the elderly population. Immunol Cell Biol. 2000;78(5):523–531. doi: 10.1111/j.1440-1711.2000.t01-14-.x. [DOI] [PubMed] [Google Scholar]
- 20.Passos GS, et al. Effects of moderate aerobic exercise training on chronic primary insomnia. Sleep Med. 2011;12(10):1018–1027. doi: 10.1016/j.sleep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 21.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008;8(3):205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 22.Brown BM, Peiffer JJ, Martins RN. Multiple effects of physical activity on molecular and cognitive signs of brain aging: Can exercise slow neurodegeneration and delay Alzheimer’s disease? Mol Psychiatry. 2013;18(8):864–874. doi: 10.1038/mp.2012.162. [DOI] [PubMed] [Google Scholar]
- 23.Erickson KI, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee IM, et al. Lancet Physical Activity Series Working Group Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallal PC, et al. Lancet Physical Activity Series Working Group Global physical activity levels: Surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 26.Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273(6 Pt 2):R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- 27.Farajnia S, et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32(17):5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu K, Harper DG, Shea SA, Stopa EG, Scheer FA. Noninvasive fractal biomarker of clock neurotransmitter disturbance in humans with dementia. Sci Rep. 2013;3:2229. doi: 10.1038/srep02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsieh WH, et al. Simulated shift work in rats perturbs multiscale regulation of locomotor activity. J R Soc Interface. 2014;11(96):20140318. doi: 10.1098/rsif.2014.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu K, Scheer FA, Buijs RM, Shea SA. The circadian pacemaker generates similar circadian rhythms in the fractal structure of heart rate in humans and rats. Cardiovasc Res. 2008;80(1):62–68. doi: 10.1093/cvr/cvn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leise TL, et al. Voluntary exercise can strengthen the circadian system in aged mice. Age (Dordr) 2013;35(6):2137–2152. doi: 10.1007/s11357-012-9502-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fonken LK, Nelson RJ. Dim light at night increases depressive-like responses in male C3H/HeNHsd mice. Behav Brain Res. 2013;243:74–78. doi: 10.1016/j.bbr.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 33.Power A, Hughes AT, Samuels RE, Piggins HD. Rhythm-promoting actions of exercise in mice with deficient neuropeptide signaling. J Biol Rhythms. 2010;25(4):235–246. doi: 10.1177/0748730410374446. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AT, Piggins HD. Feedback actions of locomotor activity to the circadian clock. Prog Brain Res. 2012;199:305–336. doi: 10.1016/B978-0-444-59427-3.00018-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.