Significance
For the management of prostate cancer it has remained a significant clinical challenge to identify biomarkers that can be used as prognostic indicators to facilitate early treatment decisions and indicate patients at risk for castrate-resistant bone-metastatic prostate cancer in need of more aggressive treatment. In this report, serum antibodies to alpha-2–Heremans–Schmidt glycoprotein (fetuin-A) were demonstrated to display increased reactivity with concomitant development of metastatic castrate-resistant disease in a large cohort of prostate cancer patients. Furthermore, metastatic prostate cancer cell lines and bone metastasis samples displayed robust fetuin-A expression. To our knowledge, this is the first report to indicate that serum autoantibodies reactive to fetuin-A show utility as a prognostic indicator for prostate cancer patients at risk for progressing to metastatic disease.
Keywords: cancer biomarker, peptide library, prostate cancer, phage display
Abstract
In response to an urgent need for improved diagnostic and predictive serum biomarkers for management of metastatic prostate cancer, we used phage display fingerprinting to analyze sequentially acquired serum samples from a patient with advancing prostate cancer. We identified a peptide ligand, CTFAGSSC, demonstrating an increased recovery frequency over time. Serum antibody reactivity to this peptide epitope increased in the index patient, in parallel with development of deteriorating symptoms. The antigen mimicking the peptide epitope was identified as alpha-2–Heremans–Schmid glycoprotein, also known as fetuin-A. Metastatic prostate cancer cell lines and bone metastasis samples displayed robust fetuin-A expression, and we demonstrated serum immune reactivity to fetuin-A with concomitant development of metastatic castrate-resistant disease in a large cohort of prostate cancer patients. Whereas fetuin-A is an established tumor antigen in several types of cancer, including breast cancer, glioblastoma, and pancreas cancer, this report is to our knowledge the first study implicating fetuin-A in prostate cancer and indicating that autoantibodies specific for fetuin-A show utility as a prognostic indicator for prostate cancer patients prone to progress to metastatic disease.
Prostate cancer accounts for nearly 27,000 deaths annually, with end-stage bone metastases representing a leading cause of morbidity and mortality (1). The introduction of diagnostic serum biomarkers into clinical practice, such as prostate-specific antigen (PSA), has greatly improved early detection of the disease (2). However, the lack of reliable methods for prediction of progression beyond early-stage disease and the paucity of treatment options for patients with bone metastasis results in many patients with localized disease subjected to aggressive treatment with sequelae including incontinence and impotence (3). Thus, identification of biomarkers to improve the accuracy of clinical assessment and stratification of patients needing conservative versus aggressive treatment would constitute a major advance in the management of this disease.
Antibodies specific for tumor-associated antigens are detectable in the serum of cancer patients and have been studied as diagnostic and prognostic markers (4). Simultaneous quantification of autoantibodies and PSA was proposed as a new approach to improve diagnosis and prognosis of prostate cancer (5). After autoantibodies against Huntingtin interacting protein-1 were identified in prostate cancer patients, combining serum reactivity with PSA values led to a screening discrimination with 97% specificity (6).
Using combinatorial peptide phage libraries, we developed a fingerprinting method based on targeting of circulating tumor-associated antibodies isolated from cancer patients (7, 8). Specific autoantibodies and their cognate tumor-associated antigens have been characterized, e.g., GRP78 for prostate cancer (9), HSP90 for ovarian cancer (8), ubiquilin 1 in lung cancer (10), and annexin XI-A in breast cancer (11). In addition, phage-based screening approaches have been developed for high-throughput profiling of immunogenic antigens for prostate cancer (12).
In this study, we analyzed clinically annotated serum samples obtained from an index patient at time points from his initial diagnosis, presenting with androgen-dependent, localized prostate cancer, until his death with androgen-independent metastatic multifocal bone disease 7 y after the initial banked serum sample. A unique peptide, CTFAGSSC, was identified, for which autologous serum IgG showed increasing reactivity. We identified alpha-2–Heremans–Schmid glycoprotein (AHSG, also known as fetuin-A) as the putative protein corresponding to the peptide mimic. We demonstrated increased serum antibody reactivity to fetuin-A during progression of disease in the index patient, as well as strong serum reactivity in a large cohort of metastatic prostate cancer patients. Reactivity to fetuin-A, identified years before the onset of metastatic disease in the index patient, indicates that serum antibodies constitute potential predictive biomarkers for metastatic prostate cancer and might facilitate relevant early treatment decisions.
Results
Combinatorial Peptide Screening.
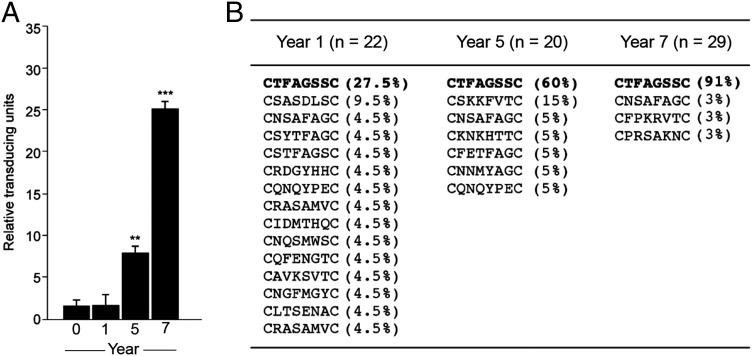
Profiling of the humoral response during cancer progression can provide insights into changing antigenicity of a tumor and may identify serum autoantibodies of diagnostic and prognostic value. We applied a phage display fingerprinting approach (7, 8) on isolated antibodies from sequentially acquired serum samples from an index patient and performed three rounds of selection for each time point. Relative phage recovery was very similar from serum obtained at initial diagnosis of castrate-sensitive prostate adenocarcinoma (year 0) and serum obtained during androgen ablation treatment, in which PSA was undetectable (year 1) (Fig. 1A). An increase in relative phage recovery was observed when the patient demonstrated elevated serum PSA and multifocal bone metastasis (year 5) (Fig. 1A). Further increased phage recovery was evident in the samples from year 7, when the patient presented with further elevated serum PSA concentration, increased number of bone lesions, and a biopsy confirmation of a right clavicle metastasis, consistent with prostate cancer metastasis (Fig. 1A).
Fig. 1.
Fingerprinting the antibody repertoire with combinatorial phage display libraries. (A) Selection of peptide phage on isolated immunoglobulins from sequentially acquired serum [year 0 (diagnosis), year 1, year 5, and year 7] from a prostate cancer patient. An increase in selectivity relative to the serum of age-matched control men was observed over time. The third round of selection is shown, and bars represent mean values for phage-transducing units ± SEM, from triplicates. **P < 0.01, ***P < 0.001 by t test (two-tailed). (B) Peptide sequences identified from randomly selected phage clones from the third round of selection on serum from year 1, 5, and 7 samples.
Sequence analysis of phage clones selected with patient IgG from years 1, 5, and 7 demonstrated enrichment of a single peptide, CTFAGSSC, increasing in relative recovery frequency from 27.5% in year 1 to 91% in year 7 (Fig. 1B). We also identified peptide sequences unique for each serum collection date (Fig. 1B).
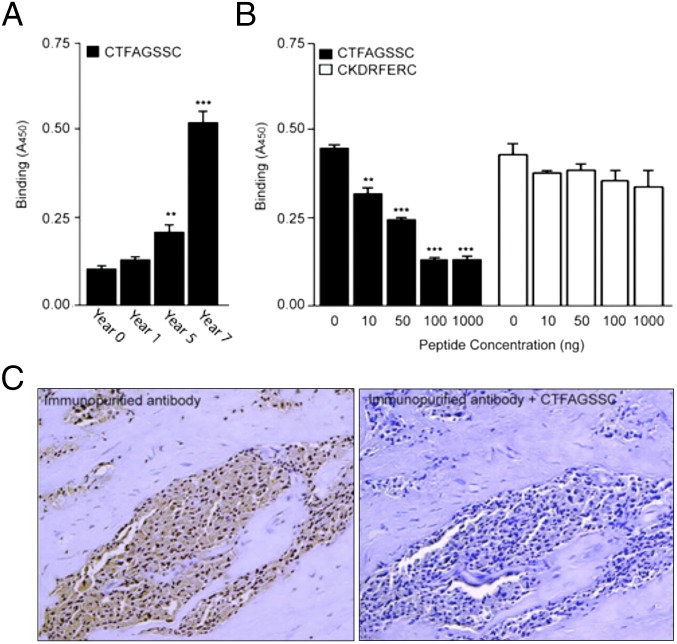
Because the enrichment of CTFAGSSC phage was concomitant with development of advanced metastatic prostate cancer, we next sought to validate the selectivity and specificity of the CTFAGSSC peptide motif as a candidate marker of disease progression. We evaluated binding of the isolated serum antibodies from the different time points to the CTFAGSSC peptide (Fig. 2A) and found an increase in reactivity against the peptide over time, with the sample from year 7 producing the most robust binding. This trend mimicked the phage enrichment profile (Fig. 1A). Binding of year 7 antibodies to CTFAGSSC could be inhibited in a concentration-dependent manner with preincubation of the synthetic peptide CTFAGSSC, whereas preincubation with a control peptide, CKDRFERC, did not inhibit binding (Fig. 2B). Immunostaining with autologous purified antibodies on prostate cancer biopsies from the index patient showed reactivity (Fig. 2C, Left) that could be inhibited by preincubation with the synthetic peptide CTFAGSSC (Fig. 2C, Right). These data confirm the specificity of the isolated antibodies for the peptide CTFAGSSC.
Fig. 2.
CTFAGSSC peptide reactivity is acquired in metastatic prostate cancer. (A) Immunoreactivity of isolated antibodies from the index patient to the peptide sequence CTFAGSSC increased over time. (B) Specific inhibition of antibody binding to immobilized peptide phage by the biotinylated peptide CTFAGSSC (black bars). No inhibition was seen with an unrelated biotinylated peptide (white bars) **P < 0.01, ***P < 0.001 by t test (two-tailed). (C) Immunoreactivity on prostate cancer biopsy samples from the index patient with the autologous isolated antibodies (Ab, Left). Binding was inhibited by preincubation with the synthetic peptide CTFAGSSC (Right). Bars represent mean ± SEM, from triplicates.
Fetuin-A Is the Antigen Mimicked by CTFAGSSC.
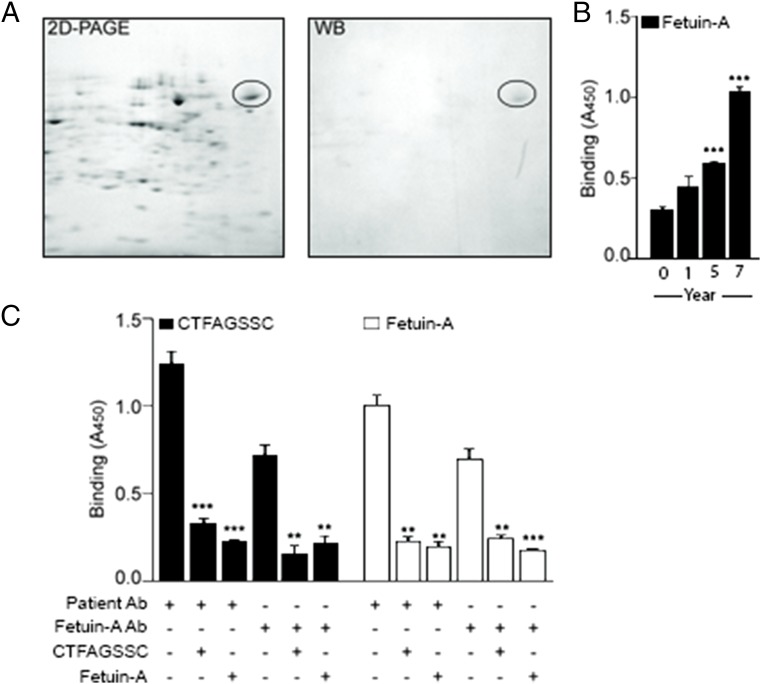
After demonstrating specific binding of CTFAGSSC to the antibodies present during metastatic disease, we next identified the antigen mimicked by the peptide. Using the metastatic LNCaP prostate cancer cell line, we performed 2D PAGE on whole-cell lysates and subsequent Western blotting with purified antibodies from year 7 (Fig. 3A). A single band was identified by the antibodies and was excised for protein sequence analysis by matrix-assisted laser desorption/ionization–time-of-flight (MALDI-TOF) mass spectrometry. Amalgamation of mass spectrometry data with results from BLAST similarity searches of CTFAGSSC identified AHSG, also known as fetuin-A, as the putative antigen recognized by the antibodies.
Fig. 3.
Fetuin-A is the antigen mimicked by CTFAGSSC. (A) Two-dimensional PAGE and Western analysis on lysates from LNCaP prostate cancer cells revealed a single band with the purified antibodies from serum collected in year 7. (B) Increasing reactivity to fetuin-A was observed with isolated serum antibodies. (C) Cross-inhibition studies confirmed binding selectivity of patient antibodies or anti–fetuin-A antibody to fetuin-A protein and CTFAGSSC. Bars represent mean ± SEM, from triplicates. **P < 0.01, ***P < 0.001 by t test (two-tailed).
To confirm fetuin-A as the antigen, we first determined that the index patient’s antibodies bound to fetuin-A and exhibited increasing reactivity over time (Fig. 3B). These data paralleled the initial screening profile with temporally increased reactivity against the peptide CTFAGSSC. We next performed cross-inhibition experiments with isolated antibodies from the year 7 sample, polyclonal anti–fetuin-A antibodies, synthetic CTFAGSSC peptide, and purified fetuin-A (Fig. 3C). Binding of the isolated patient antibodies from year 7 to CTFAGSSC could be inhibited by preincubation with either fetuin-A or the cognate peptide. Similar results were obtained with an anti–fetuin-A antibody.
Fetuin-A Is Expressed in Prostate Cancer.
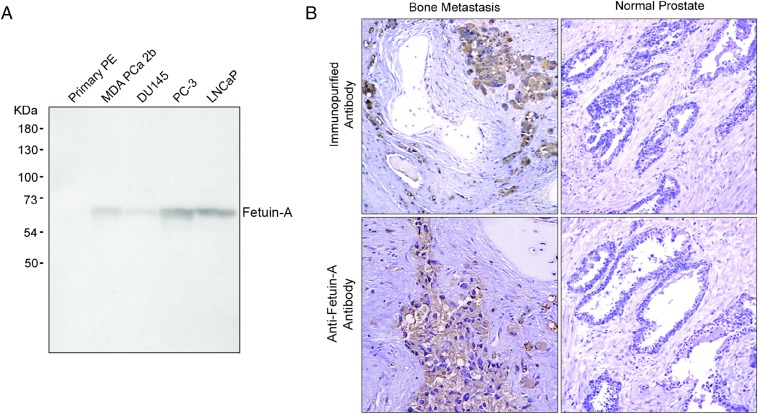
Subsequently, we investigated the specificity of fetuin-A for prostate cancer by analysis of protein expression in a panel of prostate cancer cells lines and tissue samples. Western blot analysis of four prostate cancer cell lines, LNCaP, DU145, PC-3, and MDA PCa 2b, revealed expression of fetuin-A. The densest band was observed for highly metastatic PC-3 cells. Normal prostate epithelium exhibited no detectable expression of fetuin-A (Fig. 4A). Detection of fetuin-A in tissue samples by immunohistochemistry revealed robust and specific staining of the tumor cells in prostate cancer bone metastases, whereas minimal staining was observed in normal prostate tissue. Similarly, purified antibodies from the index patient’s sample from year 7 post initial diagnosis generated a strong staining in bone metastases and minimal staining in normal prostate samples (Fig. 4B). In summary, fetuin-A is expressed in primary prostate cancer as well as bone metastases and elicits fetuin-A–specific serum IgG.
Fig. 4.
Fetuin-A is expressed in prostate cancer cells. (A) Western blot analysis of MDA PCa 2b, DU145, PC-3, and LNCaP metastatic prostate cancer cells demonstrated expression of fetuin-A. In contrast, no expression was detected in normal prostate epithelium (PE). (B) Anti–fetuin-A or purified serum antibodies from the index patient revealed staining of prostate cancer bone metastases (Left), whereas minimal staining was observed in normal prostate tissue (Right).
Fetuin-A Autoantibodies as a Marker of Prostate Cancer Progression.
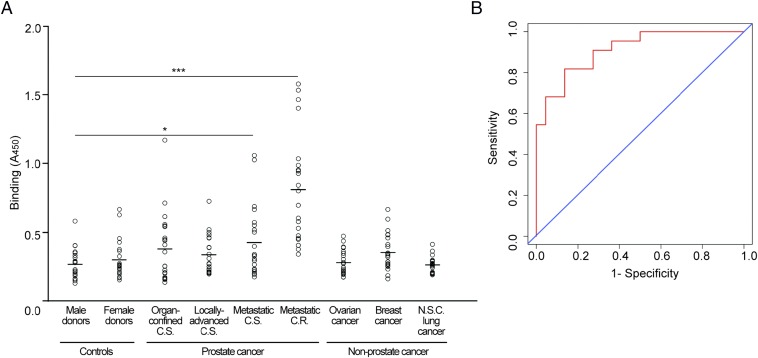
We next assessed the utility of fetuin-A autoantibodies for early detection of metastatic prostate cancer. We evaluated fetuin-A reactivity in a large population of patients at different stages of prostate cancer and in normal donor controls (n = 22 per group). We observed strong serum reactivity in metastatic, castrate-resistant prostate cancer patients and a trend toward increasing reactivity from organ-confined prostate cancer to metastatic disease (Fig. 5A). We also evaluated serum reactivity in three nonprostate cancers and observed low reactivity from patients with non–small-cell lung cancer, metastatic breast cancer, and advanced ovarian cancer (Fig. 5A).
Fig. 5.
Fetuin-A autoantibodies identify metastatic prostate cancer patients. (A) Strong reactivity to fetuin-A was observed in sera from prostate cancer patients with metastatic castrate-resistant disease. Normal donor controls and three non–prostate-cancer types exhibited low reactivity. C.S., castrate-sensitive; C.R., castrate-resistant; N.S.C., non–small-cell. Small open circles represent individual donor sera, and the lines represent average absorbance for each group. *P < 0.05, ***P < 0.001 by t test (two-tailed). (B) Sensitivity and specificity assessment by receiver-operating characteristic (ROC) curve analysis of fetuin-A reactivity exhibited an area under the curve of 0.91 (95% confidence, 0.830–0.992) for metastatic prostate cancer patients and normal donor controls (n = 22 per group).
To evaluate the performance of fetuin-A autoantibodies as markers of metastatic prostate cancer, we performed receiver-operating characteristic (ROC) analysis for antibody detection in normal donor controls and metastatic prostate cancer patients (n = 22 per group) (Fig. 5B). The analysis yielded an area under the ROC curve (AUC) of 0.91 (95% confidence interval, 0.830–0.992). These data indicate that serum fetuin-A reactivity could serve to identify patients with metastatic prostate cancer.
Discussion
Using a combinatorial phage display approach, we analyzed the autoantibody profile from a patient with prostate cancer over a 7-y period during which the cancer progressed from localized disease to low-burden metastatic disease and, finally, to castrate-resistant, diffusely metastatic cancer. We identified an antibody epitope, CTFAGSSC, which showed increasing binding to the patient’s serum antibodies concomitantly to disease progression. The peptide mimicked AHSG, also known as fetuin-A, and we suggest that serum reactivity to fetuin-A shows utility as a sensitive and specific indicator of metastatic disease. Whereas fetuin-A has been implicated as a tumor antigen in several cancer types such as breast cancer (13), glioblastoma (14), and pancreas cancer (15), to our knowledge this report is the first to show involvement of fetuin-A in prostate cancer. We investigated the expression of fetuin-A in a panel of prostate cancer cell lines and observed robust expression of fetuin-A for the highly metastatic PC-3 cells, indicating that expression levels might correlate with metastatic potential as found previously for angiogenic factors (16).
Although microarray and serologic analysis of recombinant cDNA expression libraries of human tumors with autologous serum have identified immunogenic molecules in various tumors, validation of putative antigens and biomarkers is often complicated by factors such as posttranslational modifications and differential cellular locations (17–19). Fingerprinting of enriched serum antibodies with phage display offers a more direct combinatorial screening for antibody epitopes and has identified numerous biologically relevant antigens (7–12). Moreover, studies from other laboratories have indicated clinical relevance in cancer diagnostics for antibodies against molecules such as osteopontin (20, 21), isoprostanes (22), and CXCL13 (23), among others. The concept of a global “autoantibody-ome” may apply, related to target discovery profiles for personalized therapeutics.
Fetuin-A is a liver secretory protein that accumulates in bone (24, 25). Fetuin-A also appears to be expressed in bone, as transcripts have been detected in chondrocytes and osteoblasts (26). Moreover, it has been suggested that fetuin-A is a transforming growth factor-β type II receptor mimic and acts as an antagonist of TGF-β and TGF-β–related bone morphogenetic protein (BMP) activities (27). Multiple investigations of fetuin-A–deficient mice on various genetic backgrounds suggest that fetuin-A has a role as a systemically acting inhibitor of ectopic bone formation (28–31). Bone lesions in prostate cancer are predominantly osteoblastic and cause a relative increase in bone deposition (32). In view of functions of fetuin-A as an inhibitor of bone morphogenetic proteins and ectopic bone formation, it is possible that anti–fetuin-A antibodies detected in the serum from metastatic castrate-resistant prostate cancer patients in this study have a role in the bone-metastatic pathogenic process by neutralization of fetuin-A in serum, thus precluding control of bone deposition.
This study supports the notion that profiling of the humoral response could identify molecules indicating disease well before clinical manifestations. We have shown that autoantibodies against fetuin-A, present in the serum of prostate cancer patients, constitute a putative early indicator of metastatic disease. The combination of the clinical history and development of the antibodies provides strong evidence that fetuin-A autoantibodies could serve as a predictive biomarker for patients at risk for developing metastases.
Materials and Methods
Patient Samples.
Written informed consent was obtained from all subjects and was approved by the Institutional Review Board (IRB) at the University of Texas M. D. Anderson Cancer Center (UTMDACC). Tissue and serum samples were obtained from the UTMDACC Specialized Program of Research Excellence (SPORE) Tissue Resource and Pathology Core. Control samples were obtained from blood-donor age-matched volunteers. The index patient had serum banked in 1993 (defined as year 0). After response to chemical castration, his disease was detected in bone 3 y after initially banking serum and continued to progress in this site until his death 7 y after the initial serum sample. Serum samples from years 0, 1, 5, and 7 were obtained. All blood samples were allowed to clot at room temperature, after which they were centrifuged, separated into aliquots, and frozen.
Cell Culture and Reagents.
DU145, PC-3, LNCaP, and MDA PCa 2b prostate cancer cells were purchased from the American Type Culture Collection (ATCC). Primary prostate epithelial cells were purchased from Cambrex. Synthetic peptides were produced by Genemed. Anti–fetuin-A antibody (M-17) was purchased from Santa Cruz Biotechnology.
Purification of Immunoglobulins.
Biotinylated peptides were coated (50 µg/mL) on Neutravidin plates (Pierce) for 1 h at room temperature (RT). Wells were blocked with PBS containing 4% (wt/vol) milk, 1% BSA, and 0.05% Triton X-100 for 30 min at 37 °C, after which they were washed with PBS containing 0.05% Triton X-100. Serum antibodies (1:100) were incubated in wells for 2 h at RT, followed by washing. Bound IgGs were removed with IgG elution solution (Pierce), followed by pH neutralization. Purified antibodies were pooled and quantified by absorbance.
Targeting Isolated Immunoglobulins with a Phage Peptide Library.
We used a previously established fingerprinting procedure (7, 8) to target isolated antibodies from cancer patients. In brief, we precleared a CX6C (C, cysteine; X, any residue) phage-displayed peptide library on a pool (50 samples) of protein G-purified antibodies from age-matched healthy donor men to remove nonspecific peptides. Next, we individually incubated the precleared library with purified serum IgG from each time point (year 0, and years 1, 5, and 7) and performed three rounds of selection as described previously (7). Random phage clones from the third round of panning were selected for DNA sequence analysis as previously described (7, 8, 33).
Peptide Binding and Competitive Inhibition Assays.
Binding of purified serum antibodies to the selected peptide and its corresponding mimic antigen was studied by ELISA. Briefly, biotinylated CTFAGSSC peptide or purified fetuin-A was used to coat 96-well plates at 10 µg/mL, followed by an overnight incubation at 4 °C. Purified antibodies (20 µg/mL) were added to the coated plates, and binding proceeded at RT for 2 h. Wells were washed four times with PBS containing 0.01% Tween-20. Secondary anti-human IgG conjugated to horseradish peroxidase (HRP) were added (1:5,000) and incubated at RT for 1 h. After several washes, the wells were incubated with 3,3′,5,5′-tetramethylbenzidine (TMB; Calbiochem) for 15 min. Reactions were stopped with 0.5 M H2SO4, and absorbance at 450 nm was measured with a Bio-Tek ELISA plate reader.
For inhibition studies, phage displaying the peptide CTFAGSSC was coated on 96-well plates. Purified antibodies were incubated with either CTFAGSSC or an unrelated peptide CKDRFERC for 1 h at 37 °C, before addition to coated wells. Analysis was as described above. For the cross-inhibition study, 96-well plates were coated with 10 µg/mL purified fetuin-A or biotinylated CTFAGSSC in 100 mM NaHCO3, overnight at 4 °C. The wells were washed and blocked with 1% milk (wt/vol), 0.05% Tween-20 in PBS for 2 h at 37 °C. To investigate specificity by inhibition, we incubated purified patient antibodies (1 µg) or anti–fetuin-A antibody (1:500) with purified fetuin-A (10 µg) or synthetic peptide CTFAGSSC (50 µg). The mixtures were incubated for 1 h at 37 °C, before addition to the coated wells. After 1 h of incubation at RT, the wells were washed several times. Addition of HRP-conjugated antibody and enzymatic detection proceeded as described above.
Protein Purification and Analysis.
LNCaP prostate cancer cells were grown to ∼70% confluence, washed with PBS, treated with 100 mM Tris⋅HCl, 2 mM MgCl2, and 1% Triton X-100, and subsequently sheared. Lysates were separated and analyzed by 2D polyacrylamide gel electorphoresis (PAGE). Analytical gels were either stained with Coomassie Brilliant Blue or probed with the immunopurified serum antibodies (50 µg/mL), the latter detected by enhanced chemiluminescence (ECL; Pharmacia). Protein spots were excised from the gel and were used for protein sequence analysis by MALDI-TOF mass spectrometry.
Immunoreactivity Against Fetuin-A.
IgGs were purified from individual serum samples, using protein G beads as previously described (7). Serum samples were obtained from the following groups: male donors, female donors, organ-confined disease prostate cancer (PCa), locally advanced PCa, metastatic androgen-dependent PCa, and metastatic androgen-independent PCa. In addition, serum samples from metastatic lung, breast, and advanced ovarian cancers were also purified and analyzed (n = 22 per group). In brief, 10 µg/mL of the fetuin-A protein was coated on multiwell plates in duplicates, after which the wells were blocked with 1% milk in PBS for 45 min at 37 °C. Isolated antibodies (2 µg) were added for 90 min incubation at RT. Washing, incubation with HRP-conjugated anti-human IgG, and detection with TMB proceeded as described for the peptide-binding ELISA.
Immunohistochemistry and Western Blot Analysis.
Formalin-fixed biopsy samples from the index patient or archived human bone metastasis and normal prostate tissue sections were deparaffinized in xylene, rehydrated in decreasing gradients of ethanol, treated for antigen retrieval, and blocked with a protein-blocking buffer (DAKO) (8). Staining was performed with a DAKO Labelled Streptavidin-Biotin (LSAB+) System peroxidase kit and counterstaining with hematoxylin, as described in ref. 8. Expression of fetuin-A was evaluated with Anti–fetuin-A antibody (1:20) and purified serum IgG. For the competition assay, purified patient IgG samples were preincubated with the synthetic peptide CTFAGSSC. The immunostained tissue sections were examined under an inverted optical microscope (Olympus IX70). Whole-cell lysates were made from the following cell lines: DU145, PC-3, LNCaP, and MDA PCa 2b and from primary prostate epithelial cells. Proteins (10 µg) were resolved on 4–20% SDS-polyacrylamide gels, transferred to nitrocellulose, and probed with an anti–fetuin-A antibody (1:100). Immune complexes were detected by ECL.
Acknowledgments
The authors thank Dr. Ricardo Giordano for critical reading of the manuscript, Jessica Sun for assistance with statistical analyses, and Mr. Leslie Misrock for inspiring this project. This work was supported by NIH funding for R.P. and W.A.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rubin MA. Targeted therapy of cancer: New roles for pathologists—prostate cancer. Mod Pathol. 2008;21(Suppl 2):S44–S55. doi: 10.1038/modpathol.2008.11. [DOI] [PubMed] [Google Scholar]
- 2.Brawer MK, et al. Screening for prostatic carcinoma with prostate specific antigen. J Urol. 1992;147(3 Pt 2):841–845. doi: 10.1016/s0022-5347(17)37401-3. [DOI] [PubMed] [Google Scholar]
- 3.Schover LR, et al. The use of treatments for erectile dysfunction among survivors of prostate carcinoma. Cancer. 2002;95(11):2397–2407. doi: 10.1002/cncr.10970. [DOI] [PubMed] [Google Scholar]
- 4.Madrid FF, Maroun MC. Serologic laboratory findings in malignancy. Rheum Dis Clin North Am. 2011;37(4):507–525. doi: 10.1016/j.rdc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie C, et al. A novel multiplex assay combining autoantibodies plus PSA has potential implications for classification of prostate cancer from non-malignant cases. J Transl Med. 2011;9(43):1–11. doi: 10.1186/1479-5876-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley SV, et al. Serum antibodies to huntingtin interacting protein-1: A new blood test for prostate cancer. Cancer Res. 2005;65(10):4126–4133. doi: 10.1158/0008-5472.CAN-04-4658. [DOI] [PubMed] [Google Scholar]
- 7.Mintz PJ, et al. Fingerprinting the circulating repertoire of antibodies from cancer patients. Nat Biotechnol. 2003;21(1):57–63. doi: 10.1038/nbt774. [DOI] [PubMed] [Google Scholar]
- 8.Vidal CI, et al. An HSP90-mimic peptide revealed by fingerprinting the pool of antibodies from ovarian cancer patients. Oncogene. 2004;23(55):8859–8867. doi: 10.1038/sj.onc.1208082. [DOI] [PubMed] [Google Scholar]
- 9.Arap MA, et al. Cell surface expression of the stress response chaperone GRP78 enables tumor targeting by circulating ligands. Cancer Cell. 2004;6(3):275–284. doi: 10.1016/j.ccr.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Chen G, et al. Autoantibody profiles reveal ubiquilin 1 as a humoral immune response target in lung adenocarcinoma. Cancer Res. 2007;67(7):3461–3467. doi: 10.1158/0008-5472.CAN-06-4475. [DOI] [PubMed] [Google Scholar]
- 11.Fernández-Madrid F, et al. Autoantibodies to Annexin XI-A and other autoantigens in the Diagnosis of breast cancer. Cancer Res. 2004;64(15):5089–5096. doi: 10.1158/0008-5472.CAN-03-0932. [DOI] [PubMed] [Google Scholar]
- 12.Wang X, et al. Autoantibody signatures in prostate cancer. N Engl J Med. 2005;353(12):1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 13.Dowling P, et al. Analysis of acute-phase proteins, AHSG, C3, CLI, HP and SAA, reveals distinctive expression patterns associated with breast, colorectal and lung cancer. Int J Cancer. 2012;131(4):911–923. doi: 10.1002/ijc.26462. [DOI] [PubMed] [Google Scholar]
- 14.Petrik V, et al. Serum alpha 2-HS glycoprotein predicts survival in patients with glioblastoma. Clin Chem. 2008;54(4):713–722. doi: 10.1373/clinchem.2007.096792. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, et al. Profiling the potential tumor markers of pancreatic ductal adenocarcinoma using 2D-DIGE and MALDI-TOF-MS: Up-regulation of Complement C3 and alpha-2-HS-glycoprotein. Pancreatology. 2013;13(3):290–297. doi: 10.1016/j.pan.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Aalinkeel R, et al. Gene expression of angiogenic factors correlates with metastatic potential of prostate cancer cells. Cancer Res. 2004;64(15):5311–5321. doi: 10.1158/0008-5472.CAN-2506-2. [DOI] [PubMed] [Google Scholar]
- 17.Jia HL, et al. Gene expression profiling reveals potential biomarkers of human hepatocellular carcinoma. Clin Cancer Res. 2007;13(4):1133–1139. doi: 10.1158/1078-0432.CCR-06-1025. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–226. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 19.Zhang R, Pan X, Huang Z, Weber GF, Zhang G. Osteopontin enhances the expression and activity of MMP-2 via the SDF-1/CXCR4 axis in hepatocellular carcinoma cell lines. PLoS ONE. 2011;6(8):e23831. doi: 10.1371/journal.pone.0023831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tilli TM, et al. Expression analysis of osteopontin mRNA splice variants in prostate cancer and benign prostatic hyperplasia. Exp Mol Pathol. 2012;92(1):13–19. doi: 10.1016/j.yexmp.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 21.Milne GL, Dai Q, Roberts LJ., 2nd . Biochim Biophys Acta. 2014. The isoprostanes—25 years later. , 10.1016/j.bbalip.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammirante M, Shalapour S, Kang Y, Jamieson CA, Karin M. Tissue injury and hypoxia promote malignant progression of prostate cancer by inducing CXCL13 expression in tumor myofibroblasts. Proc Natl Acad Sci USA. 2014;111(41):14776–14781. doi: 10.1073/pnas.1416498111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: An expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188(1):22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen KO. Fetuin, a new globulin isolated from serum. Nature. 1944;154:575. [Google Scholar]
- 25.Triffitt JT, Gebauer U, Ashton BA, Owen ME, Reynolds JJ. Origin of plasma alpha2HS-glycoprotein and its accumulation in bone. Nature. 1976;262(5565):226–227. doi: 10.1038/262226a0. [DOI] [PubMed] [Google Scholar]
- 26.Yang F, et al. Alpha 2-HS-glycoprotein: Expression in chondrocytes and augmentation of alkaline phosphatase and phospholipase A2 activity. Bone. 1991;12(1):7–15. doi: 10.1016/8756-3282(91)90048-n. [DOI] [PubMed] [Google Scholar]
- 27.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271(22):12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 28.Szweras M, et al. alpha 2-HS glycoprotein/fetuin, a transforming growth factor-beta/bone morphogenetic protein antagonist, regulates postnatal bone growth and remodeling. J Biol Chem. 2002;277(22):19991–19997. doi: 10.1074/jbc.M112234200. [DOI] [PubMed] [Google Scholar]
- 29.Schafer C, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112(3):357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rittenberg B, et al. Regulation of BMP-induced ectopic bone formation by Ahsg. J Orthop Res. 2005;23(3):653–662. doi: 10.1016/j.orthres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Seto J, et al. Accelerated growth plate mineralization and foreshortened proximal limb bones in fetuin-A knockout mice. PLoS One. 2012;7(10):e47338. doi: 10.1371/journal.pone.0047338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berruti A, et al. Differential patterns of bone turnover in relation to bone pain and disease extent in bone in cancer patients with skeletal metastases. Clin Chem. 1999;45(8 Pt 1):1240–1247. [PubMed] [Google Scholar]
- 33.Scott JK, Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249(4967):386–390. doi: 10.1126/science.1696028. [DOI] [PubMed] [Google Scholar]